Abstract
Cap hydrolysis is a critical step in several eukaryotic mRNA decay pathways and is carried out by the evolutionarily conserved decapping complex containing Dcp2 at the catalytic core. In yeast, Dcp1 is an essential activator of decapping and coactivators such as Edc1 and Edc2 are thought to enhance activity, though their mechanism remains elusive. Using kinetic analysis we show that a crucial function of Dcp1 is to couple the binding of coactivators of decapping to activation of Dcp2. Edc1 and Edc2 bind Dcp1 via its EVH1 proline recognition site and stimulate decapping by 1000-fold, affecting both the KM for mRNA and rate of the catalytic step. The C-terminus of Edc1 is necessary and sufficient to enhance the catalytic step, while the remainder of the protein likely increases mRNA binding to the decapping complex. Lesions in the Dcp1 EVH1 domain or the Edc1 proline-rich sequence are sufficient to block stimulation. These results identify a new role of Dcp1, which is to link the binding of coactivators to substrate recognition and activation of Dcp2.
Keywords: decapping, coactivators, 5′ to 3′ decay, proline recognition, EVH1
INTRODUCTION
Messenger RNA degradation plays a critical role in organism development (Schier 2007), cell growth and proliferation (Lindstein et al. 1989), differentiation (Shaw and Kamen 1986), response to stress (Hilgers et al. 2006), the adaptive immune system (Chowdhury and Novina 2005), and mRNA quality control (Isken and Maquat 2007). Removal of the 5′ N7-methylguanosine (m7G) cap structure by Dcp2 is an irreversible step that commits an mRNA to destruction by 5′-3′ exonucleases (Stevens and Maupin 1987), and is the penultimate step in a number of 5′-3′ decay pathways including bulk decay (Coller and Parker 2004; Franks and Lykke-Andersen 2008), nonsense-mediated decay (NMD) (LeJeune et al. 2003; Amrani et al. 2006), AU-rich element mediated decay (ARE) (Fenger-Gron et al. 2005), miRNA mediated decay (Behm-Ansmant et al. 2006; Eulalio et al. 2007), and 3′ uridylation (Song and Kiledjian 2007; Rissland and Norbury 2009).
Decapping is a highly regulated process involving an extensive network of protein–protein interactions acting upon Dcp2, requiring both general and pathway specific activators (Coller and Parker 2004; Parker and Song 2004; Krogan et al. 2006; Eulalio et al. 2007; Garneau et al. 2007; Isken and Maquat 2007; Parker and Sheth 2007; Franks and Lykke-Andersen 2008). For example, in budding yeast, all mRNA decapping and 5′-3′ decay requires the general activator Dcp1, whereas bulk decay requires Dhh1 and the Pat1/Lsm1-7 complex, and nonsense mediated decay requires the Upf proteins (Beelman et al. 1996; He et al. 1997; Tharun et al. 2000; Coller et al. 2001; He and Jacobson 2001; Chowdhury and Tharun 2009). In Saccharomyces cerevisiae, the enhancer of decapping protein family (Edc1-3) activates decapping as part of an adaptive response to carbon source shifts or by promoting decay of specific transcripts (DeRisi et al. 1997; Dunckley et al. 2001; Schwartz et al. 2003; Kshirsagar and Parker 2004; Badis et al. 2004; Dong et al. 2007). In metazoans, additional coactivators direct decapping of miRNA targets through interactions between Dcp1, Edc3, the Dhh1 homolog p54/Rck and Hedls (Eulalio et al. 2007). Moreover, decapping of mRNA containing AU-rich elements requires the sequence specific RNA binding proteins Tristetraproline and Hedls, which promotes the interaction between Dcp1 and Dcp2 (Fenger-Gron et al. 2005). These data are consistent with the idea that Dcp2 is the catalytic core of a decapping mRNP, which is highly regulated and uniquely configured for different 5′-3′ decay pathways.
Four observations suggest that Dcp1 acts as a critical protein interaction module that couples coactivators of decapping to substrate recognition or activation of Dcp2. First, Dcp1 is essential for decapping in yeast (Beelman et al. 1996). Second, codepletion of Dcp1 and coactivators by RNAi reduces miRNA decay in metazoans (Rehwinkel et al. 2005; Eulalio et al. 2007). Third, biochemical and biophysical studies of yeast proteins indicate that Dcp1 promotes the closed, active form of Dcp2 and contributes 10-fold to the rate-limiting catalytic step of decapping in vitro (Deshmukh et al. 2008; Floor et al. 2008, 2010; She et al. 2008). Finally, Dcp1 has an EVH1 fold, a protein interaction module that recognizes proline-rich sequences through a surface exposed aromatic triad (Ball et al. 2002; She et al. 2004). Lesions within the aromatic triad and surrounding residues of Dcp1 increase the half-life of reporter mRNA in vivo (Tharun and Parker 1999), suggesting that this may be a binding site for regulators of decapping. Therefore, identification of factors that directly bind the proline recognition site may provide insight into the control of decapping.
In S. cerevisiae, Edc1 and Edc2 were discovered as high-copy suppressors of mutations in Dcp1 and Dcp2 (Dunckley et al. 2001). Edc1 and 2 are primarily expressed under conditions of cellular stress, localize to mRNA processing bodies and play a role in translation as well as mRNA decapping (DeRisi et al. 1997; Dunckley et al. 2001; Schwartz et al. 2003; Neef and Thiele 2009). Edc1 is an RNA binding protein that copurifies with Dcp1 and Dcp2 from yeast and contains short segments with homology to Edc2 (Dunckley et al. 2001). The last 30 residues of Edc1 and Edc2 contain the region of highest sequence identity and deletion of these residues in Edc1 abrogated its ability to enhance decapping both in vivo and in vitro (Schwartz et al. 2003). Recombinant purified Edc1 can stimulate decapping by Dcp1/Dcp2 in vitro, suggesting that Edc1 and 2 may directly interact with Dcp1 or Dcp2 (Schwartz et al. 2003; Steiger et al. 2003).
To begin to understand the mechanism of decapping coactivators, we have focused on Edc1 and Edc2 as a model system. Unlike the Pat1/Lsm1-7 complex and the Upf1-3 complex (He et al. 1997; Tharun 2008), Edc1 and 2 are single polypeptide chains and can be purified in large quantities, allowing detailed characterization of their mechanism. Here we show that Edc1 and 2 are proline-rich ligands of Dcp1. Phage display and peptide SPOT array analysis identify a consensus binding sequence for Dcp1. Mutations in the Edc proline-rich sequence (PRS) or Dcp1 PRS binding site disrupt the interaction and reduce coactivation by Edcs. Kinetic analyses reveal that Edc1 affects both the catalytic step of decapping and substrate binding, contributing roughly 1000-fold to the overall catalytic efficiency of Dcp1–Dcp2. Deletion analyses indicate that Edc1 and Edc2 are modular proteins, containing separate Dcp1 binding and activation regions. We suggest that proline-rich ligand binding by Dcp1 is widespread and provides a general mechanism for coupling coactivators of decapping to substrate recruitment and activation of Dcp2.
