Abstract
Specific quantitative techniques have been used to measure the cytoplasmic estradiol-binding protein (EBP) in human mammary carcinoma tissue specimens. Sucrose gradient centrifugation reveals EBP to sediment at 8S and 4S. Variable quantities of non-specific estradiol binding occurs in the 4S region of the sucrose gradient necessitating controls to insure specificity of the estradiol protein interaction.
Using dextran-coated charcoal to separate bound from free estradiol Scatchard analysis finds the dissociation constant of the estradiol EBP interaction to be ∼ 2.6×10-10 M, indicative of the very high affinity of the ligand for the EBP. Quantitation of EBP sites in 64 primary and metastatic human breast tumors demonstrates a continuous spectrum of values from 0 to 612 fmol per mg of cytoplasmic protein. Specific 8S binding in the sucrose gradient centrifugation was not detected in specimens containing less than 9.0 fmol EBP per mg cytoplasmic protein.
Since data from animal breast tumors and preliminary evidence from human breast tumors indicates an excellent correlation between the presence of abundant tumor EBP and endocrine-induced breast cancer regressions, precise quantitation of EBP in all human primary tumors may prove to be an excellent prognosticator of endocrine therapy in metastatic breast cancer.
Full text
PDF

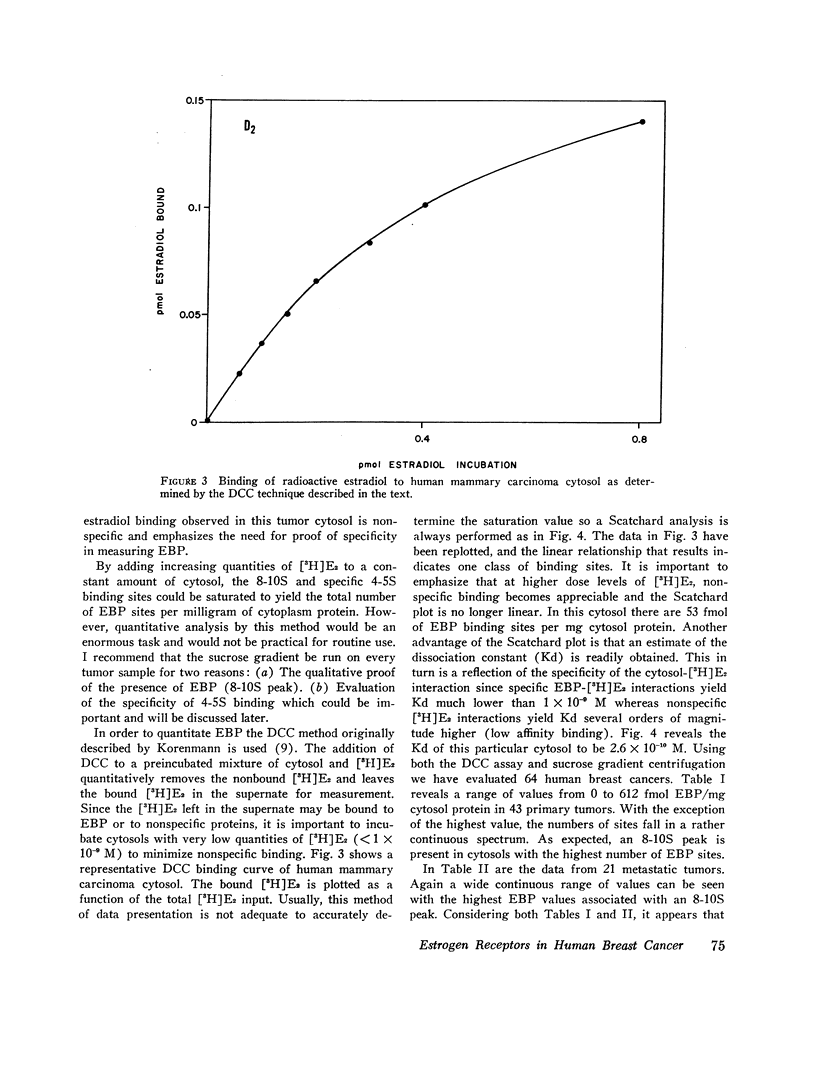
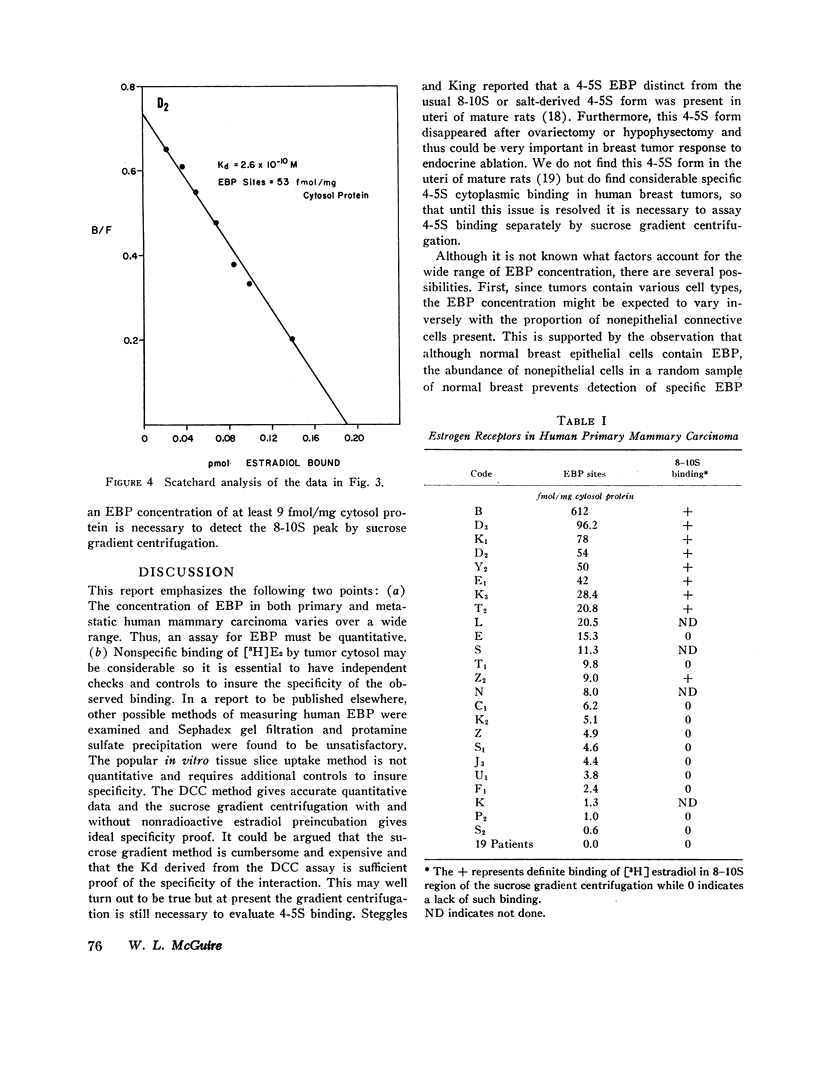

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chamness G. C., McGuire W. L. Estrogen receptor in the rat uterus. Physiological forms and artifacts. Biochemistry. 1972 Jun 20;11(13):2466–2472. doi: 10.1021/bi00763a013. [DOI] [PubMed] [Google Scholar]
- Feherty P., Farrer-Brown G., Kellie A. E. Oestradiol receptors in carcinoma and benign disease of the breast: an in vitro assay. Br J Cancer. 1971 Dec;25(4):697–710. doi: 10.1038/bjc.1971.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGINS C., BERGENSTAL D. M. Inhibition of human mammary and prostatic cancers by adrenalectomy. Cancer Res. 1952 Feb;12(2):134–141. [PubMed] [Google Scholar]
- Hähnel R., Twaddle E., Vivian A. B. Estrogen receptors in human breast cancer. 2. In vitro binding of estradiol by benign and malignant tumors. Steroids. 1971 Dec;18(6):681–708. doi: 10.1016/0039-128x(71)90030-4. [DOI] [PubMed] [Google Scholar]
