Abstract
The lack of an effective medical treatment for gastroparesis has pushed the research of new techniques of gastric electrical stimulation (GES) for nearly half a century of experimentation with a large variety of electrical stimuli delivered to the gastric wall of animals and patients with gastroparesis. Three principal methods are currently available: gastric low-frequency/high-energy GES with long pulse stimulation, high-frequency/low-energy GES with short pulse stimulation and neural sequential GES. The first method aims to reset a regular slow wave rhythm, but has variable effects on contractions and requires devices with large and heavy batteries unsuitable for implantation. High-frequency/low-energy GES, although inadequate to restore a normal gastric electro-mechanical activity, improves dyspeptic symptoms, such as nausea and vomiting, giving patients a better quality of life together with a more satisfactory nutritional status and is suitable for implantation. Unfortunately, the numerous clinical studies using this type of GES, with the exception of two, were not controlled and there is a need for definitive verification of the effectiveness of this technique to justify the cost and the risks of this procedure. The last method, which is neural sequential GES, consists of a microprocessor-controlled sequential activation of a series of annular electrodes along the distal two thirds of the stomach and is able to induce propagated contractions causing forceful emptying of the gastric content. The latter method is the most promising, but has been used only in animals and needs to be tested in patients with gastroparesis before it is regarded as a solution for this disease.
Keywords: Gastric electrical stimulation, Gastric emptying, Gastric motility, Gastric myoelectric activity, Gastroparesis, Prokinetic drugs
INTRODUCTION
Gastroparesis is a chronic disorder characterized by a severe functional delay in gastric emptying (GE), which not only causes distressing symptoms, such as upper abdominal discomfort or pain, a sense of epigastric fullness after meals, early satiety, nausea, and vomiting, but may also lead to nutritional depletion requiring enteral or parenteral nutrition. The treatment of this condition represents a clinical challenge and is one of the most disappointing areas in medicine. The current available medical therapy is represented, by dietary modifications and administration of prokinetic agents, such as domperidone, metoclopramide and derivatives, cholinomimetics, such as neostigmine, macrolides, such as clarithromycin, erythromycin, and “motilides” and, more recently, the 5HT4 selective agonists, such as prucalopride[1], while cisapride has been withdrawn from most markets due to its dangerous side effects which affect the heart. However, some patients with gastroparesis can not undergo chronic treatment with prokinetic drugs, due to the occurrence of severe side-effects, such as nervous disturbances caused by metoclopramide, hyperprolactinemia due to domperidone, and antibiotic activity with erythromycin. In addition, tachyphylaxis may occur with some drugs, such as domperidone and erythromycin, and refractoriness to prokinetic agents is observed in a significant number of patients. The intrapyloric endoscopic injection of botulinum toxin seems to relieve the symptoms of gastroparesis[2,3]. However, a preliminary controlled double-blind study apparently failed to confirm previous results with regard to symptoms, although showed a significant improvement in solid GE[4]. If all of these treatments are unsuccessful and nutrition “per os” is insufficient, patients must undergo enteral nutrition. Surgical jejunostomy performed by laparoscopy and percutaneous endoscopic jejunostomy are indicated for patients with refractory gastroparesis unable to maintain sufficient nutrition “per os”, in order to provide nutrients, fluids and medications[5], on condition that there are no motor disturbances of the intestine, such as pseudo-obstruction. If enteral nutrition is not possible, the patient is usually referred to the surgeon for partial or even subtotal gastrectomy with Roux en Y reconstruction[6]. If gastric resection is risky, or refused by the patient, or does not resolve the nutritional problems, the patient must undergo permanent enteral or parenteral nutrition. No alternative to surgery and chronic artificial nutrition was imaginable until 1963, when investigators hoped that gastric electrical stimulation was a new way to cure ileus[7]. Following cardiac stimulation with a pacemaker, these authors thought that it would be sufficient to deliver an electrical stimulus to the gut wall to restore an efficient contraction. However, this simple idea turned out to be more difficult than expected and remained a dream for decades, because the electrical activity, that governs the motor function of the stomach, is much more complex than that of the heart.
The present paper presents a critical overview of various methods of gastric electrical stimulation used in animals and humans aiming to restore efficient gastric motor function and improve dyspeptic symptoms in gastroparesis.
GASTRIC MYOELECTRICAL ACTIVITY
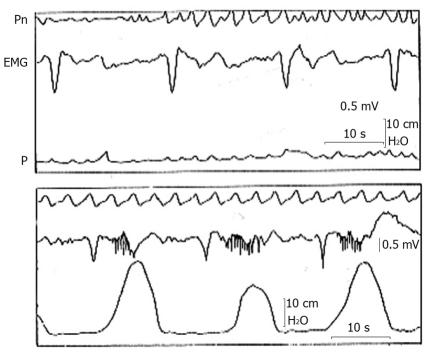
To understand the mechanism of gastric electrical stimulation it is necessary to know what gastric myoelectrical activity is. This consists of an uninterrupted sequence of electrical potential variations called “slow waves”, that spring out continuously, at a frequency of about 3/min in man, from a small zone of the proximal gastric corpus near the great curvature (pacemaker area), and propagate distally along the gastric wall toward the pylorus in the form of incomplete depolarization-repolarization annular bands. When the depolarization reaches a determined threshold, the smooth muscle cell membrane depolarizes completely with consequent contraction and another kind of electrical activity, called “spike potentials”, appears superimposed on the second part of the slow wave[8,9] (Figure 1). The origin of slow waves lies in the interstitial cells of Cajal type I (ICC), a series of highly ramified cells located between the longitudinal and circular muscle coats, making close contacts with the Auerbach plexus and the smooth muscle cells of both layers mediating the cholinergic excitatory and nitrergic inhibitory inputs[10]. These cells, also called myoneural, have the property of automatically generating and transmitting to smooth muscle cells, the slow waves with an intrinsic frequency decreasing caudally[10,11]. The absence of ICC is associated to the absence of coordinated slow waves[12] and depletion of these cells in pathologic conditions, such as diabetic gastroparesis, may interrupt the propagation of both spontaneous and artificially paced slow waves[13].
Figure 1.
Gastric myoelectric and pressure activities recorded with an intraluminal electromyographic and manometric technique from the gastric antrum of a healthy subject during a period of absence of pressure waves (top tracing) and a period of contractile activity (bottom tracing). Note the slow waves of normal morphology and the frequency of 3 cycles/min that in correspondence with the contractions are followed by bursts of spikes[16]. EMG: Gastric myoelectric; Pn: Pneumogram; P: Pressure.
Hence, the orad area generates the most frequent slow wave activity and functions as a pacemaker. Slow waves initiated at proximal areas migrate caudally and “capture” (“entrain”) contiguous distal areas of less frequent intrinsic activity, driving them at their own rate (“coupling”). The slow waves propagate from one cell to another through special contacts in the cell membrane called “nexuses”, which provide a pathway of low electrical resistance regulated by the neuro-humoral control system. From these data one can understand the complexity of gastric myoelectrical activity and the importance of its role in gastric motor function. It represents the end point of the motility control system, on which neurocrine, endocrine and paracrine systems operate, and, as it establishes frequency, direction and propagation velocity of peristaltic waves, it may be considered the indispensable condition (“conditio sine qua non”) of any coordinated motor activity of the stomach[14].
