Summary
Background and objectives
Most deaths in autosomal dominant polycystic kidney disease (ADPKD) are attributable to cardiovascular disease (CVD). We examined novel CVD biomarkers in different stages of ADPKD.
Design, setting, participants, & measurements
We recruited 50 hypertensive subjects with ADPKD with estimated GFR (eGFR) of >60 ml/min per 1.73 m2; 52 hypertensive subjects with ADPKD with eGFR of 25 to 60 ml/min per 1.73 m2; 42 normotensive subjects with ADPKD and eGFR of >60 ml/min per 1.73 m2; and 50 healthy controls. We assayed serum C-reactive protein and IL-6 as markers of inflammation; plasma 8-epi-prostaglandin F2α (8-epi-PGF2α) and superoxide dismutase (SOD) as markers of oxidative stress; and homeostasis model assessment (HOMA) as a measure of insulin resistance.
Results
The hypertensive ADPKD eGFR of 25 to 60 group had higher levels of C-reactive protein and IL-6 than controls, normotensive ADPKD with eGFR of >60, and hypertensive ADPKD with eGFR of >60. The normotensive ADPKD eGFR >60, hypertensive ADPKD eGFR >60, and hypertensive ADPKD eGFR 25 to 60 groups had higher 8-epi-PGF2α and lower SOD than controls, with no difference between the ADPKD groups. There was no difference in HOMA levels between any of the groups. Adjustment for age, race, gender, and body mass index did not alter these relationships.
Conclusions
Inflammation and oxidative stress are evident early in ADPKD even with preserved kidney function. Inflammation exhibits a graded relationship with levels of kidney function, whereas oxidative stress demonstrates a threshold effect. These pathways may be therapeutic targets for CVD risk mitigation.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common life-threatening genetic disease, affecting more than 600,000 Americans. The major outcomes in this patient population are progression to kidney failure and cardiovascular disease. Cardiovascular disease is the leading cause of premature mortality in patients with ADPKD, with over 80% of deaths attributable to coronary artery disease (1,2).
Hypertension is an early event in the natural history of ADPKD and precedes the development of reduced kidney function. It is present in approximately half of all patients aged 24 to 30 years and in all patients who reach kidney failure (3). The high prevalence of hypertension may contribute to the excess risk of cardiovascular disease in patients with ADPKD (4–6). In addition, experimental data from studies in animal models suggest that processes such as inflammation, oxidative stress, and insulin resistance may be involved in the pathogenesis and progression of ADPKD (7,8). These novel risk factors may directly contribute to the vascular pathology seen in these patients or may be pathway factors via which hypertension leads to kidney disease progression and cardiovascular disease (9–11). The objective of this study was to characterize inflammation, oxidative stress, and insulin resistance in different stages of APDKD.
Materials and Methods
Study Population
The ongoing Halt Progression of Kidney Disease (HALT-PKD) trial consists of two concurrent randomized clinical trials designed to evaluate the effects of renin-angiotensin-aldosterone suppression on the progression of ADPKD (12). Study A recruits patients with an estimated GFR (eGFR) of >60 ml/min per 1.73 m2, and Study B recruits patients with an eGFR of 25 to 60 ml/min per 1.73 m2. All of the participants had hypertension or high normal BP defined as three separate readings of systolic BP of >130 mmHg and/or a diastolic BP of >80 mmHg over the past year or current use of antihypertensive medications. Age limits were 15 to 49 years for Study A and 18 to 64 years for Study B. Major exclusion criteria for both studies include renal vascular disease, diabetes, and history of severe heart failure.
We recruited 43 Study A and 35 Study B subjects from the Tufts Medical Center site of the HALT-PKD trial. We also recruited 24 subjects (seven with eGFR >60 ml/min per 1.73 m2 and 17 with eGFR 25 to 60 ml/min per 1.73 m2) who were screened but ineligible for the HALT-PKD trial due to age restrictions or inability to tolerate study medications. Our response rate for subjects from the HALT-PKD study was 100% with every eligible person agreeing to participate in the ancillary study. In addition, we recruited 50 healthy volunteers (using flyers and advertisements) and 42 subjects with ADPKD who had preserved kidney function (eGFR of >60 ml/min per 1.73 m2) and normal BP (defined as BP of <135/85 mmHg during the 6 months preceding study entry and not on antihypertensive medications). Our four study groups were: healthy controls, normotensive ADPKD eGFR >60 ml/min per 1.73 m2, hypertensive ADPKD eGFR >60 ml/min per 1.73 m2, and hypertensive ADPKD eGFR 25 to 60 ml/min per 1.73 m2.