RESULTS
The C-terminal region of Edc1 binds Dcp1
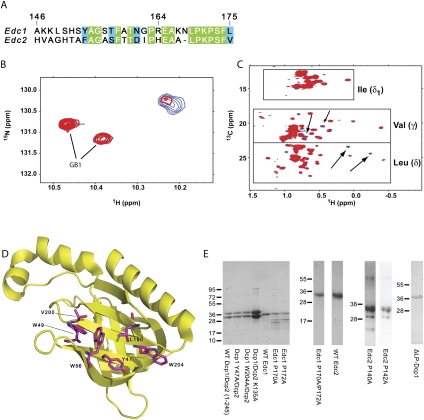
Previous studies indicated that the last 30 residues of Edc1 are essential for enhancing decapping by Dcp1–Dcp2 in vitro and in yeast, suggesting a critical protein–protein interaction surface resides within the C-terminal region (CTR, residues 146–175) (Fig. 1A; Schwartz et al. 2003). Since the Edc1–CTR contains a proline-rich region, we hypothesized it would bind Dcp1. To address this possibility, we recorded HSQC spectra on 15N, 13C ILV labeled Dcp1 alone and in the presence of excess unlabeled Edc1–CTR. We observed a limited number of specific chemical shift changes that were induced upon addition of the CTR (Fig. 1B,C). Dcp1 contains three tryptophan residues in its sequence, all of which correspond to conserved residues in the putative proline-rich sequence (PRS) binding site (Fig. 1D). Interestingly, the most prominent changes to the 15N-HSQC spectrum occurred in a region where crosspeaks from the indole nitrogen of tryptophan often appear (Fig. 1B). We also observed specific chemical shift changes in the 13C ILV HSQC, which likely correspond to leucine and valine residues in the vicinity of the PRS binding site (Fig. 1C). These data provide evidence that the Edc1–CTR binds Dcp1 and forms a specific interaction with the Dcp1 PRS binding site. However, poor solubility of Dcp1 precluded resonance assignment and a definitive mapping of the binding site.
FIGURE 1.
The CTR of Edc1 binds Dcp1. (A) Sequence alignment of Edc1 and Edc2 C-terminal regions depicting residues 146–175 of Edc1 and 117–145 of Edc2. Residues invariant between Edc1 and Edc2 are highlighted in green and similar residues are in blue. (B) Overlay of 15N HSQC depicting the putative tryptophan indole region of GB1–Dcp1 alone (blue) and in the presence of a 1.5 molar excess of GB1–Edc1–CTR (red). Dcp1 and Edc1 were expressed and purified as GB1 fusions to increase protein solubility. Resonances corresponding to the GB1 tag are indicated. (C) Overlay of 13C ILV HSQC comparing the chemical shifts of apo GB1–Dcp1 (blue) to that bound to GB1–Edc1–CTR (red) indicates specific shifts in the leucine and valine methyl group region. Key spectral changes are marked with arrows. (D) The structure of S. cerevisiae Dcp1 is shown with conserved aromatic and leucine and valine residues in the PRS-binding site depicted in magenta (PDB entry 1Q67) (She et al. 2004). (E) SDS-PAGE analysis of recombinant wild-type and mutant GB1–Dcp1/Dcp2 complexes, GB1–ΔLP–Dcp1, and wild-type and mutant GB1–Edc1 and Edc2 proteins used in this study. Gels were stained with Coomassie Brilliant Blue.
Dcp1 recognizes a proline-rich consensus sequence of Edc1
Since Edc1 and Edc2 share a region of high sequence homology within the CTR, we reasoned that this region contains a consensus sequence that is recognized by Dcp1 (Fig. 1A). To test this idea, we performed a substitution analysis on residues 164–175 of the Edc1–CTR covalently tethered to a peptide SPOT membrane, and probed with GST–Dcp1 (Fig. 2A). We observed that Dcp1 binds a proline-rich consensus sequence of [E/D]x3[L/F/W]PxP[S/T][F/W], with proline being strictly required at positions corresponding to P170 and P172 on Edc1. Edc2 contains a similar sequence and SPOT analysis confirms that Edc2 also binds Dcp1 (data not shown). Additionally, E165 in Edc1 also appears to be important for the interaction and may form contacts with Dcp1 outside the PRS-binding region. This is consistent with a previously observed EVH1–ligand interaction which exhibits a similar extended binding epitope, [D/E]FPPPPX[D/E] (Holt and Koffer 2001).
FIGURE 2.
Peptide SPOT membrane analysis and phage display reveal the consensus sequence recognized by Dcp1. (A) Substitution analysis of Edc1 (164–175) peptide. All possible single site substitution variants of the peptide were synthesized on a nitrocellulose membrane. The sequence of the wild-type peptide is indicated by the single letter code displayed in the first column. The row defines the position of the substitution within the peptide, and the code above each column indicates the amino acid that replaces the corresponding wild-type residue. The membrane was incubated with GST–Dcp1, and bound protein was detected with an anti-GST primary antibody and a horseradish peroxidase coupled secondary antibody. The relative spot intensities correlate qualitatively with the binding affinities (Kramer et al. 1999). (B) List of ligands selected after four rounds of panning with an X9 peptide library against GST–Dcp1. Proline residues in the consensus motif are highlighted in red, basic residues are highlighted in blue, serine and threonine residues are shown in green, and aliphatic residues are colored yellow. Amino acids from the phage pVIIII protein or the remainders of the signal sequence are depicted in italics.
Our SPOT analysis contains information on individual amino acid substitutions but does not take into account nonadditive effects. Therefore, we used a randomized nonapeptide library presented on the capsid of the filamentous phage M13 to analyze the pool of peptides that interact with Dcp1. Following four rounds of panning, we observed specific enrichment of phages bound to GST–Dcp1 compared to the GST control. Analysis of 20 sequences for GST–Dcp1 revealed a clear preference for sequences of the signature FPRP[S/T][F/W] (Fig. 2B). The preference for a basic residue between the prolines is interesting, and may point to a general motif common to coactivators of Dcp1. The fact that 85% of the obtained sequences are shifted to the N-terminus of the library peptide and border at the phage-derived residues of pVIII protein (ending with the dipeptide EF) suggests a role for the phenylalanine at position −1, which coincides with the known core consensus FPPPP of the EVH1 domain Mena (Niebuhr et al. 1997; Prehoda et al. 1999). Phage display also revealed a preference for sequences of primarily hydrophobic residues that extend beyond the C-terminus of Edc1 and Edc2. Taken together, these data demonstrate that Dcp1 binds to certain peptides that contain 5–6 critical residues of which two are proline. This is similar to the interaction of other proline-rich binding domains (Freund et al. 2008), and it is expected that the promiscuity of the motif allows additional activators of Dcp2 to utilize this signature for colocalization with the enzyme.
The PRS of Edc1 and Edc2 is required for activation of decapping
Given that the proline-rich sequence (PRS) of Edc1 plays a central role in Dcp1 ligand recognition, we postulated that the mutation of one or both proline residues in Edc1 or Edc2 may inhibit stimulation of the decapping complex by Edcs. Therefore, we mutated Edc1 P170 and P172 and Edc2 P140 and P142, which form the core of the consensus motifs, to alanine. We previously developed a single-turnover kinetic assay to investigate RNA decapping by the Dcp1–Dcp2 complex (Jones et al. 2008). To study coactivation by Edcs, we incubated the Dcp1–Dcp2 complex with Edc1 or Edc2 prior to adding the protein to full-length, cap-radiolabeled MFA2 RNA. These assays were performed under kmax/KM conditions, where the concentration of Dcp1–Dcp2 is less than the KM and with subsaturating Edc, since higher concentrations of enzyme or coactivator resulted in rates that were too fast to follow by manual pipetting. A control experiment in the absence of Dcp1–Dcp2 indicates that Edc1 and Edc2 do not exhibit decapping activity on their own (Fig. 3A).
FIGURE 3.
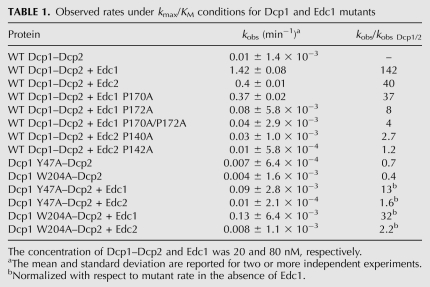
Proline recognition is required for coactivation by Edc1 and 2. (A) Representative TLC plate showing the decapping activity of WT Dcp1–Dcp2 and Edc proteins. Edc1 and Edc2 exhibit no decapping activity on their own, while Dcp1–Dcp2 in the presence of Edc1 and Edc2 exhibit enhanced decapping. (B) Fold activation in the presence of wild-type or mutant Edc1 and Edc2 is measured with respect to the observed rate of decapping by 20 nM wild-type Dcp1–Dcp2 alone. Rate constants were obtained at a single concentration of Dcp1–Dcp2 (20 nM) and Edc (80 nM), and were measured over two or more independent experiments.