- James F., James V. H., Carter A. E., Irvine W. T. A comparison of in vivo and in vitro uptake of estradiol by human breast tumors and the relationship to steroid excretion. Cancer Res. 1971 Sep;31(9):1268–1272. [PubMed] [Google Scholar]
- Jensen E. V., Block G. E., Smith S., Kyser K., DeSombre E. R. Estrogen receptors and breast cancer response to adrenalectomy. Natl Cancer Inst Monogr. 1971 Dec;34:55–70. [PubMed] [Google Scholar]
- Johansson H., Terenius L., Thorén L. The binding of estradiol-17beta to human breast cancers and other tissues in vitro. Cancer Res. 1970 Mar;30(3):692–698. [PubMed] [Google Scholar]
- Kennedy B. J. Hormone therapy for advanced breast cancer. Cancer. 1965 Dec;18(12):1551–1557. doi: 10.1002/1097-0142(196512)18:12<1551::aid-cncr2820181206>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Korenman S. G., Dukes B. A. Specific estrogen binding by the cytoplasm fof human breast carcinoma. J Clin Endocrinol Metab. 1970 May;30(5):639–645. doi: 10.1210/jcem-30-5-639. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewison E. F. Castration in the treatment of advanced breast cancer. Cancer. 1965 Dec;18(12):1558–1562. doi: 10.1002/1097-0142(196512)18:12<1558::aid-cncr2820181207>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McGuire W. L., Huff K., Jenning A., Chamness G. C. Mammary carcinoma: a specific biochemical defect in autonomous tumors. Science. 1972 Jan 21;175(4019):335–336. doi: 10.1126/science.175.4019.335. [DOI] [PubMed] [Google Scholar]
- McGuire W. L., Julian J. A., Chamness G. C. A dissociation between ovarian dependent growth and estrogen sensitivity in mammary carcinoma. Endocrinology. 1971 Oct;89(4):969–973. doi: 10.1210/endo-89-4-969. [DOI] [PubMed] [Google Scholar]
- McGuire W. L., Julian J. A. Comparison of macromolecular binding of estradiol in hormone-dependent and hormone-independent rat mammary carcinoma. Cancer Res. 1971 Oct;31(10):1440–1445. [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Sander S. The in vitro uptake of oestradiol in biopsies from 25 breast cancer patients. Acta Pathol Microbiol Scand. 1968;74(2):301–302. doi: 10.1111/j.1699-0463.1968.tb03481.x. [DOI] [PubMed] [Google Scholar]
- Steggles A. W., King R. J. The use of protamine to study [6,7-3H] oestradiol-17-beta binding in rat uterus. Biochem J. 1970 Aug;118(5):695–701. doi: 10.1042/bj1180695. [DOI] [PMC free article] [PubMed] [Google Scholar]



