Summary
Background and objectives
Renal function is an important predictor of survival in cirrhosis and liver transplantation. GFR estimates using serum cystatin C (CysC) are proposed as better predictors of renal function than ones on the basis of serum creatinine (Cr). Our aims were: (1) evaluate correlations between serum CysC and different methods of creatinine measurements; (2) compare CysC and Cr GFR formulas with 51Cr-EDTA; and (3) evaluate liver-related parameters potentially influencing GFR.
Design, setting, participants, & measurements
254 blood samples in 65 patients with cirrhosis correlating CysC with four Cr methods were used; another 74 patients comparing 51Cr-EDTA GFR to Modification of Diet in Renal Disease and Larsson and Hoek formulas for CysC were also included. Agreement was assessed using Bland-Altman plots and concordance correlation coefficients. Multivariate linear regression analysis was used for GFR predictors.
Results
Serum CysC correlated modestly with O'Leary modified Jaffe, compensated kinetic Jaffe, enzymatic creatinine, and standard kinetic Jaffe 0.72/0.71/0.72/0.72 (all P < 0.001). Bland-Altman agreement with 51Cr-EDTA GFR was poor; the best agreement was Modification of Diet in Renal Disease (concordance 0.61; 95% CI, 0.47 to 0.71); the worst agreement was the Hoek formula (concordance 0.46; 95% CI, 0.27 to 0.61). A new GFR formula including the Child-Pugh score improved the accuracy of Cr GFR formulas compared with 51Cr-EDTA GFR.
Conclusions
Estimated GFR in cirrhosis is not better with CysC formulas compared with creatinine ones: specific formulas may be necessary.
Introduction
Renal dysfunction is a well established predictor of increased mortality in both acute liver failure and cirrhosis, particularly after the development of complications, such as sepsis (1), and after liver transplantation (2). Serum creatinine (Cr) is only an indirect marker of renal function, i.e., of GFR. Measurement of Cr suffers from a variety of interferences and is not standardized (3,4). We and others previously reported (4,5) that different methods used for measuring Cr give significantly different values. Recently we found a lack of agreement in creatinine values (6) between different laboratories that used the same method of measurement. We also reported (7) that Cr values significantly overestimate renal function in women. Other factors affecting Cr are also known (8). Thus the most frequently used GFR formulas (8), the Cockcroft-Gault (C-G) (9) and Modification of Diet in Renal Disease (MDRD) (10), use several corrections for age, gender, ethnicity, and body weight. Different creatinine-based formulas were evaluated (11) in 1447 patients with cirrhosis, all transplant candidates, using 125I-iothalamate clearance as a reference standard; the four-, five-, and six-variable MDRD equations were similar and had greater accuracy than the C-G formula, but the concordance with 125I-iothalamate clearance was lower than the equivalent MDRD estimations in other populations.
Inulin clearance and other direct methods using injected exogenous radiolabeled substances (51Cr-EDTA,125I-iothalamate, and 99mTc-DTPA) are the most accurate to assess renal function. The 51Cr-EDTA method is an accepted substitute of the “gold standard” inulin clearance (12).
Serum cystatin C (CysC) is a low molecular weight protein functioning as an extracellular inhibitor of cysteine proteases (13). CysC is freely filtered by the renal glomeruli and subsequently metabolized in the proximal tubules. Given these features and the reported independence of CysC from age, gender, and body composition (14), it has been considered a more sensitive indicator of renal function compared with Cr in several disease groups (15) including cirrhosis (16–18).
Thus several CysC-based GFR equations have been derived (19), all in nonliver disease patients. Despite being more accurate than Cr-based formulas (15), they still lacked significant correlation with direct methods of GFR estimation. To date only one study evaluated (20) CysC GFR, using the Larsson et al. (21) and Hoek et al. (22) formulas in patients with cirrhosis, comparing them with MDRD (10) and C-G (9). Inulin clearance was the gold standard. The CysC formulas were more accurate than the Cr formulas but had significantly different values of GFR in comparison with inulin clearance.
In cirrhosis, CysC has been proposed as a marker of liver disease stage (23). Significant differences were found in CysC values but not Cr values between Child-Pugh class A, B, and C patients (18). Cr and CysC concentrations correlated well with the severity of liver disease in 180 patients (24), but a gold standard to assess renal function was not used. Increased CysC values in cirrhosis may be related to increased production, secondary to active inflammatory and fibrotic processes (25), decreased renal function, or both.
Our aims were: (1) to assess correlations between serum CysC and different Cr measurement methods and to evaluate whether bilirubin (Bil) concentration affects CysC; (2) to compare “true” GFR using a gold standard method and estimated GFR (eGFR) using Cr and CysC; and (3) to investigate whether a new formula to estimate GFR in cirrhosis using parameters related to liver function could be derived.
Materials and Methods
We used 256 consecutive blood samples obtained during routine clinical care from 65 patients with cirrhosis, at the Royal Free Hospital, being part of a previously reported cohort (4) and samples from a separate cohort of 74 patients, with cirrhosis candidates for liver transplantation who had 51Cr-EDTA GFR measurement (C51Cr-EDTA) as pretransplant assessment. We compared C51Cr-EDTA with Cr and CysC-GFR formulas. In the 74 transplant candidates, stepwise multivariate linear regression analysis was used to identify any liver disease-specific factors influencing renal function to derive a new GFR model. We tested bilirubin interference with CysC with dilution/titration curves in blood samples taken from five patients with a median bilirubin of 582 μmol/L (range, 303 to 639 μmol/L).
