Summary
Background and objectives
Iron (Fe) overload may complicate parenteral Fe therapy used to enhance the efficacy of erythropoietic-stimulating agents in the treatment of anemia of chronic kidney disease. However, serum Fe markers are influenced by inflammation or malignancy and may not accurately reflect the amount of body Fe.
Design, setting, participants, & measurements
We studied the relationship between parenteral Fe therapy, conventional serum Fe markers, and liver iron concentration (LIC) measured using magnetic resonance R2 relaxometry (FerriScan) in 25 Fe-deficient predialysis chronic kidney disease patients before and 2 and 12 weeks after single high-dose intravenous Fe and in 15 chronic hemodialysis patients with elevated serum ferritin (>500 μg/L).
Results
In predialysis patients, there was strong dose dependency between the administered Fe dose and changes in LIC at weeks 2 and 12; however, no dose dependency between Fe dose and changes in ferritin or transferrin saturation (TSAT) were observed. In hemodialysis patients, LIC correlated with the cumulative Fe dose and duration of dialysis but not with current ferritin or TSAT. The cumulative Fe dose remained a significant independent predictor of LIC in a multiple regression model. Two dialysis patients who received >6 g parenteral Fe had substantially elevated LIC >130 μmol/g, which is associated with hemochromatosis.
Conclusions
In Fe-deficient predialysis patients, intravenous Fe therapy is associated with increases in LIC unrelated to changes in conventional Fe markers. In hemodialysis patients, TSAT and ferritin are poor indicators of body Fe load, and some patients have LICs similar to those found in hemochromatosis.
Introduction
Chronic kidney disease (CKD) is commonly accompanied by the development of anemia that is characterized by poor intestinal iron (Fe) absorption, low ferritin levels, and requirement for parenteral Fe supplementation (1–3). There is good evidence that intravenous Fe therapy should be administered as a standard therapy for Fe deficiency (ferritin < 100 μg/L, transferrin saturation [TSAT] < 20%) in conjunction with or before therapy with erythropoietic-stimulating agents in anemia of CKD and to maintain adequate Fe stores in dialysis patients (4,5). Hemoglobin and ferritin concentrations have been shown to increase significantly in CKD patients after intravenous Fe compared with oral Fe therapy (6). For ease and convenience, intravenous Fe 500 to 1500 mg can be administered over a 4-hour period (7). Dialysis patients regularly require 50 to 200 mg monthly of intravenous Fe to maintain their Fe stores (8,9). Although it is generally assumed that restoration of hemoglobin toward the target range is a good outcome of this therapy, it is well known that Fe overload and Fe toxicity may be adverse consequences of this therapy. Both the American and Australian guidelines (4,5) recommend caution with the routine administration of intravenous Fe if the serum ferritin is >500 μg/L. However, the upper limit of a safe serum ferritin level remains unresolved (10). Serum ferritin levels between 300 and 1200 μg/L are associated with the lowest mortality risk after adjusting for malnutrition and inflammation (11), and only serum ferritin levels >2000 μg/L have been associated with hemochromatosis in dialysis patients (3). Unfortunately, serum Fe markers do not accurately reflect the amount of Fe in the body, because factors such as infections, inflammatory diseases, or malignancy can alter ferritin levels in the body. Thus, some authors have suggested that no specific level of serum ferritin should be stated as an upper limit for Fe treatment (10).
Recently, a noninvasive magnetic resonance imaging (MRI)-based R2 relaxometry method (FerriScan) was developed that accurately measures liver Fe concentration (LIC) in a range of diseases (12). LICs >130 μmol/g dry weight are associated with increased risks of liver injury (13), whereas cardiac toxicity occurs when LIC exceeds 270 μmol/g (14). Liver biopsy is rarely performed in subjects with CKD, and retrospective quantitative phlebotomy as a direct measure of total body Fe burden is not possible because of the anemic status of the patients and their consequent limited tolerance of phlebotomy. Given the uncertainties surrounding the accuracy of serum Fe biochemistry as a direct measure of Fe status, R2 relaxometry presents a readily available and validated noninvasive method of measuring LICs in vivo to assess the relationship between conventional biochemical markers of Fe stores, intravenous Fe therapy, and liver Fe load in CKD patients. The aims of this study were to (1) prospectively evaluate the effects of a single high-dose Fe infusion on LIC in Fe-deficient CKD stage 3 to 5 patients who had previously not received parenteral Fe and (2) characterize the extent of hepatic Fe overload in hemodialysis patients receiving regular intravenous Fe replacement.
