Abstract
AIM: To analyze outcomes of delayed single-stage necrosectomy after early conservative management of patients with infected pancreatic necrosis (IPN) associated with severe acute pancreatitis (SAP).
METHODS: Between January 1998 and December 2009, data from patients with SAP who developed IPN and were managed by pancreatic necrosectomy were analyzed.
RESULTS: Fifty-nine of 61 pancreatic necrosectomies were performed by open surgery and 2 laparoscopically. In 55 patients, single-stage necrosectomy could be performed (90.2%). Patients underwent surgery at a median of 29 d (range 13-46 d) after diagnosis of acute pancreatitis. Sepsis and multiple organ failure accounted for the 9.8% mortality rate. Pancreatic fistulae (50.8%) predominantly accounted for the morbidity. The median hospital stay was 23 d, and the median interval for return to regular activities was 110 d.
CONCLUSION: This series supports the concept of delayed single-stage open pancreatic necrosectomy for IPN. Advances in critical care, antibiotics and interventional radiology have played complementary role in improving the outcomes.
Keywords: Necrosectomy, Infected necrosis, Pancreas, Severe acute pancreatitis, Inflammation
INTRODUCTION
Severe acute pancreatitis (SAP) is a disease with high morbidity and mortality[1,2]. In the absence of specific effective therapy, management revolves around supportive care[3,4].
While the overall reported mortality of acute pancreatitis (AP) varies between 5% and 12%[2,5], SAP, which comprises around 10%-20% of AP, continues to have a high mortality rate of around 25%[6,7] due to organ failure and sepsis arising from infected pancreatic necrosis (IPN).
Management of IPN has been widely studied over the last few decades[6,8-20]. Data on indications, timing and technique of debridement for IPN are varied. However, while recent reports reflect the common theme of delayed surgery in IPN[21], the ideal debridement technique continues to be debated[6,12,15].
Reports regarding minimally invasive surgery for IPN are now being published[22-25]. Thus, if we are to develop evidence-based guidelines for the management of IPN, rather than comparing outcomes with the relatively higher mortality encountered in some reports published a few decades ago, a more balanced comparison should particularly include results from larger series, and include some of the more recent series in which surgery for IPN has been complemented by advances in critical care, interventional radiology and broader spectrum antibiotics.
We have been performing open necrosectomy in a uniform manner for the last 10 years with conventional abdominal drainage without post-operative peritoneal lavage.
The purpose of our study was to analyze the feasibility and outcome of performing open necrosectomy for IPN in a delayed fashion.
MATERIALS AND METHODS
During the time period between January 1998 and December 2009, patients with SAP who developed IPN and were referred to the authors’ center for surgical management were analyzed for this report.
At admission, patients were scored for severity, based on the APACHE II scoring system[1,26], and were managed with resuscitation and intensive (supportive) care strategies. SAP was defined clinically by the presence of associated organ failure and/or local complications such as necrosis, abscess, or pseudocyst[27]. In addition, the patients were also defined as having SAP if the APACHE II score was ≥ 9.
At admission, all the patients classified as having SAP were admitted to the intensive care unit (ICU) where resuscitation was commenced. The patients were started on antibiotics, which were usually fluoroquinolones and metronidazole during the initial few years. However, the choice of antibiotic was changed to carbapenems (meropenem 500 mg 6 hourly, for 7 to 14 d) thereafter, owing to the sensitivity of the local microbiological flora and based on the reports of the ability of carbapenems to penetrate the necrosum[28]. Antifungals were commenced if the duration of antimicrobial therapy went beyond 7 d.
In patients with SAP, contrast-enhanced computed tomography (CECT) scan was performed for assessing the local severity by the Balthazar computed tomography (CT) severity index[29]. A CT severity index of ≥ 7 was considered indicative of SAP. Nutrition was maintained by nasojejunal intubation and feeding. Percutaneous interventions were performed when clinically indicated, particularly in unstable patients as a temporizing measure or a bridge to surgery. The aim was to try and delay any intervention beyond the first 21 d.
Indications for surgery[23,30,31]: (1) Sepsis syndrome - clinical deterioration that is progressive with or without organ system failure and accompanied by fever and leucocytosis; (2) IPN - confirmed by fine needle aspiration (FNA) cytology and microbiological examination; (3) CECT showing extensive pancreatic necrosis with air pockets diagnostic of IPN; and (4) “Persisting unwellness” - in the form of abdominal pain, malaise, inability to tolerate a diet, general lack of well being, and continuing weight loss.