Clinical, hematologic, and biochemical data were collected on the day of the C51Cr-EDTA. The Child-Pugh (CPT) (26) and Model for End-stage Liver Disease (MELD) (http://www.unos.org) scoring systems were calculated. Cirrhosis, ascites, encephalopathy, and hepatocellular carcinoma (HCC) were diagnosed by clinical, imaging, and histologic criteria. Patients consented for the 51Cr-EDTA clearance results to be used. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Analyses in the First Cohort
For serum creatinine measurement we evaluated four commonly used methods in the UK and the USA (27): (1) O'Leary modified Jaffe (mJCr): potassium ferricyanide was used to oxidize bilirubin to biliverdin (prestep), and an increase in absorbance was measured at 505 nm and blanking at 570 nm; (2) compensated (rate blanked) kinetic Jaffe (cJCr): measured increase in absorbance at 505 nm with blanking at 570 nm; (3) enzymatic creatinine (ECr): we used a creatininase/creatinase/sarcosine oxidase system with detection at 546 nm and absorbance blanking at 700 nm, and a Roche Modular P unit (Roche Diagnostics, Ltd., Lewes, UK) was used for all three assays, calibrated using a lyophilized human serum-based Cfas calibrator (Roche Diagnostics) standardized using the isotope dilution mass spectrometry method; and (4) standard kinetic Jaffe (JCr): performed on an Olympus AU2700 analyzer (Olympus Diagnostic Systems, Southall, UK); calibration was by the manufacturer's recommended Olympus System calibrator (traceable to the National Institute of Standards and Technology Standard Reference Material [909b, level 2]). It measures increased absorbance at 520 nm and blanking at 800 nm.
CysC was analyzed by immunonephelometry using a BN-ProSpec analyzer (Dade Behring BN-ProSpec). The manufacturer's reference interval for healthy subjects is 0.53 to 0.95 mg/L. Assay sensitivity was 0.005 mg/L; intra-assay and interassay coefficients of variation were 2% and 3.6%, respectively.
Analyses in the Second Cohort
The cJCr and ECr methods when bilirubin >171 μmol/L were used to measure serum creatinine, and the same methodology was used to measure CysC.
The C51Cr-EDTA was performed by sampling blood after intravenous injection of tracer at 2, 4, and 6 hours. GFR was calculated using the slope-intercept technique, correcting for body surface area, and the fast exponential curve recommended by the British Nuclear Medicine Society guidelines (28).
Cr-based GFR was calculated using the four variable MDRD formulas, which are considered the best in adults (12,29) and in cirrhosis (11,20): GFR (mL/min per 1.73 m2) = 186 × (Cr, using the automated compensated [rate blanked] kinetic Jaffe, in μmol/L per 88.4)−1.154 × (age in years)−0.203 × 0.742 (if female) × 1.210 (if African American). Body weight is not required because the results are normalized to 1.73 m2 body surface area.
The two CysC-GFR formulas were: Hoek formula: GFR = −4.32 + 80.35 × 1/CysC in mg/L (22), and Larsson formula: GFR = 77.239 × CysC in mg/L−1.2623 (21). To convert Cr and Bil in mg/dl to μmol/L, we multiplied by 88.4 and 17.1, respectively.
Statistical Analyses
All of the data were analyzed using Medcalc software (Mariakerke, Belgium). Normality was assessed with the Kolmogorov-Smirnov test. Quantitative variables were expressed as the mean values ± SD when parametric or as median values (range) when nonparametric. Before multiple parametric comparisons, the Levene's Test for equality of variances was performed. Parametric data were compared using unpaired t tests and ANOVA tests and nonparametric data by the Mann-Whitney's and Kruskal-Wallis tests. For parametric post tests pairwise subgroup comparison a Student-Newman-Keul's test was used, and the Bonferroni correction was used for nonparametric comparisons. Nonparametric correlations were evaluated by Spearman, and parametric ones were evaluated by Pearson correlation. The chi-square test and Fischer's exact test (when the total number of observations was <20) were used as qualitative tests.
Bland and Altman analysis (30) and the concordance correlation coefficient (31) were used to evaluate agreement. Significance testing was two-sided and set to <0.05.
Results
Comparison of Serum Cystatin C with Different Serum Creatinine Measurements
There were 65 patients with cirrhosis (60% male) with 254 blood samples (Table 1). Median values of bilirubin and INR were 166 μmol/L (range, 8 to 913; 95% CI, 149 to 192) and 1.8 (range, 1 to 8; 95% CI, 1.6 to 1.9), respectively. The median mJCr, cJCr, ECr, and JCr values were 110 μmol/L (range, 56 to 1280; 95% CI, 104 to 122), 81 μmol/L (range, 36 to 1339; 95% CI, 75 to 87), 74 μmol/L (range, 35 to 1146; 95% CI, 70 to 79), and 94 μmol/L (range, 40 to 1212; 95% CI, 87 to 99), respectively (all P < 0.001). The median CysC value was 1.14 mg/dl (range, 0.3 to 4.8; 95% CI, 104 to 122) (Table 1). Correlations between CysC and the four Cr measurements methods were reasonable being 0.72, 0.71, 0.72, and 0.72, respectively, for JCr, cJCr, ECr, and mJCr (all P < 0.001).
