Abstract
AIM: To investigate the role of heme oxygenase-1 (HO-1) in pathogenesis of experimental hepatorenal syndrome (HRS).
METHODS: Rats were divided into liver cirrhotic group, zinc protoporphyrin IX (ZnPP) treatment group, cobalt protoporphyrin (CoPP) treatment group and sham group. Biliary cirrhosis was established by bile duct ligation in the first three groups. Rats in the ZnPP and CoPP treatment groups received intraperitoneal injection of ZnPP and CoPP, respectively, 24 h before sample collection. Expression of HO-1 mRNA in kidney was detected by reverse-transcription polymerase chain reaction, while protein expression was determined by immunohistochemical analysis. Hematoxylin and eosin staining was performed to observe liver cirrhosis and renal structure. Renal artery blood flow, mean arterial pressure and portal vein pressure, 24 h total urinary volume, serum and urine sodium concentrations, and creatinine clearance rate (Ccr) were also measured.
RESULTS: The HO-1 mRNA and protein expression levels in kidney, 24 h total urinary volume, renal artery blood flow, serum and urine sodium concentration and Ccr were lower in cirrhotic group than in sham group (P < 0.05). However, they were significantly lower in ZnPP treatment group than in cirrhotic group and significantly higher in CoPP treatment group than in cirrhotic group (P < 0.05).
CONCLUSION: Low HO-1 expression level in kidney is an important factor for experimental HRS.
Keywords: Heme oxygenase-1, Carbon monoxide, Hepatorenal syndrome, Zinc protoporphyrin IX, Cobalt protoporphyrin, Bile duct ligation, Biliary cirrhosis
INTRODUCTION
Renal dysfunction is very common in patients with advanced liver disease or cirrhosis. Its severity ranges from electrolyte-balance disturbances and water retention to hepatorenal syndrome (HRS)[1], which is a unique form of renal failure associated with liver cirrhosis or portal hypertension[2]. Although HRS represents a functional form and sometimes a reversible form of renal failure without significant changes in renal histology during the course of decompensated cirrhosis[3,4], it is a poor prognostic indicator for patients with liver cirrhosis, who show an increased risk of morbidity and mortality[2,3]. So far, no effective strategies are available for the treatment or prevention of HRS. Instead, patients are usually managed by maintaining their adequate hemodynamic status and intravascular volume. A better understanding of the pathophysiological mechanism underlying HRS helps to guide its treatment.
It is currently believed that marked renal vasoconstriction and predominant peripheral arterial vasodilation play a critical role in the pathogenesis of HRS[5-7]. Previous studies have shown that nitric oxide (NO), a potent vasodilator, plays an important role in the development of hyperdynamic syndrome and peripheral vasodilation during cirrhosis[8]. Increased NO level and synthetase activity in patients with liver cirrhosis have adverse effects on the functions of renal tubules and glomeruli[9], and inhibition of NO synthetase prevents the development of renal failure in an animal model of HRS[10,11]. Carbon monoxide (CO), a byproduct of heme oxygenase-1 (HO-1), shares many characteristics with NO. Endogenous CO is an activator of soluble guanylate cyclase and relaxes vascular smooth muscle in a cGMP-dependent or a cGMP-independent manner[12,13]. Studies have shown that the HO-1/CO system plays an important role in the control of vascular tone and that inhibition of HO-1 blocks vasodilation induced by heme[12-14]. HO-1 is also involved in the prevention of renal failure after renal ischemia[15] or glycerol-induced acute renal injury in rats[16].
Metalloporphyrins constitute a class of compounds in which the central iron of heme is replaced by other metals such as cobalt and zinc[17]. These metalloporphyrins inhibit or induce HO-1. This study was to evaluate the expression of HO-1 in kidneys of rats with experimental HRS and the functional role of HO-1 in the pathogenesis of HRS by manipulating its activity via intraperitoneal injection of either zinc protoporphyrin IX (ZnPP), a specific HO-1 enzyme inhibitor, or cobalt protoporphyrin (CoPP), a specific HO-1 enzyme inducer.
MATERIALS AND METHODS
Animals
Healthy male Sprague Dawley rats, weighing 200-220 g, were obtained from the Laboratory Animal Center of Dalian Medical University.
Reagents
ZnPP and CoPP (Sigma, St Louis, MO, USA) were dissolved in 0.2 mol/L of NaOH, adjusted to a pH of 7.4 and diluted in 0.85% NaCl with a final concentration of 1 mg/mL as previously described[18]. Rabbit anti-mouse HO-1 antibody (Boster Biological Technology, Wuhan, China), anti-rabbit IgG (MaxVisionTM2, Maixin Biotechnology, Fuzhou, China), TaKaRa RNA polymerase chain reaction (PCR) kit (AMV) Version 3.0 (TaKaRa Biotechnology, Dalian, China) were used in the study.
