Summary
Background and objectives
The significance of renal parenchymal volume and the factors that influence it are poorly understood.
Design, setting, participants, & measurements
Renal parenchymal volume (RPV) was measured on contrast-enhanced CT scans after exclusion of sinus fat and vessels in 224 healthy subjects evaluated as kidney donors and in a separate cohort of 22 severely obese individuals before and after 6 months of weight loss. GFR was measured by iohexol clearance in 76 of the transplant donors. RPV was correlated with age, GFR, and various anthropometric parameters.
Results
In potential transplant donors, RPV correlated with body surface area (BSA; r = 0.68) and was 7% larger in men but did not vary with age or race. Gender and body size were independent determinants of RPV. RPV correlated well with GFR (r = 0.62) and accounted for almost all of the variability in a model of GFR that included age, race, gender, and body surface area. GFR correlated more strongly with RPV than with creatinine-based equations. The same relationship between RPV and BSA was observed in obesity, and RPV decreased with weight loss.
Conclusions
In healthy adults younger than 65 years, renal parenchymal volume is governed by body size and gender but not age or race and is strongly correlated with GFR. This indicates that renal parenchymal volume varies to meet metabolic demand and is closely linked to renal function.
Introduction
Detection and assessment of kidney disease is usually through measurement or estimation of renal function and examination of the urine. However, renal parenchymal volume is another parameter that may have clinical utility. For instance, a reduction can occur in chronic kidney disease and is a frequently used parameter for decisions about biopsy, whereas renal enlargement can occur in a number of disorders. Renal enlargement in diabetics is a risk factor for nephropathy (1,2), and the size of donor kidneys influences the outcome after transplantation (3–5). A reduced quantity of nephrons at birth may contribute to the development of hypertension (6,7) and may be detectable as differences in renal parenchymal volume because kidney weight correlates closely with nephron number (8) in healthy humans. The use of kidney volume as a clinical or research tool has been limited by inaccuracy in its measurement in vivo, uncertainty about its determinants, and a lack of normative data. Sonographic measurements of kidney volume have been used in most studies but are very inaccurate (9–12). Although it is generally accepted that kidney size should be corrected for body size, the magnitude of this correction is unknown, and the influence of other parameters such as gender and age is unclear.
The determinants of renal parenchymal volume were identified in adults without kidney disease undergoing contrast-enhanced computed tomography (CT) scans as part of an evaluation for kidney donation. Volume was also measured in a cohort of obese subjects before and after weight loss therapy to better define the relationship with body size.
Materials and Methods
Subjects
Two cohorts of patients were studied. The first was selected between January 2003 and February 2006 from individuals undergoing inpatient evaluation as kidney donors, which included contrast-enhanced computed tomography of the abdomen. The potential donors were screened before admission by telephone interview only for a history of hypertension or kidney disease. In 224 subjects, adequate CT scans were available and showed structurally normal kidneys. Of these, 80 were identified prospectively and agreed to a measurement of GFR. The second cohort consisted of 21 female patients undergoing CT scanning as part of a study of metabolic changes during weight loss. Weight loss was produced by Roux-en-Y gastric bypass in 16 patients, gastric banding in two, and diet in four. Patients underwent noncontrast CT scans before and 1, 6, and 24 months after weight loss therapy to quantitate mesenteric fat.
Renal Parenchymal Volume
Transverse images from CT scans were exported as TIFF files into ScionImage software (Scion Corp., Frederick, MD) for tracing and measurement of the cross-sectional kidney areas. For total kidney area, the outline of the kidney was traced, and where the renal border is discontinuous (renal hilum), the kidney was enclosed by extending a straight line tangential to each hilar lip. For renal parenchymal area, the tracing excluded sinus fat, vessels, and cysts. The number of enclosed pixels was counted and converted to a cross-sectional area after calibration of pixel size. Volume was determined by summing the cross-sectional areas and multiplying by the slice interval. Most of the CT scans were performed with a slice interval of 5 mm. To determine whether a larger interval could be used to reduce the amount of tracing, 13 kidneys were traced at 5-mm intervals, and the volume was compared using every slice (5-mm interval) and every other slice (10-mm interval). Because there was no significant difference (0.4% ± 0.3%), 10-mm intervals were used. Some scans were performed with 7-mm intervals or 1-mm intervals, in which case every image (7-mm interval) or every tenth image (10-mm interval) was used. Measurements were performed by one of five investigators. Each underwent a period of training, and consistency was checked with test cases in a blinded fashion. We have previously reported intraobserver and interobserver variabilities of 3 and 8% for the measurement of renal parenchymal volume (13), and others have reported an interscan correlation of 0.94 (14).
