Summary
Background and objectives
Vitamin D deficiency is highly prevalent among patients with chronic kidney disease (CKD). The benefits and harms of vitamin D supplementation (ergocalciferol or cholecalciferol) were assessed in patients with nondialysis-dependent CKD, dialysis-dependent CKD, and renal transplant recipients.
Design, setting, participants, & measurements
MEDLINE (1966 to September 2009), SCOPUS (September 2009), and nephrology conference proceedings were searched for relevant observational and randomized controlled trials (RCTs). Treatment effects were summarized as mean differences (MDs) with 95% confidence intervals (CIs) using a random effects model. Separate analyses were conducted for observational studies and RCTs.
Results
Twenty-two studies (17 observational and 5 RCTs) were included. There was a significant improvement in 25-hydroxyvitamin D (MD 24.1 ng/ml, 95% CI 19.6 to 28.6) and an associated decline in parathyroid hormone (PTH) levels (MD −41.7 pg/ml, 95% CI −55.8 to −27.7) among observational studies. PTH reduction was higher in dialysis patients. Among RCTs, there was a significant improvement in 25-hydroxyvitamin D (MD 14 ng/ml, 95% CI 5.6 to 22.4) and an associated decline in PTH levels (MD −31.5 pg/ml, 95% CI −57 to −6.1). A low incidence of hypercalcemia and hyperphosphatemia was reported with vitamin D supplementation. Cardiovascular and skeletal effects of vitamin D supplementation have not been studied. Included studies were mostly of low to moderate quality.
Conclusions
Available evidence from low-to-moderate quality observational studies and fewer RCTs suggests that vitamin D supplementation improves biochemical endpoints. However, whether such improvements translate into clinically significant outcomes is yet to be determined.
Introduction
The adequacy of body vitamin D stores is best assessed by the measurement of the serum level of 25-hydroxyvitamin D (25(OH)D). More than a billion people worldwide are estimated to be vitamin D deficient or insufficient. Although there is no consensus, most experts define levels <20 to 30 ng/ml as vitamin D deficient (1). Prevalence of vitamin D deficiency increases with extremes of age, postmenopausal state, African-American race, and presence of chronic kidney disease (CKD) (2). Studies have reported varying prevalence rates of vitamin D deficiency in CKD with 70% to 80% prevalence in some parts of the world (3,4). Vitamin D plays a central role in calcium and phosphorus homeostasis. However, 25(OH)D needs to undergo 1-α hydroxylation for it to be converted into the active form, 1,25-dihydroxyvitamin D (1,25(OH)2D). Although kidneys are the primary site for 1-α hydroxylation of vitamin D, the enzyme has been found in many extrarenal sites including the parathyroid gland (5). Thus, despite the loss of renal mass with progressive CKD, there has been a renewal in interest in studying the mineral effects of supplementation with calciferols in CKD patients with low vitamin D levels.
This interest has been amplified by studies that have demonstrated several potential nonskeletal benefits of vitamin D (6). Vitamin D deficiency is associated with albuminuria and a higher prevalence of cardiovascular disease and mortality in the Third National Health and Nutrition Examination Survey (NHANES III) cohort (7,8). Several other studies have shown an association between vitamin D deficiency and other traditional cardiovascular risk factors such as hypertension, insulin resistance, diabetes, and dyslipidemia (9,10). Finally, an association between mortality and vitamin D deficiency has been shown in dialysis- and nondialysis-dependent CKD (7,11). Because the risk of cardiovascular disease is higher in CKD than non-CKD patients, the potential benefits of vitamin D supplementation (from here on this refers to vitamin D2-ergocalciferol or D3-cholecalciferol supplementation) may be greater in CKD than non-CKD individuals (12).
In the general population, exogenous administration of vitamin D has been shown to improve bone mineral density and muscle strength, to reduce risk for fractures and falls, and to correct abnormalities related to calcium, phosphorus, and secondary hyperparathyroidism (1). A recent systematic review of vitamin D repletion in healthy individuals identified lower vitamin D status to be associated with increased risk for developing hypertension and cardiovascular disease with unclear effect of supplementation on clinical outcomes (13). Another systematic review reported that vitamin D supplements at moderate to high doses could reduce cardiovascular disease risk (14).
National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) guidelines currently recommend vitamin D supplementation in patients with stage 3 to 4 CKD with 25(OH)D levels <30 ng/ml (15). Recently released Kidney Disease Improving Global Outcome guidelines recommend using vitamin D in patients with stage 3 to 5 CKD (not on dialysis) who are vitamin D deficient and who have parathyroid hormone (PTH) levels above the normal range (16). These guidelines are predominantly based on the extrapolation of data from the general population and from observational studies in CKD. We performed a systematic review to assess the benefits and harms of vitamin D supplementation in CKD, including end-stage renal disease patients.
Materials and Methods
Study Inclusion Criteria
Randomized controlled trials (RCTs) and observational studies of vitamin D supplementation in patients with various forms of CKD (nondialysis-dependent CKD, hemodialysis, peritoneal dialysis, and renal transplant recipients) were included. Studies evaluating vitamin D preparations administered at doses available in over-the-counter preparations such as 400 or 800 IU and that assessed active vitamin D analogs (such as calcitriol) were excluded. Studies that did not report baseline vitamin D levels were also excluded.
Data Sources and Search Strategy
MEDLINE (1966 to September 2009), and SCOPUS (1980 to September 2009) were searched using optimally sensitive search strategies for relevant studies (Supplemental Appendix 1). We also reviewed reference lists of all included studies for any additional relevant studies. Abstracts presented at American Society of Nephrology, National Kidney Foundation, and World Congress of Nephrology meetings from 2003 to 2009 were searched for additional unpublished data. Studies of at least 4 weeks in duration were selected for the purposes of this systematic review. Studies were considered without language restriction.
Data Extraction
Two reviewers (P.K. and M.D.) screened the search results according to inclusion criteria. Studies that did not meet the inclusion criteria were excluded at this stage. The same reviewers independently extracted relevant data regarding study design and setting, participant characteristics, and outcome measures using a standardized data extraction form from the included studies. In circumstances in which more than one publication of one trial existed, only the latest publication with the most complete data were included. We contacted the original authors for any further information by written correspondence and any relevant information obtained was included in the review. Disagreements were resolved in consultation with an arbitrator (S.D.N.).