Kinetic analysis revealed that the addition of Edc1 or Edc2 to the wild-type decapping complex results in a 140-fold or 40-fold increase, respectively, in the first order rate constant, kobs. (Fig. 3B; Table 1). As expected, the proline mutants displayed reduced the ability to enhance the rate of decapping for all Edc mutants except Edc1 P170A, with a greater than 20-fold reduction in the ability to activate the decapping complex compared to wild-type Edc1 (Fig. 3B; Table 1). This indicates that the proline residues play a critical role in the Dcp1–Edc interaction. Interestingly, Edc1 P170A still displays a modest ability to stimulate decapping. This is consistent with the phage display data, which suggest that Dcp1 may recognize some ligands with alanine at this position (Fig. 2B). Thus, we conclude that a consensus motif comprised of at least one proline residue is required for Edc1 and Edc2 to enhance decapping.
TABLE 1.
Observed rates under kmax/KM conditions for Dcp1 and Edc1 mutants
The Dcp1 PRS binding site is required for coactivation
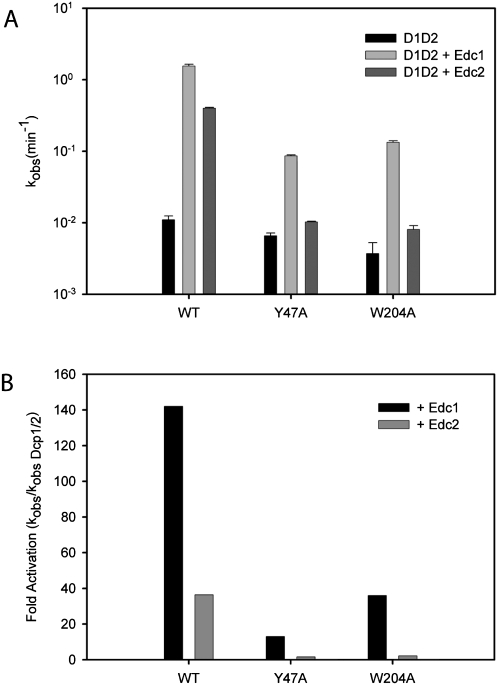
NMR binding experiments suggest that the Edc1–CTR binds the PRS binding site of Dcp1 (Fig. 1B–D). To determine whether the PRS binding site is required for coactivation by Edc1 and Edc2, we mutated two conserved residues of Dcp1, Y47 and W204, and tested the ability of Edc1 to enhance decapping in vitro. Mutation of Y47 or W204 to alanine reduced the ability of Edc1 to enhance decapping by roughly 18- and 12-fold, respectively. Coactivation by Edc2, which stimulated wild-type Dcp1–Dcp2 by 40-fold, was almost completely abolished in the mutant Dcp1 (Fig. 4; Table 1). In contrast, the Y47A and W204A mutants of Dcp1 had reduced rates of decapping of 1.4-fold and 2.5-fold, respectively, in the absence of Edc1, indicating the decreased ability of Edc1 to stimulate these mutants is not due to a general loss of function of Dcp1 (Fig. 4A; Table 1). Importantly, it was previously reported that mutation of Y47 and W204 to alanine resulted in reduced decapping activity in vivo (Tharun and Parker 1999). Although W56 is also conserved, we did not mutate it since it has been previously demonstrated that mutation of the corresponding residue in the Mena EVH1 domain alters its structure (Ball et al. 2000). These findings suggest that this conserved region of Dcp1 modulates the interaction between the decapping complex and PRS-containing coactivators.
FIGURE 4.
Mutation of conserved aromatic residues in the Dcp1 PRS-binding site reduces coactivation by Edc1 and 2. (A) Bar graph of observed rate constants for wild-type Dcp1–Dcp2, Dcp1 Y47A–Dcp2, and Dcp1 W204A–Dcp2 in the presence of wild-type Edc1 and Edc2 are plotted on a log scale. Assays were performed at a single concentration using 20 nM Dcp1–Dcp2 and 80 nM Edc1 or 2. Error bars for kobs are the standard error for the rate measured in two or more independent experiments. (B) Fold activation in the presence of Edc1 and 2 is normalized with respect to WT and mutant Dcp1–Dcp2 complexes in the absence of Edcs.
Lesions in Dcp1 and Edc1 impair binding
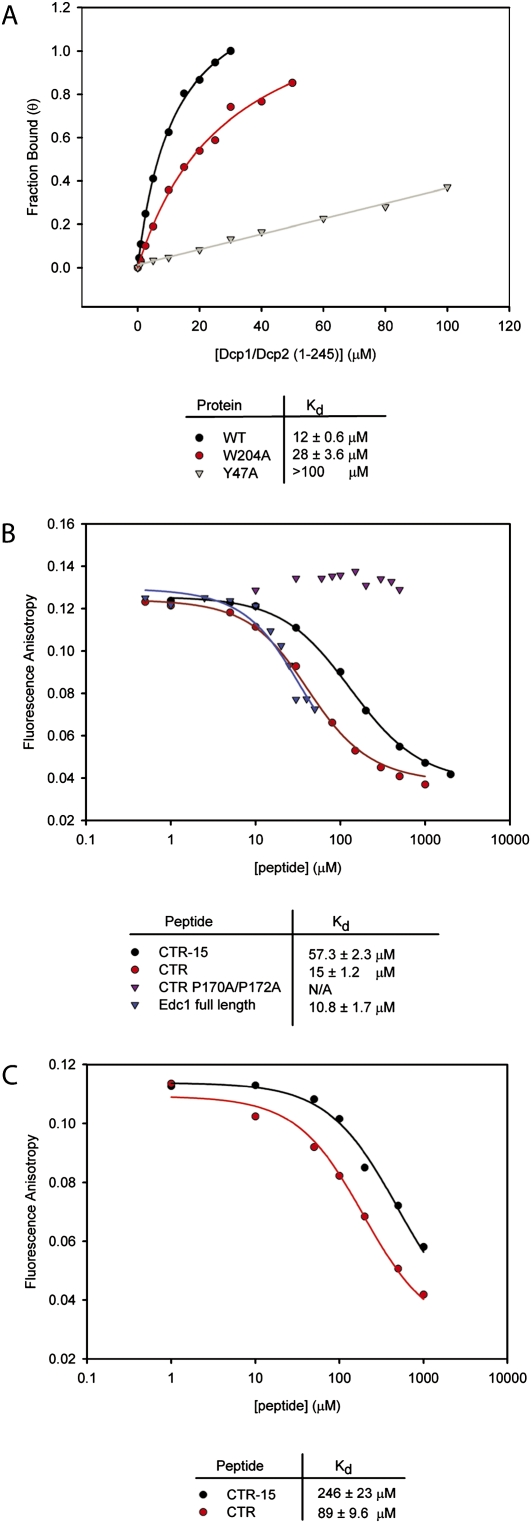
The mutational analyses above suggest that reduced activity may be correlated with a decrease in binding affinity. We therefore developed a fluorescence anisotropy assay to measure binding affinities of wild-type Dcp1–Dcp2 and PRS binding site mutants for a peptide containing the Edc PRS sequence. We used the last 15 residues of Edc1 N-terminally labeled with fluorescein (Fluor–CTR-15) (Table 2). Titration of this peptide with Dcp1–Dcp2 resulted in a fourfold increase in fluorescence anisotropy. Fitting of the data was performed using previously described methods (Roehrl et al. 2004). The anisotropy data were converted to fraction bound, and the Kd of the labeled peptide with wild-type Dcp1–Dcp2 was determined to be ∼12 μM (Fig. 5A). Mutations in the Dcp1 PRS binding site reduced the affinity of Dcp1–Dcp2 for the peptide by slightly more than twofold for the W204A mutant. The reduction in binding affinity for Y47A was too large to allow accurate determination of Kd (Fig. 5A). These experiments are in line with the NMR and kinetic analysis and suggest direct binding of the CTR to the Dcp1 PRS binding site.
TABLE 2.
Edc1 peptides used in fluorescence and deletion studies
FIGURE 5.