GFR
GFR was measured as the plasma clearance of the iohexol (approximately 145 ml at 350 mg of iodine/ml) that was administered intravenously for the CT scan. The bottle of iohexol was weighed before and after the iohexol was withdrawn, and residual solution in the syringe and tubing was weighed to determine the precise dose. At least four and usually five heparinized blood samples were collected at approximately 30-minute intervals beginning 2 hours after iohexol administration, a period during which there is a single exponential decline in plasma iohexol concentration (15). Plasma iohexol concentration was measured by reverse-phase HPLC after deproteination with perchloric acid (15) with detection by ultraviolet absorption, and the larger of the two peaks was used to calculate the concentration. Clearance was calculated from the slope of the linear regression of the logarithm of iohexol concentration as a function of time as described by Gaspari et al. (15) using a one-compartment model corrected by the method of Bröchner-Mortensen (16). This technique gives an excellent correlation with inulin clearance over a wide range (15). The data were rejected if the correlation coefficient for the regression was less than 0.97, which occurred in four of the 80 subjects in whom GFR was measured.
Other Measurements
Height and weight were measured in the hospital, and body surface area was estimated using the formula of DuBois and DuBois (17). Lean body mass was obtained by subtracting fat mass (measured by air plethysmography) from total weight. Serum creatinine and urine creatinine were measured in the clinical laboratory of the hospital and used to calculate creatinine clearance. GFR was estimated by the modification of diet in renal disease (MDRD2) formula as described previously (18).
Data Analyses
Univariate data are presented as Pearson correlation coefficients. Multivariate regression was performed using Statistical Excel Add-in software, version 1.6 (StatistiXL, Perth, Australia). A P value of 0.05 was considered statistically significant.
Results
Of the 224 potential transplant donors studied, 125 (56%) were female, 155 (69%) were Caucasian, 47 (21%) were black, and 9 (4%) were Hispanic. Other characteristics are shown in Table 1. All of the parameters appeared to be distributed evenly about their mean. Serum creatinine was greater than 1.2 in four subjects, all of whom had a creatinine clearance greater than 120 ml/min. Creatinine clearance was less than 80 ml/min in eight subjects, all of whom had a serum creatinine concentration of 1.0 or less. Two subjects had an admission diastolic BP above 100 mmHg but had no evidence of renal disease on the basis of serum creatinine levels of 0.7 and 0.8 mg/dl, creatinine clearances of 109 and 116 ml/min, and 24-hour urine protein excretions of less than 250 mg.
Table 1.
Clinical characteristics of the potential kidney donors (n = 224)
| Parameter | Mean ± SD | Median | Range |
|---|---|---|---|
| Age (yr) | 39 ± 11 | 38 | 18 to 66 |
| Serum creatinine (mg/dl) | 0.86 ± 0.17 | 0.9 | 0.5 to 1.8 |
| Creatine clearance (ml/min per 1.73 m2) | 131 ± 35 | 128 | 40 to 266 |
| Mean blood pressure (mmHg) | 92 ± 9 | 92 | 71 to 127 |
| Weight (kg) | 79 ± 16 | 77 | 44 to 122 |
| Body surface area (m2) | 1.90 ± 0.23 | 1.87 | 1.37 to 2.47 |
| Body mass index (kg/m2) | 27.2 ± 4.6 | 27.0 | 18.8 to 39.4 |
| Combined renal parenchymal volume (ml) | 357 ± 73 | 348 | 233 to 601 |
Prior studies have measured total kidney volume, which includes tissue such as the renal sinus that is devoid of nephrons and does not contribute to renal function. To determine the relationship between total renal volume and renal parenchymal volume, initial measurements included both total kidney volume and parenchymal volume. In 20 subjects, the relationship between the two varied considerably, with renal parenchymal volume (RPV) ranging from 55 to 98% of total kidney volume. Because of the desire to measure functional renal tissue and the poor correlation with total renal volume, renal parenchymal volume rather than total kidney volume was measured in the remainder of the subjects.