We estimated GFR from serum creatinine using the four-variable MDRD equation (13). A trained nurse assessed BP using an automated oscillometric monitor (Dinamap DPC120X-EN) following standard guidelines. Measurements were made with patients in a seated position after 5 to 10 minutes of quiet rest.
Measurement of Biomarkers
Fasting blood draws were obtained and stored at −70°C for subsequent batch analysis. The HALT-PKD participants had a 2- to 4-week drug washout period before the study visit, during which time BP was controlled using labetolol or clonidine. Non-HALT-PKD subjects withheld medications for 12 hours before the study visit.
High sensitivity C-reactive protein (CRP) and IL-6 were measured in serum using ELISA kits from R&D Systems Inc (Minneapolis, MN). Plasma 8-epi-prostaglandin F2α (8-epi-PGF2α) and superoxide dismutase (SOD) were assayed by Cayman Chemicals (Ann Arbor, MI). 8-epi-PGF2α was measured using an enzyme immunoassay kit. The SOD assay utilizes a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine and measures all three types of SOD (copper/zinc, manganese, and FeSOD). The intra- and interassay coefficients of variation were 12.6 and 10.5% for the 8-epi-PGF2α assay and 3.7 and 3.2% for the SOD assay. Insulin was measured with an automated immunochemiluminometric assay (ADVIA Centaur insulin assay from Bayer Diagnostics, Pittsburgh, PA). Insulin resistance (IR) at baseline was estimated using the homeostasis model assessment (HOMA-IR) method using the formula: HOMA-IR (mmol/L × μIU/ml) = fasting glucose (mmol/L) × fasting insulin (μIU/ml)/22.5 (14).
Statistical Analyses
We present summary statistics according to study group (healthy controls, normotensive ADPKD eGFR >60 ml/min per 1.73 m2, hypertensive ADPKD eGFR >60 ml/min per 1.73 m2, and hypertensive ADPKD eGFR 25 to 60 ml/min per 1.73 m2) as percentages for categorical data, means (± SD) for approximately normally distributed continuous variables, and medians (interquartile range) for skewed continuous variables. We tested for differences in baseline characteristics between the groups using the chi-squared test, one-way ANOVA, and the Kruskall-Wallis test as appropriate.
All of the biomarkers were log transformed for analyses because they had skewed distributions. We performed a one-way ANOVA test to compare differences in biomarker levels between study groups with the Bonferroni method of post hoc comparisons. The Bonferroni method of multiple post hoc comparisons was also used in a general linear model ANOVA procedure to compare the means of biomarkers with adjustment for age, race, gender, and body mass index.
We tested for a linear trend in levels of biomarkers between the study groups using the linear by linear association chi-squared test statistic. We examined univariate correlations of biomarkers with eGFR using Spearman's rank correlations. We used SPSS (Chicago, IL) version 14.0 for all analyses.
Sensitivity Analyses
We repeated the analyses combining the two ADPKD groups with preserved kidney function (normotensive ADPKD eGFR >60 ml/min per 1.73 m2 and the hypertensive ADPKD eGFR >60 ml/min per 1.73 m2 groups) so as to maximize statistical power to evaluate whether ADPKD with preserved GFR was associated with differences in levels of biomarkers in comparison with controls.
To assess the consistency of our results, we also repeated the analyses after excluding the 24 non-HALT participants. These participants were slightly different in that they withheld anti-hypertensive medications for 12 hours before the blood draw as opposed to the HALT subjects, who had a 2- to 4-week drug washout period.