Table 1.
Characteristics of 65 patients with cirrhosis (254 samples) at the Royal Free Hospital
| Characteristics | Patients with Cirrhosis (n = 65) | Samples (n = 254) |
|---|---|---|
| Age (yrs) | 51 (26 to 92) | |
| Gender (female/male) | 26/39 | |
| Cause of liver disease (n [%]) | ||
| alcohol | 21 (32) | |
| viral (hepatitis B or C) | 17 (26) | |
| autoimmune | 1 (2) | |
| cryptogenic/NASH | 8 (12) | |
| PBC/PSC | 9 (14) | |
| others | 9 (14) | |
| MELD (using the mJCr) | 24 (7 to 43) | |
| INR (n.r. 0.8 to 1.2) | 1.8 (1 to 8) | |
| Bilirubin (μmol/L) (n.r. <18) | 166 (8 to 913) | |
| mJCr (μmol/L) (n.r. 49 to 120) | 110 (59 to 1.280) | |
| cJCr (μmol/L) | 81 (36 to 1.339) | |
| ECr (μmol/L) | 74 (35 to 1.146) | |
| JCr (μmol/L) | 94 (40 to 1.212) | |
| CysC (mg/dl) (n.r. 0.53 to 0.95) | 1.14 (0.31 to 4.84) |
All of the values are expressed as medians (range) or number (%). MELD, model for end stage liver disease; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; n.r., normal range.
Relationship of Serum Bilirubin to Serum Cystatin C
The groups were subdivided according to bilirubin values: (1) Bil <100 μmol/L (n = 60, 23.6%); (2) between 100 and 199 μmol/L (n = 89, 35%); (3) between 200 and 399 μmol/L (n = 57, 22.4%); or (4) >400 μmol/L (n = 48, 18.9%). The median values for mJCr, cJCr, ECr, JCr, and CysC according to bilirubin concentrations are shown in Table 2. Comparisons of CysC median values between the Bil >400 group and every other group were significant (P < 0.0001 for Bil <100; P < 0.0001 for Bil ≥100 and <200; and P < 0.04 for ≥200 and <400).
Table 2.
Median values of serum creatinine (μmol/L) in 254 samples of 65 patients with cirrhosis as measured by four different methods and of cystatin C (mg/dl) for different categories of raised bilirubin concentrations
| mJCr (n = 254) | cJCr (n = 254) | ECr (n = 254) | JCr (n = 254) | CysC (n = 254) | P | |
|---|---|---|---|---|---|---|
| Bil <100 μmol/L (n = 60) | 84 (59 to 159) | 72 (36 to 171) | 66 (35 to 158) | 79 (48 to 160) | 0.99 (0.3 to 2.6) | All P < 0.0083 except JCr to mJCr, JCr to cJCr, mJCr to ECr, and ECr to cJCr |
| Bil ≥100 and <200 μmol/L (n = 89) | 100 (73 to 404) | 77 (39 to 439) | 73 (37 to 456) | 92 (40 to 416) | 0.99 (0.5 to 3.7) | All P < 0.0083 except ECr to cJCr |
| Bil ≥200 and <400 μmol/L (n = 57) | 129 (82 to 294) | 81 (38 to 289) | 79 (35 to 256) | 94 (56 to 283) | 1.15 (0.4 to 3.1) | All P < 0.0083 except ECr to cJCr and mJCr to JCr |
| Bil ≥400 μmol/L (n = 48) | 174 (102 to 1280) | 106 (46 to 1339) | 91 (36 to 1146) | 119 (67 to 1212) | 1.37 (0.5 to 4.8) | All P < 0.0083 except JCr to cJCr and mJCr to JCr |
| P | All P < 0.001 | All P < 0.001 | All P < 0.001 | All P < 0.001 | All P < 0.001 |
All of the values are expressed as medians (range). The P values after Bonferroni correction are set to <0.0083 comparing between Bil subgroups and for comparisons within each Bil subgroup of the four Cr measurements.
The correlation between CysC and bilirubin was poor (r = 0.42, P < 0.001). Dilution/titration analysis showed that serum CysC concentration was not influenced by bilirubinemia with values as high as 639 μmol/L. The poor correlation of CysC with bilirubin and a much stronger one with Cr indicate that the increase seen with CysC as bilirubin increased is mostly influenced by renal function and not by liver disease. As we previously reported, correlations between the four different creatinine and bilirubin measurements were poor (4) being 0.56, 0.42, 0.38, and 0.44, respectively, for mJCr, cJCr, ECr, and JCr (all P < 0.0001).
Evaluation of 51Cr-EDTA GFR in Cirrhosis and Comparison with MDRD and Cystatin C GFR Formulas
In the 74 patients with cirrhosis, the mean MELD score was 12 ± 5.5, range 2 to 25, and the mean CPT score was 8.2 ± 2.4, range 5 to 14 (Child A/B/C, 22/27/25). Liver disease etiology was: alcohol, 12; viral hepatitis (B/C), 28; cryptogenic/nonalcoholic-steatohepatitis, 13; primary biliary cirrhosis/primary sclerosing cholangitis, 14; autoimmune hepatitis, 3; and other, 4. Fifteen patients (20.3%) had HCC. Median Bil was 38 μmol/L (range, 6 to 957).