Animal model and grouping
The rats were randomly divided into sham group (n = 8), cirrhotic group (n = 10), ZnPP treatment group (n = 9) and CoPP treatment group (n = 8). They were well fed and housed for 3 d before any experimental protocols. Biliary cirrhosis was induced by bile duct ligation (BDL)[19,20] in rats of the cirrhotic group, ZnPP and CoPP treatment groups. The surgical procedures were approved by the Animal Care and Use Committee of Dalian Medical University. Laparotomy was performed under anesthesia with ether. The bile duct was isolated and double-ligated with a 3-0 silk suture. The abdominal wall and skin were closed with a 4-0 silk suture, and the antibiotic benzathine benzylpenicillin powder was sprinkled over the closed incision. The rats were continuously fed and housed for a further 4-wk period after surgery, and samples were collected. Rats in sham group underwent laparotomy with the bile duct isolated but not ligated. Rats in ZnPP and CoPP treatment groups received an intraperitoneal injection with ZnPP[21] or CoPP (30 mg/kg body weight) once, 24 h before sample collection. Rats in the 4 groups were housed in metabolic cages for the last 24 h, and urine was collected to measure its volume and the sodium and creatinine (Cre) levels.
Sample collection
Four weeks after surgery, the rats were anesthetized with ether and their portal vein, right carotid artery, and renal artery were isolated. Renal artery blood flow was measured by ultrasound (LOGIQ7, GE, USA). A catheter, connected to a pressure transducer (BL-420F biological experimental system, Chengdu Technology and Market Co. Ltd., China), was placed in the carotid artery for measurement of mean arterial pressure (MAP), then 1 mL of arterial blood was collected in a heparinized syringe through an arterial catheter to measure carboxyhemoglobin (COHb) using a RapidLab 1245 blood gas analyzer (Siemens, USA), as an index for the CO level in arterial blood. The catheter was placed in the portal vein to measure portal vein pressure (PVP). Then, 4 mL of blood was collected from the rats to measure serum levels of bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), Cre and sodium with a Hitachi 7600-110 automatic biochemical analyzer (Hitachi Co., Tokyo, Japan). Urine levels of Cre and sodium were also measured using this machine. The Cre clearance rate (Ccr) was calculated as urine Cre × urine volume/serum Cre. The left kidney and one liver lobe were excised, some of their tissues were fixed in a 10% neutral formalin solution and embedded in paraffin, while the remaining tissues were preserved at -80°C for PCR.
Reverse-transcription PCR analysis
Total RNA was extracted from kidneys following a standard guanidinium phenol-chloroform extraction protocol. The quantity of RNA was determined by measuring the optical density at 260 nm (A260 nm = 1 for 40 μg/mL RNA), and the purity of RNA was assessed by determining the ratio of the optical density obtained at 260 and 280 nm (pure RNA: A260 nm/A280 nm = 2.0) using a Shimadzu UV-1206 spectrophotometer (Shimadzu, Japan). The primer sequences for HO-1 are 5'-ACTTTCAGAAGGGTCAGGTGTCC-3' (forward) and 5'-TTGAGCAGGAAGGCGGTCTTAG-3' (reverse) and the product size is 524 bp, while the primer sequences for β-actin are 5'-GGAGTCAACGGATTTGGT-3' (forward), 5'-GTGATGGGATTTCCATTG-3' (reverse) and the product size is 226 bp. An aliquot of each mixture was used for reverse-transcription (RT)-PCR amplification using reagents purchased from Takara Bio Inc (Dalian, China). PCR products were separated by 2.5% agarose gel electrophoresis. The product bands were photographed and the density of each product band was quantified. The results were expressed as the ratio of the band density for HO-1 mRNA to that of β-actin mRNA.
Immunohistochemical analysis
Kidney and liver tissues were fixed in a 10% neutral formalin solution and embedded in paraffin wax and cut into sections. Some sections were routinely stained with HE while the other sections underwent deparaffinization, rehydration and inactivation, and were incubated with rabbit-anti-mouse HO-1 monoclonal antibody (1:50) at room temperature for 60 min, and then with secondary antibody (MaxVisionTM2) at room temperature for 15 min. The sections were mounted after staining. The primary antibody was replaced by phosphate-buffered saline to serve as a negative control. Five high-power microscopic fields were randomly chosen per slide and the yellow material in cytoplasm was considered to represent a HO-1-positive cell. Cell staining was assigned to 4 scores: 4 = > 75% positive cells, 3 = 50%-75% positive cells, 2 = 25%-50% positive cells, and 1 = < 25% positive cells. Cell staining intensity was scored based on its color as follows: 0 = no staining, 1 = faint yellow, 2 = light brown, and 3 = dark brown[22]. The final score was defined as staining intensity × percentage of positive cells. The mean score of five fields was used to compare the four groups.