Outcome Measures
We considered following outcomes: (1) biochemical endpoints: change in serum (a) 25(OH)D (ng/ml), (b) intact PTH (pg/ml), (c) 1,25(OH)2D (pg/ml), (d) calcium (mg/dl), and (e) phosphorous (mg/dl) levels; (2) patient-centered endpoints: (a) cardiovascular events, (b) outcomes related to bone disease, and (c) mortality; (3) adverse effects: incidence of hypercalcemia (defined as serum calcium >10.2 mg/dl or as defined by study authors) and hyperphosphatemia (defined as serum phosphorus >4.6 mg/dl or as defined by study authors); and (4) miscellaneous outcomes as reported by authors.
Study Quality
Observational Studies.
For observational studies, the Newcastle–Ottawa scale was used to assess the study quality (17). A quality score was calculated based on three major components: (1) selection of the groups of study (0 to 4 points), (2) quality of the adjustment for confounding (0 to 2 points), and (3) ascertainment of the outcome of interest in the cohorts (0 to 3 points). The maximum score could be 9 points, representing the highest methodological quality.
RCTs.
The quality of RCTs was assessed without blinding to authorship or journal using a prespecified checklist (18). The quality items assessed were allocation concealment; intention-to-treat analysis; completeness of follow-up; and blinding of investigators, participants, and outcome assessors.
Data Analyses
The results of the individual studies were reported as mean and SD or median and range. When data were provided as median and range, we contacted authors to obtain actual mean and SD. For two studies (19,20) we converted median and range to mean and SD using appropriate formulas because we could not obtain additional details from the authors (21). For observational studies, we extracted pre- and postdata of the treated cohort, whereas the end of treatment values of the treatment and control groups from RCTs were used for the analyses. Where continuous scales of measurement were used to assess the effects of treatment (PTH, calcium, phosphorus, 25(OH)D level, 1,25(OH)2D level), results were expressed using the mean difference (MD) with 95% confidence intervals (95% CIs). For dichotomous outcomes (hypercalcemia, hyperphosphatemia, fracture rates, cardiovascular events, and mortality), we planned to report results as relative risk with 95% CI. Results were reported using a random effects model. Heterogeneity was analyzed using a χ2 test on n − 1 degrees of freedom, with α = 0.05 used for statistical significance and with the I2 test (22). We conducted separate analyses for observational studies and RCTs.
Subgroup analyses to explore possible sources of heterogeneity were also performed. These included participant-specific characteristics such as baseline PTH levels and baseline vitamin D levels, treatment-specific characteristics such as dose of vitamin D used (KDOQI recommended dose versus others), and study-specific characteristics such as duration of vitamin D use and sample size. Separate analysis for individual outcomes according to the stage of kidney disease was also performed. All analyses were undertaken in RevMan 5 (The Nordic Cochrane Centre, Copenhagen, Denmark) and Comprehensive Meta-analysis (Biostat).
Results
Search Results
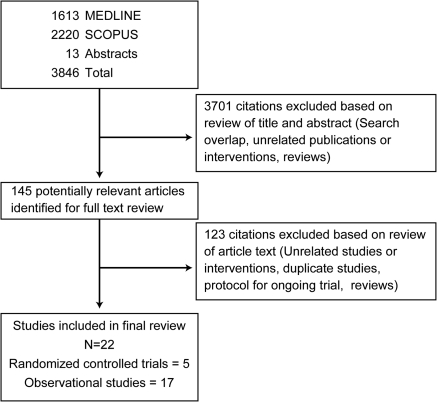
Details of flow of study identification are shown in Figure 1. The combined search of MEDLINE, SCOPUS, and nephrology conference proceedings identified 3846 citations, of which 3701 were excluded after title and abstract review. Full-text assessment of 145 potentially relevant articles identified 22 studies (5 RCTs [see references 23–27] and 17 observational studies [see references 19,20,28–42]) enrolling 1593 patients. Nine of the 11 authors who were contacted for additional data or clarification responded.
Figure 1.
Study flow diagram: Included studies and reasons for exclusion of studies.
Study Characteristics
Observational Studies.
Of the 17 observational studies, 11 studies (19,28–37) were published and 6 were unpublished studies from conference proceedings (20,38–42) evaluating 1329 patients with mean age of 61.2 ± 8.8 years. Patients with CKD stages 3 to 5 including dialysis and renal transplant recipients were identified. Baseline 25(OH)D levels ranged from <7 to 20.3 ng/ml. Dose of vitamin D ranged from 4000 to 50,000 IU daily. The average treatment and follow-up duration was 6.4 ± 4.7 months. Most studies reported outcomes on 25(OH)D, PTH, calcium, and phosphorus levels before and after supplementation. Nine studies reported data relating to 1,25(OH)2D levels. Most published studies reported the type of assay used to assess PTH and 25(OH)D levels (predominantly RIA method), with one study mentioning the degree of sensitivity used for the assay. Four studies reported using vitamin D on the basis of KDOQI guidelines but none of the studies reported the mean doses of vitamin D actually administered to the patients. Other characteristics of the included observational studies are detailed in Table 1.
Table 1.
