Abstract
Objective
The current paper provides an analysis of the use of artificial sweeteners, caffeine, and excess fluids in patients diagnosed with anorexia nervosa.
Method
Seventy subjects with anorexia nervosa (AN) were recruited to participate in an ecologic momentary assessment study which included nutritional analysis using the Nutrition Data Systems for Research (NDS-R), a computer based dietary recall system.
Results
When subtypes were compared, AN-restricting subtype (AN-R) subjects and AN-Binge-Purge (AN-B/P) subjects did not differ in quantity of aspartame, caffeine, or water consumed. Daily water consumption was related to daily vomiting frequency in AN-B/P but not to daily exercise frequency in either AN-R or AN-B/P subjects.
Conclusion
Caffeine, water, and aspartame consumption can be variable in AN patients and the consumption of these substances appears to be only modestly related to purging behavior.
Previous empirical and case studies have documented patterns of excessive consumption of caffeine, artificial sweeteners, and fluids in individuals with eating disorders. Specifically, individuals with eating disorders appear to have a tendency to consume high amounts caffeine through soft drinks, energy drinks, coffee, and/or caffeine pills as a means to increase energy levels and suppress appetite.1 Excessive fluid intake often takes the form of carbonated beverages, coffee, and water. Similarly, artificial sweeteners in the form of sugar-free candies or chewing gum, foods, and drinks have been reported to be used to suppress appetite in this population.2, 3 Understanding the relationship between fluid intake, caffeine use and artificial sweetener use is challenging because these substances are often found in combination in the same products. For example, beverages such as diet soda, diet energy drinks, coffee, and tea often contain caffeine and artificial sweeteners.
The medical repercussions of the misuse of these substances can be significant. Caffeine overuse can lead to anxiety and tremor and discontinuation can result in withdrawal symptoms including headache and concentration difficulties.4 Excessive use of sweeteners, such as Sorbitol or Malitol, can lead to gastrointestinal symptoms such as pain, bloating, and loose stools, while excessive fluid consumption can result in electrolyte imbalances and seizures. In combination with these medical problems, compensatory behaviors (e.g., vomiting, restriction, laxative abuse) may result in additional adverse medical sequelae, including hypokalemia, hypochloremia, hyponatremia, and edema.5,6,7,8 In addition, patients with anorexia nervosa (AN) have impaired osmoregulation and difficulty concentrating urine when dehydrated.9 The relationship between this complication and excess fluid consumption is not well understood.
Overuse of caffeine has been reported by several investigators. Research has suggested that caffeine use may vary over the course of an eating disorder. In comparing AN and control subjects, Striegel-Moore and colleagues observed that caffeine intake increased sharply in individuals with AN after they were first diagnosed and decreased subsequently.10 More specifically, intake of fluids such as coffee, tea, and soda increased after AN onset, but intake of chocolate food (containing caffeine) decreased.
The research related to the overuse of caffeine has yielded mixed results when examining AN-B/P, AN-R, and bulimia nervosa (BN) subjects. Some research has suggested that BN subjects do not show increased caffeine consumption when compared to AN subjects, although it was unclear as to whether these AN subjects were AN-R or AN-B/P subtype.11 Others studies suggest that BN, AN-B/P and EDNOS patients who binge eat and purge show increased caffeine consumption compared to AN-R subjects and other subgroups who do not purge.1, 12 Additionally, Stock and colleagues observed that AN-B/P, BN, and EDNOS patients with purging behavior overused caffeine (i.e., consumed three or more caffeinated drinks per day), compared to AN-R patients.13 Although the exact cause of increase caffeine consumption is unknown, consuming excessive amounts of caffeine containing fluids may serve as a weight control method to mask hunger, to aid in purging behavior, or increase energy levels.1 Alternatively, consumption may be higher in those with AN-B/P or BN because of the overall greater amount of food and beverage intake in those individuals due to binge eating behavior.