Materials and Methods
Prospective Study in Iron-Deficient CKD Subjects
The primary objective of this substudy was to prospectively assess LIC before and after a single high-dose intravenous Fe infusion and to compare LIC with conventional markers of Fe stores. This cohort comprised patients with stage 3 to 5 CKD (estimated GFR, 10–60 ml/min per 1.73 m2), who were scheduled to receive 10 to 20 mg/kg of intravenous Fe-polymaltose (Ferrosig, Sigma Pharmaceuticals, Croydon, Australia) if they had (1) anemia, using World Health Organization definitions of hemoglobin <120 g/L for women and <130 g/L for men (15), (2) Fe deficiency (TSAT < 20% and/or serum ferritin < 100 μg/L), (3) not previously received parenteral Fe, and (4) no contraindications to MRI. The first MRI assessment of LIC was scheduled on the day of intravenous Fe therapy just before infusion; postinfusion scans and biochemical studies were obtained at weeks 2 and 12. The primary endpoint was calculated as the change in mean LIC from baseline to follow-up. A sample size of 20 was expected to provide 80% power to detect a change in mean LIC over time of 7.7 μmol/g dry weight, equating to an effect size of 66%, using a paired t test with a two-tailed α level of 0.05 (16). This was based on an SD of LIC of 11.7 μmol/g reported in hemodialysis patients (17).
Cross-Sectional Study in Hemodialysis Subjects
The primary objective of this substudy was to assess whether LIC in hemodialysis patients with serum ferritin levels above the upper recommended guidelines was best predicted by ferritin, TSAT, or cumulative Fe dose. This cohort comprised chronic hemodialysis patients treated between September 2009 and February 2010. The inclusion criteria were (1) maintenance hemodialysis of ≥12 months, (2) native arteriovenous fistula, (3) parenteral Fe therapy (Ferrosig, usually 50 to 200 mg monthly) over the past 12 months, (4) serum ferritin >500 μg/L on two consecutive occasions 3 months apart, (5) full history of total parenteral Fe treatment since start of dialysis, (6) alcohol consumption of less than two standard drinks per day, and (7) exclusion of significant liver disease. Serologic testing was used to exclude chronic viral hepatitis B or C infection. Serum Fe markers and LIC-R2 relaxometry were measured at least 2 weeks after the last intravenous Fe dose. These studies were approved by the Hospital Ethics Committee, and all subjects consented to participation in the study.
MRI
MRI was conducted on a 1.5-T whole body imaging unit (Siemens MAGNETOM Vision Plus) using a recently described method (12). This method measures the mean liver proton transverse relaxation rate (R2), which has a high sensitivity and specificity for prediction of LIC (12). Images were acquired in partial Fourier mode with a multislice single-spin-echo pulse sequence, with the pulse repetition time = 2500 ms, spin echo times = 6, 9, 12, 15, and 18 ms, and slice thickness = 5 mm. All spin-echo sequences were performed using fixed gain control. Subjects were scanned together with a bag of normal saline, which acted as a long T2 standard to enable correction of any gain drift between different image acquisitions. Subjects were positioned to locate the liver central to the chest coil. Eleven slices were collected, with the gap between slices being 5 mm. R2 values were calculated throughout the largest cross-section of the liver by curve fitting the equation for the bi-exponential decay in transverse magnetization after an single-spin-echo pulse sequence to the voxel intensity data as a function of echo time with radiofrequency field intensity–weighted spin density projection (18). Mean LIC was calculated from mean R2 values as previously reported (12). The accepted normal range of LIC in healthy subjects is <30 μmol/g (Figure 1) (19). Serum Fe and ferritin concentrations and TSAT were measured or derived using standard methods.
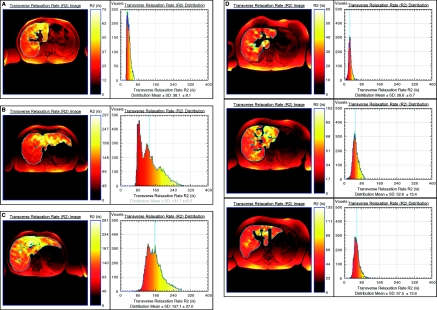
Figure 1.