Mutations in Dcp1 and Edc1 reduce binding affinity of a Dcp1–Dcp2–Edc complex. (A) Binding curves of Fluor–CTR-15 to wild-type Dcp1–Dcp2, W204A, and Y47A mutants. The measured binding affinities are indicated below. (B) Competitive binding assay with wild-type Dcp1–Dcp2 measuring the displacement of Fluor–Edc-15 by unlabeled Edc1 peptides. Kd values were estimated based on a best fit of the data to a three state binding model. (C) Competitive binding assay with W204A mutant.
EVH1 ligands often achieve specificity for their target domain by binding regions outside the aromatic region (Peterson et al. 2007). We utilized a fluorescence anisotropy-based competition assay to determine if ligands of Dcp1 exhibit an extended binding epitope. We titrated increasing concentrations of 15 or 30 residue Edc1 CTR peptides against a fixed concentration of wild-type Dcp1–Dcp2 and Fluor–CTR-15 and observed a decrease in anisotropy, indicating displacement of the labeled peptide (Fig. 5B). The Kd of unlabeled CTR-15 is about fivefold greater than the fluorescein labeled peptide. This is likely because the hydrophobic fluorescein label interacts nonspecifically with the EVH1 binding surface on Dcp1, but this does not affect the interpretation of Kd determined from the competition assay. The 30 residue CTR peptide demonstrated an approximately fourfold greater affinity compared with Edc1 CTR-15. However, a double proline mutant of this peptide was unable to bind Dcp1–Dcp2 in any measurable quantity (Fig. 5B). Interestingly, the affinity of full-length Edc1 for Dcp1–Dcp2 is comparable to the 30 residue peptide, suggesting that all the binding determinants reside within the last 30 residues. We also tested peptides with lengths of 20 and 25 residues and found that they bind Dcp1–Dcp2 affinities similar to that of the Edc1–CTR (data not shown).
Consistent with the activity assays (Fig. 4), the binding experiments showed that Dcp1 W204A has a higher affinity than Y47A. Since W204A is outside of the canonical EVH1 aromatic triad, this raised the possibility that longer ligands might demonstrate a more substantial loss in binding affinity. We thus performed competition experiments using Dcp1 W204A-Dcp2 with unlabeled CTR-15 and CTR and found that binding affinity was sixfold weaker for the longer peptide compared to wild-type and about fourfold weaker for CTR-15 (Fig. 5C). Overall, the data from the anisotropy experiments demonstrate that mutation of conserved aromatic residues on Dcp1 or prolines in the PRS of Edc1 substantially impair binding. These data, in addition to the kinetic assays, indicate that binding to Dcp1 is a critical step in the mechanism of some coactivators of Dcp1–Dcp2.
Edc1 and Edc2 enhance the catalytic step and reduce KM
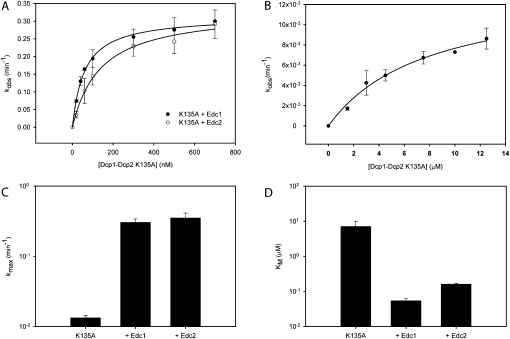
In principle, Edc1 and Edc2 could enhance the catalytic step of decapping, promote a tighter interaction between Dcp1–Dcp2 and the mRNA substrate, or both. To test these possibilities, we performed single-turnover kinetics at several concentrations of Dcp1–Dcp2 in the presence of saturating Edc1 or Edc2, and extracted kmax and KM (Fig. 6A,C,D; Table 3). We used a catalytically compromised mutant, Dcp2 K135A (Fig. 6B; Deshmukh et al. 2008; She et al. 2008), since reactions were too fast to reliably measure rates by manual pipetting for wild-type Dcp1–Dcp2 in the presence of Edc1 at enzyme concentrations higher than 80 nM. The K135A mutant was chosen since previous studies suggest that K135 plays a role in the chemical step of decapping, consistent with the observation that a related Nudix enzyme MutT has a lysine at this position that stabilizes departure of the leaving group (Mildvan et al. 2005; Deshmukh et al. 2008; She et al. 2008).
FIGURE 6.
Edc1 and Edc2 enhance both the catalytic step and substrate binding. (A) Graph of kobs versus enzyme concentration for Dcp1–Dcp2 K135A in the presence of saturating (5 μM) Edc1 (•) or Edc2 (○) used to obtain kmax and KM. The standard error was determined in two or more independent experiments. (B) Graph of kobs versus enzyme concentration for Dcp1–Dcp2 K135A with a 323-nt MFA2 RNA as substrate. The standard error is indicated. (C,D) Bar graphs of kmax and KM values for Dcp1–Dcp2 K135A alone and in the presence of saturating Edc1 or Edc2.
TABLE 3.
Kinetic rate constants for Dcp1–Dcp2 K135A alone and in the presence of Edcs obtained from single turnover kinetic assaysa
Addition of 5 μM Edc1 or Edc2 substantially increases rates of decapping, enhancing the catalytic step and reducing KM by 20- and 100-fold, respectively (Fig. 6C,D; Table 3). Increasing the concentration of Edc by twofold did not increase the observed rate of decapping, indicating saturating conditions (data not shown). The 100-fold reduction in KM agrees with previous studies showing that Edc1 and 2 preferentially bind RNA (Schwartz et al. 2003), and suggest that Edc1 and Edc2 enhance decapping by promoting a tighter enzyme–substrate complex, possibly by providing an additional RNA binding surface. Taken together, the catalytic efficiency (kmax/KM) of Dcp1–Dcp2 K135A is enhanced more than 1000-fold with Edc2 and more than 3000-fold with Edc1 (Table 3).
Edc1 and 2 are modular proteins
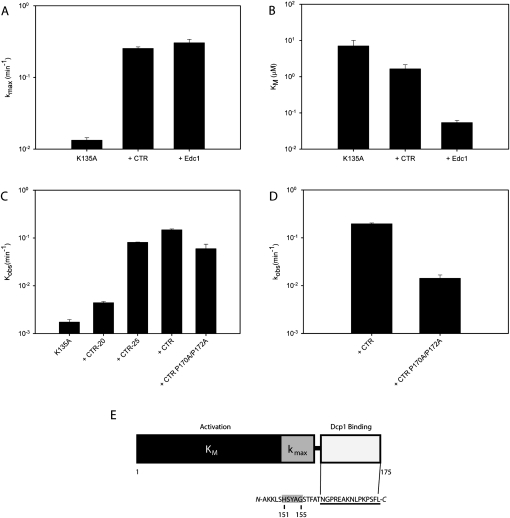
In a previous study it was reported that the Edc1–CTR is necessary to stimulate decapping by Dcp1–Dcp2 (Schwartz et al. 2003). We found that this peptide is sufficient to confer activation and performed kinetic analysis with Dcp1–Dcp2 K135A to determine the step affected. Surprisingly, the CTR of Edc1 enhances the catalytic step to nearly the same degree as full-length Edc1, stimulating kmax by roughly 20-fold (Fig. 7A; Table 3). In contrast, the CTR of Edc1 reduces KM by only fourfold, compared to the 100-fold reduction measured for the full-length protein (Fig. 7B; Table 3). We conclude the Edc1–CTR is primarily responsible for binding to Dcp1 and enhancing the catalytic step, whereas the preceding residues contribute most of the decrease in KM.
FIGURE 7.
The Edc1–CTR is necessary and sufficient to stimulate the catalytic step. (A,B) Bar graphs comparing kmax and KM values for Dcp1–Dcp2 K135A alone and in the presence of 100 μM CTR peptide or 5 μM full-length Edc1. (C) Bar graph of observed rate constants of Dcp1–Dcp2 K135A in the presence of Edc1–CTR peptides of increasing length. Assays were performed using 1.5 μM Dcp1–Dcp2 K135A and 200 μM Edc1 peptides. (D) K135A rate in the presence of Edc1–CTR and double proline mutant CTR measured at 20 μM peptide. (E) Schematic depicting Edc1 modularity. The Dcp1 binding region is shown in white. The consensus motif is underlined. The region associated with activation of the catalytic step is highlighted in gray and the region associated with reducing KM is shown in black.