51Cr-EDTA varied between 15 and 156 ml/min per 1.73 m2. The patients were classified into two groups: 51Cr-EDTA more (n = 48) or less (n = 26) than 70 ml/min per 1.73 m2, similar to a previous publication (32) (Table 3). The median creatinine and cystatin C values differed significantly, both being increased in the group with GFR <70 ml/min per 1.73 m2 (P < 0.0001).
Table 3.
Characteristics of patients with cirrhosis assessed for liver transplantation and GFR measured by 51Cr-EDTA
| Characteristic | Total (n = 74) | GFR > 70 (n = 48) | GFR ≤ 70 (n = 26) | P |
|---|---|---|---|---|
| Age (yrs) | 49 (9.2) | 49 (9.8) | 50 (8.3) | 0.7 |
| Gender (female/male) | 28/46 | 10/16 | 18/30 | |
| Race | ||||
| white | 61 | 39 | 22 | |
| black | 2 | 2 | 0 | |
| Indian | 11 | 7 | 4 | |
| Weight (kg) | 77 (13.9) | 76.5 (12.6) | 78 (16.2) | 0.6 |
| Height (cm) | 170 (8) | 170 (8) | 169 (8) | 0.6 |
| Body surface area (m2) | 26.5 (4.5) | 1.86 (0.19) | 1.94 (0.2) | 0.1 |
| CPT score | 8.2 (2.4) | 8.1 (2.6) | 8.4 (2.2) | 0.6 |
| MELD score | 12 (5.5) | 10.7 (4.6) | 15.5 (5.8) | 0.0003a |
| Creatinine (μmol/L) | 67.5 (28 to 234) | 64 (28 to 90) | 109 (53 to 234) | <0.0001a |
| Cystatin C (mg/dl) | 0.83 (0.36 to 2.9) | 0.81 (0.3 to 1.0) | 1.11 (0.5 to 2.9) | <0.0001a |
| C51Cr-EDTA (ml/min per 1.73 m2) | 81.7 (30.8) | 100 (17.8) | 47.6 (17.5) | |
| Ascites | ||||
| not present or mild to moderate (n [%]) | 57 (77) | 41 (85) | 16 (62) | 0.001a |
| severe | 17 (23) | 7 (15) | 10 (38) | 0.6 |
| Encephalopathy | ||||
| not present (n [%]) | 42 (57) | 32 (67) | 10 (38) | 0.001a |
| mild to moderate | 24 (32) | 10 (21) | 14 (54) | 0.5 |
| severe | 8 (11) | 6 (12) | 2 (8) | 0.2 |
| Bilirubin (μmol/L) (n.r. <18) | 38 (6 to 957) | 54 (6 to 277) | 35 (7 to 957) | 0.1 |
| AST (UI/L) (n.r. <19) | 69.5 (17 to 266) | 76 (27 to 266) | 56 (17 to 222) | 0.02a |
| ALT (UI/L) (n.r. <23) | 49.5 (11 to 508) | 58 (15 to 225) | 38 (11 to 508) | 0.01a |
| Albumin (mg/L) (n.r. 36 to 50) | 33.8 (5.8) | 33.5 (6.2) | 34.2 (5.1) | 0.6 |
| Prothrombin time (sec) | 18.7 (12 to 39) | 18.7 (12 to 39) | 19 (13 to 25) | 0.9 |
| INR (n.r 0.0.8 to 1.2) | 1.4 (0.9 to 3.2) | 1.4 (0.9 to 3.2) | 1.4 (1 to 2) | 0.9 |
| CRP (mg/L) (n.r. 0.0.06 to 8) | 5.5 (1 to 80) | 8 (1 to 80) | 4.5 (1 to 64) | 0.3 |
| Platelets (n.r. 0.136 to 423/nl) | 97.5 (21 to 917) | 92.5 (33 to 917) | 120 (21 to 328) | 0.08 |
All of the values are expressed as the medians (range), means (± SD), or numbers (%). The P values are for comparisons between the GFR subgroups (more than 70 or less than 70). AST, aspartate aminotransferase; ALT, alanine aminotransferase; INR, international normalized ratio; CRP, C-reactive protein; MELD, model for end stage liver disease; n.r., normal range.
Statistically significant results.
The liver-related variables that were significantly different between these groups were encephalopathy, which was worse, and lower levels of both transaminases, in the GFR group <70 ml/min per 1.73 m2.
The 74 patients were also subdivided according to CPT stage (A/B/C); without significant differences for Cr, CysC, or GFR measured by 51Cr-EDTA, MDRD, Larsson, and Hoek (Table 4). Correlations of the single values of Cr, CysC, and 51Cr-EDTA with bilirubin were NS.
Table 4.