Statistical analysis
Data analysis was performed using the SPSS 10.0 software (Chicago, IL, USA). Analysis of variance or Wilcoxon statistical methods were used to determine statistical significance. All measurements in this study were expressed as mean ± SD. P < 0.05 was considered statistically significant.
RESULTS
Biochemical examination
The serum level of AST in biliary cirrhotic group was 237.2 ± 95.13 IU/L, which was significantly higher than that (156.8 ± 18.28 IU/L) in sham group (P < 0.05). The serum level of Cre was significantly higher and the Ccr was significantly lower in biliary cirrhotic group than in sham group (P < 0.05). The serum Cre level was significantly higher in ZnPP treatment group (P < 0.05) and slightly lower in CoPP treatment group than in cirrhotic group (P > 0.05). The serum and urine sodium levels were significantly lower in cirrhotic group than in sham group (P < 0.05). The serum sodium concentration was significantly lower in ZnPP treatment group and significantly higher in CoPP treatment group than in cirrhotic group (P < 0.05, Table 1).
Table 1.
Effect of bile duct ligation and cobalt protoporphyrin or zinc protoporphyrin IX treatment on serum and urine levels of creatinine and sodium, and creatinine clearance rate (mean ± SD, n = 8-10 per group)
| Sham group | Cirrhosis group | CoPP group | ZnPP group | |
| Serum Cre (μmol/L) | 30.4 ± 1.81 | 36.3 ± 6.27a | 33.5 ± 5.98 | 45.3 ± 8.92c |
| Urine Cre (μmol/L) | 7.18 ± 1.15 | 8.08 ± 2.50 | 4.49 ± 1.51 | 6.31 ± 1.20 |
| Serum sodium (mmol/L) | 142.86 ± 3.44 | 138.75 ± 0.96a | 142.64 ± 5.43c | 136.57 ± 1.40c |
| Urine sodium (mmol/L) | 91.50 ± 12.12 | 71.33 ± 10.07a | 109.15 ± 64.93 | 66.25 ± 11.8 |
| Ccr (mL/min) | 0.23 ± 0.02 | 0.12 ± 0.05a | 0.14 ± 0.04 | 0.07 ± 0.01c |
| AST (IU/L) | 156.8 ± 18.28 | 237.2 ± 95.13a | 467.14 ± 222.28c | 209.11 ± 65.77 |
P < 0.05 vs sham group;
P < 0.05 vs cirrhotic group. CoPP: Cobalt protoporphyrin; ZnPP: Zinc protoporphyrin IX; Cre: Creatinine; Ccr: Creatinine clearance rate; AST: Aspartate aminotransferase.
Hemodynamic parameters and arterial blood gas levels
The PVP was significantly higher and the MAP was significantly lower in cirrhotic group than in sham group (P < 0.01). However, no significant difference was found in PVP and MAP in ZnPP and CoPP treatment groups compared with cirrhotic group. The COHb level in arterial blood was significantly higher in cirrhotic group than in sham group (P < 0.05) while significantly lower in ZnPP treatment group and significantly higher in CoPP treatment group than in cirrhotic group (P < 0.05). The renal artery blood flow was significantly lower in cirrhotic group than in sham group (P < 0.01), while significantly lower in ZnPP treatment group and significantly higher in CoPP treatment group than in cirrhotic group (P < 0.05). Furthermore, the 24 h urine volume was significantly smaller in cirrhotic group than in sham group (P < 0.05), while significantly smaller in ZnPP treatment group and significantly larger in CoPP treatment group than in cirrhotic group (P < 0.05, Table 2).
Table 2.
Effects of bile duct ligation and cobalt protoporphyrin or zinc protoporphyrin IX treatment on hemodynamic parameters, carboxyhemoglobin and 24-h urine volume (mean ± SD, n = 8-10 per group)
| Sham group | Cirrhosis group | CoPP group | ZnPP group | |
| PVP (mmHg) | 9.24 ± 0.76 | 15.56 ± 2.36b | 17.28 ± 1.20 | 13.71 ± 1.39 |
| MAP (cmH2O) | 118.83 ± 8.09 | 59.23 ± 12.19b | 52.75 ± 5.76 | 67.76 ± 7.66 |
| COHb (%) | 0.23 ± 0.05 | 0.50 ± 0.20a | 0.83 ± 0.39c | 0.23 ± 0.06c |
| RABF(mL/min•100 g) | 3.89 ± 0.09 | 3.58 ± 0.04b | 3.76 ± 0.06c | 3.50 ± 0.08c |
| Urine (mL/24 h) | 15.00 ± 2.23 | 10.93 ± 1.92a | 13.5 ± 1.10c | 8.50 ± 1.10c |
P < 0.05,
P < 0.01 vs sham group;
P < 0.05 vs cirrhosis group. CoPP: Cobalt protoporphyrin; ZnPP: Zinc protoporphyrin IX; PVP: Portal vein pressure; MAP: Mean arterial pressure; COHb: Carboxyhemoglobin; RABF: Renal arterial blood flow.