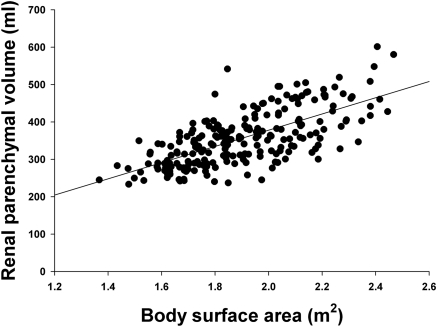
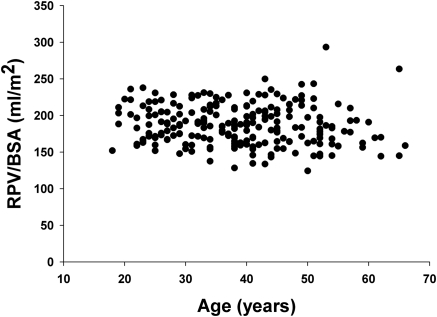
Pearson correlation coefficients for potential determinants of RPV in the transplant donors are shown in Table 2. Of the various anthropometric indices, body surface area and body weight correlated best with RPV. The relationship between RPV and body surface area is shown in Figure 1. The correlation between BSA and total kidney volume was slightly better (r = 0.77), but this analysis was limited to 14 subjects. RPV was also correlated with gender, and this was not due to differences in body size because RPV/BSA was 7% less in women than in men (181 ± 2.4 versus 195 ± 2.7 ml/m2, P < 0.001). As shown in Figure 2, there was no significant correlation with age (r = −0.15, P > 0.05), but there were few donors above age 60. There was also no correlation with age in the subgroup of 41 subjects aged 50 or greater. Race did not correlate with RPV, and in particular RPV/BSA was the same in blacks and other races (193 ± 5 versus 185 ± 2 ml/m2). The results of these univariate analyses were confirmed by a multivariate regression (Table 3) in which only body surface area and gender were significantly correlated with RPV. The adjusted r2 for this model was 0.51.
Table 2.
Univariate correlations between renal parenchymal volume and anthropometric parameters in potential kidney donors (n = 224)
| Parameter | Correlation Coefficient | P |
|---|---|---|
| Weight | 0.66 | <0.0001 |
| Height | 0.52 | <0.0001 |
| Body surface area | 0.68 | <0.0001 |
| Body mass index | 0.39 | <0.0001 |
| Age | −0.10 | NS |
| Gender | 0.55 | <0.0001 |
| Race | 0.098 | NS |
Figure 1.
Relationship between renal parenchymal volume and body surface area in potential kidney donors (n = 224). The regression equation is RPV = 217 × BSA − 55 (r = 0.68; P < 0.0001).
Figure 2.
Plot of age and renal parenchymal volume in potential kidney donors showing no relationship (n = 224).
Table 3.