Characteristics of observational studies included in this systematic review
| Study, Year | Country | Type of Study | Sample Size | Mean Age (years) | Stage of CKD | Baseline 25(OH)D/ PTH Level | Intervention | Study Duration (months) | Outcomes Reported |
|---|---|---|---|---|---|---|---|---|---|
| Published studies | |||||||||
| Al Aly, 2007 (28) | United States | Prospective cohort | 66 | 70 | 3 and 4 | 16.6/231 | 50,000 IU of ergocalciferol once a week for 12 weeks, then once a month for an additional 3 months. | 6 | 25(OH)D, PTH, calcium, phosphorus |
| Bagnis, 1998 (29) | Italy | Prospective cohort | 15 | 61 | 5 (PD) | 7/157 | Calcidiol (25 hydroxyvitamin D3) 100 μg/d (= 4000 IU/d) | 1 | 25(OH)D, 1,25(OH)2D calcium, phosphorus |
| Blair, 2008 (30) | United States | Retrospective | 318 | 62 | 5 (HD) | 18.35/453 | 50,000 IU of ergocalciferol once weekly | 6 | PTH, calcium, phosphorus |
| Bouchard, 2008 (31) | Canada | Prospective cohort | 27 | NR | 5 (PD) | 12.5/596 | 4440 IU ergocalciferol once weekly | 1 | 25(OH)D, 1,25(OH)2D, PTH |
| Courbebaisse, 2009 (19) | France | Prospective cohort | 94 | 46 | Transplant | 14/76 | Four oral doses of 100,000 IU of cholecalciferol once every 2 weeks from 4 to 6 months after renal transplantation (intensive phase), then every 2 months until 12 months (maintenance phase). | 12 | 25(OH)D, PTH, calcium, phosphorus |
| Deville, 2006 (32) | United States | Prospective cohort | 85 | 67 | 3 to 5 | 18/178 | 50,000 IU of ergocalciferol twice weekly for 8 weeks followed by 800 IU/day. Patients with 25(OH)D levels between 16 and 30 ng/ml (40 and 75 nmol/L) were prescribed 50,000 IU/month for 2 months, followed by 800 IU/day. Patients with 25(OH)D levels >30 ng/ml (75 nmol/L) were prescribed 800 IU/day. | 12 | 25(OH)D, PTH, calcium |
| Jean, 2009 (33) | France | Prospective cohort | 107 | 66 | 5 (HD) | 13.3/307.9 | Cholecalciferol (100,000 IU) monthly | 15 | 25(OH)D, 1,25(OH)2D, PTH, calcium, phosphorus |
| Saab, 2007 (34) | United States | Retrospective | 118 | 59 | 5 (HD) | 16.9/304.2 | Ergocalciferol (50,000 IU) monthly by the nursing staff during the HD. | 6 | 25(OH)D, PTH, calcium, phosphorus |
| Shah, 2005 (35) | United States | Prospective cohort | 23 | 47 | 5 (PD) | 6.9/302.1 | 50,000 IU once weekly | 1 | 25(OH)D, 1,25(OH)2D, PTH, calcium, phosphorus |
| Tokmak, 2008 (36) | Germany | Prospective cohort | 64 | NR | 5 (HD) | 6.9/213 | 20,000 IU cholecalciferol once weekly | 9 | 25(OH)D, PTH, calcium, phosphorus |
| Zisman, 2007 (37) | United States | Prospective cohort | 52 | 72 | 3 to 5 | 19.5/159.5 | 50,000 IU weekly for 4 weeks depending on severity of deficiency followed by 1200 IU/d | 12 | 25(OH)D, 1,25(OH)2D, PTH, calcium, phosphorus |
| Unpublished studies | |||||||||
| Balon, 2009 (38) | Slovenia | Prospective cohort | 97 | NR | HD | 11.4/280.7 | 40,000 IU of cholecalciferol monthly | 3 | 25(OH)D, PTH, calcium, phosphorus |
| Finn, 2007 (39) | United States | Retrospective | 20 | NR | CKD 3 | 15.7/184 | 50,000 IU once weekly | 3 | 25(OH)D, 1,25(OH)2D, PTH, calcium, phosphorus |
| Matias, 2009 (40) | Portugal | Prospective cohort | 158 | 62.8 | HD | 22.3/267 | Oral cholecalciferol per KDOQI guidelines | 12 | 25(OH)D, 1,25(OH)2D, PTH, calcium, phosphorus |
| Pesenson, 2007 (41) | United States | Prospective cohort | 14 | NR | Transplant | 17.7/137.2 | 50,000 IU daily | 1 | 25(OH)D, 1,25(OH)2D, PTH, calcium, phosphorus |
| Shannon, 2007 (42) | United States | Prospective cohort | 38 | NR | Nondialysis-dependent CKD | 34.43/NR | 50,000 IU weekly for 4 to 12 weeks, then monthly | NR | 25(OH)D, Epo |
| Lopes, 2009 (20) | Brazil | Prospective cohort | 33 | NR | Nondialysis-dependent CKD | 11.3/116 | 50,000 IU weekly until 25(OH)D level >30 ng/ml and then monthly | 6 | 25(OH)D, 1,25(OH)2D, PTH, calcium, phosphorus |
Vitamin D levels are shown in ng/ml and PTH levels are shown in pg/ml. HD, hemodialysis; PD, peritoneal dialysis; Epo, Epogen usage per week; NR, not reported.
RCTs.
Five RCTs (four published studies [23–26] and one unpublished study [27]) included 264 patients with a mean age of 50.7 ± 10.1 years. Patients with CKD stage 2 to 5 including dialysis and renal transplant recipients were included. Baseline 25(OH)D levels ranged from 8.5 to 24.5 ng/ml. Doses of vitamin D ranged from 20,000 IU once a week to 25,000 IU once a month. The average treatment and follow-up duration was 6.2 ± 5.4 months. Most studies reported outcomes on biochemical endpoints such as serum 25(OH)D, 1,25(OH)2D, PTH, calcium, and phosphorus levels before and after supplementation. None of the studies followed KDOQI guidelines for vitamin D repletion. Other characteristics of the included RCTs are detailed in Table 2.
Table 2.
Characteristics of RCTs included in the systematic review
| Study, Year | Country | Sample Size | Mean Age | Stage of CKD | Baseline 25(OH)D/ PTH Level (mean) | Intervention | Study Duration (months) | Outcomes Reported |
|---|---|---|---|---|---|---|---|---|
| Chandra, 2008 (24) | United States | 20 | 61 | 3 and 4 | 20/368 | 50,000 IU of cholecalciferol or placebo once weekly | 3 | 25(OH)D, 1,25(OH)2Da PTH, calcium, phosphorus |
| Dogan, 2008 (25) | Turkey | 40 | 49 | 3 to 4 | 8.5/368 | 300,000 IU cholecalciferol versus placebo in a single dose orally | 1 | 25(OH)D, PTH, calcium, phosphorus |
| Oksa, 2008 (23) | Slovakia | 87 | 66 | 2 to 4 | 19.5/68.3 | Oral cholecalciferol 5000 IU/wk (group A) or 20,000 IU/wk (group B) | 12 | 25(OH)D, 1,25(OH)2D PTH, calcium, phosphorus |
| Wissing, 2005 (26) | Belgium | 79 | 43 | Transplant | 24.5/127 | Patients were randomized to receive cholecalciferol (25,000 IU) or no treatment in addition to calcium supplementation. | 12 | 25(OH)D, 1,25(OH)2D, PTH, calcium, phosphorus |
| Siebert, 2007 (27) | Germany | 38 | NA | HD | 11.8/192.3 | Oral cholecalciferol—dose not mentioned | 3 | 25(OH)D, PTH, calcium, phosphorus |
Vitamin D levels are shown in ng/ml and PTH levels are shown in pg/ml. HD, hemodialysis; not applicable.