Histopathological analysis of liver and kidney in cirrhotic and sham rats
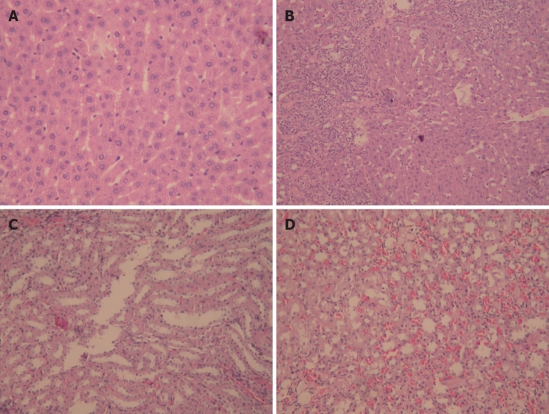
Liver and kidney tissue samples from cirrhotic and sham rats were stained with HE to examine the histopathological changes. The bridging necrosis of hepatic cells was observed in livers of rats 4 wk after BDL, particularly in portal areas, nodular regeneration of hepatocytes, collapse, and disorganization of the hepatic lobular structure, numerous lymphocytes infiltrating the portal area and around the central vein, and the formation of pseudolobules surrounded by fibrous septa. In contrast, except for vascular dilation and congestion of the mesenchyme, no obvious pathological changes were observed in kidneys of cirrhotic group compared with sham group (Figure 1).
Figure 1.
Representative photomicrographs of rats in cirrhotic and sham groups (magnification 200 ×, HE staining). A: Normal liver structure; B: Liver cirrhosis; C: Normal kidney structure; D: Renal structure in cirrhotic group.
Renal HO-1 mRNA expression level
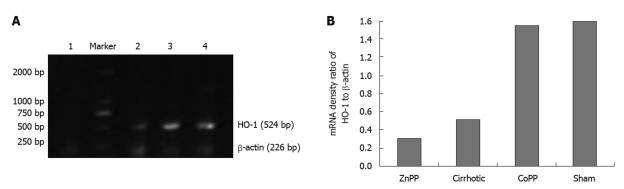
RT-PCR showed that the expression level of HO-1 mRNA in kidney was significantly lower in cirrhotic group than in sham group (P < 0.01). Furthermore, renal HO-1 mRNA expression was significantly decreased in ZnPP treatment group (P < 0.05) and significantly higher in CoPP treatment group than in cirrhotic group (P < 0.05) (Figure 2).
Figure 2.
Expression of heme oxygenase-1 mRNA in kidney. A: Representative reverse-transcription polymerase chain reaction data showing the heme oxygenase-1 (HO-1) mRNA expression levels in kidneys from zinc protoporphyrin IX (ZnPP) treatment group (lane 1), cirrhotic group (lane 2), cobalt protoporphyrin (CoPP) treatment group (lane 3), and sham group (lane 4); B: Quantitative data showing the ratio of band density of the corresponding HO-1 mRNA to that of β-actin mRNA.
Immunohistochemical detection of HO-1 protein in kidney and liver
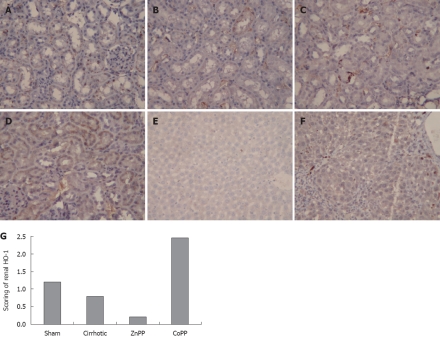
To localize the HO-1 protein expression in kidneys, immunohistochemistry was performed using specimens from the four groups. The HO-1 protein was mainly expressed in the distal renal tubules, which is similar to reported findings[23] (Figure 3). The intensity and percentage of cells expressing HO-1 protein in kidney were also detected. Mild staining was observed in renal tissue samples from sham group, with a score of 1.21 ± 0.33. The HO-1 score was 0.79 ± 0.25 in cirrhotic group, which was significantly lower than that in sham group (P < 0.01). The HO-1 score (0.21 ± 0.25) was lower in ZnPP treatment group and higher in CoPP treatment group (2.46 ± 0.46) than in cirrhotic group (P < 0.05). The staining intensity and percentage of cells expressing HO-1 protein were also evaluated in liver of cirrhotic and sham groups. Unlike the HO-1 protein expression in kidney, the mean hepatic HO-1 score was significantly higher in cirrhotic group than in sham group (4.63 ± 0.74 vs 0.63 ± 0.52, P < 0.01).