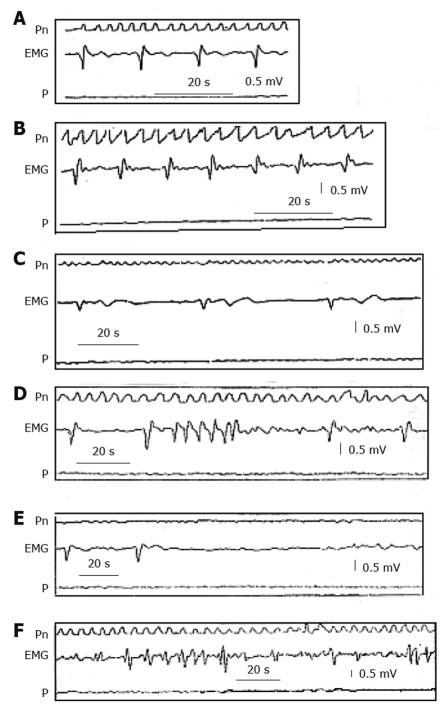
In gastroparesis, there are more or less severe alterations in gastric myoelectric activity[15], which may be recorded with intraluminal, serosal and cutaneous electrodes[16,17]. The electrogastrographic alterations consist of various kinds of arrhythmias (Figure 2), very similar to those observed on the electrocardiogram in some cardiac diseases, such as tachygastria, tachyarrhythmia, bradyarrhythmia, asystolia (electrical silence), and gastric fibrillation[16]. The latter is a complete disorganization of gastric electrical activity due to impairment of coupling and propagation of gastric slow waves. All these alterations result in a lack of propagated gastric contractions with a more or less severe delay in GE. However, it is also possible that, despite a regular slow wave rhythm, the gastric wall is unable to contract (electro-mechanical dissociation), because of alterations in the smooth muscle cell contractile system activation and operation.
Figure 2.
Electrogastrographic alterations. Series of alterations in the gastric myoelectric activity recorded with an electromyographic and manometric technique from the gastric antrum of patients with severe gastroparesis in confront with (A) normal myoelectric activity recorded in a healthy subject showing slow waves of normal morphology and frequency of 3 cycles/min. B: Tachygastria; C: Bradygastria; D: Run of high frequency tachygastria; E: Bradyarrhythmia; F: Complete disorganization of myoelectric activity (“gastric fibrillation”). Note that all these alterations are associated with absence of gastric contractions[1]. EMG: Gastric myoelectric; Pn: Pneumogram; P: Pressure.
It is paramount to remember that the motility structures of the gastric wall, such as the smooth muscle cell contractile system, interstitial cells of Cajal pacemaker network, enteric neurons (motor, sensory, integratory) and afferent and efferent fibres connected with the CNS, work using a depolarization-repolarization mechanism. An electrical stimulus delivered to the gastric wall may influence the electrical activity of these structures with consequent modifications of their function and its effect depends on the characteristics of excitability of the target tissues and on the stimulus parameters.
GASTRIC ELECTRICAL STIMULATION
Gastric electrical stimulation (GES) consists of the delivery of electrical stimuli by means of electrodes implanted in the musculature of the gastric wall which are connected to a stimulator device in order to restore effective gastric contractions with normal GE and improve the symptoms of refractory gastroparesis.
Since the 1960s, many investigators have tried to re-establish normal gastric myoelectrical activity to generate coordinated peristaltic activity in patients with refractory gastroparesis[18]. They used a large variety of electrical stimuli differing in pulse width, amplitude and frequency with diverse approaches and various results. It is the difference in frequency of the electrical stimuli from 3-4 cycles/min (cpm) to 50 cycles/s (Hz), which is mainly responsible for the different effects on the target structures of the gut wall.
Two principal types of GES are available: (1) Low-frequency/high-energy GES with long pulse stimulation, the frequency of which is just above that of the native slow wave with a pulse duration in the order of some tenths of a second; and (2) High-frequency/low-energy GES with short pulse stimulation, the frequency of which is markedly above that of the native slow wave with a pulse duration less than one thousandth of a second, delivered singly or in bursts of various length.
Low-frequency/high-energy GES with long pulse (gastric electrical pacing)
The electrical stimulus likely activates the interstitial cells of Cajal and/or muscle cells directly without involving intramural cholinergic nerves, because the administration of atropine does not block the appearance of electrically-induced slow waves[19].
This stimulation is called low-frequency/high-energy GES, because the frequency is slightly above that of the slow wave and its request for energy is high, due to prolonged delivery of a pulse current, for which it is also called “long pulse stimulation”. It may be properly defined as “gastric electrical pacing” (GEP), as it refers to electrical stimuli which are aimed to induce propagated slow waves that replace the spontaneous waves.
If a pulse stimulus with a constant current of 2-4 mA lasting 30-500 ms is given to the gastric wall during a non-refractory period, an extra slow wave is induced, which propagates along the gastric wall both in oral and aboral directions, depending on the site of stimulation[20,21]. When a series of stimuli is given, a series of slow waves is induced (entrainment) only if the stimulus frequency is slightly above that of the intrinsic frequency, but not more that 4.7 cpm in man[18,20,22] (Figure 3). The entrainment, however, is not sufficient to re-establish a propagated contraction in all cases and consequently to improve GE, especially if the neuromuscular structures are severely damaged[20]. In fact, GEP in dogs following stimulation of 100-300 ms duration, 5-7 cpm frequency and 2-4 mA amplitude was able to entrain slow waves, even after an artificial gastroparesis induced by the association of vagotomy and glucagon, but the effect on gastric contraction and GE was obtained only in some cases[18,23-27]. However, in patients with gastroparesis, GEP performed by means of an external device and transcutaneous electrodes fixed in the proximal corpus, with a frequency of 3-3.3 cpm, an amplitude of 2-4 mA and a pulse duration of 30-300 ms, was able to induce a regular rhythm in most patients, and in some cases restored efficient contractions, accelerated GE and improved symptoms[28-32].
Figure 3.
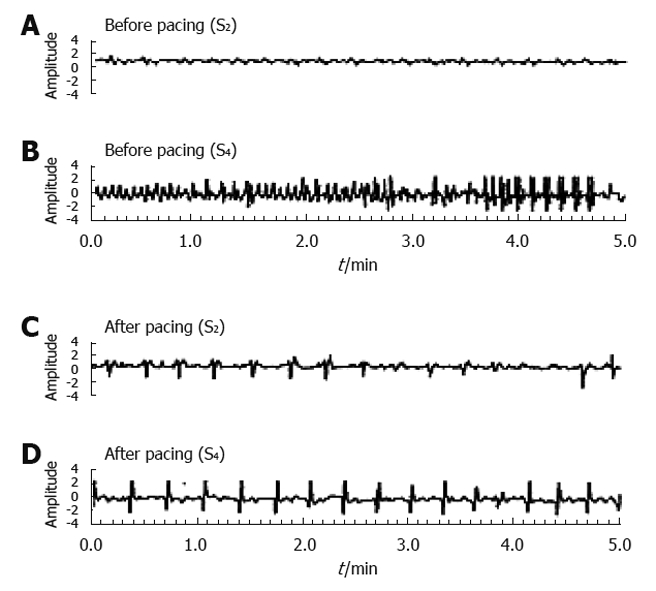
Normalization of ectopic tachygastria with low-frequency/high-energy gastric electrical stimulation [pacing frequency: 3.2 cycles/min (cpm), pulse width: 300 ms, amplitude: 4 mA] in a patient with gastroparesis. The pacing was carried out in the proximal corpus and the recording electrodes were in the mid gastric corpus (S2) and in the gastric antrum (S4). Before pacing a slow wave of about 3.5 cpm was recorded at S2 (A) and tachyarrythmias at S4 (B), whereas the pacing with a frequency of 3.2 cpm entrained gastric slow waves at S2 (C) and S4 (D)[44].
In conclusion, besides these limitations this kind of extrinsic pacing may re-establish a normal frequency of slow wave activity in gastric dysrhythmias, but does not guarantee the appearance of true contractions and consequent improvement in GE, especially in conditions of severe gastric atony, and has little effect on vomiting. Unfortunately, long duration pulses require high energy which must be provided by batteries too heavy and large to be implanted in a patient for long-term treatment.