Data were not available.
Study Quality
Study quality was evaluated only for the full-text published studies. The quality of the observational studies varied from 3 to 6 points, with a mean of 4.6 points. Most studies were of low to moderate quality. Allocation concealment was unclear in the included RCTs, and participants, investigators, and outcome assessors were not blinded except for one study (24). None of the trials were analyzed on an intention-to-treat basis. There were no dropouts in the treatment or control groups of the RCTs.
Outcome Measures
25(OH)D Levels: Observational Studies.
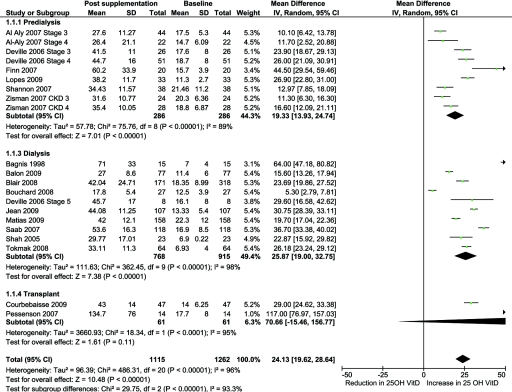
There was a significant increase in 25(OH)D levels with vitamin D supplementation in CKD (17 studies, 1115 patients, MD 24.1 ng/ml, 95% CI 19.6 to 28.6, P < 0.0001) with significant heterogeneity between the studies (χ2 = 486.31, P < 0.001; I2 = 96%) (Figure 2). The increase was higher in transplant recipients than in dialysis- and nondialysis-dependent CKD. The higher levels noted in transplant recipients were secondary to the results of the study by Pesenson et al. (41), which showed an increase in 25(OH)D levels by 117 ng/ml.
Figure 2.
Effect of vitamin D supplementation on 25(OH)D levels at the end of treatment period among observational studies in CKD.
25(OH)D Levels: RCTs.
There was a significant increase in 25(OH)D levels with vitamin D versus placebo in CKD (three studies, 71 patients, MD 13.9 ng/ml, 95% CI 5.6 to 22.4, P = 0.001) with moderate heterogeneity between the studies (χ2 = 4.96, P = 0.08, I2 = 60%). One study compared 5000 IU with 20,000 IU and revealed the higher dose to be significantly more effective (MD, 37 versus 28 ng/ml) (23). One unpublished study reported significant improvement with vitamin D supplementation but did not provide values for the placebo group (27).
PTH: Observational Studies.
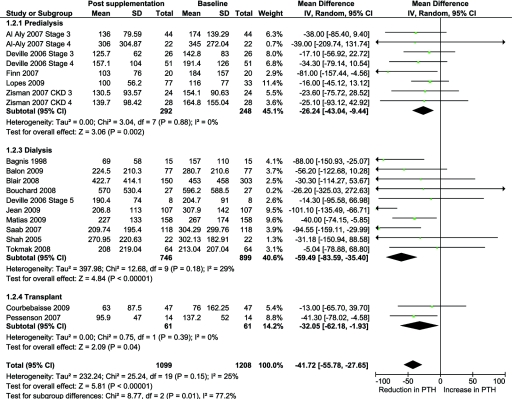
There was a significant decrease in PTH levels with vitamin D supplementation (16 studies, 1099 patients, MD −41.7 pg/ml, 95% CI −55.8 to −27.7, P < 0.00001) with mild heterogeneity between the studies (χ2 = 25.24, P = 0.15, I2 = 25%) (Figure 3). The benefit was higher in dialysis patients compared with the nondialysis-dependent CKD and transplant recipients (P = 0.01 for subgroup differences) (Figure 3).
Figure 3.
Effect of vitamin D supplementation on PTH levels at the end of treatment period among observational studies in CKD.
PTH: RCTs.
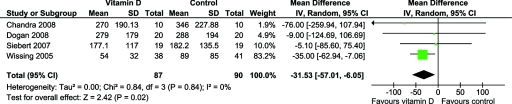
There was a significant decrease in PTH levels with vitamin D supplementation (four studies, 90 patients, MD −31.5 pg/ml, 95% CI −57.0 to −6.1, P = 0.02) with no significant heterogeneity between the studies (χ2 = 0.84, P = 0.84, I2 = 0%) (Figure 4). One study (23) compared 5000 IU with 20,000 IU and revealed a similar and significant reduction in PTH levels between both groups.
Figure 4.
Effect of vitamin D supplementation on PTH levels at the end of treatment period among RCTs in CKD.
1,25(OH)2D Levels: Observational Studies.
Overall there was a significant improvement in the levels of 1,25(OH)2D with vitamin D supplementation (nine studies, 449 patients, MD 6.9 pg/ml, 95% CI 6.0 to 7.8, P < 0.00001) with significant heterogeneity between the studies (χ2 = 121.64, P < 0.001; I2 = 93%).
1,25(OH)2D Levels: RCTs.
Although three of the five RCTs reported 1,25(OH)2D levels, data were not available for calculation from one study (24) and another study (23) compared 5000 IU with 20,000 IU precluding pooling of the data. Both of these studies reported a nonsignificant increase in 1,25(OH)2D levels. Wissing et al. (26) compared calcium supplementation and calcium and vitamin D supplementation among new transplant recipients and found significant improvements in 1,25(OH)2D levels among both groups.
Calcium Levels: Observational Studies.
There was no significant change in serum calcium levels after vitamin D supplementation (16 studies, 1071 patients, MD 0.07 mg/dl, 95% CI −0.03 to 0.17, P = 0.19).
Calcium Levels: RCTs.
There was no significant change in serum calcium levels with vitamin D supplementation (three studies, 77 patients, MD 0.23 mg/dl, 95% CI −0.31 to 0.77, P = 0.40). One study (23) compared 5000 IU with 20,000 IU vitamin D supplementation and revealed a similar and nonsignificant change in calcium levels.
Phosphorus Levels: Observational Studies.
There was no significant change in serum phosphorus levels with vitamin D supplementation (15 studies, 986 patients, MD 0.05 mg/dl, 95% CI −0.11 to 0.22, P = 0.53).
Phosphorus Levels: RCTs.