Liver R2 images and distributions for four subjects with different degrees of iron (Fe) overload and pathologic conditions: (A) healthy control, (B) hereditary hemochromatosis, (C) ESKD patient with >6 g cumulative Fe dose, and (D) Fe deficient CKD patient before and 2 and 12 weeks after 1 g intravenous Fe (top to bottom). Note that the liver R2 images are superimposed on standard spin-echo images for registration purposes. Note that to enable visualization of the heterogeneity of R2 within each liver, the color scale within each liver is adjusted for each image such that zero corresponds to voxel R2 of zero, whereas the maximum of the color scale is scaled to the maximum R2 value within the liver.
Statistical Analyses
Statistical analysis was performed with Systat software package version 10 (SPSS, Chicago, IL). Results are presented as mean ± SD for continuous variables and number and percentage for categorical variables. Differences between subjects were tested by nonparametric Mann-Whitney U test statistic for continuous data and Fisher exact test for categorical data. ANOVA was used to compare within-group changes over time in the first substudy. Non-CKD patients with secondary Fe overload and LIC >60 μmol/g are considered eligible for chelation therapy, whereas those with LIC >130 μmol/g are at increased risk of liver injury (13). Thus, these values were used as threshold levels for determination of the number of dialysis patients above and below these limits. All P values quoted are two tailed.
Results
Prospective Study in Iron-Deficient CKD Subjects
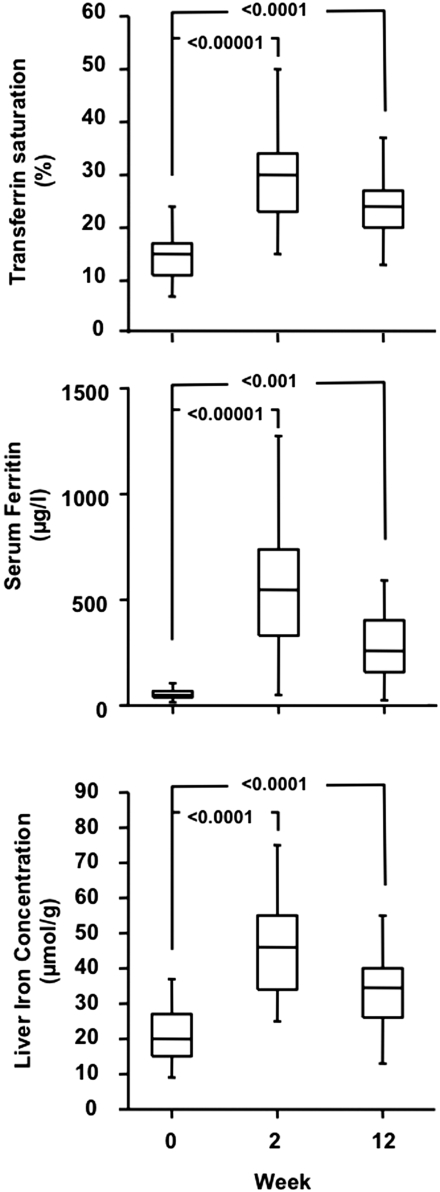
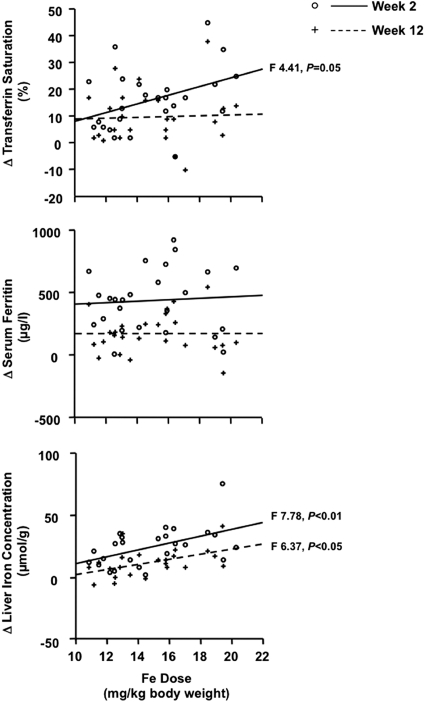
Twenty-five CKD patients (stage 3, n = 6; stage 4, n = 14; stage 5, n = 5) qualified for intravenous Fe infusion according to the hospital protocol and completed the three follow-up visits. Baseline characteristics of these patients are summarized in Table 1. The dose of intravenous Fe ranged from 1000 to 1500 mg, and the average Fe dose per body weight was 15 ± 3 mg/kg. At week 2, the hemoglobin averaged 113 ± 12 g/L (P = not significant versus baseline), TSAT averaged 31 ± 12% (P < 0.00001 versus baseline), and ferritin averaged 563 ± 282 μg/L (P < 0.0001 versus baseline), and at week 12, the mean hemoglobin was 120 ± 13 g/L (P < 0.00001 versus baseline), TSAT