High-frequency/low-energy GES with short pulse (gastric neuro-stimulation)
This type of stimulation is called “high-frequency GES” (HF-GES), because the frequency of the stimulation is well above the intrinsic frequency, and is also called “high-frequency/low-energy” GES, because it requires a low quantity of energy, and, being short the length of pulse, is also called “short pulse stimulation”.
If a series of stimuli of 2-5 mA amplitude is delivered to the gastric wall with a frequency higher than 4.8 cpm cycles/min at 2-5 mA, no slow waves are induced, because the frequency stimulus is above the “maximum driven frequency” of the stomach. The native slow wave continues to spread with its own frequency and slight modifications, while the effects on contractions and GE are variable[27,33-38]. Stimulation may be performed with a single pulse of constant current[35] of short duration (approximately 300 μs) or by a couple of pulses of 300 μs at 70 μs intervals[34] or by a burst of pulses of high frequency (up to 50 Hz)[33] and variable length. As the power consumption is low, this system does not require unwieldy batteries and allows the implantation of a portable device.
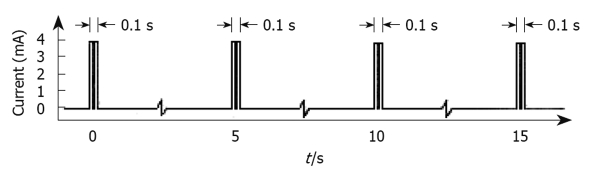
The type of HF-GES most used is performed with an implantable stimulator called Enterra (Medtronics, Minneapolis, MN, USA). It delivers electrical stimuli consisting of couples of pulses with a frequency of 14 Hz, amplitude 5 mA, duration 330 μs, which are delivered for 0.1 s at a frequency of 12 cpm[39] (Figure 4). The electrodes are positioned via laparotomy or laparoscopy in the musculature of the gastric corpus, whereas the pulse generator is inserted in a subcutaneous pocket[34,40,41]. This method of stimulation is approved by the USA Food and Drug Administration (FDA) within certain limits on humanitarian grounds and in a few selected centres of research with the approval of the Institutional Preview Board, to be used in patients with diabetic or idiopathic refractory gastroparesis, but has not been authorized by NICE in the UK. Moreover, some centres in the USA have discontinued the implantation of this device, because of few benefits to patients in terms of cost and risks.
Figure 4.
Type of electrical stimulation used by the Enterra system. Short bursts of short duration rectangular pulses (330 μs each) with amplitude of 4 mA were given at a frequency of 14 Hz in each burst. Bursts in turn lasted 0.1 s and were delivered every 5 s[39].
With regard to the effects of HF-GES on gastric electro-mechanical activity, the slow waves remain practically unchanged[19,24,26,27,35,37,38], whereas the effects on contractions are contradictory[19,26,27,37]. This may be due to the fact that none of these investigators considered the possible spontaneous occurrence of activity fronts of the migrating motor complex that may increase the motility index casually in correspondence with the period of stimulation. GE was found to be unchanged, worsened or improved, sometimes after months or years of stimulation[26,34,35,38,42-51]. However, these studies were not controlled and in some cases the patients continued to take prokinetic drugs during the period of stimulation[35,38]. Some investigators solved the problem of little effect on GE by adding a pyloroplasty to GES obtaining an obvious improvement in GE[52]. Other investigators devised a dual stimulation protocol alternating pulses of short duration (0.3 ms) with pulses of long duration (500 ms) every 10 s with the aim of obtaining not only an antiemetic effect, but also to correct dysrhythmias and improve GE[53]. In conclusion, the effect of HF-GES on slow waves and contractions is absent or at least dubious, while there is a slight and inconstant effect on GE.
With regard to the effects of HF-GES on symptoms of gastroparesis, the first uncontrolled trials from a small number of centres reported significant and prolonged gastric symptoms improvement in both diabetic and idiopathic gastroparesis with about 80% reduction in nausea and vomiting[34,46,54-59]. One further study[48] from three regional centres regarding 214 patients carrying the device for an average of 4 years, reported a continued improvement of at least one of the gastroparesis symptoms in 50% to 92% of patients. However, no symptom score and quality of life measurements at baseline and at follow-up were carried out with respect to a control group of 25 non-implanted patients. In addition, no survival benefits were observed in implanted patients with respect to non-implanted patients.
The major fault in all these studies was the absence of a double-blind randomized crossover design, with the exception of one complete study[60] and one abstract[61]. In the first study which included the Enterra system, 33 patients (17 diabetic and 16 idiopathic) were randomized to ON and OFF stimulation for 1 mo periods in a double-blind crossover design, followed by a non-blinded ON period of 6-12 mo. With regard to the results there were, however, as noted in a follow-up “Letter to the Editor”[62], discrepancies between the initial submission to the USA FDA, where a decrease in vomiting frequency was reported without significant differences between the ON and OFF periods, and the subsequent publication in Gastroenterology, where a reduction in vomiting frequency was observed in diabetic patients during the ON period and not during the OFF period. The decrease in vomiting frequency continued during the uncontrolled phase of stimulation, confirming the results of other studies[58], but no significant decreases in postprandial fullness, early satiety, pain and bloating were observed. Due to these limited results, the authors announced in a reply to the previously reported “Letter to the Editor”[62], that a new controlled double-blind multicentre trial of GES with Enterra was underway.
The results of a new prospective multicentric double-blind randomized controlled crossover study with Enterra was presented at the DDW of 2010[61] on the effects observed in 32 patients with idiopathic gastroparesis. After 6 wk of stimulation, a double-blind randomized consecutive 3-mo crossover period with the device ON or OFF was followed by an unblinded ON period up to 12 mo after implantation. However, during the crossover period there was a non-significant reduction in weekly vomiting frequency with a median of 9.8 episodes during the OFF period vs 6.4 during the ON period. At one year after implantation, symptoms and quality of life were significantly improved as well as GE at 2 h, but not at 4 h.
In patients treated with this system the decrease in vomiting was associated with an improvement in some nutritional parameters, such as body weight and serum albumin, and with a decrease in necessity for parenteral or enteral nutrition. In addition, the need for visits and hospitalization and the use of prokinetic and antiemetic drugs were significantly decreased, whereas in diabetic gastroparesis the glycaemic control was improved, as well as the health-related quality of life[34,46,49,50,56,57,60]. A comparison between medical therapy for gastroparesis and the necessity for health care resources was determined, however, this was carried out only in one study with a very small randomized control group and without a detailed indication of the drugs and doses used[55].
Other studies on the effect of GES were performed in other types of patients with gastroparesis, such as those who underwent a partial gastric resection with or without Roux en Y gastric bypass and those who underwent esophagectomy or heart-lung and kidney-pancreas transplant procedures[42,63,64], who reported a decrease in symptoms for long periods of time. However, the energy requirement for successful stimulation was higher in patients with postsurgical gastroparesis with respect to any other type of gastroparesis[65].
The major problem with this procedure is the scarce responsiveness which may occur in patients with prominent bloating or pain[58], and in idiopathic with respect to diabetic gastroparesis[58,60]. Also, patients with interstitial cell of Cajal loss showed little response to HF-GES[66]. The possible causes of a poor response may lie in the incorrect positioning of electrodes and in opiate use at the time of implantation which may blunt the response to stimulation[58]. Consequently, some investigators suggest an intraoperative endoscopic ultrasound to confirm the correct positioning of the electrodes within the gastric muscle layer[67]. To test the response before the implantation of a permanent stimulatory device, other investigators placed percutaneous stimulating electrodes at the time of gastrostomy or used a PEG technique[68]. Self anchoring percutaneous electrodes have been used[69,70], which may allow prolonged stimulation up to 2 mo[71]. Other investigators propose to adjust the stimulation parameters using hand-held programming devices to increase the voltage or pulse frequency.