Multivariate model of renal parenchymal volume in potential kidney donors (n = 224)
| Parameter | Coefficient | Standardized β | P |
|---|---|---|---|
| Body surface area (m2) | 182 | 0.58 | <0.0001 |
| Gender (female) | −27 | −0.19 | 0.002 |
| Age (yr) | −0.39 | 0.06 | 0.22 |
| Race (white) | −6.0 | −0.07 | 0.156 |
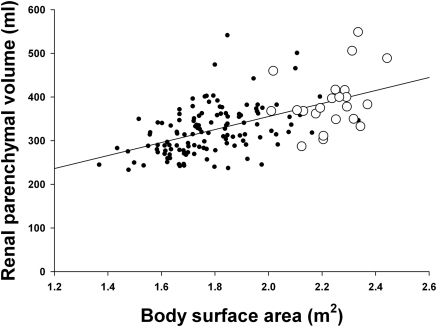
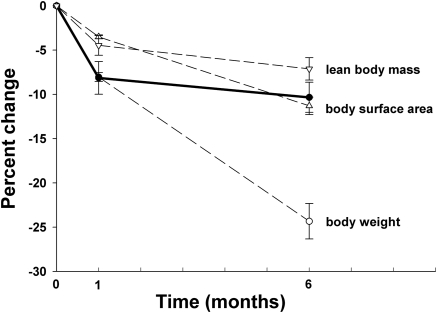
The relationship between body size and kidney volume was also examined in 22 severely obese individuals undergoing weight loss therapy. The mean age was 39 years (range, 25 to 57 years), the mean body mass index was 47 (range, 35 to 55), and all were female. The baseline RPV/BSA was not significantly different than in female potential kidney donors (180 ± 5 ml/m2 versus 183 ± 2.5 ml/m2, P < 0.05). This is apparent in Figure 3, in which the obese subjects and the female transplant donors appear to fall on the same regression line for RPV/BSA. RPV was also measured 1 month (19 subjects) and 6 months (18 subjects) after initiation of weight-loss therapy. All of the patients lost weight after 1 month and further weight after 6 months. As shown in Figure 4, RPV decreased significantly in the first month (8.1 ± 1.8%) with a smaller decrease over the next 5 months (to 10.3 ± 1.7%). RPV decreased in all but four patients at 1 month and in all but two patients at 6 months. The initial loss of RPV matched the loss in body weight, but subsequent weight loss was much greater than the decrease in RPV. The initial loss of RPV was greater than the decrease in BSA, but these two values were equal after 6 months. Although the loss of lean body mass was less than the loss of RPV at both time points, the patterns matched.
Figure 3.
Relationship between renal parenchymal volume and body surface area in female potential kidney donors (n = 122; solid symbols) and in severely obese women (n = 22; open symbols) at baseline.
Figure 4.
Decrease in renal parenchymal volume with weight loss in severe obesity. The thick line indicates renal parenchymal volume. The data are the means of 19 subjects at baseline at 1 month and 18 subjects at 6 months. The error bars are standard errors.
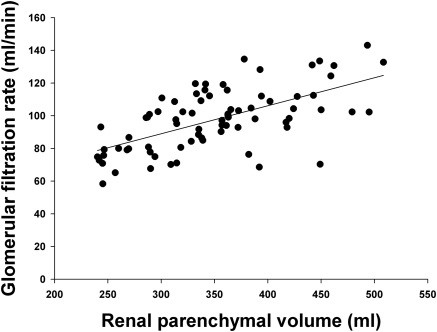
To determine whether renal function is related to renal parenchymal volume, GFR was measured in 76 of the potential kidney donors. As shown in Figure 5, there was a strong correlation between GFR and RPV (r = 0.62, P < 0.0001). Common methods to estimate GFR were also compared with measured GFR to determine whether RPV is a better indicator. The correlation between GFR and RPV was stronger than the correlation between GFR and creatinine clearance (r = 0.48) or the Cockcroft-Gault formula for creatinine clearance (r = 0.47) and considerably stronger than the correlation between GFR (normalized to BSA) and the MDRD formula (r = 0.35) or the MDRD2 formula (r = 0.31). The relationship between GFR and RPV remained highly significant in a multivariate regression model that included age, gender, race, and BSA (Table 4). The adjusted r2 for the inclusive model (0.39) was only slightly better than that for the correlation between GFR and RPV alone (0.38).
Figure 5.
Relationship between GFR and renal parenchymal volume in potential kidney donors (n = 76). The equation for the regression line is: GFR = 0.17 × RPV + 38; r = 0.62; P < 0.0001.
Table 4.