There was no significant difference in serum phosphorus levels with vitamin D supplementation (three studies, 80 patients, MD 0.15 mg/dl, 95% CI −0.19 to 0.49, P = 0.38). One study compared 5000 IU with 20,000 IU vitamin D supplementation and revealed a similar change in phosphorus levels (23). Data from one study were not available (24).
Incidence of Hypercalcemia and Hyperphosphatemia
Observational Studies.
Of the 17 observational studies, hypercalcemia and hyperphosphatemia incidence were reported in nine and six studies, respectively. Of the nine studies that reported hypercalcemia details, only three studies provided the definition of hypercalcemia, and the definitions varied among the studies. Similarly, only three of six studies that reported hyperphosphatemia provided the definition of hyperphosphatemia. There were 14 patients with hypercalcemia among 554 patients (2%) and 3 patients with hyperphosphatemia among 348 patients (0.8%) treated with vitamin D, which were corrected by withholding the vitamin D or addition of phosphate binders.
RCTs.
Of the five randomized studies, hypercalcemia and hyperphosphatemia data were reported in four and two studies, respectively. Definition of hypercalcemia was reported only in three studies and hyperphosphatemia in one study. Five patients with hypercalcemia among 148 patients (3%) and six patients with hyperphosphatemia among 85 patients (7%) treated with vitamin D were reported, all of which resolved by withholding the active vitamin D or phosphate binders.
Other Outcomes
Other outcomes reported in the included studies were hemoglobin, estimated Kt/V, normalized protein catabolic rate, urinary calcium-to-creatinine ratio, use of erythropoietin stimulating agents, albumin, bone alkaline phosphatase, urinary calcium levels, and estimated GFR (Table 3). None of the studies reported outcomes related to cardiovascular disease, bone disease, or mortality.
Table 3.
Miscellaneous outcomes reported in the included studies
| Study, Year | Outcomes Analyzed | Results |
|---|---|---|
| Bagnis, 1998 (29) | BAP, osteocalcin | Reduction in osteocalcin but not BAP |
| Courbebaisse, 2009 (19) | UCa/Cr, urinary calcium, eGFR | NS change with intervention |
| Chandra, 2008 (24) | BAP, C telopeptide, TRAP 5b | NS change with intervention |
| Dogan, 2008 (25) | Albumin, UCa/Cr, BAP, eGFR | NS change with intervention |
| Blair, 2008 (30) | Albumin, Hb, eKt/V, nPCR, SF 36 | Significant improvement in Hb, nPCR |
| Finn, 2009 (39) | Albumin, eGFR | NS change with intervention |
| Jean, 2009 (33) | Albumin, Hb, eKt/V, nPCR, ESA requirement | NS change with intervention |
| Lopes, 2009 (20) | BAP, urinary calcium, FGF23, | Significant reduction in BAP and increase in Urinary calcium |
| Mathias, 2009 (40) | ESA requirement | NS change with intervention |
| Saab, 2007 (34) | ESA requirement | NS change with intervention |
| Oksa, 2008 (23) | Urinary calcium | NS change with intervention |
| Wissing, 2005 (26) | Urinary calcium | NS change with intervention |
| Zisman, 2007 (37) | eGFR | NS change with intervention |
BAP, bone alkaline phosphatase; UCa/Cr, urinary calcium-to-creatinine ratio; eGFR, estimated GFR; eKtV, estimated KtV; TRAP 5B, tartarate-resistant acid phosphatase isoform 5 b; Hb, hemoglobin; nPCR, protein catabolic rate; SF 36, Standard Form 36 questionnaire; ESA, erythropoietin stimulating agents; NS, not significant; FGF23, fibroblast growth factor-23.
Sensitivity Analyses
We explored the reasons for heterogeneity noted in the analysis of 25(OH)D and PTH levels. Some covariates such as baseline vitamin D level and dose of vitamin D used were significant effect modifiers and accounted for the heterogeneity noted in the 25(OH)D analysis (Table 4). We also reported the results for different stages of kidney disease wherever data were available (Figure 2 and 3). Blair et al. reported approximately 50% lost to follow-up and therefore had a different number of patients at baseline and again at follow-up. Exclusion of this study from all of the analyses (of observational studies) did not alter the results (30).
Table 4.
Subgroup analyses to explore the reasons for heterogeneity in observational studies
| Variable | 25(OH)D Level |
PTH Level |
||
|---|---|---|---|---|
| MD (95% CI); n Trials or Comparisons | P | MD (95% CI); n Trials or Comparisons | P | |
| Baseline vitamin D level | ||||
| <10 ng/ml | 34.23 (20.19 to 47.55); 3 | 0.008 | −46.93 (−102.71 to 8.84); 3 | 0.81 |
| 10 to 20 ng/ml | 25.43 (18.98 to 31.89); 12 | −46.12 (−66.28 to −25.96); 12 | ||
| 20 to 30 ng/ml | 14.98 (9.20 to 20.75); 3 | −35.07 (−63.63 to −6.51); 2 | ||
| Baseline PTH level | ||||
| <100 pg/ml | 29.00 (24.61 to 33.38); 1 | 0.14 | −13.00 (−65.70 to −39.70); 1 | 0.17 |
| 101 to 200 pg/ml | 32.32 (23.07 to 41.57); 7 | −33.37 (−49.73 to −17.01); 7 | ||
| >200 pg/ml | 21.27 (14.65 to 27.89); 9 | −56.97 (−81.45 to −32.49); 9 | ||
| Study duration | ||||
| <3 months | 32.05 (21.60 to 42.49); 7 | 0.22 | −47.49 (−70.46 to −24.52); 7 | 0.90 |
| 3 to 6 months | 22.29 (13.19 to 31.40); 5 | −38.49 (−72.92 to −4.07); 4 | ||
| >6 months | 21.94 (16.63 to 27.26); 6 | −42.06 (−71.91 to −12.21); 6 | ||
| Vitamin D dose | ||||
| KDOQI recommended | 18.57 (13.24 to 23.90); 4 | 0.04 | −31.07 (−51.13 to −11.00); 4 | 0.19 |
| others | 26.99 (20.89 to 33.09); 14 | −50.50 (−71.87 to −29.12); 13 | ||
| Number of study participants | ||||
| <50 | 27.11 (18.08 to 36.13); 9 | 0.31 | −31.76 (−47.41 to −16.12); 8 | 0.09 |
| 50 to 100 | 21.04 (14.85 to 27.23); 5 | −69.71 (−110.76 to −38.67); 7 | ||
| >100 | 27.67 (20.67 to 34.67); 4 | −32.05 (−62.17 to −1.92); 2 | ||
Discussion
We performed a systematic review of the available literature on the effects of vitamin D supplementation at therapeutic doses on biochemical endpoints in patients with vitamin D deficiency and varying degrees of CKD. Our analysis revealed an improvement in biochemical endpoints with a significant increase in serum 25(OH)D and an associated significant decline in PTH levels. These effects were similar for observational and randomized studies in the direction of the effect but were different in magnitude, with RCTs showing smaller effects. In observational studies, the decline in PTH levels was higher in the dialysis patients than in the nondialysis-dependent CKD patients and renal transplant recipients. There was no effect on serum calcium and phosphorus levels, and no significant increase in hypercalcemia and hyperphosphatemia was noted. However, none of the available studies explored the effect of vitamin D supplementation on bone mineral density, fracture risk, cardiovascular disease, and mortality in CKD.