Results
Baseline Characteristics of the Study Cohort by Study Group
Subjects in the hypertensive ADPKD eGFR 25 to 60 ml/min per 1.73 m2 group were older, with the other three groups having similar age distributions (Table 1). Patients with APDKD were more likely to be white than the healthy control group. Gender distribution was similar between healthy controls and the hypertensive ADPKD eGFR >60 ml/min per 1.73 m2 and the hypertensive ADPKD eGFR 25 to 60 ml/min per 1.73 m2 groups; however, the normotensive ADPKD eGFR >60 ml/min per 1.73 m2 subjects were predominantly women. Mean body mass index was higher in the hypertensive ADPKD eGFR 25 to 60 ml/min per 1.73 m2 group compared with the three other study groups.
Table 1.
Baseline characteristics by study group
| Healthy Control (n = 51) | ADPKD |
Overall P | |||
|---|---|---|---|---|---|
| eGFR >60 No HTN (n = 42)a | eGFR >60 and HTN (n = 50) | eGFR 25 to 60 and HTN (n = 52) | |||
| Age (years) | 37 ± 11 | 36 ± 10 | 39 ± 10 | 49 ± 8 | <0.001 |
| Male (%) | 45 | 17 | 46 | 44 | 0.01 |
| White (%) | 61 | 83 | 96 | 92 | <0.001 |
| Smoking (%) | 8 | 14 | 10 | 4 | 0.34 |
| Total cholesterol (mg/dl) | 185.7 ± 34.2 | 187.4 ± 30.4 | 196.2 ± 34.6 | 194.0 ± 40.8 | 0.31 |
| Body mass index (kg/m2) | 24.6 ± 4.6 | 24.5 ± 4.3 | 25.8 ± 3.9 | 27.5 ± 4.7 | 0.02 |
| Systolic BP (mmHg) | 118.2 ± 15.5 | 121.0 ± 13.9 | 137.4 ± 14.6 | 132.3 ± 17.5 | <0.001 |
| Diastolic BP (mmHg) | 70.2 ± 8.9 | 74.3 ± 9.0 | 80.7 ± 11.5 | 78.5 ± 9.6 | <0.001 |
| eGFR (ml/min per 1.73 m2) | 101.5 ± 11.5 | 91.7 ± 20.7 | 88.6 ± 16.8 | 40.8 ± 13.2 | <0.001 |
| Fasting glucose (mg/dl) | 84.5 ± 10.9 | 81.6 ± 7.9 | 81.9 ± 6.3 | 84.0 ± 8.7 | 0.11 |
The data are the means ± SD.
Autosomal dominant polycystic kidney disease with glomerular filtration rate >60 ml/min per 1.73 m2 and blood pressure <135/85 mmHg.
Because the groups were chosen on the basis of BP and eGFR, they differed significantly in these variables. However, the mean systolic/diastolic BP were 137/81 mmHg in the hypertensive ADPKD eGFR >60 ml/min per 1.73 m2 group and 132/79 mmHg in the hypertensive ADPKD eGFR 25 to 60 ml/min per 1.73 m2 group, suggesting that BP was well controlled in these patients and did not increase significantly during the 2-week drug washout period. None of the healthy controls and none of the ADPKD eGFR >60 ml/min per 1.73 m2 subjects were on any medications. In the hypertensive ADPKD eGFR >60 ml/min per 1.73 m2 group, 18% were on beta blockers, 8% were on calcium channel blockers, 62% were on angiotensin converting enzyme (ACE) inhibitors, 20% were on angiotensin receptor blockers, 14% were on diuretics, 4% were on statins, and none were on aspirin. In the hypertensive ADPKD eGFR 25 to 60 ml/min per 1.73 m2 group, 33% were on beta blockers, 21% were on calcium channel blockers, 58% were on ACE inhibitors, 29% were on angiotensin receptor blockers, 31% were on diuretics, 19% were on statins, and 13% were on aspirin.