Figure 3.
Expression of heme oxygenase-1 protein in kidney and liver. Immunohistochemical staining of renal heme oxygenase-1 (HO-1) protein in rats of sham group (A), cirrhotic group (B), zinc protoporphyrin IX (ZnPP) treatment group (C), cobalt protoporphyrin (CoPP) treatment group (D), and immunohistochemical staining of hepatic HO-1 protein in rats of sham group (E) and cirrhotic group (F) in the upper part (magnification 200 ×), and quantitative scoring (G) of immunohistochemical staining of renal HO-1 protein expression in each group in the lower part.
DISCUSSION
Renal dysfunction is very common in patients with advanced cirrhosis, and its severity varies from electrolyte-balance disturbances and water retention to HRS. HRS is considered a functional renal failure because no structural damage has been observed in kidney, and can be reversed in some cases[4]. However, it carries a worse prognosis of patients with cirrhosis and increases their risk of mortality[2,3]. It has been reported that the annual incidence of HRS in patients with liver cirrhosis and ascites is about 8%[24]. Typical features of HRS include oliguria, hyponatremia, azotemia, and hyponatruria. Although the pathophysiological mechanism underlying HRS is still incompletely understood, marked renal vasoconstriction in the presence of splanchnic and systemic vasodilation may play an important role, and may thus reduce the renal arterial blood flow and the glomerular filtration rate, resulting in oliguria and an increased serum Cre concentration[6-8]. Studies have shown that the HO-1/CO system plays an important role in the control of vascular tone and that inhibition of HO-1 blocks vasodilation induced by heme[12-14]. In addition, HO-1 is also involved in the prevention of renal failure after renal ischemia[15] or glycerol-induced acute renal injury in rats [16]. In this study, the relation between expression of HO-1 in kidney and renal arterial blood flow and renal function was investigated in cirrhotic rats.
To evaluate the hepatic, renal, and systemic changes in rats 4 wk after BDL, the MAP, PVP, COHb in arterial blood, serum levels of AST and ALT, Cre and sodium, urine sodium and Cre were measured. The changes in hepatic and renal histology were also examined, and the expression levels of HO-1 mRNA and protein in liver and kidney were evaluated. The Ccr level was also measured as an index of glomerular filtration rate and renal function. The serum level of AST was significantly higher in cirrhotic group than in sham group, indicating that BDL causes marked liver injury, with liver cirrhosis confirmed with HE staining of liver specimens. In addition, the MAP level was significantly lower in BDL rats than in time-matched sham rats, indicating that a hyperdynamic state occurs. Furthermore, the PVP was significantly higher in BDL rats than in time-matched sham rats, indicating that portal hypertension exists. Oliguria, hyponatremia, hyponaturia, increased Cre concentrations and decreased Ccr were also observed in BDL rats. All these findings show that experimental HRS was established in BDL rats. Accompanied with the decreased renal arterial blood flow and renal function, the HO-1 mRNA and protein levels in kidney of rats were significantly lower in cirrhotic group than in sham group, indicating that production of CO is decreased in kidney, because CO is mainly generated by degrading heme due to HO. HO has constitutive and inducible isoforms[25,26]. HO-1, a 32-kDa inducible protein[27], catalyzes the rate-limiting step in oxidative degradation of heme to biliverdin, releasing equimolar amounts of CO and iron[26]. The HO-1/CO system plays a vital role in many activities, including anti-oxidative stress, anti-inflammation, inhibition of cellular proliferation, and regulation of cytokine expression. CO, a gaseous messenger similar to NO, can activate soluble guanylate cyclase leading to production of cGMP[28] which mediates various physiological functions[29] including vasodilation[30]. CO can also relax vascular smooth muscle in a cGMP-independent manner[12,13]. HO-1 activity is the primary source of circulating CO[31], and HO-1 contributes to vasodilation mainly through HO-1-derived CO[32]. Thus, the declined HO-1 expression in kidney may be responsible for a decrease in vasodilation. In addition, oxidants can cause localized renal vasoconstriction[33]. Thus, the antioxidant action of HO-1 and its products can preserve renal arterial blood flow. Decreased HO-1 expression in kidney of BDL rats impairs their ability to buffer locally produced oxidants, thus leading to decreased renal arterial blood flow and deteriorated renal function.