Comparison between serum creatinine and cystatin C, and the various GFR formulae grouped according to CPT stage
| CPT Class A | CPT Class B | CPT Class C | P | |
|---|---|---|---|---|
| Cystatin C | 0.84 (0.47 to 2.9) | 1.01 (0.39 to 2.67) | 0.82 (0.36 to 1.27) | 0.5 |
| Creatinine | 69.5 (48 to 234) | 67 (40 to 228) | 68 (28 to 150) | 0.7 |
| C51Cr-EDTA | 84.8 (38) | 83.2 (32) | 77.4 (21.8) | 0.4 |
| MDRD | 96.7 (36.1) | 95 (36.8) | 110 (48.7) | 0.7 |
| Larsson | 89.3 (41.4) | 105 (53.7) | 106 (44) | 0.4 |
| Hoek | 84.2 (33.5) | 95.9 (41.7) | 97.9 (32.1) | 0.4 |
All of the values are expressed as medians (range) or means (± SD).
Correlation between 51Cr-EDTA and MDRD was moderately good (r = 0.72; 95% CI, 0.59 to 0.81; P < 0.0001), whereas between 51Cr-EDTA and Larsson and Hoek it was not (r = 0.46; 95% CI, 0.25 to 0.62; P < 0.0001; and r = 0.49; 95% CI, 0.29 to 0.64; P < 0.0001, respectively). Overall agreement with 51Cr-EDTA was better for MDRD than for CysC formulas (concordance correlation coefficient: MDRD 0.61; 95% CI, 0.47 to 0.71; Larsson, 0.38; 95% CI, 0.20 to 0.52; and Hoek, 0.46; 95% CI, 0.27 to 0.61). Separating patients into those with ascites and those without, the correlations between the single CysC values, Hoek, or Larsson formulas and 51Cr-EDTA GFR were better in those without ascites than with ascites, being 0.76 versus 0.52 (P = NS), 0.75 versus 0.31 (P = 0.0001), and 0.72 versus 0.28 (P = 0.0002), respectively. The correlations for Cr did not differ significantly in the two groups, for neither the single values nor the estimated GFR values using the MDRD formula. The latter findings suggest that ascites may significantly influence the estimated GFR when the CysC formulas are used but not the Cr formula.
Degree of Agreement between Different Formulas for GFR in Cirrhosis
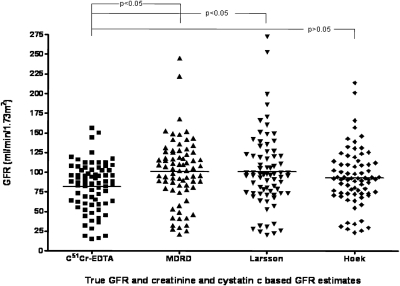
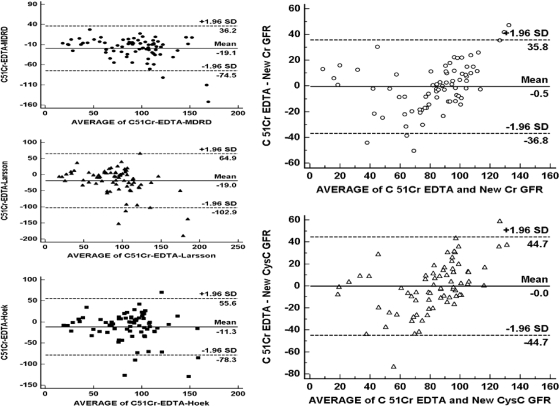
The differences between true GFR and eGFRs were significantly different (F = 3.38, P = 0.01) (Figure 1). In a pairwise comparison, differences between 51Cr-EDTA and MDRD and 51Cr-EDTA and Larsson were significant (P < 0.05 for each) but not between 51Cr-EDTA and Hoek (P > 0.05). Bland-Altman plots (Figure 2) showed that the most accurate method was Hoek, and the most precise method was MDRD. However, both methods overestimated GFR in comparison with 51Cr-EDTA especially for values <70 ml/min per 1.73 m2, and thus neither CysC GFR formula had good accuracy.
Figure 1.
Scatter plot diagram showing GFR in each of 74 patients with cirrhosis measured with the gold standard method 51Cr-EDTA and estimated methods using creatinine (MDRD) or cystatin C (Larsson and Hoek). The mean for each method is represented by a line.
Figure 2.
Bland-Altman plots comparing GFR measurements with 51Cr-EDTA (gold standard method) with two creatinine-based formulas of estimated GFR (MDRD and new Cr eGFR) and three cystatin C-based formulas (Larsson, Hoek, and new CysC eGFR).
Multivariate Analyses and Evaluation of Improved Accuracy of Modified GFR Formulas
We performed a stepwise multivariate linear regression analysis of liver parameters in two analyses using Cr and CysC separately. With creatinine 51Cr-EDTA, the factors used in stepwise order were: age, gender, CPT score, albumin, bilirubin, ascites, encephalopathy, international normalized ratio, platelets, aspartate aminotransferase, alanine aminotransferase, C-reactive protein, and the presence of HCC. Partial correlations and relative P values are shown in Table 5.
Table 5.