Very few investigations have examined fluid intake in eating disorder patients. Research suggests that eating disorder patients may consume excessive amounts of fluids and that this might be used as an aid in vomiting.1,5 Water loading can also be a way to “make weight” for a clinical visit. However, Lowinger and colleagues presented descriptions of several cases studies of AN-R subjects who severely limited fluid consumption, purportedly to avoid the feelings of fullness, or the perceived calories in beverages, including water.14
The use of artificial sweeteners such as saccharin and aspartame has also received limited attention. Ohlrich and colleagues offered case reports of sorbitol abuse through chewing gum.2 The authors suggested that sorbitol-based gum was used as a purging technique, a way to control binge-eating episodes, or as a means to limit hunger.2 Most of these patients experienced gastrointestinal symptoms (e.g., bloating, cramping, loose stools, and distention). The use of artificial sweetener packets has been reported to be less common in AN-R individuals compared to AN-B/P individuals, while those with AN-B/P have been shown to consume significantly more artificial sweetener than controls.3
The purpose of the current study was to examine the intake of caffeine, water, and artificial sweeteners throughout the day in a group of AN subjects. It was hypothesized that AN-B/P subjects would consume higher levels of caffeine, water, and sweeteners compared to those individuals with AN-R. Additionally, it was hypothesized that there would be an increase in consumption of caffeine throughout the day in both the AN-B/P and AN-R subgroups. We also anticipated that fluids would be used as an aid to purging behavior. Therefore a relationship would exist between daily caffeine consumption and exercise frequency if caffeine was used as an energy supplement, and between daily water consumption and purging behavior.
Method
Subjects (N=70) were recruited from three sites in the Midwestern region of the United States. The sample included 43 AN-R and 27 AN-B/P subjects. Subjects were diagnosed using the SCID-I patient version.15 Subjects recalled their consumption in person or over the phone on two or three separate days using the Nutrition Data Systems for Research (NDS-R), a system created at the Nutrition Coordinating Center in the Division of Epidemiology and School of Public Health at the University of Minnesota. Subjects were interviewed in the evening and data from the prior 24 hours were entered into the NDS-R by trained raters. The NDS-R system provides a nutrient and caloric breakdown of each food item consumed and has been shown to be a useful assessment tool.16,17
Purging behaviors were assessed using ecologic momentary assessment (EMA) with palm top computers.18 Subjects entered their exercise, vomiting, and laxative use episodes into the palm top computers over a two-week period when the instrument signaled them to do so. The instrument signaled participants at six semi-random times throughout the day. Participants were also instructed to record these behaviors if they occurred at a time other than when the participant was signaled. The EMA protocol coincided with the days the NDS-R was administered, allowing a temporal alignment of consumption and purging patterns for each subject. This research was reviewed and approved by an institutional review board.
Results
The mean BMI of the sample was 17.3 (SD = .9) and the mean age was 24.8 (SD=7.8). The BMI for the AN-R group (M= 17.4 SD = 1.47) and AN-B/P group (M = 17.1, SD = 1.7) did not differ significantly (p = .337). The mean age of the AN-R (M = 23.8, SD= 7.2) and AN-B/P (M = 26.6, SD = 8.5) groups did not differ significantly (p = .147). Additional demographic information is provided in Table 1.
Table 1.
Demographic Information
| Frequency (Percent) | |
|---|---|
| Marital Status | |
| Married | 8 (11.4%) |
| Single | 57 (81.4%) |
| Separated | 1 (1.4%) |
| Divorced | 2 (2.9%) |
| Cohabitating | 2 (2.9%) |
| Education | |
| Some high school | 2 (2.9%) |
| Completed High School | 2 (2.9%) |
| Some College | 39 (55.7%) |
| Completed Associate's Degree | 2 (2.9%) |
| Completed Bachelor's Degree | 11 (15.7%) |
| Some graduate school | 7 (10.0%) |
| Completed Graduate School | 4 (5.7%) |
| Other | 3 (4.3%) |
| Ethnicity | |
| Caucasian | 68 (97.1%) |
| African American | 1 (1.4%) |
| Other | 1 (1.4%) |
Subjects' entries in the NDS-R database included 785 eating episodes in the AN-R group and 431 eating episodes in the AN-B/P group. Water, caffeine, and aspartame consumption did not differ significantly between groups although the AN-B/P group consumed numerically more of each type of substance compared than the AN-R group. The results are shown in Table 2. A mixed-effects linear model was used with water, caffeine, and aspartame each serving as a dependent variable in three analyses. Diagnosis and the hour of the meal served as the independent variables. Results of the consumption patterns are found in Table 3.
Table 2.
Mean Intake of Substances
| AN-R |
AN-B/P |
|||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| Water | 392.8 | 349.6 | 0–2964.1 | 581.1 | 786.1 | 0–5085.0 |
| Aspartame | 26.8 | 70.6 | 0–558.3 | 73.0 | 175.1 | 0–1480.0 |
| Caffeine | 27.4 | 76.4 | 0–999.2 | 48.7 | 124.8 | 0–1515.5 |
Note: Water was measured in grams. Aspartame and caffeine were measured in milligrams.