Surprisingly, the COHb level was significantly higher in cirrhotic group than in sham group, suggesting that there is more CO in circulation, since it is predominately bound to hemoglobin in the form of COHb[34]. This large amount of CO may be produced by increased HO-1 expression in other organs, such as liver, because the HO-1 expression level in liver was higher in cirrhotic group than in sham group. Thus, we speculate that the decreased HO-1 expression in kidney and suppression of locally produced CO contribute to the decreased renal arterial blood flow and renal dysfunction in cirrhotic rats.
To evaluate the functional consequences of HO-1 changes, BDL rats were treated with either ZnPP or CoPP. The PVP and MAP were measured to evaluate systemic effects of ZnPP and CoPP treatment, renal arterial blood flow, 24 h total urinary volume, serum and urine levels of sodium and Cre were also measured to evaluate the effects of ZnPP and CoPP on HRS. The expression level of HO-1 mRNA and protein in kidney was lower in ZnPP treatment group and higher in CoPP treatment group than in cirrhotic group, without obvious changes in PVP and MAP, while the renal arterial blood flow was significantly lower and the renal function was more severely impaired in ZnPP treatment group than in cirrhotic group, as demonstrated by the decreased 24 h total urinary volume, Ccr, and serum level of sodium. In contrast, the renal arterial blood flow and 24 h total urinary volume were higher in CoPP treatment group than in cirrhotic group. However, unlike ZnPP treatment, CoPP treatment did not significantly affect serum Cre or Ccr compared with cirrhotic group. Nevertheless, increasing the treatment time or the CoPP dose may have elicited the different results in our study. Because ZnPP or CoPP treatment did not significantly affect MAP or PVP, changes in renal artery vascular tone were not considered to represent systemic vascular effects of HO-1 inhibition or HO-1 induction.
In conclusion, renal HO-1 expression, renal arterial blood flow, Ccr, 24 h total urinary volume, serum and urine sodium concentrations are lower in cirrhotic rats than in sham rats. Inhibition of renal HO-1 activity decreases renal arterial blood flow and aggravates renal dysfunction in rats with HRS. Meanwhile, activation of renal HO-1 activity had the opposite effects. Taken together, decreased HO-1 expression in kidney plays an important role in the pathogenesis of experimental HRS.
COMMENTS
Background
Renal dysfunction is very common in patients with advanced cirrhosis, and its severity ranges from electrolyte-balance disturbances and water retention to hepatorenal syndrome (HRS). HRS is a poor prognostic indicator for patients with liver cirrhosis, and can increase its morbidity and mortality in such patients. At present, the pathophysiological mechanism underlying HRS is still incompletely understood.
Research frontiers
Intense renal vasoconstriction in combination with peripheral arterial vasodilation plays an important role in occurrence of HRS. Studies have shown that the heme oxygenase-1 (HO-1)/carbon monoxide system is a crucial component in regulation of vascular tone and that inhibition of HO-1 blocks vasodilation induced by heme. HO-1 is also central to the prevention of renal failure after renal ischemia or glycerol-induced acute renal injury in rats. In this study, we investigated the expression of HO-1 in the kidney of experimental rats with HRS and evaluated the functional role of HO-1 in the pathogenesis of HRS.
Innovations and breakthroughs
The characteristics of HRS became evident in rats 4 wk after bile duct ligation (BDL), including reduced creatinine clearance rate and fluid retention, without changes in renal histology. The decreased renal arterial blood flow and renal function were accompanied with decreased renal expression of HO-1 at mRNA and protein levels. To evaluate the functional consequences of the changes in HO-1 expression, the authors treated BDL rats with either zinc protoporphyrin IX (ZnPP), a specific HO-1 inhibitor or cobalt protoporphyrin (CoPP), a HO-1 inducer. ZnPP treatment significantly reduced the renal arterial blood flow and further worsened the renal function, while CoPP treatment had the opposite effects. The relation between HO-1 and renal arterial blood flow and renal function was systematically evaluated by treating BDL rats with ZnPP and CoPP.
Applications
The mechanisms underlying renal dysfunction in patients with advanced liver disease or cirrhosis are complicated and remain incompletely understood. However, the findings in this study indicate that decreased renal HO-1 expression plays an important role in the pathogenesis of experimental HRS.
Terminology
HRS, a progressive renal failure that occurs in patients with chronic liver disease and advanced hepatic failure in the absence of any apparent clinical cause for renal insufficiency, corresponds to a functional alteration without histological changes in renal tissue. HO-1 is heme oxygenase-1, a rate-limiting enzyme that is also known as heat shock protein 32, and can be induced by CoPP and inhibited by ZnPP in vivo.