Differences in Biomarker Levels by Study Groups
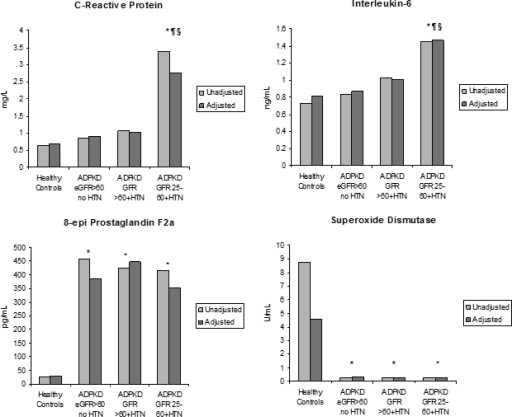
In unadjusted analyses, levels of CRP and IL-6 were significantly higher in the hypertensive ADPKD eGFR 25 to 60 ml/min per 1.73 m2 group compared with healthy controls, the normotensive ADPKD eGFR >60 ml/min per 1.73 m2 group, and the hypertensive ADPKD eGFR >60 ml/min per 1.73 m2 group (Figure 1). There was no difference, however, between the normotensive ADPKD eGFR >60 ml/min per 1.73 m2 group and the hypertensive ADPKD eGFR >60 ml/min per 1.73 m2 group in comparison with controls. For markers of oxidative stress, the levels of 8-epi-PGF2α were higher, and SOD was lower in each of the ADPKD groups compared with controls with no difference between the ADPKD groups.
Figure 1.
Unadjusted and adjusted means of biomarkers by the four study groups. The values are adjusted for age, race, gender, and body mass index. *P < 0.05 compared with control; §P < 0.05 to normotensive ADPKD eGFR >60; ¶P < 0.05 compared with hypertensive ADPKD eGFR >60.
The insulin levels were 5, 7, 6, and 7 μIU/ml, and HOMA levels were 1.14, 1.21, 1.15, and 1.20 in the four study groups (healthy controls, ADPKD eGFR >60 no hypertension [HTN], ADPKD eGFR >60 and HTN, and ADPKD eGFR 25–60 and HTN), respectively. There were no differences in insulin levels or in HOMA between any of the study groups.
We examined differences in levels of biomarkers between groups after adjustment for age, gender, race, and body mass index (Figure 1). Although the absolute levels of inflammatory markers in the study groups decreased with adjustment for these covariates, the relationship between the groups remained unaltered. Thus, the hypertensive ADPKD eGFR 25 to 60 ml/min per 1.73 m2 had significantly higher levels of CRP and IL-6 compared with healthy controls and the other two ADPKD groups. Adjustment for previously listed covariates did not alter the relationships between study groups for 8-epi-PGF2α and SOD, and patients with ADPKD had significantly different levels of these markers compared with healthy controls. Insulin levels and HOMA remained similar between study groups after covariate adjustment (data not shown).
Tests for linear trend were significant for CRP (P < 0.001) and IL-6 (P < 0.001) but not for 8-epi-PGF2α (P = 0.47), SOD (P = 0.16), insulin (P = 0.94), or HOMA (P = 0.95). In the subgroup of participants with ADPKD, the only significant univariate correlations detected were for CRP and IL-6 with eGFR (Table 2).
Table 2.
Univariate correlations of biomarkers with glomerular filtration rate in participants with polycystic kidney disease (n = 144)
| GFR Correlation Coefficient (r)a | |
|---|---|
| C-Reactive Protein (mg/L) | −0.25b |
| IL-6 (ng/ml) | −0.26b |
| 8-epi-prostaglandin F2α (pg/ml) | −0.04 |
| Superoxide dismutase (units/ml) | 0.03 |
| Insulin (μIU/ml) | 0.02 |
| Homeostasis model assessment | −0.03 |
Spearman's rho.
P < 0.001.
Sensitivity Analyses
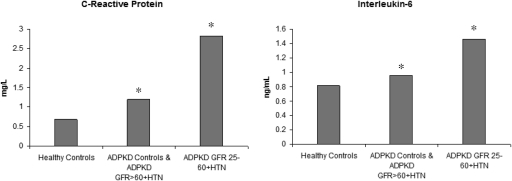
We repeated analyses combining the normotensive ADPKD eGFR >60 and hypertensive ADPKD eGFR >60 groups. In this combined group, mean ± SD systolic BP was 130 ± 16 mmHg, and diastolic BP was 78 ± 11 mmHg. In analyses with the two ADPKD groups with preserved kidney function combined, the levels of CRP (median [interquartile range (IQR)] = 1.22 [2.8]) and IL-6 (median [IQR] = 0.96 [2.0]) were significantly higher (P < 0.01 for both comparisons) in this group compared with control. These differences remained with adjustment for covariates (Figure 2). The levels of 8-epi-PGF2α and SOD were higher in the group with preserved kidney function compared with controls but not different from the group with eGFR 25 to 60 (data not shown).