Another important problem of the Enterra system is the complications that may take place in up to 20% of patients, such as infections, migration and erosion of the stimulating device[34,60], stomach wall perforation, pain due to adhesive bands from pacing wires to the abdominal wall[72], dislodgment, breakage and erosion of leads into small bowel[73], and stomach wall perforation and intestinal obstruction. All these complications require another surgical intervention and are sometimes lethal[46].
The mechanism of action of this type of HF-GES is unknown. In fact, symptom improvement is not due to an entrainment of slow waves, or to a correction of underlying slow wave dysrhythmias[56,74], or to an improvement in GE. The improvement in symptoms was associated with a decrease in gastric retention at 4 h in rare cases[43], whereas in the majority of cases there was a discrepancy between the improvement in symptoms and the disappointing results on gastric motor function. A decrease in vomiting with the Enterra system was also observed in patients with hereditary intestinal pseudo-obstruction or with simple functional dyspepsia[70,75], as well as in patients with nausea and vomiting regardless of GE rates[74,76]. These results indicate the existence of a mechanism independent of GE improvement. The fact that in a couple of controlled studies[60,61] a similar improvement in symptoms occurred during the crossover double-blind ON and OFF stimulation, suggests that this mechanism could be a placebo effect or that the surgery itself on the stomach and abdominal wall may have given rise to some kind of afferent stimuli that decreased the sensation of nausea and vomiting. In addition, a spontaneous improvement in gastric motility and dyspeptic symptoms cannot be excluded, as this was noted in patients with postviral gastroparesis and in patients with intractable diabetic and idiopathic gastroparesis under tube feeding, who resumed spontaneously with oral feeding[5,77,78]. No significant effect on the blood levels of gut hormones with gastrokinetic activity, such as motilin, gastrin, neurotensin and pancreatic polypeptide was observed. Other investigators took into consideration modifications in sympatho-vagal activity, adrenergic and cholinergic functions[79,80] or modulation of thoracic spinal neurons activity[81], as well as that of the paraventricular nucleus of the hypothalamus[82] and that of the thalamus, which were found on PET to be activated in gastroparetic patients by gastric stimulation[80]. However, the hypothesis that the device acts through vagal pathways[54,83] was disproved by the fact that HF-GES also works well in patients with vagotomy[42]. Symptom improvement is possibly due to an action on afferents fibres[84] which inhibit the vomiting centre or influence symptom perception in the brain or promote fundic relaxation through nonvagal nitrergic pathways[38,85-87]. In fact, experiments with a gastric barostat showed that HF-GES decreases sensitivity to gastric distension and enhances gastric accommodation to a meal in patients with severe idiopathic gastroparesis[85]. As to why this kind of GES improves nausea and vomiting remains an enigma.
Neural sequential GES is another type of high-frequency GES
The application of a train of electrical square waves with a duration of a few ms and a frequency > 50 Hz with an amplitude of 8-16 V for 4-16 s invokes a contraction of the gut wall at the site of the electrodes. This type of electrical stimulation induces a release of acetylcholine from the intramural cholinergic fibres, which in turn stimulates muscle cell contraction. In fact, its effect is prevented by the previous administration of atropine[88] and for this reason is called “neural GES”[89]. This contraction, however, does not propagate spontaneously, but either circumferentially and aborally[90]. To have a contraction involving a circular band of gastric musculature it is necessary to use a circular chain of electrodes, and to have a propagated contraction it is necessary to employ a series of these electrodes encircling both the corpus and the antrum, activating them sequentially (“neural sequential GES”)[88]. The spontaneous slow wave is overwhelmed by these electronically co-ordinated contractions. This system has the advantage of working both when spontaneous waves show a regular rhythm, but are unable to induce efficient pressure waves, and when slow waves are arrhythmic, uncoupled, completely disorganised and not responding to low-frequency pacing.
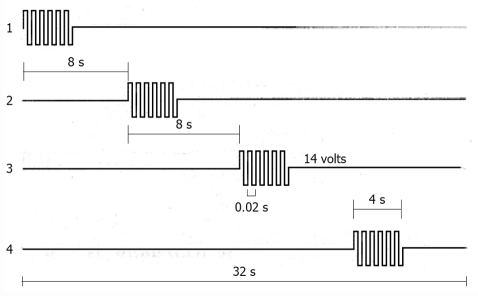
Mintchev et al[89,91] with the aid of a series of 4-6 ring electrodes placed in the corpus and antrum of dogs, sequentially activated by a microprocessor (Figure 5), was able to induce strong propagated contractions, that increased GE of both liquids and solids. The effectiveness of this type of GES was also demonstrated in a gastroparetic patient at the time of laparotomy[89]. Acute and chronic canine studies confirmed the feasibility of this microprocessor-controlled stimulation method with an implantable multichannel stimulator[92], which may be externally controlled with radiofrequency[93,94].
Figure 5.
Characteristics of one sequential gastric pacing stimulation protocol in dogs are shown from the proximal (1) to the distal (4) electrodes, that were positioned along the gastric corpus and antrum at 4 cm interval. Four second duration pulse trains with an amplitude of 14 V and a frequency of 50 Hz were delivered in synchronized fashion with a 4 s lag between adjacent stimulus sites[89].
However, before initiating studies in patients with gastroparesis, chronic experiments in animal models are necessary with an implantable device to evaluate not only the long-term efficiency of this method and the possible incidence of surgical complications, but also to assess the pathophysiologic influence of electrical current pulses on neuromuscular structures of the gastric wall and the effects of the strong antral contractions in the management of gastric content. In fact, one must bear in mind the motor function of the pylorus and duodenum when antral contractions occur. If the pylorus remains open during stimulation, the strong artificial contractions may cause a rapid GE of food particles with consequent risk of maldigestion and dumping syndrome, as suggested by Hasler[95], because the “intestinal brake” is lacking. In addition, if there is a non-propulsive motor disorder of the small intestine, an accumulation of material in the intestinal lumen may take place, which may give rise to a functional obstruction. If the pylorus does not open, as may happen in patients with diabetic gastroparesis[96], strong artificial contractions could accumulate the gastric content against the closed pylorus with consequent abnormal antral distension and possible occurrence of pain.
Finally, we are not sure that the normalization of GE will be accompanied by the disappearance of dyspeptic symptoms, if there is visceral hypersensitivity, as happens in patients with dyspepsia despite normal GE[97]. However, we believe that the problem of gastric stasis is crucial in gastroparesis and should be corrected in any case. In fact, besides the severe consequences on symptoms and nutrition and the negative effect on the glycaemic control of diabetes, it may cause “per se” gastric damage, such as gastritis (which may be erosive in diabetics), phytobezoars[98] with possible ulceration, obstruction and gastric perforation and pharmacobezoars comprised of medications[99].
From these considerations we believe that an in-depth experimentation of neural sequential GES in various pathophysiological conditions associated with gastroparesis is mandatory.
COMMENT AND CONCLUSION
Many investigators have been engaged in resolving the problem of gastric electrical stimulation in the last half century with different approaches and various outcomes.
Low-frequency/high-energy GES, known as gastric electrical pacing with long pulse stimulation, is able to induce a regular rhythm, restore efficient contractions, and improve GE and symptoms in some cases of gastroparesis, but requires a high quantity of electrical current, which can only be provided by a device too large and heavy to be implanted and for this reason is not suitable for clinical studies. However, with the progress in electronic miniaturization and in the potency of batteries this method may be considered again in the future.
High-frequency/low-energy GES with short pulse stimulation, such as the Enterra system, although it does not significantly modify slow wave and motor activity and does not consistently resolve the problem of delayed GE[34,46,60], shows, however, a good effect on nausea and vomiting with slight but significant improvement in nutritional depletion and health-related quality of life[34,48,57,100]. However, the possibility of spontaneous improvement or a placebo effect cannot be ruled out for sure and there are many considerations regarding the clinical use of this type of GES.