We next mapped the minimal region of Edc1 required to stimulate decapping by using a series of truncated peptides beginning with CTR and reducing the length by five amino acids. These experiments were carried out with 200 μM peptide to ensure saturation of the Dcp1 binding site. The last 25 residues (CTR-25) were sufficient to stimulate Dcp1–Dcp2 K135A (Fig. 7C). Further N-terminal truncations (CTR-20) have little to no stimulatory effect, although the NMR, SPOT membrane and fluorescence anisotropy experiments demonstrate that fragments of Edc1 containing the last 12 residues do bind Dcp1 (Figs. 1B,C, 2A, 5). Additionally, we also tested the ability of a double proline CTR mutant to stimulate decapping. The double mutant enhanced decapping, which is surprising given that the same peptide did not bind Dcp1–Dcp2 in the fluorescence assay (Fig. 4B). To determine if this effect was related to peptide concentration, we performed the experiment again with the CTR peptides at 20 μM and found that the double proline mutant had only slight stimulatory effects compared to wild-type (Fig. 7D). These results suggest that coactivator binding to Dcp1 is necessary, but not sufficient to activate the decapping complex. Instead, a modular organization of Dcp1 binding and decapping activation domains is required. Within the activation domain, residues 151–155 are necessary for enhancing the catalytic step, while the remainder of the protein primarily decreases KM (Fig. 7E).
DISCUSSION
In this study we provide direct biochemical evidence of decapping coactivators that regulate the catalytic activity of Dcp2 by way of the PRS-binding site on Dcp1. Edc1 and Edc2 were previously identified as coactivators of the decapping complex (Schwartz et al. 2003), and we show here that they contain a proline-rich consensus motif at their C-termini that binds Dcp1 and is required for coactivation (Figs. 1–4). Moreover, loss of coactivating function can be directly correlated with a reduction in binding affinity (Fig. 5). A key observation is that Edc1 and Edc2 enhance both the catalytic step and substrate binding, thereby increasing the catalytic efficiency (kmax/KM) of Dcp1–Dcp2 by more than 3000-fold for a Dcp1–Dcp2–Edc1 heterotrimer, and more than 1000-fold for Dcp1–Dcp2–Edc2 (Fig. 6). This suggests that coactivators play a significant role in the catalytic mechanism of decapping, not only recruiting mRNA to the Dcp1–Dcp2 complex, but by enhancing the rate-limiting catalytic step.
Our results indicate that Edc1 and 2 are modular, possessing separable Dcp1 binding and activation domains. The activation domain of Edc1 was mapped to residues 1–155, with residues 151–155 required for observing coactivation of the catalytic step in vitro, while residues 1–145 decrease the KM. Shorter peptides containing the last 12 amino acids are sufficient for Dcp1 binding but do not enhance decapping (Figs. 2, 5, 7). Previous work indicated Edc1 is an RNA binding protein and our kinetic analysis reveals that one way Edc1 and Edc2 enhance decapping is by substantially lowering the KM for the RNA substrate (Fig. 6D; Table 3), presumably by providing additional binding surface for the RNA body. While Dcp2 has been shown to bind the RNA body as well, its affinity for RNA is relatively weak and nonspecific, with KM values in the low micromolar range (Deshmukh et al. 2008). Together with Dcp1–Dcp2, Edc1 and 2 improve substrate binding by 140-fold and 40-fold, respectively. Thus, one function of Edc1 and 2 may be to enhance association of Dcp1–Dcp2 with substrate RNA during carbon source shifts when these coactivators are expressed (DeRisi et al. 1997; Schwartz et al. 2003). Interestingly, deletion of Edc1 causes a dysregulation of specific mRNAs in yeast, suggesting that Edc1 and 2 may bind specific targets (Schwartz et al. 2003). A challenge for the future is to determine whether Edc1 and Edc2 enhance decapping globally on all mRNA or whether they function on specific targets, as described for Edc3 (Badis et al. 2004; Dong et al. 2007).
All EVH1 domains possess a cluster of surface-exposed aromatic residues involved in PRS binding (Ball et al. 2002; Zarrinpar et al. 2003). It was proposed that the PRS binding site of Dcp1 may accommodate extended epitopes compared to other EVH1 domains, since Dcp1 contains additional conserved surface-exposed residues (W204) neighboring the aromatic triad (W49, W56, and Y47) (Fig. 1D; She et al. 2004). Consistent with this view, mutation of W204 impairs Edc peptide binding in competition and direct binding assays (Fig. 5A,C). Additionally, phage display and peptide array experiments reveal that Dcp1 has an extended consensus sequence [E/D]x3[L/F/W]PxP[S/T][F/W] which differs from that described for other EVH1 domains (Fig. 2; Peterson and Volkman 2009). We note that while the data indicate that Dcp1 binds ligands over an extended surface, the observed binding affinities are relatively weak (Fig. 5), and this is consistent with previous EVH1 studies (Prehoda et al. 1999). Weak but specific interactions may be crucial in the rapidly changing environment of a decapping mRNP.
It is well understood that a variety of ligands can interact with a single EVH1 domain (Prehoda et al. 1999; Boeda et al. 2007), and three observations suggest that Dcp1 conforms to this paradigm. First, the Dcp1 PRS-binding site interacts with both Edc1 and Edc2, which promotes a similar coactivating response in Dcp2 (Figs. 3, 4, 6). Second, although Edc1 and Edc2 appear to be specific to yeast, the EVH1 fold in Dcp1 is well-conserved from yeast to humans, and earlier studies of human Dcp1 have suggested that it interacts with domains of several proteins containing PRS motifs (Bai et al. 2002; Cho et al. 2009). Finally, mutation of Y47 and W204 to alanine increase the half-life of reporter mRNA in vivo under conditions when Edc1 and 2 are not expressed, suggesting that additional proteins in yeast bind Dcp1 through the PRS binding site (Tharun and Parker 1999). It is noteworthy that additional decapping coactivators, such as Pat1 and PNRC2, have proline-rich sequences that are required for function (Cho et al. 2009; Braun et al. 2010; Haas et al. 2010). The question arises whether these proline-rich sequences confer to the consensus motif found here. Future work will be needed to further characterize the interaction of the Dcp1 PRS binding site with additional coactivators.
An important observation is that the catalytic step is strongly enhanced by coactivators. Previous work has shown that the catalytic step is rate-limiting and regulated at the level of protein–protein interactions (Deshmukh et al. 2008; She et al. 2008; Floor et al. 2010). Here we show that the Edc1 and Edc2 stimulate the catalytic step, providing an ∼20-fold improvement over Dcp1–Dcp2 K135A alone (Fig. 6C; Table 3). Combined with the 10-fold effect of Dcp1, this represents a 200-fold increase in the rate of the catalytic step for Dcp2 alone (Floor et al. 2010). These findings support the idea that Dcp1 is a general activator of decapping, which functions to bind coactivators that further enhance the catalytic activity of Dcp2. Surprisingly, the CTR recapitulates nearly all of the catalytic effects of the full-length protein. Clues to how the CTR affects the catalytic step come from recent studies that suggest Dcp2 has a composite active site, where the regulatory and catalytic domains are far apart and must be brought in close proximity to each other during the catalytic step to promote efficient decapping (Floor et al. 2010). It is tempting to speculate that like Dcp1, Edc1 and Edc2 may enhance the catalytic step by promoting the transition from the open, inactive form of Dcp2 to the closed, active form required for efficient chemistry. Edc1 residues 151–155, which are N-terminal to the Dcp1 binding region, may contact the catalytic domain of Dcp2, thereby enhancing closure. Alternatively, Edc1 may contribute to the chemical step directly without enhancing closure. It is also noteworthy that the double proline mutant CTR exhibits no detectable binding in the fluorescence assay, but is able to enhance decapping at high peptide concentrations (Figs. 5B, 7C). This indicates that the CTR may bind the active complex with RNA tighter than complex in the absence of substrate. Further structural and mechanistic studies will be required to understand how coactivators promote the catalytic step in Dcp2 upon binding to Dcp1.