Peer review
This paper, written in rather good English, is quite important and interesting, which shows that decreased HO-1 expression in the kidney plays an important role in the pathogenesis of experimental HRS, as it demonstrated that the renal HO-1 expression, renal arterial blood flow, creatinine clearance rate, 24 h total urinary volume, serum and urine sodium concentrations were lower in cirrhotic rats than in sham rats, thus inhibition of renal HO-1 activity decreases renal arterial blood flow and aggravates renal dysfunction in rats with HRS.
Footnotes
Supported by National Natural Science Foundation of China, No. 30970886; and Science and Technology Project of Dalian, No. 2008E13SF193
Peer reviewer: Dr. Jeff Butterworth, MB, FRCP, Department of Gastroenterology, Shrewsbury and Telford Hospital NHS Trust, Mytton Oak Road, Shrewsbury, Shropshire, SY3 8XQ, United Kingdom
S- Editor Sun H L- Editor Wang XL E- Editor Lin YP
References
- 1.Miyazono M, Garat C, Morris KG Jr, Carter EP. Decreased renal heme oxygenase-1 expression contributes to decreased renal function during cirrhosis. Am J Physiol Renal Physiol. 2002;283:F1123–F1131. doi: 10.1152/ajprenal.00363.2001. [DOI] [PubMed] [Google Scholar]
- 2.Guevara M, Ginès P. Hepatorenal syndrome. Dig Dis. 2005;23:47–55. doi: 10.1159/000084725. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer J, Brentjens TE. Renal failure in patients with cirrhosis: hepatorenal syndrome and renal support strategies. Curr Opin Anaesthesiol. 2010;23:139–144. doi: 10.1097/ACO.0b013e32833724a8. [DOI] [PubMed] [Google Scholar]
- 4.Cárdenas A, Gines P. Hepatorenal syndrome. Clin Liver Dis. 2006;10:371–385, ix-x. doi: 10.1016/j.cld.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Moreau R, Lebrec D. Acute renal failure in patients with cirrhosis: perspectives in the age of MELD. Hepatology. 2003;37:233–243. doi: 10.1053/jhep.2003.50084. [DOI] [PubMed] [Google Scholar]
- 6.Cárdenas A. Hepatorenal syndrome: a dreaded complication of end-stage liver disease. Am J Gastroenterol. 2005;100:460–467. doi: 10.1111/j.1572-0241.2005.40952.x. [DOI] [PubMed] [Google Scholar]
- 7.Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol. 2006;1:1066–1079. doi: 10.2215/CJN.01340406. [DOI] [PubMed] [Google Scholar]
- 8.Martin PY, Ginès P, Schrier RW. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Engl J Med. 1998;339:533–541. doi: 10.1056/NEJM199808203390807. [DOI] [PubMed] [Google Scholar]
- 9.Türkay C, Yönem O, Arikan O, Baskin E. Nitric oxide and renal functions in liver cirrhosis. Turk J Gastroenterol. 2004;15:73–76. [PubMed] [Google Scholar]
- 10.Saracyn M, Wesołowski P, Nowak Z, Patera J, Kozłowski W, Wańkowicz Z. [Role of nitric oxide system in pathogenesis of experimental model of hepatorenal syndrome] Pol Merkur Lekarski. 2008;24:293–297. [PubMed] [Google Scholar]
- 11.Islas-Carbajal MC, Covarrubias A, Grijalva G, Alvarez-Rodríguez A, Armendáriz-Borunda J, Rincón-Sánchez AR. Nitric oxide synthases inhibition results in renal failure improvement in cirrhotic rats. Liver Int. 2005;25:131–140. doi: 10.1111/j.1478-3231.2005.01018.x. [DOI] [PubMed] [Google Scholar]
- 12.Kozma F, Johnson RA, Zhang F, Yu C, Tong X, Nasjletti A. Contribution of endogenous carbon monoxide to regulation of diameter in resistance vessels. Am J Physiol. 1999;276:R1087–R1094. doi: 10.1152/ajpregu.1999.276.4.R1087. [DOI] [PubMed] [Google Scholar]
- 13.Kozma F, Johnson RA, Nasjletti A. Role of carbon monoxide in heme-induced vasodilation. Eur J Pharmacol. 1997;323:R1–R2. doi: 10.1016/s0014-2999(97)00145-3. [DOI] [PubMed] [Google Scholar]
- 14.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu H, Takahashi T, Suzuki T, Yamasaki A, Fujiwara T, Odaka Y, Hirakawa M, Fujita H, Akagi R. Protective effect of heme oxygenase induction in ischemic acute renal failure. Crit Care Med. 2000;28:809–817. doi: 10.1097/00003246-200003000-00033. [DOI] [PubMed] [Google Scholar]