Figure 2.
Adjusted means of inflammatory markers in healthy controls, ADPKD with preserved kidney function, and ADPKD with eGFR 25 to 60 ml/min per 1.73 m2. The values are adjusted for age, race, gender, and body mass index. *P < 0.05 compared with control.
We repeated analyses excluding the 24 non-HALT participants. The results in this subgroup were similar to those in the entire study cohort. Median (IQR) values of CRP, IL-6, 8-epi-PGF2α, and SOD were 1.05 (1.85), 1.03 (0.75), 415.1 (302.1), and 0.3 (0.18) in the 35 non-HALT subjects in the hypertensive ADPKD eGFR >60 ml/min per 1.73 m2 group and 3.0 (4.0), 1.64 (1.78), 435.9 (268.5), and 0.25 (0.15) in the 43 non-HALT subjects in the hypertensive ADPKD eGFR 25 to 60 ml/min per 1.73 m2 group.
Discussion
Inflammation and oxidative stress are evident in this cohort of subjects at different stages of ADPKD. Inflammation is pronounced with worse kidney disease, whereas oxidative stress is apparent in subjects with preserved kidney function and appears to predate clinically apparent hypertension. We did not find differences in fasting insulin or HOMA levels between patients with APDKD and healthy controls.
Several animal studies have suggested a role for cytokines in the development of interstitial inflammation leading to kidney injury and progression in ADPKD. In a rat model of ADPKD, monocyte chemoattractant protein-1 and osteopontin mRNA expression was upregulated in cystic kidneys (7). Immunostaining revealed high levels of these proinflammatory cytokines in the cystic epithelium that correlated with interstitial macrophage accumulation. A study involving mural epithelial cells cultured from cysts of patients with ADPKD demonstrated increased urinary monocyte chemoattractant protein-1 excretion, which appeared to precede elevation in serum creatinine and development of proteinuria (15). A series of experiments using cell culture systems, murine and human kidney tissue, and embryonic kidney organ culture from mice provided strong support for a role for TNF-α in cystogenesis (16).
There is increased inflammatory activity with concomitant activation of the acute phase response in patients with CKD (17); however, data are limited in ADPKD. A small study of 10 patients with ADPKD and preserved kidney function demonstrated higher plasma levels of IL-6 and IL-8 (9). In our cohort of subjects at different stages of ADPKD, levels of inflammatory markers were highest in the group with the worst kidney function but appeared to also be elevated in patients with preserved kidney function. Our data support the hypothesis generated from animal models that inflammation is an upstream event in the pathogenesis of ADPKD and may be a contributory factor in progression (18).
Oxidative stress may be a pathway factor contributing to progression and poor outcomes in CKD (19). Uremia leads to increased formation of reactive oxygen species and reduced antioxidant capacity (20,21). The subsequent imbalance results in increased free radical activity, oxidative stress, and lipid peroxidation. We chose 8-epi-PGF2α and SOD as markers of oxidative stress; the former is an eicosanoid produced by the oxidation of tissue phospholipids by oxygen radicals (22), and the latter plays a key antioxidant role by scavenging reactive oxygen species (23).
Data regarding oxidative stress are limited in patients with ADPKD. Investigators examined mRNA expression of oxidative stress markers in a mouse and rat model of ADPDKD (8). They found upregulation of heme oxygenase-1 mRNA and downregulation of antioxidant enzyme mRNA expression, both of which correlated with disease severity. They also demonstrated decreased protein levels and enzyme activity of antioxidant enzymes glutathione peroxidase and superoxide dismutase and accumulation of lipid peroxidation byproducts in the plasma and kidneys of these animals. In a study of 27 patients with early ADPKD, plasma and urinary levels of 13-hydroxyoctadecadienoic acid, a marker of oxidative stress, were elevated compared with age-matched controls (24). In our study, we demonstrate markedly elevated plasma 8-epi-PGF2α and reduced SOD in subjects with ADPKD. Altered levels of these oxidative stress markers were apparent in early ADPKD subjects and did not appear to change with worsening kidney function or hypertension.