was 25 ± 9% (P < 0.0001 versus baseline), and ferritin was 299 ± 221 μg/L (P < 0.001 versus baseline; Figure 2). LIC increased significantly to 46.1 ± 15.6 μmol/g at week 2 and 33.7 ± 11.3 μmol/g at week 12 (Figure 2). A comparison of LIC at week 2 in subjects receiving Fe amounts below or above the median dose showed that LIC remained ≤30 μmol/g in 25% of subjects given <14.5mg /kg Fe, whereas all subjects receiving ≥14.5 mg/kg of Fe had LIC >30 μmol/g. The mean increase in LIC from baseline was 25.4 ± 15.6 μmol/g (152 ± 126%) at week 2 and 13.0 ± 11.2 μmol/g (80 ± 89%) at week 12. The change in TSAT from baseline tended to show a dose dependency at week 2 but not at week 12, whereas there was no dose dependency for changes in serum ferritin either at week 2 or 12. The increase in LIC showed a clear dependence on the administered Fe dose at both weeks 2 and 12 (Figure 3).
Table 1.
Baseline characteristics of the iron-deficient CKD cohort and the hyperferritinemic hemodialysis cohort
| Cohort | CKD | HD |
|---|---|---|
| N | 25 | 15 |
| Age (years) | 65 ± 15 | 61 ± 12 |
| Gender (male/female) | 17/8 | 10/5 |
| Serum creatinine | 296 ± 130 | — |
| Hemoglobin (g/L) | 107 ± 8 | 116 ± 9 |
| Transferrin saturation (%) | 15 ± 6 | 31 ± 10 |
| Ferritin (mg/L) | 67 ± 56 | 782 ± 170 |
| CRP (mg/L) | 1.2 ± 2.6 | 4.9 ± 4.1 |
| Transfusions (N) | 0 | 3.8 ± 2.7 |
| Time on dialysis (days) | — | 899 ± 353 |
| Cumulative Fe dose (mg) | — | 6560 ± 3098 |
| Mean monthly Fe dose (mg) | — | 217 ± 87 |
| Weekly erythropoietin dose (U/wk) | — | 7870 ± 4360 |
| Liver Fe concentration (μmol/g) | 20.6 ± 7.9 | 81.2 ± 58.3 |
Figure 2.
Baseline and follow-up serum ferritin, transferrin saturation, and liver iron concentration in 25 iron-deficient CKD patients not yet on dialysis after single high-dose (mean, 15 ± 3 mg/kg body weight) parenteral Fe administration. Boxes represent median with interquartile range, and bars represent minimum and maximum values. P values for differences versus baseline are reported.
Figure 3.
Changes, compared with baseline, in serum ferritin, transferrin saturation, and liver iron concentration in 25 iron-deficient CKD patients not yet on dialysis in relation to the administered Fe dose. Individual changes and regression line are (○/——) at week 2 and (+/-----) at week 12, respectively.