Multivariate model of glomerular filtration rate in potential kidney donors (n = 76)
| Parameter | Coefficient | Standardized β | P |
|---|---|---|---|
| Renal parenchymal volume (ml) | 0.15 | 0.56 | <0.0001 |
| Age (yr) | −0.39 | −0.20 | 0.044 |
| Body surface area (m2) | −18 | 0.06 | 0.69 |
| Race (white) | −4.7 | −0.02 | 0.87 |
| Gender (female) | 2.3 | −0.04 | 0.74 |
Discussion
Renal parenchymal volume may have both diagnostic and prognostic utility, but the lack of normative data and knowledge of the factors that affect it has limited its use. Previous studies of kidney size have been performed primarily in children and have relied on sonographic or autopsy measurements. Sonographic measurements of renal volume are very inaccurate (9–11), and autopsy weights are subject to postmortem changes. The volume of kidneys and other organs can be accurately measured by CT scanning with errors of 3% or less (19). However, studies to date have measured total kidney volume, which includes tissue that does not contribute to renal function. Initial measurements in this study showed a wide variation in the proportion of kidney volume that is parenchymal (cortex and medulla). Thus, differences in kidney volume do not accurately reflect differences in parenchymal volume. Furthermore, there was an inverse correlation between nonparenchymal volume and kidney volume (r = −0.33, P < 0.05), suggesting that the renal sinus, which is composed primarily of fat in adults, compresses or expands to accommodate changes in parenchymal volume. Despite this, total kidney volume and RPV showed similar correlations with BSA, suggesting that nonparenchymal portions of the kidney also vary with body size. Body size and gender were both independent determinants of renal parenchymal volume. The strong correlation between renal parenchymal volume and body size is consistent with previous autopsy (20,21), sonographic (22), and CT (23) studies. When measured, lean body mass has shown a slightly better correlation with renal volume than body surface area in children (24) and adults (23). In children, kidney volume correlates more strongly with body size than with age (25,26). This, together with the fact that BSA and RPV are closely linked in adults, suggests that renal enlargement during development is an adaptation to body size and that this continues into adulthood.
The mechanism by which body size affects renal parenchymal volume is unknown. Because additional glomeruli do not appear to form after birth, the increase in renal parenchymal volume with increasing body size during development results from nephron hypertrophy (8,20,27), presumably to meet increased metabolic demand. The decrease in RPV during weight loss in the obese subjects is consistent with this and indicates that renal parenchymal volume is dynamic. Although there were similar declines in RPV and BSA at 6 months, the greater decrease in RPV at 1 month suggests that food intake or metabolic rate rather than body size may affect renal parenchymal volume. While the decrease in RPV was greater than the loss of lean body mass at both 1 and 6 months, the pattern was similar.
The results of this study also clarify the influence of gender on kidney size. The larger renal size in men has been shown in previous studies (21,28), but when differences in body size were taken into account, no effect of gender was observed in other studies (20,29). We found that renal parenchymal volume is significantly lower in women, even when differences in body surface area are accounted for. Less accurate measurements that did not exclude nonparenchymal tissue may have obscured this relationship in prior studies. Whether the effect of gender is due to a direct action of sex steroids on kidney growth or is secondary to differences in body composition, diet, or physical activity is unclear. Race did not influence renal parenchymal volume, which complements autopsy data showing no racial differences in kidney weight (20).
An important finding in this study is that renal parenchymal volume does not decrease with age in healthy individuals. However, because there were only a few subjects over age 60 and none over age 65, we cannot exclude a decline after age 60. The effect of age on renal parenchymal volume has not been determined in normal individuals, and the results of sonographic or autopsy studies of total renal mass are variable, ranging from a linear decline after age 40 (30) to no decline (20). Measurements of total volume or weight can be misleading in this regard, because sinus fat increases with age (28) and, as shown in this study, varies inversely with parenchymal volume. Nevertheless, several studies of apparently healthy individuals have shown a decline in kidney size only after age 60 (8,28,29). The correlation between kidney size and renal function during aging is unclear because of uncertainty about the effect of aging on renal structure and function (31). Our data indicate that the decline in renal function noted throughout adulthood in cross-sectional studies (32,33) is not associated with a loss of renal parenchymal volume before age 60, suggesting a functional rather than structural basis. An alternative explanation for the stable renal parenchymal volume is that hypertrophy of remaining nephrons compensates for the nephron loss that may occur (8,21). However, the decline in renal function may be much slower before age 60 (34–36), and longitudinal data indicate no decline in renal function with age in many healthy individuals (37), consistent with the stable RPV noted in this study.