Partial correlations of the variables evaluated with respect to associations with 51Cr-EDTA GFR in 74 patients with cirrhosis
| Model for Creatinine |
Model for Cystatin C |
|||
|---|---|---|---|---|
| r | P | r | P | |
| Creatinine | 0.80 | P < 0.001a | ||
| Cystatin C | 0.64 | P < 0.001a | ||
| Age | 0.03 | P = 0.7 | 0.09 | P = 0.4 |
| Albumin | 0.03 | P = 0.7 | 0.05 | P = 0.6 |
| ALT | 0.17 | P = 0.1 | 0.008 | P = 0.9 |
| AST | 0.02 | P = 0.8 | 0.09 | P = 0.4 |
| Ascites | 0.16 | P = 0.1 | 0.33 | P < 0.001a |
| Bilirubin | 0.16 | P = 0.1 | 0.11 | P = 0.3 |
| CPT score | 0.22 | P = 0.003a | 0.11 | P = 0.3 |
| CRP | 0.21 | P = 0.07 | 0.07 | P = 0.5 |
| HCC | 0.07 | P = 0.5 | 0.06 | P = 0.5 |
| INR | 0.17 | P = 0.1 | 0.03 | P = 0.7 |
| Gender | 0.25 | P = 0.001a | 0.11 | P = 0.3 |
| Platelets | 0.18 | P = 0.1 | 0.09 | P = 0.4 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; INR, international normalized ratio; CRP, C-reactive protein.
Variables included in the final multivariate model.
Independently associated factors were included in GFR estimation: GFR (ml/min per 1.73 m2) = 156 − (0.53 × Cr in μmol/L) − 16.3 (if female) − (2.79 × CPT score), and R2 = 0.62, F-ratio = 41.2 (P < 0.001). Next we tested CysC using the same factors as for Cr (Table 5). The resulting equation was: GFR (ml/min per 1.73 m2) = 129.71 − (37.16 × CysC) − 17.88 (if moderate/severe ascites), and R2 = 0.45, F-ratio = 29.2 (P < 0.001).
Evaluation of New Derived GFR Formulas in Cirrhosis
Using the Bland-Altman analysis, the two new formulas were more accurate compared with the MDRD, Larsson, and Hoek formulas. The new Cr-eGFR was more precise than the new CysC-eGFR (Figure 2). We further evaluated the accuracy of the new eGFR formulas using a method proposed by the National Kidney Foundation (8) that measures the percentage of GFR estimates that fall within 10, 30, and 50% above or below the measured GFR (P10, P30, and P50, respectively). The new Cr-eGFR formula was the most accurate (Table 6). When the same evaluations were performed in the subgroup of patients with the true GFR of less than 70 ml/min per 1.73 m2, P10, P30 and P50 were 15, 48, and 70% for the new Cr-eGFR; 15, 44, and 66% for the new CysC-eGFR; 23, 42, and 53% for Hoek; and 15, 46, and 73% for MDRD.
Table 6.
Percentages of GFR estimates within 10, 30, or 50% above or below measured GFR by 51Cr-EDTA (see reference 11)
| GFR Estimates | P10 | P30 | P50 |
|---|---|---|---|
| New Cr eGFR | 39 | 78 | 89 |
| New CysC eGFR | 33 | 74 | 84 |
| MDRD | 37 | 64 | 81 |
| Hoek | 32 | 68 | 79 |
All of the values are expressed as percentages.
Discussion
We used two cohorts of patients with cirrhosis. In the first cohort we assessed correlations of serum CysC with different measurement methods for Cr and whether worsening liver function expressed by increasing bilirubin concentrations affected its values. In the second cohort we assessed whether CysC and its derived GFR equations performed better in comparison with Cr and its derived GFR equations, compared with 51Cr-EDTA GFR.
Bil correlated poorly with CysC in both cohorts, whereas CysC was strongly correlated with Cr. Similar to our results, initial studies assessing the interference of Bil with CysC measurement showed that results were not influenced by hyperbilirubinaemia up to 700 μmol/L (33). There were no significant differences across the CPT stage, at variance with a previous report of only 25 patients (18).
Both CysC formulas significantly overestimated renal clearance compared with C51Cr-EDTA. Multivariate severity of the CPT score added accuracy to a Cr-based GFR formula and the presence of ascites to a CysC-based formula. Interestingly it also showed that female gender is still an important factor affecting the Cr-based formula (7) but not the CysC-based formula. The new Cr formula resulted the best in comparison with C51Cr-EDTA rather than MDRD, Hoek, and the new CysC formulas.
The four-variable MDRD formula is derived from large patient cohorts without liver disease (8) but has only been validated in one large cohort of patients with cirrhosis (11). The CysC equations have also been derived from nonliver disease populations (93 patients with diabetes, vasculitis, and glomerulopathies (22) and 100 patients of unspecified cause [21]) and only evaluated in 44 patients with cirrhosis (20).
In our patients with cirrhosis, the Hoek CysC formula performed better than the Larsson, having less bias, and was better than the MDRD. However, the MDRD was more precise than both the Hoek and Larsson formulas. Nevertheless, each formula overestimated renal clearance significantly in comparison with C51Cr-EDTA.