In summary, we have demonstrated that the PRS-binding site of Dcp1 is an important interaction site for coactivators that play a direct role in the mechanism of decapping by enhancing the catalytic function of Dcp2. While a major function of Dcp1 is to accelerate the catalytic step in Dcp2, it plays a broader role by recruiting coactivators to the decapping mRNP, acting as a mediator between Dcp2 and other decay factors, thereby acting as an additional control point for decapping and 5′-3′ decay. The EVH1 functionality of Dcp1 could be utilized in the context of bulk decay, as well as to recruit factors that promote transcript-specific decapping. Alternatively, inhibitors of decapping could bind the PRS binding site of Dcp1, blocking the recruitment of coactivators and reducing rates of decapping. An important area of future research will be to determine how additional decapping factors impinge on the mechanism of Dcp1–Dcp2.
MATERIALS AND METHODS
Protein purification and mutagenesis
S. cerevisiae Dcp1–Dcp2 (1–245) complex was expressed and purified as described (Deshmukh et al. 2008). Mutants Y47A and W204A were made using whole plasmid PCR with divergent primers and sequences were confirmed by dideoxy sequencing. Edc1 and Edc2 were cloned into the vector pHis-GB1-parallel, containing a His tag and the B1 domain of Streptoccocal protein G (GB1) at the N-terminus (Card and Gardner 2005). Proteins were expressed in E. coli BL21(DE3) cells at 37° for 3 h following induction with IPTG. Cells were lysed by sonication, clarified at 14,000g, and the lysate was purified using a 5-mL Ni-NTA HisTrap HP column (GE Healthcare), eluting with an imidazole gradient (20–500 mM). Eluted proteins were subjected to gel filtration chromatography using a Superdex G75 column. Proteins were evaluated for purity by SDS-PAGE (Fig. 1E). Proteins were stored as described (Deshmukh et al. 2008). Edc1–CTR was made using whole plasmid PCR and divergent primers, and was expressed and purified as above. The Ni-NTA elution was cleaved with TEV protease and then subjected to HPLC using a preparative C18 column and eluting using a 0%–100% H2O-0.1% TFA/Acetonitrile gradient. The fraction containing the eluted peptide was identified by LC-MS and then lyophilized and stored. Shorter Edc1 peptides and CTR P170A/P172A were obtained from Elim Biopharmaceuticals. Peptides were synthesized without purification but were judged to be >95% pure by HPLC analysis.
NMR spectroscopy, protein expression, and purification
S. cerevisiae Dcp1 was cloned into pHis-GB1-parallel. A yeast-specific loop, which lacked density in the crystal structure (She et al. 2004), was deleted using whole plasmid PCR and divergent primers between residues 84 to 126 to improve protein solubility. Deletion of this loop does not affect decapping in vitro or in yeast (data not shown). Dcp1 was expressed in E. coli BL21(DE3) Rosetta cells grown in SBMX minimal media containing 15NH4Cl (Weber et al. 1992). 13C ILV precursors were added 30 min prior to induction as described (Medek et al. 2000). Cells were grown at 30°C for 7.5 h following induction and purified by Ni-NTA affinity chromatography as described above. Ni-NTA eluate was buffer-exchanged using a PD-10 column (GE Healthcare) into 50 mM L-Arginine, 50 mM L-Glutamate, 150 mM NaCl, 5 mM DTT, and 20 mM sodium phosphate at pH 7.6 (11.5 mM Na2HPO4 and 8.5 mM NaH2PO4). 15N and 13C HSQC experiments were performed with 70 μM Dcp1 on a Bruker Avance 800 MHz spectrometer outfitted with a cryogenic probe. For experiments with Edc1, unlabeled GB1–Edc1–CTR was added in 1.5 molar excess to GB1–Dcp1.
SPOT membrane
Single substitutions of the Edc1 C-terminal (164–175) peptide REAKNLPKPSFL were generated by semiautomated spot synthesis on Whatman 50 cellulose membranes as described (Kramer and Schneider-Mergener 1998). A spacer of three β-alanine residues was added at the C-terminus of each spotted peptide to avoid steric hindrance through the membrane surface. The membranes were incubated with GST–Dcp1 (10 μg/mL) for 1 h at room temperature. After washing, bound GST fused protein was labeled with rabbit polyclonal anti-GST antibody (Z-5, Santa Cruz Biotechnology) and HRP-coupled anti-rabbit IgG antibodies (Rockland). An enhanced chemiluminescence substrate (SuperSignal West Pico, Pierce) was used for detection on a LumiImagerTM (Roche Applied Science).
Phage display
A randomized nonapeptide library (X9) fused to the pVIII protein of the phagemid vector pC89 was used for the phage display procedure (Felici et al. 1991). Screening of the library was performed as follows: 200 μg of GST–Dcp1 fusion protein was bound to 20 μL glutathione-Sepharose 4B gel (Amersham Biosciences) and the matrix incubated for 30 min with 1010–1012 infectious particles in PBST (phosphate-buffered saline 0.05% Tween 20), supplemented with 5 mg/mL BSA, at room temperature in a total volume of 400 μL. After washing 10 times with PBST, the adherent phage was eluted by 350 μL of 100 mM glycine/HCl (pH 2.2) and the eluate neutralized with 20 μL of 2 M Tris base. Logarithmic phase E. coli XL-1Blue cells were infected by the eluate and the phages amplified using the helper phage VCSM13 (Stratagene) according to the standard protocol (Golemis 2001). After four panning rounds, the eluate was used to infect E. coli cells, and 20 individual colonies were picked and phagemid DNA sequenced.
Fluorescence anisotropy
N-terminally labeled Fluor–CTR-15 was synthesized by Elim Biopharmaceuticals. The buffer used for analysis was the same as that used for decapping assays as described (Jones et al. 2008). Measurements of FP were made using Greiner black 384 well nonbinding plates and an Analyst plate reader (LJL Biosystems). For the direct binding assays, 15 μL of labeled peptide (final concentration 20 nM) was mixed with an equal amount of GB1–Dcp1–Dcp2 at variable concentrations and incubated on ice for ≥10 min. For the competition assays, a solution containing 1.5X GB1–Dcp1–Dcp2 and labeled peptide (22.5 μM and 30 nM, respectively) was prepared. Competitor peptides were prepared at 3X final concentration and 10 μL of peptide or full-length Edc1 was mixed with 20 μL of protein solution. All samples were prepared in low-retention Eppendorf tubes. All assays were performed in triplicate and the average of the readings was plotted using Sigma Plot. Curve-fitting for estimation of Kd was performed using equations derived as previously described (Roehrl et al. 2004).
RNA preparation
Three hundred twenty-three-nucleotide MFA2 mRNA lacking a poly(A) tail was produced using T7 RNA polymerase in vitro run-off transcription from plasmid pRP802 linearized by EcoRI (LaGrandeur and Parker 1998). Transcription reactions contained 30 μL 10X buffer (400 mM Tris pH 8.0, 25 mM spermidine, 0.1% Triton), 15 μL each NTP from 100 mM stock, 11.4 μL 1 M MgCl2, 1.5 μL 1 M DTT, 1–2 μg template DNA, and H2O to a final volume of 300 μL. Preparation of cap-radiolabeled RNA was carried out as described (Jones et al. 2008).