Body size and gender together explained only about half the variability in renal parenchymal volume, and the source of the remaining variability is unknown. Although subjects with diabetes, which causes renal enlargement (38), were not included in the cohort of transplant donors, less severe metabolic abnormalities may play a role. Diet may also have an effect because protein intake can influence renal size (39). An additional variable is glomerular number, which is fixed at birth and can vary as much as eight-fold (8,21). While glomerular number is reported to correlate with kidney weight in healthy adults (8), compensatory hypertrophy could obscure this relationship as indicated by the increase in glomerular size during childhood (27) and the inverse relationship between nephron number and glomerular size in adults (27,40,41). This may be the explanation for the recent finding of substantially reduced nephron numbers yet similar kidney weights in hypertensive kidneys (7).
The correlation between kidney size and renal function has not been carefully examined. Several studies have shown a good correlation between GFR and renal size measured urographically or sonographically in children (42–45). Correlations between size and function have been found in adults with hypertension or renal disease using urography (46), in diabetics (1,38) and elderly hospitalized patients (47) using sonography, and in disease-free cancer patients using CT scanning (23). However, no correlation was found between sonographic kidney size and GFR in normal subjects (38). Our data show that there is a strong relationship between renal parenchymal volume and GFR and that this correlation remained highly significant when age, gender, race, and body size were taken into account. Almost all of the variation in GFR accounted for by these variables could be explained solely by RPV, suggesting that RPV alone may be useful in predicting GFR. In fact, GFR showed a stronger correlation with RPV than with commonly used methods to estimate renal function such as the Cockcroft-Gault and MDRD formulas and the measurement of creatinine clearance. The poor performance of these methods in subjects without renal disease has recently been documented (48). The data indicate that measurement of renal parenchymal volume may be a useful method for estimating renal function in normal individuals, but additional data will be required to determine whether this can be extrapolated to patients with renal disease.
Disclosures
None.
Acknowledgments
Supported by Grants R21 DK064740, RO3 DK067167, and MO1 RR000039 from the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Feldt-Rasmussen B, Hegedus L, Mathiesen ER, Deckert T: Kidney volume in type I (insulin-dependent) diabetic patients with normal or increased urinary albumin excretion: Effect of long-term improved metabolic control. Scand J Clin Lab Invest 51: 31–36, 1991 [PubMed] [Google Scholar]
- 2. Baumgartl HJ, Sigl G, Banholzer P, Haslbeck M, Standl E: On the prognosis of IDDM patients with large kidneys. Nephrol Dial Transpl 13: 630–634, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Kim YS, Moon JI, Kim DK, Kim SI, Park K: Ratio of donor kidney weight to recipient bodyweight as an index of graft function. Lancet 357: 1180–1181, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Saxena AB, Busque S, Arjane P, Myers BD, Tan JC: Preoperative renal volumes as a predictor of graft function in living donor transplantation. Am J Kidney Dis 44: 877–885, 2004 [PubMed] [Google Scholar]
- 5. Giral M, Nguyen JM, Karam G, Kessler M, de Ligny BH, Buchler M, Bayle F, Meyer C, Foucher Y, Martin ML, Daguin P, Soulillou JP: Impact of graft mass on the clinical outcome of kidney transplants. J Am Soc Nephrol 16: 261–268, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Brenner BM, Chertow GM: Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kid Dis 23: 171–175, 1994 [PubMed] [Google Scholar]