The necrosectomy was planned as a single stage. Surgery was defined as delayed if it was performed at least 21 d after the onset of pain, which was considered as Day 0 of the attack. Fresh imaging in the form of CECT was obtained just prior to the exploration. The areas of necrosis and fluid collection were carefully mapped. The patient underwent a laparotomy through a transverse upper abdominal incision. Free fluid was aspirated, and the lesser sac was exposed either through the transgastrocolic or transmesocolic route. All the pus and fluid were removed and sent for microbiological examination. The necrotic debris was also removed carefully with blunt finger dissection and sponge-holders, with an attempt not to damage any of the normal tissue. Particular care was taken not to divide bands across the cavity, especially in areas where known vessels could cross, e.g. middle colic artery. Copious lavage with warm normal saline was performed, which also helped to separate the necrotic tissue from the normal tissue. Bleeding was controlled with temporary packing, after which specific vessels were underrun with non-absorbable sutures. Other areas were explored, depending on the CT interpretation. These included the right and left paracolic gutters, head of the pancreas, gastrohepatic omentum, pelvis, small bowel mesentery and the splenic hilum. Two 28 Fr tube drains were placed in the lesser sac and necrotic cavity. Loop ileostomy was performed selectively in the presence of extensive pericolic necrosis. Figure 1 is an abdominal CECT showing IPN. Figure 2 shows necrotic pancreas post-necrosectomy.
Figure 1.
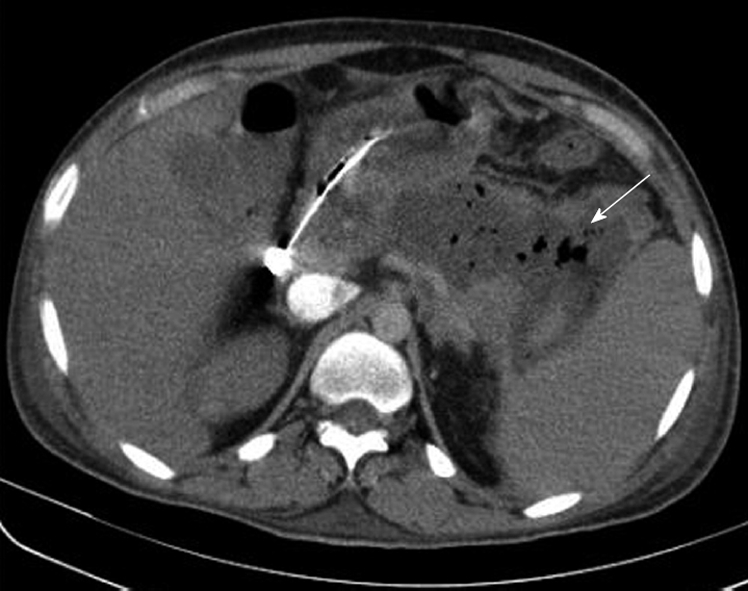
Contrast-enhanced computed tomography of the abdomen showing a large hypodense collection with air pockets in the location of the pancreatic body and tail (white arrow) indicative of an infected pancreatic necrosis.
Figure 2.
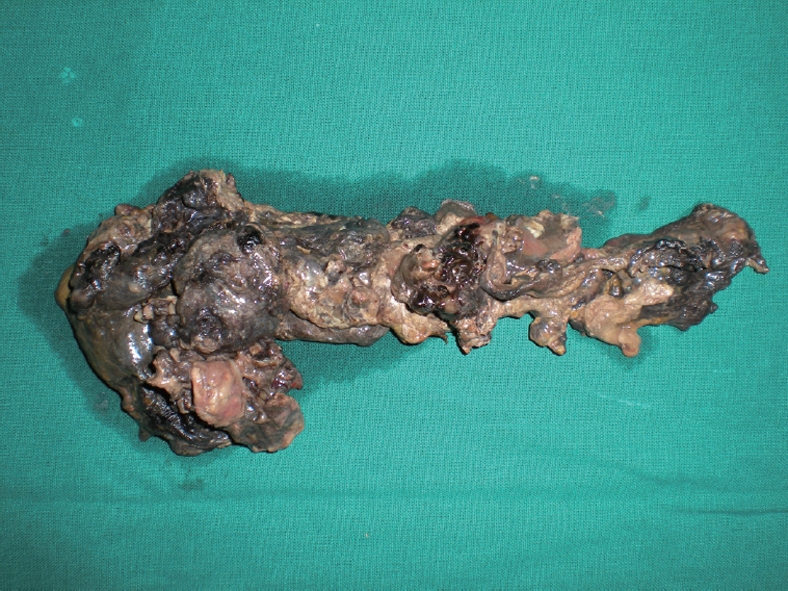
Post-operative photograph demonstrating a complete necrotic pancreas.