Cross-Sectional Study in Hemodialysis Subjects
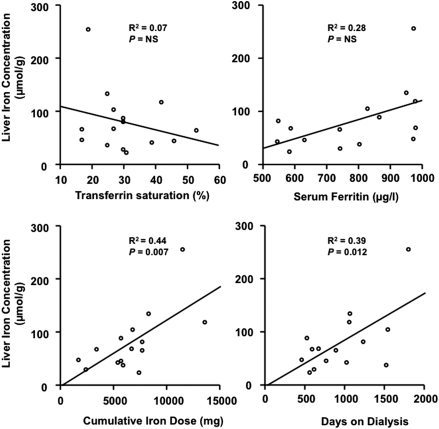
Twenty patients were screened to participate in the study, but five failed to undergo R2 relaxometry (claustrophobia, n = 1; pacemaker, n = 1; canceled two appointments, n = 2; malignancy, n = 1). Patients were dialyzed three times weekly for 272 ± 24 minutes on a Fresenius 4008 machine using FX80 high-flux dialyzers at an average blood flow of 329 ± 25 ml/min. Baseline characteristics of these patients are summarized in Table 1. LIC correlated with the cumulative Fe dose (R2 = 0.44, P < 0.01) and the duration of dialysis (R2 = 0.39, P < 0.05) but not with current ferritin or TSAT (Figure 4). The cumulative Fe dose remained a significant independent predictor of LIC (R2 = 0.69, P < 0.05) in a multiple regression model that included C-reactive protein (CRP), ferritin, TSAT, current and cumulative Fe dose, and duration of dialysis. Nine patients (60%) had LIC >60 μmol/g. All seven subjects with ≥6000 mg cumulative Fe dose had LIC >60 μmol/g compared with only two of eight subjects with cumulative Fe dose <6000 mg. Two of seven subjects with ≥6000 mg cumulative Fe dose had LIC >130 μmol/g (Figure 1).
Figure 4.
Relationship between liver iron concentration and serum ferritin, transferrin saturation, cumulative Fe dose, or duration of dialysis treatment in 15 hemodialysis patients with serum ferritin levels >500 μg/L.
Discussion
Our study showed that in predialysis Fe-deficient CKD patients and ESKD patients on hemodialysis, intravenous Fe therapy can cause significant increases in LIC that are not related to or predicted by conventional serum markers of Fe metabolism such as TSAT and ferritin. LIC was assessed using a noninvasive MRI-based technique using tissue proton transverse relaxation rates (R2). The accuracy and precision of this technology have been shown in several previous studies comparing R2-LIC with liver biopsy-LIC in non-CKD patients (12,20–22).
In the first substudy, we prospectively assessed the response of a single, high-dose Fe infusion in Fe-deficient predialysis patients. Clinically, the primary aim of this therapy is to correct anemia, and in our subjects, hemoglobin increased significantly from 107 ± 8 to 120 ± 13 g/L by 12 weeks after Fe administration, as previously reported (6). Compared with baseline, 2 weeks after intravenous Fe, there was an 11-fold increase in serum ferritin and a 2-fold increase in TSAT. However, these changes are not expected to reflect Fe overload, because changes in Fe indicators may take up to 14 days to reach steady state after intravenous Fe (23). At 12 weeks, changes in TSAT and serum ferritin compared with baseline were still significant, although all subjects had TSAT <50%, and all but one subject had serum ferritin <500 μg/L as recommended by guidelines (4,5). On the other hand, 56% of the predialysis CKD subjects had LIC in excess of the upper limit of normal (<30 μmol/g) 12 weeks after high-dose parenteral Fe, although none had levels >60 μmol/g. In our cohort, only changes in LIC, but not serum ferritin or TSAT, showed a dose dependency to the administered Fe dose. These findings suggest that, in Fe-deficient CKD patients, a single, high-dose Fe infusion only leads to a transient liver Fe loading that is dependent on the amount of Fe administered. Although increases in LIC in predialysis CKD patients after Fe infusion are of smaller magnitude and transient, and thus seem to be safe, repeated infusions over intervals <12 weeks could result in significant Fe load.
In the second substudy, we deliberately selected CKD patients on dialysis with serum ferritin levels above what is considered to be the safety threshold (4,5). Thus, one would expect the majority of these patients to show significantly increased LIC and serum ferritin to predict LIC. Our findings suggest that this may occur only in hemodialysis patients with persistently increased serum ferritin levels who have received considerable amounts of parenteral Fe. Interestingly, we found no correlation between serum Fe markers and LIC, but a strong correlation between LIC and both the cumulative Fe dose received and the time elapsed since start of dialysis. This is an interesting observation that suggests that uremia itself may progressively lead to Fe accumulation in tissues over time.