As expected, we noted an improvement in biochemical endpoints across all stages of CKD. Derangements in mineral metabolism increase steadily with decline in kidney function (43,44). A secondary analysis of NHANES that included over 19,000 patients revealed increasing prevalence of 25(OH)D deficiency (<15 ng/ml) from 9% to 14% in CKD stages 1 to 3 to 27% in CKD stages 4 to 5 (45). However, U.S. dialysis patients have a much higher prevalence rate of 79% (4). Therefore, whether similar graded improvement in biochemical endpoints with vitamin D supplementation occurs will be of interest to clinicians. In our pooled analyses of observational studies, we found a mean increase in vitamin D level of 24 ng/ml along with a decline of 41 pg/ml in PTH level. Although no graded increase in the improvement from CKD stage 3 to 4 occurred (data not shown), in observational studies the CKD stage 5 group (on dialysis) had the most benefit with vitamin supplementation that resulted in a decline in PTH of 59 pg/ml. This additional benefit noted in dialysis is of interest because calciferols might be expected to provide less benefit in dialysis secondary to the higher fibroblast growth factor 23 (FGF23) levels and lower functioning renal mass.
Elevated FGF23 levels are associated with decreased 1,25(OH)2D levels (46) because of their suppressive effect on 1-α hydroxylase. Elevated FG23 levels may also increase the degradation of 25(OH)D by increasing the activity of 25-hydroxyvitamin D-24-hydroxylase (47). Transplant recipients may constitute a special subset of CKD patients because FGF23 levels drop in the posttransplant setting because of restoration of kidney function (48). This might also explain the greater increase in 25(OH)D levels noted in this review of transplant recipients apart from the influence of differences in the study design (dose, frequency, etc.). However, this interaction between FGF23 and calciferols needs to be studied in future clinical trials because it is unclear how much of this benefit noted in these observational studies might have been influenced by other strategies used to treat secondary hyperparathyroidism.
Recently, Palmer et al. pooled data from 16 RCTs of active vitamin D analogs among CKD patients not on dialysis and 60 RCTs among CKD patients on dialysis (49,50). In each of these subgroups, authors reported a reduction in PTH levels by 49.34 pg/ml (95% CI −85.70 to −12.97) and 196.05 pg/ml (95% CI −298.43 to −93.66), respectively. Our pooled analysis of RCTs showed a net decrease of 31 pg/ml in PTH levels—whether the benefit of vitamin D repletion would be additive to that obtained with active vitamin D is unclear. A recent RCT comparing active vitamin D analog to vitamin D supplementation showed no difference in PTH reduction between the treatment groups (51). We could not explore the benefits and risks of sequential versus combined treatment with both forms of vitamin D therapy in this review.
Lower vitamin D levels are associated with incident cardiovascular disease and mortality in the general population (13). A recent meta-analysis that included observational and randomized studies of vitamin D supplementation concluded that on the basis of limited evidence, vitamin D supplements at moderate to high doses may reduce cardiovascular risk (14). However, this analysis included studies that assessed active vitamin D analog and calciferols in dialysis and the general population. In analysis restricted to RCTs, no benefit was noted with vitamin D supplementation in the general population. Low serum 25(OH)D concentrations are associated with a higher risk for hip fractures (52). However, the effects of vitamin D on vertebral and hip fractures varied with dose, type of agent, and study population in people without kidney disease (53). We did not identify any study that assessed whether vitamin D supplementation improved bone and cardiovascular outcomes in CKD. This is critical because targeting surrogate endpoints does not always translate into an improvement in long-term outcomes such as cardiovascular disease and mortality in patients with CKD (54).
Associations between low 25(OH)D level and albuminuria, a known cardiovascular risk factor, have been shown in recent studies (8,55). Intervention studies using active vitamin D analogs have shown reduction in proteinuria among CKD patients (56,57). It is plausible that potential antiproteinuric benefits might exist with vitamin D supplementation. We did not find any evidence in this review to support this notion, although this may be due to the lack of studies. Included studies also assessed other surrogate endpoints (Table 4), but the data were too sparse to make any meaningful conclusions.
Our analysis has specific strengths and few weaknesses. This analysis is based on an exhaustive and systematic search of medical databases, including abstract presentations at major conferences, data extraction, analysis, and trial quality assessment by two independent reviewers. We included observational studies and RCTs to compare and contrast the benefits seen in these studies. Our study is limited by a lack on information on long-term efficacy, effects on patient-centered outcomes, and low quality of available studies. These limitations are apart from the inherent biases associated with observational studies. Furthermore, we noted significant heterogeneity in some analyses. We conducted subgroup analyses to assess the reasons for heterogeneity and identified covariates that might be considered as potential effect modifiers for designing future trials. We also noted wider CIs for these biochemical endpoints that also suggest the need for adequately powered trials.
Vitamin D deficiency is more prevalent in African Americans than Caucasians and in women than in men. However, further analysis was not possible to assess any differential effects of vitamin D in these populations because of limited data. Definition of hypercalcemia and hyperphosphatemia were not reported in all of the studies, and the reported definitions were heterogeneous. Concomitant use of active vitamin D products can lead to increase in serum 1,25(OH)2D levels. None of the observational studies reported the use of active vitamin D products as a part of their protocol. However nonprotocol-based use was reported in two studies only in relation to hypercalcemia (34,36). We cannot confirm lack of their effect on 1,25(OH)2D or PTH levels among studies that did not explicitly prohibit their usage. Furthermore, the differences in food fortification with vitamin D might have also influenced these results. We could not rule out publication bias on the basis of the funnel plots of the 25(OH)D and PTH analysis in this review, limiting the interpretation of these results.