Post-operatively the patients were managed in the ICU. There was no attempt to perform post-operative lavage or flushing of the drains and the drains were removed once the output became minimal.
RESULTS
The 61 patients who required a necrosectomy for IPN included 49 male and 12 female patients. The mean age was 43 years (range 18-73 years). The predominant etiology for AP was gallstones (25 patients). Other etiologies included alcohol-induced (14 patients), idiopathic (13 patients), traumatic (3 patients), post-endoscopic retrograde cholangio-pancreatography (3 patients), and metabolic (3 patients).
The median time of patient transfer to our institute, which is a tertiary care center, was 9 d (range 4-40 d). No patient had surgical intervention prior to transfer, but 4 patients had already undergone percutaneous (n = 2) or endoscopic (n = 2) drainage for fluid collections prior to transfer.
Fifty-nine patients underwent an open necrosectomy while 2 patients had a laparoscopic necrosectomy. Patients underwent surgery at a median of 29 d (range 13-46 d) from the onset of symptoms. In only one patient, the necrosectomy had to be performed on day 13 for unresponsive multiple organ dysfunction syndrome (MODS). Delayed necrosectomy could be performed in the other 60 patients (98.3%). Re-exploration was required in five (5/59, 8%) patients for ongoing necrosis. In these patients further exploration was required on an average 2.4 occasions (range 2-3 occasions). The rate of re-exploration was 8%. Two patients required subsequent percutaneous drainage for residual intra-abdominal collections. Overall, a single-staged open necrosectomy was successful in 55 (90%) patients.
The microbiological cultures obtained from the necrotic tissue showed evidence of organism growth in 51 patients (83.6%). Mixed gram-positive and -negative organisms were encountered in 9 cases. Of the organisms isolated, 46 cultures were positive for gram-negative organisms, predominantly E. coli, Klebsiella, Acinetobacter and Pseudomonas and 11 grew gram-positive organisms. Fungi were isolated in 9 cases, all of which were in bacterial-positive cultures.
The various complications encountered have been listed in Table 1. The most common complication encountered was pancreatic fistula. Other complications were bowel fistulae, bleeding, recurrent sepsis, wound infection and secondary fungal infection.
Table 1.
Complications encountered in the 61 patients and their management
| Complication | n (%) | Management |
| Pancreatic fistula | 31 (50.8) | Tube drainage-20 |
| Stenting-11 | ||
| Fistulojejunostomy-1 | ||
| Distal pancreatectomy-1 | ||
| Enteric fistula | 11 (18.0) | |
| Small bowel | 2 (3.2) | Tube drainage-2 |
| Large bowel | 9 (14.7) | Defunctioning ileostomy-9 |
| Spontaneous healing-5 | ||
| Segmental colectomy-4 | ||
| Bleeding | 4 (6.5) | |
| Pseudo-aneurysm | 3 (5) | Angioembolization-3 |
| DIC | 1 (1.6) | Platelets, factor VII |
| Secondary fungal infection | 9 (14.7) | Antifungals |
| Wound infection | 18 (29.5) | Wound drainage and dressings |
| Intestinal obstruction | 3 (5) | Conservative-2 |
| Laparotomy-1 | ||
| Pseudocyst | 2 (3.2) | Cystojejunostomy-1 |
| Open drainage-1 | ||
| Pelvic abscess | 1 (1.6) | Pig tail drainage-1 |
DIC: Disseminated intravascular coagulation.