First, one must keep in mind that up to 20% of patients develop more or less severe complications, sometimes lethal, related to implantation of the device[46].
Second, 13% of patients are non-responders[60], especially those with idiopathic gastroparesis, and the improvement in nausea and vomiting may be temporary in 50% of patients[58] and does not include other dyspeptic symptoms, such as epigastric pain, which is an important disabling symptom compelling the patient to continue analgesic therapy[101].
Third, the benefit in nutritional parameters is low, as the average body weight increases were from 0.9 kg[46] to 8.4%[34] a year.
Fourth, when an improvement is obtained, this may not be any better than that obtained by a pyloric injection of botulinum toxin, which avoids the need for surgery in up to 2/3 of patients referred to the surgeon for GES[102]. Moreover, an aggressive drug treatment in suitable doses, taking care to recognize and avoid pseudo-refractoriness to these drugs, may spare implantation of GES. In fact, in most of the studies examined, including those claiming the superiority of GES over medical therapy[55,56], there was a generic statement of refractoriness to prokinetic and antiemetic drugs without specifying the kind of drugs and dosages. None of the studies considered pseudo-refractoriness due to faulty bioavailability of the drug, which may take place when it is orally administered at the usual time interval of 30 min before meals. We performed a gastroduodenal 24 h-manometric examination in a gastroparetic patient with apparent refractoriness to prokinetics and demonstrated that the drug administered 30 min before a meal took about 3 h to stimulate gastric motility (Figure 6)[103]. Gastric contractions were almost absent during this time, while the meal stagnated in the stomach causing dyspeptic symptoms. One should remember that in these patients about 80% of the gastric content is still in the stomach 2 h after ingestion[60], and that the prokinetic pill also takes this time to reach the intestine to be absorbed and stimulate gastric motility. Therefore, we decided to administer the prokinetic pill more than 2 h before meals to this patient and obtained a marked improvement in dyspeptic symptoms associated with an improvement in GE and a progressive gain in body weight[103].
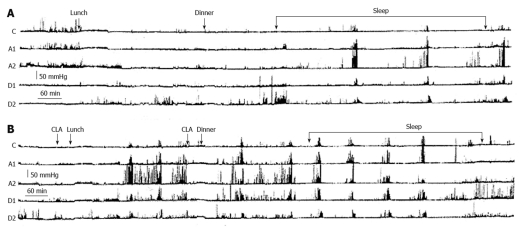
Figure 6.
Two ambulatory 24-h gastroduodenal recordings (A and B) carried out on two separate days in a patient with apparently refractory gastroparesis by means of a probe with 5 miniaturized electronic pressure transducers, 5 cm apart: one in the corpus (C), two in the antrum (A1 and A2) and two in the duodenum (D1 and D2). On the first day (A) the recording was carried out without drug administration and on the second day (B) with clarithromycin (CLA) administration. A: On the first day, the postprandial gastric motor activity was very low and only three activity fronts of the Migrating Motor Complex were observed, two during the night and one early in the morning; B: On the second day, the oral administration of clarithromycin 30 min before lunch was followed about 3 h later by a burst of powerful peristaltic contractions starting in the stomach and progressing in the duodenum, followed by two others bursts at about 80 min intervals. The oral administration of clarithromycin 30 min before dinner induced after about 2.4 h a series of six bursts of powerful peristaltic waves in the stomach and duodenum at 80-100 min intervals[103].
Fifth, among the considerations that dissuade exposing a patient with intractable gastroparesis to the Enterra system outside of a rigorous placebo-controlled study, there is also the high cost of the procedure which exceeds USD 20 000 and the existence of some limitations, such as the necessity to avoid certain metal detecting security devices and magnetic resonance imaging.
With these considerations in mind, it is advisable to discontinue the use of this type of gastric stimulator for gastroparesis outside properly designed double-blind controlled studies, because it is a costly and risky procedure that does not resolve the principal problem of gastroparesis, that is GE delay, and only improves vomiting without significantly influencing other dyspeptic symptoms, such as epigastric fullness, satiety, anorexia and epigastric pain.
Sequential neural GES, which is able to induce propagated gastric contractions with consequent acceleration of GE, is the most promising method, as it affects the core of the problem of gastroparesis which is gastric stasis, rather than just mitigate the symptoms. However, there is still much research to be carried out, since to date, this method has been used only on animals and one patient with gastroparesis[89,91], therefore it is necessary to use this type of GES in different pathophysiologic conditions of gastroparesis. The hope is that electronic technology could make possible an easily implantable device for humans, able to modulate contractile activity following physiologic necessities. Under these circumstances, GES will become able to treat gastroparesis, whereas to date none of the current technologies has been demonstrated unequivocally to consistently accelerate GE and improve all symptoms in patients with gastroparesis.
Footnotes
Peer reviewer: Dr. Chikashi Shibata, Department of Surgery, Tohoku University, 1-1 Seiryo-machi, Aoba-ku, Sendai 980-8574, Japan
S- Editor Shi ZF L- Editor Webster JR E- Editor Lin YP
References
- 1.Bortolotti M. Treatment of gastric emptying delay. Minerva Gastroenterol Dietol. 2009;55:345–377. [PubMed] [Google Scholar]
- 2.Ezzeddine D, Jit R, Katz N, Gopalswamy N, Bhutani MS. Pyloric injection of botulinum toxin for treatment of diabetic gastroparesis. Gastrointest Endosc. 2002;55:920–923. doi: 10.1067/mge.2002.124739. [DOI] [PubMed] [Google Scholar]
- 3.Miller LS, Szych GA, Kantor SB, Bromer MQ, Knight LC, Maurer AH, Fisher RS, Parkman HP. Treatment of idiopathic gastroparesis with injection of botulinum toxin into the pyloric sphincter muscle. Am J Gastroenterol. 2002;97:1653–1660. doi: 10.1111/j.1572-0241.2002.05823.x. [DOI] [PubMed] [Google Scholar]
- 4.Arts J, Caenepeel P, Degreef T, Gebruers K, Verbeke K, Janssens J, Tack J. Randomised double-blind cross-over study evaluating the effect of intrapyloric injection of botulinum toxin on gastric emptying and symptoms in patients with gastroparesis. Gastroenterology. 2005;128:A81. [Google Scholar]
- 5.Fontana RJ, Barnett JL. Jejunostomy tube placement in refractory diabetic gastroparesis: a retrospective review. Am J Gastroenterol. 1996;91:2174–2178. [PubMed] [Google Scholar]
- 6.Ejskjaer NT, Bradley JL, Buxton-Thomas MS, Edmonds ME, Howard ER, Purewal T, Thomas PK, Watkins PJ. Novel surgical treatment and gastric pathology in diabetic gastroparesis. Diabet Med. 1999;16:488–495. doi: 10.1046/j.1464-5491.1999.00086.x. [DOI] [PubMed] [Google Scholar]