There is only one published study similar to ours (20), evaluating retrospectively 44 patients with cirrhosis, comparing CysC-based (Larsson and Hoek) and Cr-based (MDRD and C-G) formulas with inulin clearance as the gold standard. The median CPT score was similar, but with a lower median Bil (29 μmol/L) but higher mean Cr (94.5 μmol/L) and mean CysC (1.21 mg/dl). All eGFR formulas overestimated the true GFR significantly. CysC-based formulas performed similarly, and both were more precise than Cr-based formulas. However, accuracies measured with the relative P10, P30, and P50 were surprisingly very low; for the best P10 it was 2.3%, for the best P30 it was 13.6%, and for the best P50 it was 20.5%. These differences are greater than in our cohort and than in patients with chronic kidney disease, in whom the MDRD formula was validated; the P30 accuracy was 92%, and the P50 accuracy was 98% (8). The accuracy of MDRD in our cohort with cirrhosis was 64% and 81%, similar to the reference study (8). For our new Cr eGFR formulas, the accuracy was 78 and 89%, although the accuracy was reduced in the subgroup with a true GFR <70 ml/min per 1.73 m2, in which the P10, P30, and P50 were 15, 48, and 70%, respectively, for the new Cr eGFR and 15, 44, and 66%, respectively, for the new CysC eGFR, with 23, 42, and 53%, respectively, for Hoek, and 15, 46, and 73%, respectively, for MDRD. This clearly demonstrates that in cirrhosis the relative accuracies for estimating GFR drop with decreasing renal function. Although these values are far better than the very low values reported by Poge et al. (20), our new formulas cannot be used in clinical practice but demonstrate that parameters of liver function improve standard formulas and need validation in large cohorts. Gonwa et al. (11) reported a P30 accuracy of 67% for the four-factor MDRD, a finding very similar to our result of 64%, which was in a much smaller cohort. This suggests that our cohort is a representative one for cirrhosis. In patients with chronic kidney disease even with a very low GFR (<30 ml/min per 1.73 m2), P30 and P50 were much better at 69 and 88%, respectively, for each of the four-, five-, and six-variable MDRD formulas (34), showing that patients with cirrhosis are a special case when GFR is low.
A limitation of this study was that the method used as the gold standard for measuring GFR has not been extensively validated in patients with ascites. However, we used the three-sample method to measure Cr-EDTA clearance, which is more accurate than the one-sample method.
Conclusions
This study shows that serum CysC does not provide a sufficient improvement over serum Cr in reflecting renal function in patients with cirrhosis, using the most widely used formulas to estimate GFR. A new GFR formula for Cr improved accuracy in estimating GFR by including the CPT score (i.e. an index of liver dysfunction) as well as female gender.
Because existing CysC-eGFRs do not reflect true GFR with great accuracy in cirrhosis and because even the new formulas of eGFR that we derived, although better than existing formulas, still did not have a good correlation with a direct method of GFR measurement, more studies are required to elucidate the usefulness of CysC as a marker of GFR and to develop accurate eGFR formulas. In cirrhosis there needs to be good evidence that CysC has significant advantages over Cr to recommend the use of CysC. This is particularly so when the GFR falls below <70 ml/min per 1.73 m2, which is known to have an adverse effect on prognosis both before (1,35,36) and after (2,37) liver transplantation.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Terra C, Guevara M, Torre A, Gilabert R, Fernandez J, Martin-Llahi M, Baccaro ME, Navasa M, Bru C, Arroyo V, Rodes J, Gines P: Renal failure in patients with cirrhosis and sepsis unrelated to spontaneous bacterial peritonitis: Value of MELD score. Gastroenterology 129: 1944–1953, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Gonwa TA, McBride MA, Anderson K, Mai ML, Wadei H, Ahsan N: Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: Where will MELD lead us? Am J Transplant 6: 2651–2659, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Seronie-Vivien S, Galteau MM, Carlier MC, Hadj-Aissa A, Hanser AM, Hym B, Marchal A, Michotey O, Pouteil-Noble C, Sternberg M, Perret-Liaudet A: Impact of standardized calibration on the inter-assay variation of 14 automated assays for the measurement of creatinine in human serum. Clin Chem Lab Med 43: 1227–1233, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Cholongitas E, Marelli L, Kerry A, Senzolo M, Goodier DW, Nair D, Thomas M, Patch D, Burroughs AK: Different methods of creatinine measurement significantly affect MELD scores. Liver Transpl 13: 523–529, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Trotter JF, Brimhall B, Arjal R, Phillips C: Specific laboratory methodologies achieve higher model for endstage liver disease (MELD) scores for patients listed for liver transplantation. Liver Transpl 10: 995–1000, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Goulding C, Cholongitas E, Nair D, Kerry A, Patch D, Akyol M, Walker S, Manas D, Mc Clure D, Smith L, Jamieson N, Oberg I, Cartwright D, Burroughs AK: Assessment of reproducibility of creatinine measurement and MELD scoring in four liver transplant units in the UK. Nephrol Dial Transplant 25: 960–966, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Cholongitas E, Marelli L, Kerry A, Goodier DW, Nair D, Thomas M, Patch D, Burroughs AK: Female liver transplant recipients with the same GFR as male recipients have lower MELD scores: A systematic bias. Am J Transplant 7: 685–692, 2007 [DOI] [PubMed] [Google Scholar]