- 16.Leung N, Croatt AJ, Haggard JJ, Grande JP, Nath KA. Acute cholestatic liver disease protects against glycerol-induced acute renal failure in the rat. Kidney Int. 2001;60:1047–1057. doi: 10.1046/j.1523-1755.2001.0600031047.x. [DOI] [PubMed] [Google Scholar]
- 17.Drummond GS. Control of heme metabolism by synthetic metalloporphyrins. Ann N Y Acad Sci. 1987;514:87–95. doi: 10.1111/j.1749-6632.1987.tb48764.x. [DOI] [PubMed] [Google Scholar]
- 18.Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR, et al. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999;104:1631–1639. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fallon MB, Abrams GA, McGrath JW, Hou Z, Luo B. Common bile duct ligation in the rat: a model of intrapulmonary vasodilatation and hepatopulmonary syndrome. Am J Physiol. 1997;272:G779–G784. doi: 10.1152/ajpgi.1997.272.4.G779. [DOI] [PubMed] [Google Scholar]
- 20.Luo B, Abrams GA, Fallon MB. Endothelin-1 in the rat bile duct ligation model of hepatopulmonary syndrome: correlation with pulmonary dysfunction. J Hepatol. 1998;29:571–578. doi: 10.1016/s0168-8278(98)80152-9. [DOI] [PubMed] [Google Scholar]
- 21.Wei CL, Lee KH, Khoo HE, Hon WM. Expression of haem oxygenase in cirrhotic rat liver. J Pathol. 2003;199:324–334. doi: 10.1002/path.1284. [DOI] [PubMed] [Google Scholar]
- 22.Remmele W, Stegner HE. [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue] Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 23.da Silva JL, Zand BA, Yang LM, Sabaawy HE, Lianos E, Abraham NG. Heme oxygenase isoform-specific expression and distribution in the rat kidney. Kidney Int. 2001;59:1448–1457. doi: 10.1046/j.1523-1755.2001.0590041448.x. [DOI] [PubMed] [Google Scholar]
- 24.Arroyo V, Fernandez J, Ginès P. Pathogenesis and treatment of hepatorenal syndrome. Semin Liver Dis. 2008;28:81–95. doi: 10.1055/s-2008-1040323. [DOI] [PubMed] [Google Scholar]
- 25.Morita T, Perrella MA, Lee ME, Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci USA. 1995;92:1475–1479. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christodoulides N, Durante W, Kroll MH, Schafer AI. Vascular smooth muscle cell heme oxygenases generate guanylyl cyclase-stimulatory carbon monoxide. Circulation. 1995;91:2306–2309. doi: 10.1161/01.cir.91.9.2306. [DOI] [PubMed] [Google Scholar]
- 27.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 28.Schraufnagel DE, Kay JM. Structural and pathologic changes in the lung vasculature in chronic liver disease. Clin Chest Med. 1996;17:1–15. doi: 10.1016/s0272-5231(05)70295-1. [DOI] [PubMed] [Google Scholar]
- 29.Hervé P, Lebrec D, Brenot F, Simonneau G, Humbert M, Sitbon O, Duroux P. Pulmonary vascular disorders in portal hypertension. Eur Respir J. 1998;11:1153–1166. doi: 10.1183/09031936.98.11051153. [DOI] [PubMed] [Google Scholar]
- 30.Chang SW, Ohara N. Pulmonary circulatory dysfunction in rats with biliary cirrhosis. An animal model of the hepatopulmonary syndrome. Am Rev Respir Dis. 1992;145:798–805. doi: 10.1164/ajrccm/145.4_Pt_1.798. [DOI] [PubMed] [Google Scholar]
- 31.Naik JS, O'Donaughy TL, Walker BR. Endogenous carbon monoxide is an endothelial-derived vasodilator factor in the mesenteric circulation. Am J Physiol Heart Circ Physiol. 2003;284:H838–H845. doi: 10.1152/ajpheart.00747.2002. [DOI] [PubMed] [Google Scholar]
- 32.Carter EP, Hartsfield CL, Miyazono M, Jakkula M, Morris KG Jr, McMurtry IF. Regulation of heme oxygenase-1 by nitric oxide during hepatopulmonary syndrome. Am J Physiol Lung Cell Mol Physiol. 2002;283:L346–L353. doi: 10.1152/ajplung.00385.2001. [DOI] [PubMed] [Google Scholar]
- 33.Bomzon A, Holt S, Moore K. Bile acids, oxidative stress, and renal function in biliary obstruction. Semin Nephrol. 1997;17:549–562. [PubMed] [Google Scholar]
- 34.Rodriguez F, Kemp R, Balazy M, Nasjletti A. Effects of exogenous heme on renal function: role of heme oxygenase and cyclooxygenase. Hypertension. 2003;42:680–684. doi: 10.1161/01.HYP.0000085785.40581.1A. [DOI] [PubMed] [Google Scholar]