The diagnosis of pancreatic fistula was based on amylase estimation of the drain fluid, which ranged from 9000 to 104 000 U/mL. The drainage tube was maintained in situ and the patient was managed on an outpatient basis. Complete healing was achieved in 20 patients after an average of 2 mo. Endoscopic stenting of the pancreatic duct was performed in 11 patients in whom the leak persisted for > 2 mo. Two patients required re-surgery in the form of a fistulojejunostomy and a distal pancreatectomy, as stent placement could not be achieved across the leak. Of the 11 patients who developed enteric fistulae in the post-operative period, 4 had undergone prophylactic ileostomy creation during the primary surgery due to the presence of extensive pericolic necrosis. Five patients required a loop ileostomy later. In 4 patients the colonic fistula healed without any sequelae, while 5 patients required segmental colectomy for colonic stricture or persistent leak for more than 6 mo. One patient died after colonic resection due to sepsis. The two patients with small bowel fistulae were managed conservatively with tube drainage. In 3 of the 4 patients who had post-operative hemorrhage, the source could be localized on angiography to pseudoaneurysms (splenic artery: 2, middle colic artery: 1) and this was managed by angioembolization. Another patient died due to coagulopathy and acidosis. Fifteen patients (24.5%) required readmission. The reasons for readmission included persistent pancreatic fistula (5 patients), colonic stricture (4 patients), intestinal obstruction (3 patients), pseudocyst formation (2 patients), and pelvic abscess formation (1 patient).
There were 6 deaths in the perioperative period with a mortality rate of 10%. These included 3 (of the five) patients who underwent re-explorations; 1 patient with post-operative hemorrhage, 1 patient with a colonic fistula and the patient who required an early necrosectomy. The cause of death was sepsis and MODS in all cases except the patient with hemorrhage. The median post-operative ICU stay was 7 d (range 3-30 d) and the median duration for which the patient required ventilatory support was 3 d (range 2-7 d). The median duration of hospital stay following surgery was 23 d (range 11-88 d). The time to return to daily activity (defined as ability to perform daily personal activities, including feeding oneself and combing hair) was 16 d (10-20 d). The average time to return to regular activity was 110 d (60-140 d).
DISCUSSION
The ideal timing for a necrosectomy for IPN is a matter of debate. In our patients, we carried out a conservative management regimen with supportive care, antibiotics, early enteral feeding, and care of the patient in the ICU.
Using this management strategy we were able to perform a delayed necrosectomy, i.e. after 21 d, with potential benefits as follows: (1) Separation of viable from non-viable tissues making the operation technically easier; (2) Operating on a more hemodynamically stable patient; (3) Reduced bleeding as only non-viable tissue is removed[12]; (4) Removal of less normal pancreas resulting in reduced long-term morbidity[9]; and (5) Reduced local complications such as erosion into blood vessels/small bowel that could lead to post-operative hemorrhage or fistulae.
Mier et al[15] had previously put forward this principle of delayed surgery for IPN. The success of the approach was subsequently confirmed by other studies[6,13,21].
With this strategy of delaying surgery, in our series necrosectomy was performed as a single stage in all but 6 patients. In patients where the initially severe clinical course improved and the patient developed signs of sepsis in the third week, CT scan was repeated to map the extent of necrosis. At this time the pancreatic and peripancreatic necrosis tended to be localized with a resolution of the changes during the acute attack, such as acute fluid collections, stranding of the mesentery, etc.
The mortality rate in our study following the performance of a delayed single-staged necrosectomy was 9.8%. This compares favorably with the mortality rate of 11%-38% reported for open, as well as minimally invasive, necrosectomy for IPN over the last few years[8,10-12,17,18,23,32,33]. The indication for intervention in our patients was not solely based on an FNA as has been described previously[12]. We feel that FNA plays a role in the early period after SAP where it helps to differentiate systemic inflammatory response syndrome (SIRS) from infection. However, since we did not operate on the patients in this period, we did not find the need to apply the use of FNA as routine. Our decision to intervene was based on clinical parameters that included features such as persistent “unwellness”, persistent pain in the abdomen, leucocytosis, appearance of a new fever especially after the second week when SIRS would not be a cause of raised temperature and infection of the pancreatic necrosis would be the only likely possibility, and the CT scan appearance of pancreatic necrosis[23,30,31].
The concept of delayed surgery has definitely been facilitated by improvements in critical care, fluid resuscitation and organ support that have contributed to the fall in the early mortality associated with SAP[34,35]. These have contributed by targeting one of the most important determinants of poor outcome in SAP, i.e. the early development and persistence of organ dysfunction[36].