We did not see any difference in levels of insulin or HOMA between controls and subjects with ADPKD. This is in contrast to previous reports in the literature. In a study of 15 patients with ADPKD with mean creatinine clearance of 102 ml/min per 1.73 m2 and 20 age- and gender-matched healthy controls, insulin sensitivity, assessed by a short insulin tolerance test, was impaired in patients with ADPKD (10). Similarly, insulin sensitivity, assessed using a frequent sampling intravenous glucose tolerance test (minimal-model technique), was lower in a cohort of 29 patients with IgA glomerulonephritis and 21 patients with ADPKD compared with controls (25). It is possible that fasting insulin and HOMA are less sensitive markers of insulin resistance, and therefore we were unable to detect differences between the study groups.
Our findings have clinical relevance. In a murine model of polycystic kidney disease, combination therapy with olemsartan and azelnidipine retarded cyst growth by reducing interstitial inflammation and oxidative stress (26). A crossover study of simvastatin in 10 normocholesterolaemic patients with ADPKD showed improvements in renal blood flow and endothelial function, actions that may involve known pleiotropic effects of statins including anti-inflammatory effects (27). Etanercept, a TNF-α inhibitor, blocked new cyst formation and retarded cyst growth in murine models of ADPKD (16).
The major limitations of this study are its cross-sectional nature and the relatively small numbers of subjects, which may have limited our statistical power. However, this is the largest study to date that has comprehensively examined these pathways in patients at different stages of ADPKD. It is possible that our results are subject to residual confounding from differences in age, race, and gender, and confounding from other factors for which adjustments were not made in this study. We were also unable to examine the effects of antihypertensive medications and hypertension per se on these markers because the different study groups were selected on the basis of BP. We acknowledge that the differences in medication use may confound the observed group differences. However, given that ACE inhibitors and statins, for example, are proposed to have anti-inflammatory effects, the fact that they are most prevalent in the hypertensive ADPKD group eGFR 25 to 60 ml/min per 1.73 m2, which also had the highest levels of inflammatory markers, suggests that, if anything, medication differences bias toward the null.
Additional studies with comparator groups of patients with hypertension and without CKD, as well as patients with nondiabetic CKD from causes other than ADPKD with and without hypertension, are necessary to put these findings into perspective and to further examine the effects of ADPKD, hypertension, and kidney function on the levels of these markers.
In summary, we show that inflammation and oxidative stress are evident in early ADPKD. Future studies are necessary to explore whether intervening in these pathways can prevent progression and improve outcomes in this patient population.
Disclosures
None.
Acknowledgments
This study was funded by National Institutes of Health, NIDDK Grants K23 DK67303, K24 DK078204, and UL1 RR025752 from the National Center for Research Resources; a Norman S. Coplon Extramural Grant from Satellite Healthcare; and a grant from the Polycystic Kidney Foundation. The HALT-PKD study site at Tufts Medical Center was supported by a cooperative agreement from the National Institutes of Health, NIDDK UO1DK62411, the NCRR GCRC RR000054, and UL1 RR025752. We acknowledge the invaluable assistance of Gertrude (Peachy) Simon, Julie Driggs, and Wendy Shinzawa.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Perrone RD, Ruthazer R, Terrin NC: Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: Contribution of extrarenal complications to mortality. Am J Kidney Dis 38: 777–784, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Fick GM, Johnson AM, Hammond WS, Gabow PA: Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5: 2048–2056, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. The Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Gabow PA, Chapman AB, Johnson AM, Tangel DJ, Duley IT, Kaehny WD, Manco-Johnson M, Schrier RW: Renal structure and hypertension in autosomal dominant polycystic kidney disease. Kidney Int 38: 1177–1180, 1990 [DOI] [PubMed] [Google Scholar]