Table 3.
Water, Aspartame, and Caffeine Consumption Across Time of Day and Diagnosis
| F | p | Estimate (SE) | |
|---|---|---|---|
| Water | |||
| Diagnosis | .230 | .631 | 44.36 (92.46) |
| Meal Hour | 15.94 | .000 | 20.12 (4.87) |
| Diognosis × Meal | 7.11 | .008 | −16.03 (6.01) |
| Aspartame | |||
| Diagnosis | .346 | .556 | −11.89 (20.21) |
| Meal Hour | 2.61 | .107 | 2.25 (1.06) |
| Diognosis × Meal | 3.26 | .071 | −2.37 (1.31) |
| Caffeine | |||
| Diagnosis | .095 | .758 | −5.03 (16.32) |
| Meal Hour | 13.59 | .000 | −1.37 (.859) |
| Diognosis × Meal | 1.20 | .273 | −1.16 (1.06) |
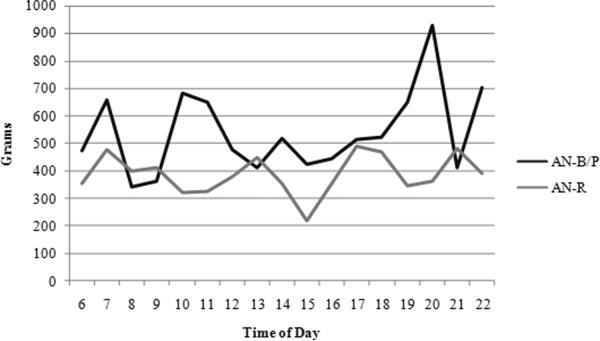
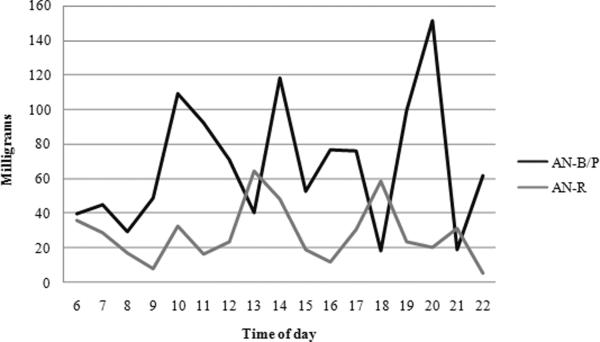
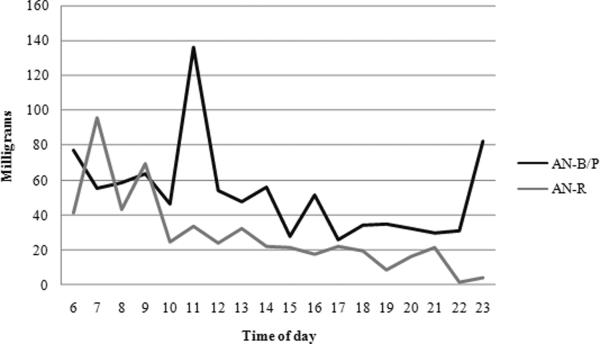
Water consumption did not significantly differ across diagnoses; however, water intake increased significantly across time of day. Most importantly, the two subtypes differed in their hourly pattern of fluid consumption. AN-R participants' consumption stayed somewhat consistent across time and AN-B/P subjects' intake increased (Figure 1). No significant difference emerged in the aspartame consumption across diagnoses or time of day (Figure 2). Caffeine use, similarly, did not differ across diagnoses but decreased in both groups as the day progressed (Figure 3).
Figure 1.
Water Consumption Patterns
Figure 2.
Aspartame Consumption Patterns
Figure 3.
Caffeine Consumption Patterns
Additional multilinear modeling was used to assess the relationship between the frequency of vomiting episodes per day and the quantity of water consumed per day in AN-B/P subjects. Results suggested that daily vomiting episodes were associated with greater water consumption. Neither caffeine nor water intake related to the number of exercise episodes of AN-R and AN-B/P subjects. Results are found in Table 5.
Discussion
Prior literature has suggested that individuals with eating disorders may use excessive amounts of water, caffeine, and/or sweeteners. The purpose of the current study was to examine daily differences in consumption of these substances among AN-R and AN-B/P subjects. Using NDS-R to assess the quantity of the substance consumed throughout the day and EMA to assess purging behavior, the results of the investigation were only partially consistent with our hypotheses. Specifically, consumption of caffeine, sweeteners, and fluids appeared to be greater in the AN-B/P group, compared to AN-R group, but the differences were not statistically significant. Contrary to our hypothesis, caffeine use decreased throughout the day in both groups. Similarly, caffeine consumption was not related to exercise in either of the AN subgroups. The findings suggest that daily water consumption is related to increased number of vomiting episodes, but not to compensation for daily exercise episodes.