- 7. Keller G, Zimmer G, Mall G, Ritz E, Amann K: Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Nyengaard JR, Bendtsen TF: Glomerular number and size in relation to age, kidney weight, and body surface in normal men. Anat Rec 232: 194–201, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Emamian SA, Nielsen MB, Pedersen JF: Intraobserver and interobserver variations in sonographic measurements of kidney size in adult volunteers. Acta Radiol 36: 399–401, 1995 [PubMed] [Google Scholar]
- 10. Bakker J, Olree M, Kaatee R, de Lange EE, Moons KGM, Beutler JJ, Beek FJA: Renal volume measurements: Accuracy and repeatability of US compared with that of MR imaging. Radiology 211: 623–628, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Sargent MA, Long G, Karmali M, Cheng SM: Interobserver variation in the sonographic estimation of renal volume in children. Pediatr Radiol 27: 663–666, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Bakker J, Olree M, Kaatee R, De Lange EE, Beek FJA: In vitro measurement of kidney size: Comparison of ultrasonography and MRI. Ultrasound Med Biol 24: 683–688, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Al-Said J, Brumback MA, Moghazi S, Baumgarten DA, O'Neill WC: Reduced renal function in patients with simple renal cysts. Kidney Int 65: 2303–2308, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Yokoyama M, Watanabe K, Inatsuki S, Ochi K, Takeuchi M: Measurement of renal parenchymal volume using computed tomography. J Comput Assisted Tomogr 6: 975–977, 1982 [DOI] [PubMed] [Google Scholar]
- 15. Gaspari F, Perico N, Ruggenenti P, Mosconi L, Amuchastegui CS, Guerini E, Daina E, Remuzzi G: Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol 6: 257–263, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Bröchner-Mortensen J: A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest 30: 271–274, 1972 [DOI] [PubMed] [Google Scholar]
- 17. DuBois D, DuBois EF: A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17: 863–871, 1916 [Google Scholar]
- 18. National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S237, 2002 [PubMed] [Google Scholar]
- 19. Heymsfield SB, Fulenwider T, Nordlinger B, Barlow R, Sones P, Kutner M: Accurate measurement of liver, kidney, and spleen volume and mass by computerized axial tomography. Ann Intern Med 90: 185–187, 1979 [DOI] [PubMed] [Google Scholar]
- 20. Kasiske BL, Umen AJ: The influence of age, sex, race, and body habitus on kidney weight in humans. Arch Pathol Lab Med 110: 55–60, 1986 [PubMed] [Google Scholar]
- 21. Hoy W, Douglas-Denton R, Hughson M, Cass A, Johnson K, Bertram JF: A stereologic study of glomerular number and volume: Preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int 63: 31–37, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Troell S, Berg U, Johansson B, Wikstad I: Renal parenchymal volume in children. Acta Radiol 29: 127–130, 1988 [PubMed] [Google Scholar]
- 23. Nawaratne S, Brien JE, Seeman E, Fabiny R, Zalcberg J, Cosolo W, Angus P, Morgan DJ: Relationships among liver and kidney volumes, lean body mass and drug clearance. Br J Clin Pharmacol 46: 447–452, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmidt IM, Molgaard C, Main KM, Michaelsen KF: Effect of gender and lean body mass on kidney size in healthy 10-year-old children. Pediatr Nephrol 16: 366–370, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Dinkel E, Ertel M, Dittrich M, Peters H, Berres M, Schulte-Wissermann H: Kidney size in childhood: Sonographical growth charts for kidney length and volume. Pediatr Radiol 15: 38–43, 1985 [DOI] [PubMed] [Google Scholar]
- 26. Han BK, Babcock DS: Sonographic measurements and appearance of normal kidneys in children. AJR Am J Roentgenol 145: 611–616, 1985 [DOI] [PubMed] [Google Scholar]
- 27. Hughson M, Farris AB, Douglas-Denton R, Hoy WE, Bertram JF: Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int 63: 2113–2122, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Emamian SA, Nielsen MB, Pedersen JF, Ytte L: Kidney dimensions at sonography: Correlation with age, sex, and habitus in 665 adult volunteers. AJR Am J Roentgenol 160: 83–86, 1993 [DOI] [PubMed] [Google Scholar]