Decapping assays
For assays performed at a single concentration under kmax/KM conditions, GB1–Dcp1–Dcp2 and GB1–Edc1 or 2 were prepared separately at 6X final concentration in 1X Dcp reaction buffer as described (Jones et al. 2008). Equal volumes of each were added together and incubated for 30 min on ice. Final concentration of GB1–Dcp1–Dcp2 was 20 nM and GB1–Edc1 or 2 was 80 nM. RNA substrate mix was prepared as described (Jones et al. 2008). Reactions were performed in an iceblock at ∼0.1°C taking time points every 15 s. Rate constants were determined by dividing the initial rate of the reaction by the endpoint. The fraction m7GDP released at the endpoint was 0.88 for these assays and was determined by incubating Dcp1–Dcp2 with the substrate for 30 min at room temperature. For the determination of kinetic constants, rates were measured at a series of enzyme concentrations using Dcp1–Dcp2 K135A in a cold room at 4°C. Assays were performed with saturating concentrations of Edc, which were 5 μM for full-length Edc1 and 2 and 100 μM Edc1–CTR. Rates were initially measured with 10 μM Edc. Reducing the concentration of Edc twofold to 5 μM did not reduce the rate. For Edc1–CTR rates were initially measured at 200 μM and a twofold reduction in concentration did not alter the rate. Rates for each enzyme concentration were calculated as described above. The endpoint for these assays was determined to be 0.95. To map the minimal region of Edc1 required for activation, Dcp1–Dcp2 K135A was incubated with saturating peptide. Reactions were measured at 4°C at a single concentration. Final concentration of K135A was 1.5 μM and peptide was 200 μM. For all assays, TLC analysis was performed and the resulting data were plotted and fitted to extract kobs, or kmax and KM as described (Jones et al. 2008). All assays were repeated at least twice and the rate for each enzyme concentration was averaged between experiments. Average rates were plotted with error bars representing the standard error of the experiment.
ACKNOWLEDGMENTS
M.S.B. received support from a National Institutes of General Medical Sciences predoctoral fellowship (1R25GM56847). This work was supported by National Institutes of Health grant R01GM78360 to J.D.G. We thank Dr. Roy Parker (University of Arizona) for providing reagents, including the MFA2 transcription template and expression plasmids for Edc1 and Edc2. We thank Kenny Ang (UCSF Small Molecule Discovery Center) for use of the Analyst plate reader and technical assistance, and Nathan Moerke (ICCB Screening Facility, Harvard Medical School) for providing the curve fitting algorithms for the fluorescence assay.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.2382011.
REFERENCES
- Amrani N, Sachs MS, Jacobson A 2006. Early nonsense: mRNA decay solves a translational problem. Nat Rev Mol Cell Biol 7: 415–425 [DOI] [PubMed] [Google Scholar]
- Badis G, Saveanu C, Fromont-Racine M, Jacquier A 2004. Targeted mRNA degradation by deadenylation-independent decapping. Mol Cell 15: 5–15 [DOI] [PubMed] [Google Scholar]
- Bai RY, Koester C, Ouyang T, Hahn SA, Hammerschmidt M, Peschel C, Duyster J 2002. SMIF, a Smad4-interacting protein that functions as a co-activator in TGFbeta signalling. Nat Cell Biol 4: 181–190 [DOI] [PubMed] [Google Scholar]
- Ball LJ, Kuhne R, Hoffmann B, Hafner A, Schmieder P, Volkmer-Engert R, Hof M, Wahl M, Schneider-Mergener J, Walter U, et al. 2000. Dual epitope recognition by the VASP EVH1 domain modulates polyproline ligand specificity and binding affinity. EMBO J 19: 4903–4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball LJ, Jarchau T, Oschkinat H, Walter U 2002. EVH1 domains: structure, function and interactions. FEBS Lett 513: 45–52 [DOI] [PubMed] [Google Scholar]
- Beelman CA, Stevens A, Caponigro G, LaGrandeur TE, Hatfield L, Fortner DM, Parker R 1996. An essential component of the decapping enzyme required for normal rates of mRNA turnover. Nature 382: 642–646 [DOI] [PubMed] [Google Scholar]
- Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde E 2006. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev 20: 1885–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeda B, Briggs DC, Higgins T, Garvalov BK, Fadden AJ, McDonald NQ, Way M 2007. Tes, a specific Mena interacting partner, breaks the rules for EVH1 binding. Mol Cell 28: 1071–1082 [DOI] [PubMed] [Google Scholar]
- Braun JE, Tritschler F, Haas G, Igreja C, Truffault V, Weichenrieder O, Izaurralde E 2010. The C-terminal α–α superhelix of Pat is required for mRNA decapping in metazoa. EMBO J 29: 2368–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card PB, Gardner KH 2005. Identification and optimization of protein domains for NMR study. Methods Enzymol 394: 3–16 [DOI] [PubMed] [Google Scholar]
- Cho H, Kim KM, Kim YK 2009. Human proline-rich nuclear receptor coregulatory protein 2 mediates an interaction between mRNA surveillance machinery and decapping complex. Mol Cell 33: 75–86 [DOI] [PubMed] [Google Scholar]
- Chowdhury D, Novina CD 2005. RNAi and RNA-based regulation of immune system function. Adv Immunol 88: 267–292 [DOI] [PubMed] [Google Scholar]
- Chowdhury D, Tharun S 2009. Activation of decapping involves binding of the mRNA and facilitation of the post-binding steps by the Lsm1-7-Pat1 complex. RNA 15: 1837–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller J, Parker R 2004. Eukaryotic mRNA decapping. Annu Rev Biochem 73: 861–890 [DOI] [PubMed] [Google Scholar]
- Coller JM, Tucker M, Sheth U, Valencia-Sanchez MA, Parker R 2001. The DEAD box helicase, Dhh1p, functions in mRNA decapping and interacts with both the decapping and deadenylase complexes. RNA 7: 1717–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRisi JL, Iyer VR, Brown PO 1997. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278: 680–686 [DOI] [PubMed] [Google Scholar]
- Deshmukh MV, Jones BN, Quang-Dang DU, Flinders J, Floor SN, Kim C, Jemielity J, Kalek M, Darzynkiewicz E, Gross JD 2008. mRNA decapping is promoted by an RNA-binding channel in Dcp2. Mol Cell 29: 324–336 [DOI] [PubMed] [Google Scholar]
- Dong S, Li C, Zenklusen D, Singer RH, Jacobson A, He F 2007. YRA1 autoregulation requires nuclear export and cytoplasmic Edc3p-mediated degradation of its pre-mRNA. Mol Cell 25: 559–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunckley T, Tucker M, Parker R 2001. Two related proteins, Edc1p and Edc2p, stimulate mRNA decapping in Saccharomyces cerevisiae. Genetics 157: 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A, Rehwinkel J, Stricker M, Huntzinger E, Yang SF, Doerks T, Dorner S, Bork P, Boutros M, Izaurralde E 2007. Target-specific requirements for enhancers of decapping in miRNA-mediated gene silencing. Genes Dev 21: 2558–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felici F, Castagnoli L, Musacchio A, Jappelli R, Cesareni G 1991. Selection of antibody ligands from a large library of oligopeptides expressed on a multivalent exposition vector. J Mol Biol 222: 301–310 [DOI] [PubMed] [Google Scholar]
- Fenger-Gron M, Fillman C, Norrild B, Lykke-Andersen J 2005. Multiple processing body factors and the ARE binding protein TTP activate mRNA decapping. Mol Cell 20: 905–915 [DOI] [PubMed] [Google Scholar]
- Floor SN, Jones BN, Gross JD 2008. Control of mRNA decapping by Dcp2: An open and shut case? RNA Biol 5: 189–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floor SN, Jones BN, Hernandez GA, Gross JD 2010. A split active site couples cap recognition by Dcp2 to activation. Nat Struct Mol Biol 17: 1096–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks TM, Lykke-Andersen J 2008. The control of mRNA decapping and P-body formation. Mol Cell 32: 605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund C, Schmalz HG, Sticht J, Kühne R 2008. Proline-rich sequence recognition domains (PRD): ligands, function and inhibition. Handb Exp Pharmacol 186: 407–429 [DOI] [PubMed] [Google Scholar]