Hart and colleagues note, “There are currently no published descriptions of fluid consumption patterns (type and amount) in patients with anorexia nervosa, bulimia nervosa, or eating disorders not otherwise specified (EDNOS)” (p. 55) prior to their study.1 Our description of consumption patterns are similar to those of Hart and colleagues who found no difference between fluid intake in eating disorder patients with and without purging behavior.1 Also consistent with Hart and colleagues, the range of fluid consumption found in this research was quite diverse both between and within AN subtypes.1 This variability suggests that some patients appear to limit intake, whereas others over-consume fluids. Indeed, fluid restriction and fluid over-consumption have both been documented in the literature.5, 14, 19
The results of this study are not consistent with previous research that suggests ED subjects (AN, BN, and EDNOS) who binge eat and purge tend to consume more caffeine.1,12 However, caffeine use decreased throughout the day in both AN-R and AN-B/P groups. This suggests that caffeine may not be used to increase energy throughout the day.
To the authors' knowledge, only one other study has examined artificial sweetener use in ED subjects and our results are similar to that study.3 In terms of sweetener consumption, the consumption of aspartame varied among AN-R and AN-B/P groups. AN-B/P subjects consumed more sweeteners than AN-R subjects. Aspartame consumption did not change significantly throughout the day. Klein and colleagues suggested that the drive for use of sweetener is likely related to “an appetitive drive in the eating-disordered populations” or “orosensory stimulation” (p. 344).3
There are several limitations to the current study. First, subject recall was used to enter data into the NDS-R system for nutritional analysis. The NDS-R system relies on accurate reporting of the patient's consumption. Accurate report of consumption is especially challenging in a population that may be overly concerned about consumption patterns. In addition, our data analysis did not include a comparison of specific foods or drinks that contained sweeteners, fluids, and caffeine. Directly comparing the specific food products consumed, as opposed to only the nutritional breakdown of the foods, may provide additional information about the reasons for consumption. For example, chocolate that contains caffeine may have been ingested for the taste, and not necessarily for the stimulant effects. Moreover, an assessment of hunger and fullness rating when patients were signaled using their palm top computer would have been advantageous in examining the relationship between consumption of each substance and purging episodes. Lastly, our study did not include aged-matched, healthy control subjects which would have provided additional information about the severity of aberrant consumption patterns in the AN participants.
Very little research has examined consumption of water, caffeine, and sweeteners among individuals with eating disorders and the inconsistency in the research findings thus far warrants addition investigation. It remains unclear as to the reasons for excessive consumption of these substances in eating disorder patients and closer analysis of patients' rationale or motivation to over-consume these nutritional components is needed. Our findings suggest that overconsumption of caffeine may not be for the purpose of increasing energy as was hypothesized by previous authors. Water consumption appeared to be related to some purging behaviors (i.e., vomit episodes), but not exercise episodes. The medical complications that can occur from overuse of the aforementioned substances speak to the clinical relevance of querying patients as to their overuse of caffeine, sweeteners, and water. Providing patient with education related to the medical sequelae secondary to the overuse of these substances is also indicated.
Table 4.
Daily Fluid Consumption and Purging Behavior in AN-B/P and AN-R
| F | p | Estimate (SE) | |
|---|---|---|---|
| Water × Vomit Frequency | |||
| AN-B/P | 8.75 | .004 | 711.25 (240.46) |
| Water × Exercise Frequency | |||
| AN-R and AN-B/P | .327 | .568 | −87.11(152.32) |
| AN-R | .416 | .520 | −104.70 (162.27) |
| AN-B/P | .107 | .745 | 98.21 (300.71) |
| Caffeine × Exercise Frequency | |||
| AN-R and AN-B/P | 3.44 | .065 | −42.14(22.73) |
| AN-R | 1.67 | .199 | −28.27(21.89) |
| AN-B/P | 2.24 | .139 | −74.25(49.65) |
Acknowledgments
Funding for this study was provided by a grant from the National Institute of Mental Health R01-MH-059674 awarded to Dr. Stephen A. Wonderlich.