- 7.Bilgutay AM, Wingrove R, Griffen WO, Bonnabeau RC Jr, Lillehei CW. Gastro-intestinal pacing: a new concept in the treatment of ileus. Ann Surg. 1963;158:338–348. doi: 10.1097/00000658-196315830-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwong NK, Brown BH, Whittaker GE, Duthie HL. Electrical activity of the gastric antrum in man. Br J Surg. 1970;57:913–916. doi: 10.1002/bjs.1800571211. [DOI] [PubMed] [Google Scholar]
- 9.Monges H, Salducci J. A method of recording the gastric electrical activity in man. Am J Dig Dis. 1970;15:271–276. doi: 10.1007/BF02233459. [DOI] [PubMed] [Google Scholar]
- 10.Ward SM, Sanders KM. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol. 2006;576:675–682. doi: 10.1113/jphysiol.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 12.Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995;269:C1577–C1585. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- 13.Bayguinov O, Ward SM, Kenyon JL, Sanders KM. Voltage-gated Ca2+ currents are necessary for slow-wave propagation in the canine gastric antrum. Am J Physiol Cell Physiol. 2007;293:C1645–C1659. doi: 10.1152/ajpcell.00165.2007. [DOI] [PubMed] [Google Scholar]
- 14.Daniel EE, Chapman KM. Electrical activity of the gastrointestinal tract as an indication of mechanical activity. Am J Dig Dis. 1963;8:54–102. doi: 10.1007/BF02233560. [DOI] [PubMed] [Google Scholar]
- 15.Telander RL, Morgan KG, Kreulen DL, Schmalz PF, Kelly KA, Szurszewski JH. Human gastric atony with tachygastria and gastric retention. Gastroenterology. 1978;75:497–501. [PubMed] [Google Scholar]
- 16.Bortolotti M, Sarti P, Barbara L, Brunelli F. Gastric myoelectric activity in patients with chronic idiopathic gastroparesis. J Gastrointest Motil. 1990;2:104–108. [Google Scholar]
- 17.Chen JD, Schirmer BD, McCallum RW. Serosal and cutaneous recordings of gastric myoelectrical activity in patients with gastroparesis. Am J Physiol. 1994;266:G90–G98. doi: 10.1152/ajpgi.1994.266.1.G90. [DOI] [PubMed] [Google Scholar]
- 18.Bortolotti M. The "electrical way" to cure gastroparesis. Am J Gastroenterol. 2002;97:1874–1883. doi: 10.1111/j.1572-0241.2002.05898.x. [DOI] [PubMed] [Google Scholar]
- 19.Sarna SK, Daniel EE. Electrical stimulation of gastric electrical control activity. Am J Physiol. 1973;225:125–131. doi: 10.1152/ajplegacy.1973.225.1.125. [DOI] [PubMed] [Google Scholar]
- 20.Kelly KA, La Force RC. Pacing the canine stomach with electric stimulation. Am J Physiol. 1972;222:588–594. doi: 10.1152/ajplegacy.1972.222.3.588. [DOI] [PubMed] [Google Scholar]
- 21.Lin ZY, McCallum RW, Schirmer BD, Chen JD. Effects of pacing parameters on entrainment of gastric slow waves in patients with gastroparesis. Am J Physiol. 1998;274:G186–G191. doi: 10.1152/ajpgi.1998.274.1.G186. [DOI] [PubMed] [Google Scholar]
- 22.Waterfall WE, Miller D, Ghista DN. Electrical stimulation of the human stomach. Dig Dis Sci. 1985;30:799. [Google Scholar]
- 23.Bellahsène BE, Lind CD, Schirmer BD, Updike OL, McCallum RW. Acceleration of gastric emptying with electrical stimulation in a canine model of gastroparesis. Am J Physiol. 1992;262:G826–G834. doi: 10.1152/ajpgi.1992.262.5.G826. [DOI] [PubMed] [Google Scholar]
- 24.Johnson B, Familoni B, Abell TL, Verkman R, Wood G. Development of a canine model for gastric pacing. Gastroenterology. 1990;98:A362. [Google Scholar]
- 25.Hinder RA, Kelly KA. The role of the antral pacesetter potential in canine gastric emptying of solids. In: Duthie HL, editor. Gastrointestinal Motility in health and disease. Lancaster: MTP press; 1978. pp. 459–468. [Google Scholar]
- 26.Eagon JC, Kelly KA. Effect of electrical stimulation on gastric electrical activity, motility and emptying. Neurogastroenterol Motil. 1995;7:39–45. doi: 10.1111/j.1365-2982.1995.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 27.Familoni BO, Abell TL, Nemoto D, Voeller G, Johnson B. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892–897. doi: 10.1023/a:1018804128695. [DOI] [PubMed] [Google Scholar]
- 28.Bellahsene BE, Schirmer BD, Updike OL, McCallum R. Effect of electrical stimulation on gastric emptying. Dig Dis Sci. 1987;32:902. [Google Scholar]
- 29.Waldhausen J, Courtney T, Schirmer B. Postoperative pacing for gastric antral distension. J Gastroint Motil. 1989;1:66. [Google Scholar]
- 30.Courtney TL, Schirmer BD, Bellahsene BE, Updike OL, McCallum JRW. Gastric electrical stimulation as a possible new therapy for patients with severe gastric stasis. Gastroenterology. 1991;100:A822. [Google Scholar]
- 31.Hocking MP, Vogel SB, Sninsky CA. Human gastric myoelectric activity and gastric emptying following gastric surgery and with pacing. Gastroenterology. 1992;103:1811–1816. doi: 10.1016/0016-5085(92)91439-b. [DOI] [PubMed] [Google Scholar]
- 32.McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998;114:456–461. doi: 10.1016/s0016-5085(98)70528-1. [DOI] [PubMed] [Google Scholar]
- 33.Song G, Hou X, Yang B, Sun Y, Liu J, Qian W, Chen JD. Efficacy and efficiency of gastric electrical stimulation with short pulses in the treatment of vasopressin-induced emetic responses in dogs. Neurogastroenterol Motil. 2006;18:385–391. doi: 10.1111/j.1365-2982.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- 34.Abell TL, Van Cutsem E, Abrahamsson H, Huizinga JD, Konturek JW, Galmiche JP, VoelIer G, Filez L, Everts B, Waterfall WE, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204–212. doi: 10.1159/000068359. [DOI] [PubMed] [Google Scholar]
- 35.Familoni BO, Abell TL, Voeller G, Salem A, Gaber O. Electrical stimulation at a frequency higher than basal rate in human stomach. Dig Dis Sci. 1997;42:885–891. doi: 10.1023/a:1018852011857. [DOI] [PubMed] [Google Scholar]
- 36.Qian LW, Peters LJ, Chen J. Effects of various electrical stimulation on gastric slow wave abnormalities induced by vasopressin. Gastroenterology. 1999:116: A970. [Google Scholar]
- 37.Wang Z, Qian L, Ueno T, Chen JDZ. Gastric myoelectrical activity and autonomic nerve system responses to various gastric electrical stimulation. Dig Dis Sci. 2000;45:1252–A39. [Google Scholar]
- 38.McCallum RW, Lin Z, Olyaee M, Sarosiek I, Forester J. High-frequency electrical stimulation of the stomach for the treatment of gastroparesis. Neurogastroenterol Motil. 2000;12:488. [Google Scholar]
- 39.Soffer E, Abell T, Lin Z, Lorincz A, McCallum R, Parkman H, Policker S, Ordog T. Review article: gastric electrical stimulation for gastroparesis--physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009;30:681–694. doi: 10.1111/j.1365-2036.2009.04082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abell TL, Minocha A. Gastroparesis and the gastric pacemaker: a revolutionary treatment for an old disease. J Miss State Med Assoc. 2002;43:369–375. [PubMed] [Google Scholar]
- 41.Al-Juburi A, Granger S, Barnes J, Voeller G, Beech D, Amiri H, Abell TL. Laparoscopy shortens length of stay in patients with gastric electrical stimulators. JSLS. 2005;9:305–310. [PMC free article] [PubMed] [Google Scholar]
- 42.McCallum R, Lin Z, Wetzel P, Sarosiek I, Forster J. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49–54. doi: 10.1016/s1542-3565(04)00605-6. [DOI] [PubMed] [Google Scholar]