Current clinical guidelines recommend reduction of PTH levels using calciferols and active vitamin D analogs with different target ranges for different stages of CKD. Vitamin D supplementation appears to improve biochemical endpoints similar to active vitamin D analogs, albeit at a smaller magnitude. Given the lower burden of costs and side effects, physicians may replenish vitamin D in the CKD population with vitamin D deficiency until further trials become available. However, measurement of serum 25(OH)D level is expensive, and there is a strong need for larger RCTs with emphasis on patient-level outcomes such as cardiovascular events, mortality, and bone outcomes using vitamin D compounds. Future studies should also identify the appropriate dose of vitamin D, target range for vitamin D level, frequency, route of administration, and its safety in various stages of CKD. Furthermore, whether combined treatment with native and active vitamin D is a better option than sequential treatment also needs exploration.
In conclusion, vitamin D supplementation appears to improve 25(OH)D and 1,25(OH)2D levels while reducing PTH levels without increasing the risk for hypercalcemia and hyperphosphatemia. However, whether such supplementation translates into better cardiovascular and skeletal outcomes needs to be evaluated in future studies.
Disclosures
S.D.N. is supported by the National Institutes of Health, the National Center for Research Resources, Multidisciplinary Clinical Research Career Development program grant RR024990. R.M. has received grant support from Amgen, Shire, and Genzyme and has served as an ad hoc consultant for Shire and Mitsubishi.
Acknowledgments
We thank Marian Simonson of the Cleveland Clinic Lerner School of Medicine who helped us in developing and conducting the search. We also thank the authors of the included studies—Drs. Al Aly, Josee Bouchard, Guillaume Jean, Patricia Matias, Adrián Oksa, Beth Piraino, Ivo Quack, Vin Tangpricha, and Micah L. Thorp—who provided additional details to conduct these analyses.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at www.cjasn.org.
References
- 1. Holick MF: Vitamin D deficiency. N Engl J Med 357: 266–281, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Ginde AA, Liu MC, Camargo CA, Jr: Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med 169: 626–632, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, Graves KL, Moe SM: Prevalence of calcidiol deficiency in CKD: A cross-sectional study across latitudes in the United States. Am J Kidney Dis 45: 1026–1033, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bhan I, Burnett-Bowie SA, Ye J, Tonelli M, Thadhani R: Clinical measures identify vitamin D Deficiency in dialysis. Clin J Am Soc Nephrol 5: 460–467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dusso A, Lopez-Hilker S, Rapp N, Slatopolsky E: Extrarenal production of calcitriol in chronic renal failure. Kidney Int 34: 368–375, 1988 [DOI] [PubMed] [Google Scholar]
- 6. Artaza JN, Mehrotra R, Norris KC: Vitamin D and cardiovascular system. Clin J Am Soc Nephrol 4: 1515–1522, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Mehrotra R, Kermah DA, Salusky IB: Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int 76: 977–983, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Boer I, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS: 25(OH)D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 50: 69–77, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC: Plasma 25(OH)D levels and risk of incident hypertension. Hypertension 49: 1063–1069, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE, Hu FB: Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 29: 650–656, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Jr, Tonelli M, Thadhani R: Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 72: 1004–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Kilickesmez KO, Abaci O, Okcun B, Kocas C, Baskurt M, Arat A, Ersanli M, Gurmen T: Chronic kidney disease as a predictor of coronary lesion morphology. Angiology 61: 344–349, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM: Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med 152: 307–314, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang L, Manson JE, Song Y, Sesso HD: Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med 152: 315–323, 2010 [DOI] [PubMed] [Google Scholar]
- 15. NKF/KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. Available at: http://www.kidney.org/professionals/KDOQI/guidelines_bone/index.htm Accessed September 3, 2010
- 16. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int 76: S50–S99, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Wells GA, Shea B, O'Connell D, Petersen J, Welch V, Losos M, Tugwell P: The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Department of Epidemiology and Community Medicine, University of Ottawa, Canada: Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm Accessed September 3, 2010 [Google Scholar]
- 18. Schulz KF, Chalmers I, Hayes RJ, Altman DG: Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273: 408–412, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Courbebaisse M, Thervet E, Souberbielle JC, Zuber J, Eladari D, Martinez F, Mamzer-Bruneel MF, Urena P, Legendre C, Friedlander G, Prié D: Effects of vitamin D supplementation on the calcium-phosphate balance in renal transplant patients. Kidney Int 75: 646–651, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Lopes MG, Pillar R, Rocha L, Canziani EM, Carvalho A, Draibe S, Cuppari L: Effect of cholecalciferol supplementation on mineral metabolism parameters in CKD patients with vitamin D deficiency [Abstract]. J Am Soc Nephrol 20: 262A, 2009 [Google Scholar]
- 21. Hozo SP, Djulbegovic B, Hozo I: Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5: 13, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JP, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ 327: 557–560, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oksa A, Spustová V, Krivosíková Z, Gazdíková K, Fedelesová V, Lajdová I, Stefíková K, Bernasovská G, Zilinská Z, Dzúrik R: Effects of long-term cholecalciferol supplementation on mineral metabolism and calciotropic hormones in chronic kidney disease. Kidney Blood Press Res 31: 322–329, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Chandra P, Binongo JN, Ziegler TR, Schlanger LE, Wang W, Someren JT, Tangpricha V: Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: A randomized controlled pilot study. Endocr Pract 14: 10–17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dogan E, Erkoc R, Sayarlioglu H, Soyoral Y, Dulger H: Effect of depot oral cholecalciferol treatment on secondary hyperparathyroidism in stage 3 and stage 4 chronic kidney diseases patients. Ren Fail 30: 407–410, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Wissing KM, Broeders N, Moreno-Reyes R, Gervy C, Stallenberg B, Abramowicz D: A controlled study of vitamin D3 to prevent bone loss in renal-transplant patients receiving low doses of steroids. Transplantation 79: 108–115, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Seibert E, Ulrich C, Seiler S, Heine GH, Kohler H, Girndt M: De novo cholecalciferol substitution in HD patients: A randomized, double-blind, placebo-controlled clinical trial [Abstract]. J Am Soc Nephrol 18: 727A, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Al-Aly Z, Qazi RA, González EA, Zeringue A, Martin KJ: Changes in serum 25(OH)D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis 50: 59–68, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Bagnis C, Dutto F, Gabella P, Vitale C, D'Ella P, Marangella M, Ramello A: Biochemical and hormonal short term effects of 25 hydroxyvitamin D3 in patients on continuous peritoneal dialysis. Ital J Min Electrol Metabol 12: 73–76, 1998 [Google Scholar]