References
- 1.Hart S, Abraham S, Luscombe G, Russell J. Fluid Intake in Patients with Eating Disorders. Int J Eat Disord. 2005;38:55–59. doi: 10.1002/eat.20155. [DOI] [PubMed] [Google Scholar]
- 2.Ohlrich E, Aughey D, Dixon R. Sorbitol Abuse among Eating Disordered Patients. Psychosomatics. 1989;30:451. doi: 10.1016/S0033-3182(89)72255-6. [DOI] [PubMed] [Google Scholar]
- 3.Klein D, Boundreau G, Devlin M, Walsh B. Artificial Sweetener Use Among Individuals with Eating Disorders. Int J Eat Disord. 2006;39:341. doi: 10.1002/eat.20260. [DOI] [PubMed] [Google Scholar]
- 4.Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; Washington, DC: 2000. p. 743. [Google Scholar]
- 5.Salkovskis P, Phil M, Jones R, Kucyj M. Water intoxication, Fluid Intake, and Nonspecific Symptoms in Bulimia Nervosa. Int J Eat Disord. 1987;6:525. [Google Scholar]
- 6.Mitchell J, Hatsukami D, Pyle R, et al. Metabolic Acidosis as a Marker for Laxative Abuse in Patients with Bulimia. Int J Eat Disord. 1987;6:557. [Google Scholar]
- 7.Mitchell J, Boutacoff L. Laxative Abuse Complicating Bulimia: Medical and Treatment Implications. Int J Eat Disord. 1986;5:325. [Google Scholar]
- 8.Turner M, Shapiro C. The Biochemistry of Anorexia Nervosa. Int J Eat Disord. 1992;12:179. [Google Scholar]
- 9.Everard F, Pinto de Cunha M, Lambert M, Devuyst O. Impaired osmoregulation in anorexia nervosa: a case-control study. Nephrol Dial Transplant. 2004;19:3034. doi: 10.1093/ndt/gfh507. [DOI] [PubMed] [Google Scholar]
- 10.Striegel-Moore RH, Franko DL, Thompson D, et al. Caffeine Intake in Eating Disorders. Int J Eat Disord. 2006;39:162. doi: 10.1002/eat.20216. [DOI] [PubMed] [Google Scholar]
- 11.Krahn DD, Hasse S, Ray A, Gosnell B, Drewnowski A. Caffeine Consumption in Patients with Eating Disorders. Hosp Community Psychiatry. 1991;42:313. doi: 10.1176/ps.42.3.313. [DOI] [PubMed] [Google Scholar]
- 12.Haug NA, Heinberg LJ, Guarda AS. Cigarette Smoking and its Relationship to Other Substance use among Eating Disordered Patients. Eat Weight Disord. 2001;6:130. doi: 10.1007/BF03339762. [DOI] [PubMed] [Google Scholar]
- 13.Stock SL, Goldberg E, Corbett S, Katzman DK. Substance Use in Female Adolescents with Eating Disorders. J Adolesc Health. 2002;31:176. doi: 10.1016/s1054-139x(02)00420-2. [DOI] [PubMed] [Google Scholar]
- 14.Lowinger K, Griffiths RA, Beumont PJ, et al. Fluid Restriction in Anorexia Nervosa: A Neglected Symptom or New Phenomenon? Int J Eat Disord. 1999;26:392. doi: 10.1002/(sici)1098-108x(199912)26:4<392::aid-eat4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 15.Spitzer R, Williams J, Gibbon M. New York State Psychiatric Institute Biometrics Research; New York: 1995. Structured Clinical Interview for DSM-IV (SCID) [Google Scholar]
- 16.Shakel SF, Sievert YA, Buzzard IM. Sources of data for developing and Maintaining a Nutrient Database. J Am Diet Assoc. 1988;10:1268. [PubMed] [Google Scholar]
- 17.Feskanich D, Sielaff BH, Chong K, Buzzard IM. Computerized collection and Analysis of Dietary Intake Information. Comput Methods Programs in Biomed. 1999;30:47. doi: 10.1016/0169-2607(89)90122-3. [DOI] [PubMed] [Google Scholar]
- 18.Engel S, Wonderlich S, Crosby R, et al. A Study of Patients with Anorexia Nervosa Using Ecologic Momentary Assessment. Int J Eat Disord. 2005;38:335. doi: 10.1002/eat.20184. [DOI] [PubMed] [Google Scholar]
- 19.Santonastaso P, Sala A, Favaro A. Water intoxication in anorexia nervosa: A case report. Int J Eat Disord. 1998;24:439. doi: 10.1002/(sici)1098-108x(199812)24:4<439::aid-eat12>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]