Almost 40% of all hemodialysis patients in Australia have “unsafe” serum ferritin levels >500 μg/L (24). The perception of toxicity of therapeutic Fe in CKD patients is based largely on a limited number of observational studies reporting an increased infection rate in Fe-deficient subjects on Fe replacement (25) or in CKD patients with high ferritin concentrations (26) and increased CVD and death in dialysis patients (27,28). Serum ferritin was shown to correlate with LIC assessed noninvasively by a superconducting quantum interference device (17). Although our study is limited by small sample size and the selection of hemodialysis patients with elevated serum ferritin and variable duration of dialysis, it is consistent with previous postmortem studies showing a lack of association between excessive tissue Fe and high serum ferritin but a strong association with duration of dialysis for >3 years and a cumulative Fe dose of between 6 and 25 g (29). Our results do not corroborate the findings by Canavese et al. (17), and it is possible that differences in timing of the parenteral Fe administration in relation to the timing of assay for TSAT and ferritin (23) may account for the observed differences between studies. A lack of correlation between serum ferritin levels and LIC has also been reported in other diseases, such as the common liver disorder nonalcoholic fatty liver disease (19). Although discrepancies between LIC and ferritin levels could be influenced by the presence of overt or subclinical inflammatory states, this seems unlikely because the mean CRP in our study (4.9 ± 4.1 mg/L) did not differ from the mean CRP in the study of Canavese et al. (6.2 ± 8.3 mg/L). Proinflammatory cytokines such as IL-1β, IL-6, and TNF-α are known to increase the synthesis of ferritin through increased translation of ferritin mRNA (30,31). It is possible that higher amounts of ferritin may trap more body Fe and protect the individual against worsening infection, because free Fe is believed to enhance the formation of free oxygen radicals, which are the mediators of cell damage in infection-associated inflammation. Hence, inflammation-induced hyperferritinemia may result in a so-called “functional Fe deficiency,” which may be useful in “acute” inflammation by Fe containment in the reticuloendothelial system sites but harmful under “chronic” inflammation by leading to anemia of chronic disease. This hypothesis is supported by recent findings of increased circulating IL-6 levels that are reduced after treatment with pentoxifylline in patients with stage 4 to 5 CKD (32).
Hemodialysis treatment can precipitate a recurrent inflammatory state (33,34), and hemodialysis patients without native vascular access are notably more prone to recurrent infection (35). It is therefore not surprising that, in our hemodialysis cohort, serum ferritin does not provide an accurate assessment of body Fe stores. Our findings of an inverse association between LIC and TSAT suggest that the latter is also not helpful in the setting of hemodialysis. Indeed, TSAT does not correlate with LIC even in the context of inherited Fe overload disorders (36). It is possible that substantial Fe overload, similar to that observed in chronically transfused patients, is occurring in CKD subjects who are receiving excessive Fe therapy. Indeed, 60% of our hemodialysis cohort had LIC >60 μmol/g, a threshold above which chelation therapy may be recommended in non-CKD patients with secondary Fe overload disorders (14), and 13% had LIC >130 μmol/g, a level associated with increased risks of liver injury and fibrosis in hemochromatosis (37) (Figure 1). Whether Fe overload in hemodialysis patients leads to end organ damage involving the heart, liver, or brain remains unknown, and thus, we cannot currently advocate chelation therapy in dialysis patients with LIC >60 μmol/g.
The most objective parameters of Fe overload are those reliant on direct measurement of organ Fe concentration. Although liver biopsy provides quantitative measurement of LIC, it is invasive, subject to sampling error, and not suited for use (nor likely to be accepted) in subjects who already have significant comorbidity. Thus, we did not perform routine liver biopsies for the purposes of this study, because there was no clinical hepatologic indication for the procedure in our study subjects. The most accurate and noninvasive methods are reliant on MRI (19,22). In nonuremic subjects with various degrees of Fe overload, an excellent correlation between R2-LIC and LIC quantified using liver biopsy specimens has been shown (12,20,21,38). It could be argued that this relationship remains to be shown in uremic patients because of the lack of liver biopsy studies. Nevertheless, MRI-based R2-relaxometry is solely dependent on the physical properties of Fe and its tissue concentration, whereas the effects of the tissue composition or the milieu are negligible. We developed and validated R2 relaxometry methods based on MRI (FerriScan) that are able to accurately quantitate hepatic Fe concentration in CKD patients and that can be undertaken in 10 minutes. Clearly, we do not advocate for R2-LIC to be routinely used in all dialysis subjects receiving erythropoietic-stimulating agent and Fe therapy; however, selected cases may benefit from R2-LIC measurement to determine whether Fe overload is present and whether Fe administration should be withheld. We suggest that this is most likely to yield clinically meaningful results in hyperferritinemic hemodialysis patients who have received large amounts of parenteral Fe (>6000 mg) and are currently requiring regular Fe administration for the management of their anemia. The issue of how to treat Fe overload in this setting is problematic with no proven therapy. Improved knowledge of key regulators of Fe metabolism should result in further study and validation of new therapies for the treatment of Fe overload in CKD.