We have used carbapenems, in particular meropenem, based on the proven efficacy of the drug for prophylaxis in patients with SAP[37]. The rationale for using antibiotics was that mortality for IPN is higher than that for sterile necrosis and antibiotic usage decreases the risk of infection[38,39]. The use of antibiotics indiscriminately, however, can lead to a 12%-35%[40,41] risk of opportunistic fungal infections, e.g. Candida albicans and Aspergillus fumigatus[42], which further increase the mortality rate[43,44]. The accepted indications for antibiotics in AP are: newly developed sepsis or SIRS, failure of two or more organ systems, proven infection, or an increase in serum C reactive protein in combination with other evidence supporting the presence of infection, e.g. CT scan[45]. We isolated fungal cultures in only 9 patients, i.e. 15% of cases, which was quite similar to the findings of Grewe et al[46] who reported similar fungal superinfections after using a four-drug regimen for a mean of 23 d.
The incidence of enteric fistulae in our study (18%) was comparable to that reported by Howard et al[47]. The incidence of developing a colonic fistula is high in patients with pericolic spread of necrosis into the left paracolic gutter, as seen in our patients, and we strongly advocate the use of prophylactic loop ileostomy in these patients. These results, along with those for pancreatic fistulae (50.8%), however, fall within the range of studies reporting post-necrosectomy gastrointestinal and pancreatic fistula rates of 1%-43% and 3%-72%, respectively[47]. The use of minimally invasive surgery has also been associated with enteric fistulae. In their series of 5 patients who underwent minimally invasive retroperitoneal necrosectomy, Lakshmanan et al[24] reported a 40% pancreatic fistula rate, while Connor et al[23] reported a 17% pancreatic fistula rate in their 24 patients. These results support the idea that such complications could largely be dependent on the nature of the disease rather than the procedure employed to treat it (open vs laparoscopy).
In our study we found that as a result of delaying the procedure beyond the first 3 wk, we were able to perform only a single, but effective, exploration in the vast majority of patients. Our re-operation rate was 8.2%, unlike that reported in other studies (22%-79%) where semi-open and open techniques of debridement, as well as early surgery, was practised[8,17,48-50].
The ideal time for intervening, as well as the number of interventions, has been shown to play a significant role on the mortality rate[6,21], as was seen in our study. Previous studies have stressed the significance of delayed necrosectomy. However, the best time for intervention continues to be controversial, though most studies have set the ideal time to be after 2-3 wk[6,13,15,51-56].
The value of a single-staged procedure is that it helps to avoid the risk of bleeding and fistula formation, as seen in patients undergoing open packing or re-operations[11,50,57]. The incidence of systemic complications is greater in patients who undergo re-operations[49].
Finally, benefit was also seen when we compared the duration of hospital stay with other studies (23 d vs 30-93 d)[8,17,23,32,40,49].
Our series provides further evidence to support the role of delayed open necrosectomy for IPN. The results are comparable, if not better, than reported smaller series using minimally invasive techniques. The results indicate that a multi-pronged conservative strategy aimed at supporting the patient, with timely intervention, may actually reduce the need for further interventions, reducing not only morbidity but also mortality in these patients.
In conclusion, this series provides further support to the concept of delayed single-stage open pancreatic necrosectomy for IPN. Advances in critical care, effective antibiotic therapy with carbapenems, the availability of interventional radiology and good supportive care have played a complementary role to surgery in improving outcomes in IPN. Prophylactic ileostomy may be considered in patients with necrosis extending into the paracolic gutters.
COMMENTS
Background
Infected pancreatic necrosis (IPN) continues to have a high morbidity and mortality. Delayed necrosectomy has been shown to have reduced mortality. This paper illustrates a method of delayed necrosectomy performed in a single stage.
Research frontiers
The ideal technique of necrosectomy continues to be debated (open, endoscopic, laparoscopic). Reports regarding minimally invasive surgery for IPN are now being published. Thus, if we are to develop evidence-based guidelines for the management of IPN, rather than comparing outcomes with the relatively higher mortality encountered in some reports published a few decades ago, a more balanced comparison should particularly include results from larger series, and include some of the more recent series in which surgery for IPN has been complemented by advances in critical care, interventional radiology and broader spectrum antibiotics.
Innovations and breakthroughs
Previously described methods of necrosectomy have employed open packing, closed packing or post-operative lavage, which have high morbidity, cost and prolonged hospital stay. This single-stage delayed necrosectomy attempts to treat the patients in a single-stage approach, helping to reduce the hospital stay and morbidity.