- 43.Lin Z, Hou Q, Sarosiek I, Forster J, McCallum RW. Association between changes in symptoms and gastric emptying in gastroparetic patients treated with gastric electrical stimulation. Neurogastroenterol Motil. 2008;20:464–470. doi: 10.1111/j.1365-2982.2007.01054.x. [DOI] [PubMed] [Google Scholar]
- 44.Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of gastroparesis with electrical stimulation. Dig Dis Sci. 2003;48:837–848. doi: 10.1023/a:1023099206939. [DOI] [PubMed] [Google Scholar]
- 45.Lin Z, Forster J, Sarosiek I, McCallum RW. Effect of high-frequency gastric electrical stimulation on gastric myoelectric activity in gastroparetic patients. Neurogastroenterol Motil. 2004;16:205–212. doi: 10.1111/j.1365-2982.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- 46.Forster J, Sarosiek I, Lin Z, Durham S, Denton S, Roeser K, McCallum RW. Further experience with gastric stimulation to treat drug refractory gastroparesis. Am J Surg. 2003;186:690–695. doi: 10.1016/j.amjsurg.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 47.Brody F, Vaziri K, Saddler A, Ali A, Drenon E, Hanna B, Akin E, Gonzalez F, Soffer E. Gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2008;207:533–538. doi: 10.1016/j.jamcollsurg.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 48.Anand C, Al-Juburi A, Familoni B, Rashed H, Cutts T, Abidi N, Johnson WD, Minocha A, Abell TL. Gastric electrical stimulation is safe and effective: a long-term study in patients with drug-refractory gastroparesis in three regional centers. Digestion. 2007;75:83–89. doi: 10.1159/000102961. [DOI] [PubMed] [Google Scholar]
- 49.Vander Voort JR, Becker JC, Dietl KH, Konturek JW, Domschke W, Pohle T. Gastric electrical stimulation results in improved metabolic control in diabetic patients suffering from gastroparesis. Exp Clin Endocrinol Diab. 2005;113:38–42. doi: 10.1055/s-2004-830525. [DOI] [PubMed] [Google Scholar]
- 50.Liu J, Qiao X, Micci MA, Pasricha PJ, Chen JD. Improvement of gastric motility with gastric electrical stimulation in STZ-induced diabetic rats. Digestion. 2004;70:159–166. doi: 10.1159/000081516. [DOI] [PubMed] [Google Scholar]
- 51.GEMS Group. Report of a multicenter study on electrical stimulation for the treatment of gastroparesis. Gastroenterology. 1997;112:A735. [Google Scholar]
- 52.Sarosiek I, Roeser K, Forster J, Hejazi RA, Sarosiek J, McCallum R. New surgery for gastroparesis-Enterra plus pyloroplasty: its efficacy in different etiolgies of gastroparesis. New Orleans: Digestive Disease Week, 1-5 May; 2010. p. W1405. [Google Scholar]
- 53.Song GQ, Hou X, Yang B, Sun Y, Qian W, Chen JD. A novel method of 2-channel dual-pulse gastric electrical stimulation improves solid gastric emptying in dogs. Surgery. 2008;143:72–78. doi: 10.1016/j.surg.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abell T, Lou J, Tabbaa M, Batista O, Malinowski S, Al-Juburi A. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. JPEN J Parenter Enteral Nutr. 2003;27:277–281. doi: 10.1177/0148607103027004277. [DOI] [PubMed] [Google Scholar]
- 55.Cutts TF, Luo J, Starkebaum W, Rashed H, Abell TL. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35–43. doi: 10.1111/j.1365-2982.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 56.Lin Z, McElhinney C, Sarosiek I, Forster J, McCallum R. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci. 2005;50:1328–1334. doi: 10.1007/s10620-005-2782-7. [DOI] [PubMed] [Google Scholar]
- 57.Lin Z, Sarosiek I, Forster J, McCallum RW. Symptom responses, long-term outcomes and adverse events beyond 3 years of high-frequency gastric electrical stimulation for gastroparesis. Neurogastroenterol Motil. 2006;18:18–27. doi: 10.1111/j.1365-2982.2005.00732.x. [DOI] [PubMed] [Google Scholar]
- 58.Maranki JL, Lytes V, Meilahn JE, Harbison S, Friedenberg FK, Fisher RS, Parkman HP. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072–2078. doi: 10.1007/s10620-007-0124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qian L, Lin X, Chen JD. Normalization of atropine-induced postprandial dysrhythmias with gastric pacing. Am J Physiol. 1999;276:G387–G392. doi: 10.1152/ajpgi.1999.276.2.G387. [DOI] [PubMed] [Google Scholar]
- 60.Abell T, McCallum R, Hocking M, Koch K, Abrahamsson H, Leblanc I, Lindberg G, Konturek J, Nowak T, Quigley EM, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 61.McCallum RWJ, Snape JM, Wo FJ, Brody FJ, Parkman HP, Novak TV, Lerew DR, Ruehlow L. Enterra Gastric electrical stimulation for idiopathic gastroparesis. Result from a multicenter randomized study. New Orleans: Digestive Disease Week, 1-5 May; 2010. p. N1065. [Google Scholar]
- 62.Jones MP. Gastric electrical stimulation for refractory gastroparesis. Gastroenterology. 2004;126:629; author 629–629; author 630. doi: 10.1053/j.gastro.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 63.Salameh JR, Schmieg RE Jr, Runnels JM, Abell TL. Refractory gastroparesis after Roux-en-Y gastric bypass: surgical treatment with implantable pacemaker. J Gastrointest Surg. 2007;11:1669–1672. doi: 10.1007/s11605-007-0331-8. [DOI] [PubMed] [Google Scholar]
- 64.Filichia LA, Cendan JC. Small case series of gastric stimulation for the management of transplant-induced gastroparesis. J Surg Res. 2008;148:90–93. doi: 10.1016/j.jss.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 65.Abidi N, Starkebaum WL, Abell TL. An energy algorithm improves symptoms in some patients with gastroparesis and treated with gastric electrical stimulation. Neurogastroenterol Motil. 2006;18:334–338. doi: 10.1111/j.1365-2982.2006.00765.x. [DOI] [PubMed] [Google Scholar]
- 66.Forster J, Damjanov I, Lin Z, Sarosiek I, Wetzel P, McCallum RW. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9:102–108. doi: 10.1016/j.gassur.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Raju GS, Forster J, Sarosiek I, Rosenthal SJ, Lin Z, McCallum R. EUS guidance in gastric pacemaker implantation. Gastrointest Endosc. 2002;55:728–730. doi: 10.1067/mge.2002.123275. [DOI] [PubMed] [Google Scholar]
- 68.Ayinala S, Batista O, Goyal A, Al-Juburi A, Abidi N, Familoni B, Abell T. Temporary gastric electrical stimulation with orally or PEG-placed electrodes in patients with drug refractory gastroparesis. Gastrointest Endosc. 2005;61:455–461. doi: 10.1016/s0016-5107(05)00076-3. [DOI] [PubMed] [Google Scholar]
- 69.Sallam HS, Chen JD, Pasricha PJ. Feasibility of gastric electrical stimulation by percutaneous endoscopic transgastric electrodes. Gastrointest Endosc. 2008;68:754–759. doi: 10.1016/j.gie.2008.04.060. [DOI] [PubMed] [Google Scholar]
- 70.Elfvin A, Andersson S, Abrahamsson H, Edebo A, Simrén M, Lönroth H. Percutaneous implantation of gastric electrodes - a novel technique applied in animals and in patients. Neurogastroenterol Motil. 2007;19:103–109. doi: 10.1111/j.1365-2982.2006.00858.x. [DOI] [PubMed] [Google Scholar]
- 71.Abrahamsson H, Lönroth H, Simrén M. Progress in gastric electrical stimulation. Gastrointest Endosc. 2008;67:1209–1210; author reply 1210-1211. doi: 10.1016/j.gie.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 72.Brody F, Chand B, Brodsky J, Soffer E. Laparoscopic revision of gastric pacing wires. J Laparoendosc Adv Surg Tech A. 2004;14:187–189. doi: 10.1089/1092642041255388. [DOI] [PubMed] [Google Scholar]