- 30. Blair D, Byham-Gray L, Lewis E, McCaffrey S: Prevalence of vitamin D [25(OH)D] deficiency and effects of supplementation with ergocalciferol (vitamin D2) in stage 5 chronic kidney disease patients. J Ren Nutr 18: 375–382, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Bouchard J, Ouimet D, Vallée M, Leblanc M, Pichette V: Effect of vitamin D supplementation on calcidiol and parathyroid hormone levels. Perit Dial Int 28: 565, 2008 [PubMed] [Google Scholar]
- 32. DeVille J, Thorp ML, Tobin L, Gray E, Johnson ES, Smith DH: Effect of ergocalciferol supplementation on serum parathyroid hormone and serum 25(OH)D in chronic kidney disease. Nephrology (Carlton) 11: 555–559, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Jean G, Souberbielle JC, Chazot C: Monthly cholecalciferol administration in haemodialysis patients: A simple and efficient strategy for vitamin D supplementation. Nephrol Dial Transplant 24: 3799–3805, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Saab G, Young DO, Gincherman Y, Giles K, Norwood K, Coyne DW: Prevalence of vitamin D deficiency and the safety and effectiveness of monthly ergocalciferol in hemodialysis patients. Nephron Clin Pract 105: 132–138, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Shah N, Bernardini J, Piraino B: Prevalence and correction of 25(OH) vitamin D deficiency in peritoneal dialysis patients. Perit Dial Int 25: 362–366, 2005 [PubMed] [Google Scholar]
- 36. Tokmak F, Quack I, Schieren G, Sellin L, Rattensperger D, Holland-Letz T, Weiner SM, Rump LC: High-dose cholecalciferol to correct vitamin D deficiency in haemodialysis patients. Nephrol Dial Transplant 23: 4016–4020, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Zisman AL, Hristova M, Ho LT, Sprague SM: Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol 27: 36–43, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Balon BP, Jakopin E, Bevc S, Knehtl M, Gorenjak M: High-dose cholecalciferol supplementation for vitamin D deficiency in haemodialysis patients [Abstract]. World Congress of Nephrology, 2009. Available at:http://www.abstracts2view.com/wcn/view.php?nu=WCN09L_1185 Accessed September 3, 2010
- 39. Finn WF: Effect of ergocalciferol on calcium and 1,25-dihydroxyvitamn D levels in chronic kidney disease patients [Abstract]. J Am Soc Nephrol 18: 529A–530A, 2007 [Google Scholar]
- 40. Matias P, Jorge C, Ferreira C: Cholecalciferol supplementation in hemodialysis patients: Effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol 5: 905–911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pesenson A, Leca N, Szpiro AA, Kendrick EA, Davis CL: Effect of vitamin D supplementation with ergocalciferol in kidney transplantation [Abstract]. J Am Soc Nephrol 18: 31A, 2007 [Google Scholar]
- 42. Shannon YM, Khambati N, Ho LT: Effects of ergocalciferol on ESA requirements in nondialysis CKD patients [Abstract]. J Am Soc Nephrol 18: 938A, 2007 [Google Scholar]
- 43. Kestenbaum B, Belozeroff V: Mineral metabolism disturbances in patients with chronic kidney disease. Eur J Clin Invest 37: 607–622, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Gutiérrez OM, Isakova T, Andress DL, Levin A, Wolf M: Prevalence and severity of disordered mineral metabolism in blacks with chronic kidney disease. Kidney Int 73: 956–962, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Mehrotra R, Kermah D, Budoff M, Salusky IB, Mao SS, Gao YL, Takasu J, Adler S, Norris K: Hypovitaminosis D in chronic kidney disease. Clin J Am Soc Nephrol 3: 1144–1151, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M: Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T: FGF23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Met 19: 429–435, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Bhan I, Shah A, Holmes J, Isakova T, Gutierrez O, Burnett SM, Jüppner H, Wolf M: Posttransplant hypophosphatemia: Tertiary hyper-phosphotinism? Kidney Int 70: 1486–1494, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF: Vitamin D compounds for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev 4: CD008175, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF: Vitamin D compounds for people with chronic kidney disease requiring dialysis. Cochrane Database Syst Rev 4: CD005633, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Moe SM, Saifullah A, LaClair RE, Usman SA, Yu Z: A randomized trial of cholecalciferol versus doxercalciferol for lowering parathyroid hormone in chronic kidney disease. Clin J Am Soc Nephrol 5: 299–306, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cauley JA, Lacroix AZ, Wu L, Horwitz M, Danielson ME, Bauer DC, Lee JS, Jackson RD, Robbins JA, Wu C, Stanczyk FZ, LeBoff MS, Wactawski-Wende J, Sarto G, Ockene J, Cummings SR: Serum 25(OH)D concentrations and risk for hip fractures. Ann Intern Med 149: 242–250, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. MacLean C, Newberry S, Maglione M, McMahon M, Ranganath V, Suttorp M, Mojica W, Timmer M, Alexander A, McNamara M, Desai SB, Zhou A, Chen S, Carter J, Tringale C, Valentine D, Johnsen B, Grossman J: Systematic review: Comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med 148: 197–213, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R; TREAT Investigators A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 55. Holden RM, Morton AR, Garland JS, Pavlov A, Day AG, Booth SL: Vitamins K and D status in stages 3 to 5 chronic kidney disease. Clin J Am Soc Nephrol 5: 590–597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fishbane S, Chittineni H, Packman M, Dutka P, Ali N, Durie N: Oral paricalcitol in the treatment of patients with CKD and proteinuria: A randomized trial. Am J Kidney Dis 54: 647–665, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Agarwal R: Vitamin D, proteinuria, diabetic nephropathy, and progression of CKD. Clin J Am Soc Nephrol 4: 1523–1528, 2009 [DOI] [PubMed] [Google Scholar]