Applications
This procedure helps to treat patients who have IPN with a high success rate, in addition to acceptable morbidity and low mortality. As it is carried out in delayed fashion, the necrotic debris has separated out and is well organized, leading to less intraoperative bleeding. This helps to pave the way for minimally invasive methods to further improve results.
Peer review
This is a well conducted retrospective study in agreement with the standard surgical approach to necrotic pancreatitis.
Acknowledgments
We wish to thank Professor John Windsor for healthy discussion.
Footnotes
Peer reviewer: Antonio Basoli, Professor, General Surgery “Paride Stefanini”, Università di Rome - Sapienza, Viale del Policlinico 155, Rome 00161, Italy
S- Editor Sun H L- Editor Logan S E- Editor Zheng XM
References
- 1.Barreto SG, Rodrigues J. Comparison of APACHE II and Imrie Scoring Systems in predicting the severity of Acute Pancreatitis. World J Emerg Surg. 2007;2:33. doi: 10.1186/1749-7922-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barreto SG, Rodrigues J. Acute pancreatitis in Goa--a hospital-based study. J Indian Med Assoc. 2008;106:575–576, 578. [PubMed] [Google Scholar]
- 3.Barreto SG, Carati CJ, Schloithe AC, Mathison R, Davison JS, Toouli J, Saccone GT. The efficacy of combining feG and galantide in mild caerulein-induced acute pancreatitis in mice. Peptides. 2010;31:1076–1082. doi: 10.1016/j.peptides.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Barreto SG, Carati CJ, Schloithe AC, Toouli J, Saccone GT. The combination of neurokinin-1 and galanin receptor antagonists ameliorates caerulein-induced acute pancreatitis in mice. Peptides. 2010;31:315–321. doi: 10.1016/j.peptides.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Lowenfels AB, Maisonneuve P, Sullivan T. The changing character of acute pancreatitis: epidemiology, etiology, and prognosis. Curr Gastroenterol Rep. 2009;11:97–103. doi: 10.1007/s11894-009-0016-4. [DOI] [PubMed] [Google Scholar]
- 6.Hartwig W, Maksan SM, Foitzik T, Schmidt J, Herfarth C, Klar E. Reduction in mortality with delayed surgical therapy of severe pancreatitis. J Gastrointest Surg. 2002;6:481–487. doi: 10.1016/s1091-255x(02)00008-2. [DOI] [PubMed] [Google Scholar]
- 7.McKay CJ, Evans S, Sinclair M, Carter CR, Imrie CW. High early mortality rate from acute pancreatitis in Scotland, 1984-1995. Br J Surg. 1999;86:1302–1305. doi: 10.1046/j.1365-2168.1999.01246.x. [DOI] [PubMed] [Google Scholar]
- 8.Ashley SW, Perez A, Pierce EA, Brooks DC, Moore FD Jr, Whang EE, Banks PA, Zinner MJ. Necrotizing pancreatitis: contemporary analysis of 99 consecutive cases. Ann Surg. 2001;234:572–579; discussion 579-580. doi: 10.1097/00000658-200110000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aultman DF, Bilton BD, Zibari GB, McMillan RW, McDonald JC. Nonoperative therapy for acute necrotizing pancreatitis. Am Surg. 1997;63:1114–1117; discussion 1117-1118. [PubMed] [Google Scholar]
- 10.Bradley EL 3rd, Allen K. A prospective longitudinal study of observation versus surgical intervention in the management of necrotizing pancreatitis. Am J Surg. 1991;161:19–24; discussion 24-25. doi: 10.1016/0002-9610(91)90355-h. [DOI] [PubMed] [Google Scholar]