- 8. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 9. Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 10. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Gonwa TA, Jennings L, Mai ML, Stark PC, Levey AS, Klintmalm GB: Estimation of glomerular filtration rates before and after orthotopic liver transplantation: Evaluation of current equations. Liver Transpl 10: 301–309, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Lamb EJ, Tomson CR, Roderick PJ: Estimating kidney function in adults using formulas. Ann Clin Biochem 42: 321–345, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Newman DJ: Cystatin C. Ann Clin Biochem 39: 89–104, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martinez-Bru C, Grubb A: Cystatin C as a marker of GFR–history, indications, and future research. Clin Biochem 38: 1–8, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Zahran A, El-Husseini A, Shoker A: Can cystatin C replace creatinine to estimate glomerular filtration rate? A literature review. Am J Nephrol 27: 197–205, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Woitas RP, Stoffel-Wagner B, Flommersfeld S, Poege U, Schiedermaier P, Klehr HU, Spengler U, Bidlingmaier F, Sauerbruch T: Correlation of serum concentrations of cystatin C and creatinine to inulin clearance in liver cirrhosis. Clin Chem 46: 712–715, 2000 [PubMed] [Google Scholar]
- 17. Orlando R, Mussap M, Plebani M, Piccoli P, De Martin S, Floreani M, Padrini R, Palatini P: Diagnostic value of plasma cystatin C as a glomerular filtration marker in decompensated liver cirrhosis. Clin Chem 48: 850–858, 2002 [PubMed] [Google Scholar]
- 18. Ustundag Y, Samsar U, Acikgoz S, Cabuk M, Kiran S, Kulah E, Aydemir S: Analysis of glomerular filtration rate, serum cystatin C levels, and renal resistive index values in cirrhosis patients. Clin Chem Lab Med 45: 890–894, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Grubb A, Nyman U, Bjork J, Lindstrom V, Rippe B, Sterner G, Christensson A: Simple cystatin C-based prediction equations for glomerular filtration rate compared with the Modification of Diet in Renal Disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem 51: 1420–1431, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Poge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP: Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant 21: 660–664, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Larsson A, Malm J, Grubb A, Hansson LO: Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest 64: 25–30, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Hoek FJ, Kemperman FA, Krediet RT: A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant 18: 2024–2031, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Takeuchi M, Fukuda Y, Nakano I, Katano Y, Hayakawa T: Elevation of serum cystatin C concentrations in patients with chronic liver disease. Eur J Gastroenterol Hepatol 13: 951–955, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Chu SC, Wang CP, Chang YH, Hsieh YS, Yang SF, Su JM, Yang CC, Chiou HL: Increased cystatin C serum concentrations in patients with hepatic diseases of various severities. Clin Chim Acta 341: 133–138, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Chen TY, Hsieh YS, Yang CC, Wang CP, Yang SF, Cheng YW, Chiou HL: Relationship between matrix metalloproteinase-2 activity and cystatin C levels in patients with hepatic disease. Clin Biochem 38: 632–638, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R: Transection of Esophagus for Bleeding Esophageal Varices. Br J Surg 60: 646–649, 1973 [DOI] [PubMed] [Google Scholar]
- 27. Miller WG, Myers GL, Ashwood ER, Killeen AA, Wang E, Thienpont LM, Siekmann L: Creatinine measurement: state of the art in accuracy and interlaboratory harmonization. Arch Pathol Lab Med 129: 297–304, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Fleming JS, Nunan TO: The new BNMS guidelines for measurement of glomerular filtration rate. Nucl Med Commun 25: 755–757, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Poggio ED, Nef PC, Wang X, Greene T, Van Lente F, Dennis VW, Hall PM: Performance of the Cockcroft-Gault and Modification of Diet in Renal Disease equations in estimating GFR in ill hospitalized patients. Am J Kidney Dis 46: 242–252, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Bland JM, Altman DG: Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310, 1986 [PubMed] [Google Scholar]
- 31. Liao JJZ, Lewis JW: A note on concordance correlation coefficient. PDA J Pharmaceutical Sci Tech 54: 23–26, 2000 [PubMed] [Google Scholar]
- 32. Gerbes AL, Gulberg V, Bilzer M, Vogeser M: Evaluation of serum cystatin C concentration as a marker of renal function in patients with cirrhosis of the liver. Gut 50: 106–110, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, Price CP: Serum cystatin-C measured by automated immunoassay: A more sensitive marker of changes in GFR than serum creatinine. Kidney Int 47: 312–318, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Kuan Y, Hossain M, Surman J, El Nahas AM, Haylor J: GFR prediction using the MDRD and Cockcroft and Gault equations in patients with end-stage renal disease. Nephrol Dial Transplant 20: 2394–2401, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Cardenas A, Gines P, Uriz J, Bessa X, Salmeron JM, Mas A, Ortega R, Calahorra B, de Las Heras D, Bosch J, Arroyo V, Rodes J: Renal failure after upper gastrointestinal bleeding in cirrhosis: Incidence, clinical course, predictive factors, and short-term prognosis. Hepatology 34: 671–676, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Fraley DS, Burr R, Bernardini J, Angus D, Kramer DJ, Johnson JP: Impact of acute renal failure on mortality in end-stage liver disease with or without transplantation. Kidney Int 54: 518–524, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Baliga P, Merion RM, Turcotte JG, Ham JM, Henley KS, Lucey MR, Schork A, Shyr Y, Campbell DA, Jr: Preoperative risk factor assessment in liver transplantation. Surgery 112: 704–710, 1992 [PubMed] [Google Scholar]