- 5. Gonzalo A, Gallego A, Rivera M, Orte L, Ortuno J: Influence of hypertension on early renal insufficiency in autosomal dominant polycystic kidney disease. Nephron 72: 225–230, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Bardaji A, Martinez-Vea A, Valero A, Gutierrez C, Garcia C, Ridao C, Oliver JA, Richart C: Cardiac involvement in autosomal-dominant polycystic kidney disease: A hypertensive heart disease. Clin Nephrol 56: 211–220, 2001 [PubMed] [Google Scholar]
- 7. Cowley BD, Jr., Ricardo SD, Nagao S, Diamond JR: Increased renal expression of monocyte chemoattractant protein-1 and osteopontin in ADPKD in rats. Kidney Int 60: 2087–2096, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Maser RL, Vassmer D, Magenheimer BS, Calvet JP: Oxidant stress and reduced antioxidant enzyme protection in polycystic kidney disease. J Am Soc Nephrol 13: 991–999, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Merta M, Tesar V, Zima T, Jirsa M, Rysava R, Zabka J: Cytokine profile in autosomal dominant polycystic kidney disease. Biochem Mol Biol Int 41: 619–624, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Vareesangthip K, Tong P, Wilkinson R, Thomas TH: Insulin resistance in adult polycystic kidney disease. Kidney Int 52: 503–508, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Fliser D, Pacini G, Engelleiter R, Kautzky-Willer A, Prager R, Franek E, Ritz E: Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int 53: 1343–1347, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Chapman AB, Torres VE, Perrone RD, Steinman TI, Bae KT, Miler JP, Miskulin DC, Oskoui FR, Masoumi A, Hogan MC, Winklhofer FT, Braun W, Thompson PA, Meyers CM, Kelleher C, Schrier RW: The HALT polycystic kidney disease trials: Design and implementation. Clin J Am Soc Nephrol 5: 102–109, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 15. Zheng D, Wolfe M, Cowley BD, Jr., Wallace DP, Yamaguchi T, Grantham JJ: Urinary excretion of monocyte chemoattractant protein-1 in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 14: 2588–2595, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Li X, Magenheimer BS, Xia S, Johnson T, Wallace DP, Calvet JP, Li R: A tumor necrosis factor-alpha-mediated pathway promoting autosomal dominant polycystic kidney disease. Nat Med 14: 863–868, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herbelin A, Urena P, Nguyen AT, Zingraff J, Descamps-Latscha B: Elevated circulating levels of interleukin-6 in patients with chronic renal failure. Kidney Int 39: 954–960, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Grantham JJ: Mechanisms of progression in autosomal dominant polycystic kidney disease. Kidney Int Suppl 63: S93–S97, 1997 [PubMed] [Google Scholar]
- 19. Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM: The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 62: 1524–1538, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Handelman GJ, Walter MF, Adhikarla R, Gross J, Dallal GE, Levin NW, Blumberg JB: Elevated plasma F2-isoprostanes in patients on long-term hemodialysis. Kidney Int 59: 1960–1966, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Ceballos-Picot I, Witko-Sarsat V, Merad-Boudia M, Nguyen AT, Thevenin M, Jaudon MC, Zingraff J, Verger C, Jingers P, Descamps-Latscha B: Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic Biol Med 21: 845–853, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Morrow JD, Frei B, Longmire AW, Gaziano M, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ: Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med 332: 1198–1203, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Miao L, St. Clair DK: Regulation of superoxide dismutase genes: Implications in disease. Free Radic Biol and Med 47: 344–356, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang D, Strandgaard S, Borresen ML, Luo Z, Connors SG, Yan Q, Wilcox CS: Asymmetric dimethylarginine and lipid peroxidation products in early autosomal dominant polycystic kidney disease. Am J Kidney Dis 51: 184–191, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Harrap SB, Davies DL, Macnicol AM, Dominiczak AF, Fraser R, Wright AF, Watson ML, Briggs JD: Renal, cardiovascular and hormonal characteristics of young adults with autosomal dominant polycystic kidney disease. Kidney Int 40: 501–508, 1991 [DOI] [PubMed] [Google Scholar]
- 26. Tanifuji C, Suzuki Y, Geot WM, Horikoshi S, Takahashi H, Tomino Y: Beneficial effects of combination therapy with olmesartan and azelnidipine in murine polycystic kidneys. Kidney Blood Press Res 32: 239–249, 2009 [DOI] [PubMed] [Google Scholar]
- 27. van Dijk MA, Kamper AM, van Veen S, Souverijn JHM, Blauw GJ: Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 16: 2152–2157, 2001 [DOI] [PubMed] [Google Scholar]