Disclosures
T.G.S. is a shareholder of Resonance Health Ltd. and is a member of the Board of Directors of Resonance Health Ltd., the company that provides the FerriScan® service.
Acknowledgments
This work was supported by a Nephrology Research Grant, Roche, Australia. J.K.O. is the recipient of a National Health and Medical Research Council of Australia Practitioner Fellowship.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Carter RA, Hawkins JB, Robinson BH: Fe metabolism in the anaemia of chronic renal failure. Effects of dialysis and of parenteral iron. BMJ 3: 206–210, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Milman N: Fe absorption measured by whole body counting and the relation to marrow Fe stores in chronic uremia. Clin Nephrol 17: 77–81, 1982 [PubMed] [Google Scholar]
- 3. Kalantar-Zadeh K, Rodriguez RA, Humphreys MH: Association between serum ferritin and measures of inflammation, nutrition and Fe in haemodialysis patients. Nephrol Dial Transplant 19: 141–149, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Roger S: The CARI guidelines. Haematological targets. Iron Nephrol (Carlton) 11[Suppl 1]: S217–S229, 2006 [DOI] [PubMed] [Google Scholar]
- 5. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis 47: S11–S145, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Charytan C, Qunibi W, Bailie GR: Comparison of intravenous Fe sucrose to oral Fe in the treatment of anemic patients with chronic kidney disease not on dialysis. Nephron Clin Pract 100: c55–c62, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Auerbach M, Witt D, Toler W, Fierstein M, Lerner RG, Ballard H: Clinical use of the total dose intravenous infusion of Fe dextran. J Lab Clin Med 111: 566–570, 1988 [PubMed] [Google Scholar]
- 8. Macdougall IC, Tucker B, Thompson J, Tomson CR, Baker LR, Raine AE: A randomized controlled study of Fe supplementation in patients treated with erythropoietin. Kidney Int 50: 1694–1699, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Taylor JE, Peat N, Porter C, Morgan AG: Regular low-dose intravenous Fe therapy improves response to erythropoietin in haemodialysis patients. Nephrol Dial Transplant 11: 1079–1083, 1996 [PubMed] [Google Scholar]
- 10. Kalantar-Zadeh K, Lee GH: The fascinating but deceptive ferritin: To measure it or not to measure it in chronic kidney disease? Clin J Am Soc Nephrol 1[Suppl 1]: S9–S18, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Kalantar-Zadeh K, Regidor DL, McAllister CJ, Michael B, Warnock DG: Time-dependent associations between Fe and mortality in hemodialysis patients. J Am Soc Nephrol 16: 3070–3080, 2005 [DOI] [PubMed] [Google Scholar]
- 12. St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK, Pootrakul P, Robins E, Lindeman R: Noninvasive measurement and imaging of liver Fe concentrations using proton magnetic resonance. Blood 105: 855–861, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Cartwright GE, Edwards CQ, Kravitz K, Skolnick M, Amos DB, Johnson A, Buskjaer L: Hereditary hemochromatosis. Phenotypic expression of the disease. N Engl J Med 301: 175–179, 1979 [DOI] [PubMed] [Google Scholar]
- 14. Olivieri NF, Brittenham GM: Iron-chelating therapy and the treatment of thalassemia. Blood 89: 739–761, 1997 [PubMed] [Google Scholar]
- 15. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B: Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr 12: 444–454, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Lenth RV: Statistical power calculations. J Anim Sci 85: E24–E29, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Canavese C, Bergamo D, Ciccone G, Longo F, Fop F, Thea A, Martina G, Piga A: Validation of serum ferritin values by magnetic susceptometry in predicting Fe overload in dialysis patients. Kidney Int 65: 1091–1098, 2004 [DOI] [PubMed] [Google Scholar]