- 73.Cendan JC, Hocking MP. Erosion of gastric pacemaker lead into small bowel. Surg Obes Relat Dis. 2006;2:531–532. doi: 10.1016/j.soard.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 74.Islam S, Vick LR, Runnels MJ, Gosche JR, Abell T. Gastric electrical stimulation for children with intractable nausea and gastroparesis. J Pediatr Surg. 2008;43:437–442. doi: 10.1016/j.jpedsurg.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Andersson S, Lönroth H, Simrén M, Ringström G, Elfvin A, Abrahamsson H. Gastric electrical stimulation for intractable vomiting in patients with chronic intestinal pseudoobstruction. Neurogastroenterol Motil. 2006;18:823–830. doi: 10.1111/j.1365-2982.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- 76.Gourcerol G, Leblanc I, Leroi AM, Denis P, Ducrotte P. Gastric electrical stimulation in medically refractory nausea and vomiting. Eur J Gastroenterol Hepatol. 2007;19:29–35. doi: 10.1097/01.meg.0000250584.15490.b4. [DOI] [PubMed] [Google Scholar]
- 77.Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis--clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501–1504. [PubMed] [Google Scholar]
- 78.Vandenbroucke K, Kindt S, Demedts J, Tack J. outcome of percutaneous jejunal feeding tube placement for refractory idiopathic severe gastroparesis: a retrospective review. Acta Gastroenterol Belg. 2006;69:D14. [Google Scholar]
- 79.GEMS Group. Long-term results of gastric stimulation four times higher than the slow wave frequency in patients with drug refractory gastroparesis. Gastroenterology. 1999;116:A949. [Google Scholar]
- 80.Mc Callum RW, Dusing RW, Sarosiek J, Cocjin J, Forster J, Lin Z. Mechanisms of high-frequency electrical stimulation of the stomach in gastroparetic patients. Conf Proc IEEE Eng Med Biol Soc. 2006;1:5400–5403. doi: 10.1109/IEMBS.2006.260115. [DOI] [PubMed] [Google Scholar]
- 81.Qin C, Chen JD, Zhang J, Foreman RD. Modulatory effects and afferent pathways of gastric electrical stimulation on rat thoracic spinal neurons receiving input from the stomach. Neurosci Res. 2007;57:29–39. doi: 10.1016/j.neures.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang M, Zhang J, Chen JD. Central mechanisms of gastric electrical stimulation involving neurons in the paraventricular nucleus of the hypothalamus in rats. Obes Surg. 2006;16:344–352. doi: 10.1381/096089206776116372. [DOI] [PubMed] [Google Scholar]
- 83.Lin Z, Cocjin J, Saroziek I, Roeser K, McCallum RW. Influence of high-frequency electrical stimulation on gastric electrical activity, autonomic function and symptoms in gastroparetic patients. Neurogastroenterol Motil. 2005;17 Suppl 2:S81–S82. [Google Scholar]
- 84.Tougas G, Huizinga JD. Gastric pacing as a treatment for intractable gastroparesis: shocking news? Gastroenterology. 1998;114:598–601. doi: 10.1016/s0016-5085(98)70544-x. [DOI] [PubMed] [Google Scholar]
- 85.Tack J, Coulie B, Van Cutsem E, Ryden J, Janssens KU. The influence of gastric electrical stimulation on proximal gastric motor and sensory function in severe idiopathic gastroparesis. Gastroenterology. 1999;116:G4733. [Google Scholar]
- 86.Xing JH, Brody F, Brodsky J, Larive B, Ponsky J, Soffer E. Gastric electrical stimulation at proximal stomach induces gastric relaxation in dogs. Neurogastroenterol Motil. 2003;15:15–23. doi: 10.1046/j.1365-2982.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- 87.Liu J, Qiao X, Chen JD. Vagal afferent is involved in short-pulse gastric electrical stimulation in rats. Dig Dis Sci. 2004;49:729–737. doi: 10.1023/b:ddas.0000030081.91006.86. [DOI] [PubMed] [Google Scholar]
- 88.Mintchev MP, Bowes KL. Production of propagated antral contractions by electrical stimulation. Dig Dis Sci. 1996;41:1890. [Google Scholar]
- 89.Mintchev MP, Sanmiguel CP, Otto SJ, Bowes KL. Microprocessor controlled movement of liquid gastric content using sequential neural electrical stimulation. Gut. 1998;43:607–611. doi: 10.1136/gut.43.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mintchev MP, Bowes K. Computer model of gastric electrical stimulation. Ann Biomed Engineering. 1997;25:726–730. doi: 10.1007/BF02684849. [DOI] [PubMed] [Google Scholar]
- 91.Mintchev MP, Sanmiguel CP, Amaris M, Bowes KL. Microprocessor-controlled movement of solid gastric content using sequential neural electrical stimulation. Gastroenterology. 2000;118:258–263. doi: 10.1016/s0016-5085(00)70207-1. [DOI] [PubMed] [Google Scholar]
- 92.Lin Y, Sanmiguel C, Turner LE, Soffer E, Mintchev MP. Hardware-software co-design of portable functional gastrointestinal stimulator system. J Med Eng Technol. 2003;27:164–177. doi: 10.1080/0309190031000081546. [DOI] [PubMed] [Google Scholar]
- 93.Jalilian E, Onen D, Neshev E, Mintchev MP. Implantable neural electrical stimulator for external control of gastrointestinal motility. Med Eng Phys. 2007;29:238–252. doi: 10.1016/j.medengphy.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 94.Jurkov AS, Arriagada A, Mintchev MP. Implantable functional gastrointestinal neurostimulation. Conf Proc IEEE Eng Med Biol Soc. 2009;1:4615–4618. doi: 10.1109/IEMBS.2009.5332682. [DOI] [PubMed] [Google Scholar]
- 95.Hasler WL. The brute force approach to electrical stimulation of gastric emptying: A future treatment for refractory gastroparesis? Gastroenterology. 2000;118:433–436. doi: 10.1016/s0016-5085(00)70226-5. [DOI] [PubMed] [Google Scholar]
- 96.Mearin F, Camilleri M, Malagelada JR. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology. 1986;90:1919–1925. doi: 10.1016/0016-5085(86)90262-3. [DOI] [PubMed] [Google Scholar]
- 97.Mearin F, Cucala M, Azpiroz F, Malagelada JR. The origin of symptoms on the brain-gut axis in functional dyspepsia. Gastroenterology. 1991;101:999–1006. doi: 10.1016/0016-5085(91)90726-2. [DOI] [PubMed] [Google Scholar]
- 98.Emerson AP. Foods high in fiber and phytobezoar formation. J Am Diet Assoc. 1987;87:1675–1677. [PubMed] [Google Scholar]
- 99.Stack PE, Thomas E. Pharmacobezoar: an evolving new entity. Dig Dis. 1995;13:356–364. doi: 10.1159/000171515. [DOI] [PubMed] [Google Scholar]
- 100.McCallum R, Abell TL, Hocking M, Koch , K , Abrahamsson H, Le Blanc I, Lindberg G, Konturek JH, Oibaise K, et al. Results of long term high frequency gastric electrical stimulation (GES) for treatment of gastroparesis refractory to standard medical therapy. Gastroenterology. 2001;120:A98. [Google Scholar]
- 101.Cherian D, Sachdeva P, Fisher RS, Parkman P. Abdominal pain: as under appreciated symptom in gastroparesis. New Orleans: Digestive Disease Week, 1-5 May; 2010. p. M1321. [Google Scholar]
- 102.Hasler W. Nonoperative management of patients with gastroparesis referred for gastric electrical stimulator implantation. Neurogastroenterol Motil. 2005;17:480. [Google Scholar]
- 103.Bortolotti M, Gentilini L, Morselli C, Giovannini M, Miglioli M. Gastroparesis refractory to prokinetics: neuromuscolar unresponsiveness or faulty bioavailability of the drug? Dig Dis Sci. 2005;50:882–884. doi: 10.1007/s10620-005-2659-9. [DOI] [PubMed] [Google Scholar]