- Garneau NL, Wilusz J, Wilusz CJ 2007. The highways and biways of mRNA decay. Nat Rev Mol Cell Biol 8: 113–126 [DOI] [PubMed] [Google Scholar]
- Golemis E 2001. Protein-protein interactions: A molecular cloning manual, pp. 143–166 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Haas G, Braun JE, Igreja C, Tritschler F, Nishihara T, Izaurralde E 2010. HPat provides a link between deadenylation and decapping in metazoa. J Cell Biol 189: 289–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Jacobson A 2001. Upf1p, Nmd2p, and Upf3p regulate the decapping and exonucleolytic degradation of both nonsense-containing mRNAs and wild-type mRNAs. Mol Cell Biol 21: 1515–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Brown AH, Jacobson A 1997. Upf1p, Nmd2p, and Upf3p are interacting components of the yeast nonsense-mediated mRNA decay pathway. Mol Cell Biol 17: 1580–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgers V, Teixeira D, Parker R 2006. Translation-independent inhibition of mRNA deadenylation during stress in Saccharomyces cerevisiae. RNA 12: 1835–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt MR, Koffer A 2001. Cell motility: proline-rich proteins promote protrusions. Trends Cell Biol 11: 38–46 [DOI] [PubMed] [Google Scholar]
- Isken O, Maquat LE 2007. Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev 21: 1833–1856 [DOI] [PubMed] [Google Scholar]
- Jones BN, Quang-Dang DU, Oku Y, Gross JD 2008. A kinetic assay to monitor RNA decapping under single-turnover conditions. Methods Enzymol 448: 23–40 [DOI] [PubMed] [Google Scholar]
- Kramer A, Schneider-Mergener J 1998. Synthesis and screening of peptide libraries on continuous cellulose membrane supports. Methods Mol Biol 87: 25–39 [DOI] [PubMed] [Google Scholar]
- Kramer A, Reineke U, Dong L, Hoffmann B, Hoffmuller U, Winkler D, Volkmer-Engert R, Schneider-Mergener J 1999. Spot synthesis: observations and optimizations. J Pept Res 54: 319–327 [DOI] [PubMed] [Google Scholar]
- Krogan NJ, Cagney G, Yu H, Zhong G, Guo X, Ignatchenko A, Li J, Pu S, Datta N, Tikuisis AP, et al. 2006. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440: 637–643 [DOI] [PubMed] [Google Scholar]
- Kshirsagar M, Parker R 2004. Identification of Edc3p as an enhancer of mRNA decapping in Saccharomyces cerevisiae. Genetics 166: 729–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGrandeur TE, Parker R 1998. Isolation and characterization of Dcp1p, the yeast mRNA decapping enzyme. EMBO J 17: 1487–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeJeune F, Li X, Maquat LE 2003. Nonsense-mediated mRNA decay in mammalian cells involves decapping, deadenylating, and exonucleolytic activities. Mol Cell 12: 675–687 [DOI] [PubMed] [Google Scholar]
- Lindstein T, June CH, Ledbetter JA, Stella G, Thompson CB 1989. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science 244: 339–343 [DOI] [PubMed] [Google Scholar]
- Medek A, Olejniczak ET, Meadows RP, Fesik SW 2000. An approach for high-throughput structure determination of proteins by NMR spectroscopy. J Biol NMR 18: 229–238 [DOI] [PubMed] [Google Scholar]
- Mildvan AS, Xia Z, Azurmendi HF, Saraswat V, Legler PM, Massiah MA, Gabelli SB, Bianchet MA, Kang LW, Amzel LM 2005. Structures and mechanisms of Nudix hydrolases. Arch Biochem Biophys 433: 129–143 [DOI] [PubMed] [Google Scholar]
- Neef DW, Thiele DJ 2009. Enhancer of decapping proteins 1 and 2 are important for translation during heat stress in Saccharomyces cerevisiae. Mol Microbiol 73: 1032–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebuhr K, Ebel F, Frank R, Reinhard M, Domann E, Carl UD, Walter U, Gertler FB, Wehland J, Chakraborty T 1997. A novel proline-rich motif present in ActA of Listeria monocytogenes and cytoskeletal proteins is the ligand for the EVH1 domain, a protein module present in the Ena/VASP family. EMBO J 16: 5433–5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R, Sheth U 2007. P bodies and the control of mRNA translation and degradation. Mol Cell 25: 635–646 [DOI] [PubMed] [Google Scholar]
- Parker R, Song H 2004. The enzymes and control of eukaryotic mRNA turnover. Nat Struct Mol Biol 11: 121–127 [DOI] [PubMed] [Google Scholar]
- Peterson FC, Volkman BF 2009. Diversity of polyproline recognition by EVH1 domains. Front Biosci 14: 833–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson FC, Deng Q, Zettl M, Prehoda KE, Lim WA, Way M, Volkman BF 2007. Multiple WASP-interacting protein recognition motifs are required for a functional interaction with N-WASP. J Biol Chem 282: 8446–8453 [DOI] [PubMed] [Google Scholar]
- Prehoda KE, Lee DJ, Lim WA 1999. Structure of the enabled/VASP homology 1 domain-peptide complex: a key component in the spatial control of actin assembly. Cell 97: 471–480 [DOI] [PubMed] [Google Scholar]
- Rehwinkel J, Behm-Ansmant I, Gatfield D, Izaurralde E 2005. A crucial role for GW182 and the DCP1:DCP2 decapping complex in miRNA-mediated gene silencing. RNA 11: 1640–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissland OS, Norbury CJ 2009. Decapping is preceded by 3′ uridylation in a novel pathway of bulk mRNA turnover. Nat Struct Mol Biol 16: 616–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrl MH, Wang JY, Wagner G 2004. A general framework for development and data analysis of competitive high-throughput screens for small-molecule inhibitors of protein-protein interactions by fluorescence polarization. Biochemistry 43: 16056–16066 [DOI] [PubMed] [Google Scholar]
- Schier AF 2007. The maternal-zygotic transition: death and birth of RNAs. Science 316: 406–407 [DOI] [PubMed] [Google Scholar]
- Schwartz D, Decker CJ, Parker R 2003. The enhancer of decapping proteins, Edc1p and Edc2p, bind RNA and stimulate the activity of the decapping enzyme. RNA 9: 239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G, Kamen R 1986. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell 46: 659–667 [DOI] [PubMed] [Google Scholar]
- She M, Decker CJ, Sundramurthy K, Liu Y, Chen N, Parker R, Song H 2004. Crystal structure of Dcp1p and its functional implications in mRNA decapping. Nat Struct Mol Biol 11: 249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- She M, Decker CJ, Svergun DI, Round A, Chen N, Muhlrad D, Parker R, Song H 2008. Structural basis of dcp2 recognition and activation by dcp1. Mol Cell 29: 337–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MG, Kiledjian M 2007. 3′ Terminal oligo U-tract-mediated stimulation of decapping. RNA 13: 2356–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger M, Schwartz DC, Kiledjian M, Parker R 2003. Analysis of recombinant yeast decapping enzyme. RNA 9: 231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A, Maupin MK 1987. A 5′-3′ exoribonuclease of Saccharomyces cerevisiae: size and novel substrate specificity. Arch Biochem Biophys 252: 339–347 [DOI] [PubMed] [Google Scholar]
- Tharun S 2008. Purification and analysis of the decapping activator Lsm1p-7p-Pat1p complex from yeast. Methods Enzymol 448: 41–55 [DOI] [PubMed] [Google Scholar]
- Tharun S, Parker R 1999. Analysis of mutations in the yeast mRNA decapping enzyme. Genetics 151: 1273–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharun S, He W, Mayes AE, Lennertz P, Beggs JD, Parker R 2000. Yeast Sm-like proteins function in mRNA decapping and decay. Nature 404: 515–518 [DOI] [PubMed] [Google Scholar]
- Weber DJ, Gittis AG, Mullen GP, Abeygunawardana C, Lattman EE, Mildvan AS 1992. NMR docking of a substrate into the X-ray structure of staphylococcal nuclease. Proteins 13: 275–287 [DOI] [PubMed] [Google Scholar]
- Zarrinpar A, Bhattacharyya RP, Lim WA 2003. The structure and function of proline recognition domains. Sci STKE 2003: RE8 doi: 10.1126/stke.2003.179.re8 [DOI] [PubMed] [Google Scholar]