- 18. St Pierre TG, Clark PR, Chua-Anusorn W: Single spin-echo proton transverse relaxometry of iron-loaded liver. NMR Biomed 17: 446–458, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Olynyk JK, Gan E, Tan T: Predicting Fe overload in hyperferritinemia. Clin Gastroenterol Hepatol 7: 359–362, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Papakonstantinou OG, Maris TG, Kostaridou V, Gouliamos AD, Koutoulas GK, Kalovidouris AE, Papavassiliou GB, Kordas G, Kattamis C, Vlahos LJ, Papavassiliou CG: Assessment of liver Fe overload by T2-quantitative magnetic resonance imaging: correlation of T2-QMRI measurements with serum ferritin concentration and histologic grading of siderosis. Magn Reson Imaging 13: 967–977, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Clark PR, St Pierre TG: Quantitative mapping of transverse relaxivity (1/T(2)) in hepatic Fe overload: A single spin-echo imaging methodology. Magn Reson Imaging 18: 431–438, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Clark PR, Chua-Anusorn W, St Pierre TG: Proton transverse relaxation rate (R2) images of liver tissue: Mapping local tissue Fe concentrations with MRI. Magn Reson Med 49: 572–575, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Van Wyck DB, Roppolo M, Martinez CO, Mazey RM, McMurray S: A randomized, controlled trial comparing IV Fe sucrose to oral Fe in anemic patients with nondialysis-dependent CKD. Kidney Int 68: 2846–2856, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Polkinghorne K, McDonald S, Excell L, Livingston B, Dent H: Ferritin and transferrin saturation. 2009 ANZDATA Registry Annual Data Report. Available at: http://www.anzdata.org.au/anzdata/AnzdataReport/32ndReport/Ch05.pdf Accessed August 27, 2010
- 25. Murray MJ, Murray AB, Murray MB, Murray CJ: The adverse effect of Fe repletion on the course of certain infections. BMJ 2: 1113–1115, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seifert A, von Herrath D, Schaefer K: Fe overload, but not treatment with desferrioxamine favours the development of septicemia in patients on maintenance hemodialysis. Q J Med 65: 1015–1024, 1987 [PubMed] [Google Scholar]
- 27. Bregman H, Gelfand MC: Fe overload in patients on maintenance hemodialysis. Int J Artif Organs 4: 56–57, 1981 [PubMed] [Google Scholar]
- 28. Drueke T, Witko-Sarsat V, Massy Z, Descamps-Latscha B, Guerin AP, Marchais SJ, Gausson V, London GM: Fe therapy, advanced oxidation protein products, and carotid artery intima-media thickness in end-stage renal disease. Circulation 106: 2212–2217, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Gokal R, Millard PR, Weatherall DJ, Callender ST, Ledingham JG, Oliver DO: Fe metabolism in haemodialysis patients. A study of the management of Fe therapy and overload. Q J Med 48: 369–391, 1979 [PubMed] [Google Scholar]
- 30. Rogers J, Lacroix L, Durmowitz G, Kasschau K, Andriotakis J, Bridges KR: The role of cytokines in the regulation of ferritin expression. Adv Exp Med Biol 356: 127–132, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Harrison PM, Arosio P: The ferritins: Molecular properties, Fe storage function and cellular regulation. Biochim Biophys Acta 1275: 161–203, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Ferrari P, Mallon D, Trinder D, Olynyk JK: Pentoxifylline improves haemoglobin and interleukin-6 levels in chronic kidney disease. Nephrology 15: 344–349, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Bossola M, Sanguinetti M, Scribano D, Zuppi C, Giungi S, Luciani G, Torelli R, Posteraro B, Fadda G, Tazza L: Circulating bacterial-derived DNA fragments and markers of inflammation in chronic hemodialysis patients. Clin J Am Soc Nephrol 4: 379–385, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Samouilidou EC, Grapsa EJ, Kakavas I, Lagouranis A, Agrogiannis B: Oxidative stress markers and C-reactive protein in end-stage renal failure patients on dialysis. Int Urol Nephrol 35: 393–397, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Xue JL, Dahl D, Ebben JP, Collins AJ: The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis 42: 1013–1019, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Bassett ML, Halliday JW, Ferris RA, Powell LW: Diagnosis of hemochromatosis in young subjects: Predictive accuracy of biochemical screening tests. Gastroenterology 87: 628–633, 1984 [PubMed] [Google Scholar]
- 37. Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, Allen CJ, Farrell DE, Harris JW: Efficacy of deferoxamine in preventing complications of Fe overload in patients with thalassemia major. N Engl J Med 331: 567–573, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, Coates TD: MRI R2 and R2* mapping accurately estimates hepatic Fe concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 106: 1460–1465, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]