- 29. Miletic D, Fuckar Z, Sustic A, Mozetic V, Stimac D, Zauhar G: Sonographic measurement of absolute and relative renal length in adults. J Clin Ultrasound 26: 185–189, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Tauchi H, Tsuboi K, Okutomi J: Age changes in the human kidney of the different races. Gerontologia 17: 87–97, 1971 [DOI] [PubMed] [Google Scholar]
- 31. Epstein M: Aging and the kidney. J Am Soc Nephrol 7: 1106–1122, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Davies DF, Shock NW: Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest 29: 496–507, 1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Foundation NK: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Part 4. Definition and classification of stages of chronic kidney disease. Am J Kidney Dis 39: S46–S75, 2002 [PubMed] [Google Scholar]
- 34. Fliser D, Zeier M, Nowack R, Ritz E: Renal functional reserve in healthy elderly subjects. J Am Soc Nephrol 3: 1371–1377, 1993 [DOI] [PubMed] [Google Scholar]
- 35. Hollenberg NK, Rivera A, Meinking T, Martinez G, McCullough M, Passan D, Preston M, Taplin D, Vicaria-Clement M: Age, renal perfusion and function in island-dwelling indigenous Kuna Amerinds of Panama. Nephron 82: 131–138, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Fliser D, Franek E, Joest M, Block S, Mutschler E, Ritz E: Renal function in the elderly: Impact of hypertension and cardiac function. Kidney Int 51: 1196–1204, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Lindeman RD, Tobin J, Shock NW: Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33: 278–285, 1985 [DOI] [PubMed] [Google Scholar]
- 38. Christiansen JS, Gammelgaard J, Frandsen M, Parving H-H: Increased kidney size, glomerular filtration rate and renal plasma flow in short-term insulin-dependent diabetics. Diabetalogia 20: 451–456, 1981 [DOI] [PubMed] [Google Scholar]
- 39. Skov AR, Toubro S, Bulow J, Krabbe K, Parving H-H, Astrup A: Changes in renal function during weight loss induced by high vs low-protein low fat diets in overweight subjects. Int J Obes 23: 1170–1177, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Hinchliffe SA, Lynch MRJ, Sargent PH, Howard CV, vanVelzen D: The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gyn 99: 296–301, 1992 [DOI] [PubMed] [Google Scholar]
- 41. Manalich R, Reyes L, Herrera M, Melendi C, Fundora I: Relationship between weight at birth and the number and size of renal glomeruli in humans: A histomorphometric study. Kidney Int 58: 770–773, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Andersen MJF, Mogensen CE: Relationship between renal size and function in normal subjects. Acta Radiol Diagn 14: 209–213, 1973 [DOI] [PubMed] [Google Scholar]
- 43. Aperia A, Broberger O, Ekengren K, Wikstad I: Relationship between area and function of the kidney in well-defined childhood nephropathies. Acta Radiol Diagn 19: 186–196, 1978 [DOI] [PubMed] [Google Scholar]
- 44. Haugstvedt S, Jacobsson B, Bjure J, Cappelen-Smith J, Granerus G: Kidney split function in children: A comparitive study between renography and planimetry from urography. Scand J Urol Nephrol 14: 269–274, 1980 [DOI] [PubMed] [Google Scholar]
- 45. Troell S, Berg U, Johansson B, Wikstad I: Comparison between renal parenchymal sonographic volume, renal parenchymal urographic area, glomerular filtration rate and renal plasma flow in children. Scand J Urol Nephrol 22: 207–214, 1988 [DOI] [PubMed] [Google Scholar]
- 46. Ladefoged J, Pedersen F: Relationship between roentgenological size of the kidney and the kidney function. J Urol 99: 239–240, 1968 [DOI] [PubMed] [Google Scholar]
- 47. Burkhardt H, Hahn T, Gladisch R: Is kidney size a useful predictor of renal function in the elderly? Clin Nephrol 59: 415–422, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Lin J, Knight EL, Hogan ML, Singh AK: A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol 14: 2573–2580, 2003 [DOI] [PubMed] [Google Scholar]