- 11.Branum G, Galloway J, Hirchowitz W, Fendley M, Hunter J. Pancreatic necrosis: results of necrosectomy, packing, and ultimate closure over drains. Ann Surg. 1998;227:870–877. doi: 10.1097/00000658-199806000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Büchler MW, Gloor B, Müller CA, Friess H, Seiler CA, Uhl W. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann Surg. 2000;232:619–626. doi: 10.1097/00000658-200011000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernández-del Castillo C, Rattner DW, Makary MA, Mostafavi A, McGrath D, Warshaw AL. Débridement and closed packing for the treatment of necrotizing pancreatitis. Ann Surg. 1998;228:676–684. doi: 10.1097/00000658-199811000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isenmann R, Rau B, Beger HG. Early severe acute pancreatitis: characteristics of a new subgroup. Pancreas. 2001;22:274–278. doi: 10.1097/00006676-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Mier J, León EL, Castillo A, Robledo F, Blanco R. Early versus late necrosectomy in severe necrotizing pancreatitis. Am J Surg. 1997;173:71–75. doi: 10.1016/S0002-9610(96)00425-4. [DOI] [PubMed] [Google Scholar]
- 16.Mutinga M, Rosenbluth A, Tenner SM, Odze RR, Sica GT, Banks PA. Does mortality occur early or late in acute pancreatitis? Int J Pancreatol. 2000;28:91–95. doi: 10.1385/IJGC:28:2:091. [DOI] [PubMed] [Google Scholar]
- 17.Rau B, Bothe A, Beger HG. Surgical treatment of necrotizing pancreatitis by necrosectomy and closed lavage: changing patient characteristics and outcome in a 19-year, single-center series. Surgery. 2005;138:28–39. doi: 10.1016/j.surg.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Reddy M, Jindal R, Gupta R, Yadav TD, Wig JD. Outcome after pancreatic necrosectomy: trends over 12 years at an Indian centre. ANZ J Surg. 2006;76:704–709. doi: 10.1111/j.1445-2197.2006.03835.x. [DOI] [PubMed] [Google Scholar]
- 19.Reddy MS, Singh S, Singh R, Singh K, Singh G. Morphological and functional outcome after pancreatic necrosectomy and lesser sac lavage for necrotizing pancreatitis. Indian J Gastroenterol. 2007;26:217–220. [PubMed] [Google Scholar]
- 20.Tsiotos GG, Luque-de León E, Söreide JA, Bannon MP, Zietlow SP, Baerga-Varela Y, Sarr MG. Management of necrotizing pancreatitis by repeated operative necrosectomy using a zipper technique. Am J Surg. 1998;175:91–98. doi: 10.1016/s0002-9610(97)00277-8. [DOI] [PubMed] [Google Scholar]
- 21.van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491–1502. doi: 10.1056/NEJMoa0908821. [DOI] [PubMed] [Google Scholar]
- 22.Bucher P, Pugin F, Morel P. Minimally invasive necrosectomy for infected necrotizing pancreatitis. Pancreas. 2008;36:113–119. doi: 10.1097/MPA.0b013e3181514c9e. [DOI] [PubMed] [Google Scholar]
- 23.Connor S, Ghaneh P, Raraty M, Sutton R, Rosso E, Garvey CJ, Hughes ML, Evans JC, Rowlands P, Neoptolemos JP. Minimally invasive retroperitoneal pancreatic necrosectomy. Dig Surg. 2003;20:270–277. doi: 10.1159/000071184. [DOI] [PubMed] [Google Scholar]
- 24.Lakshmanan R, Iyer SG, Lee VT, Chang SK, Madhavan K. Minimally invasive retroperitoneal pancreatic necrosectomy in the management of infected pancreatitis. Surg Laparosc Endosc Percutan Tech. 2010;20:e11–e15. doi: 10.1097/SLE.0b013e3181c8f340. [DOI] [PubMed] [Google Scholar]
- 25.Sakorafas GH, Lappas C, Mastoraki A, Delis SG, Safioleas M. Current trends in the management of infected necrotizing pancreatitis. Infect Disord Drug Targets. 2010;10:9–14. doi: 10.2174/187152610790410936. [DOI] [PubMed] [Google Scholar]
- 26.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 27.Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 28.Røkke O, Harbitz TB, Liljedal J, Pettersen T, Fetvedt T, Heen LØ, Skreden K, Viste A. Early treatment of severe pancreatitis with imipenem: a prospective randomized clinical trial. Scand J Gastroenterol. 2007;42:771–776. doi: 10.1080/00365520601173855. [DOI] [PubMed] [Google Scholar]
- 29.Balthazar EJ, Ranson JH, Naidich DP, Megibow AJ, Caccavale R, Cooper MM. Acute pancreatitis: prognostic value of CT. Radiology. 1985;156:767–772. doi: 10.1148/radiology.156.3.4023241. [DOI] [PubMed] [Google Scholar]
- 30.Rau B, Pralle U, Uhl W, Schoenberg MH, Beger HG. Management of sterile necrosis in instances of severe acute pancreatitis. J Am Coll Surg. 1995;181:279–288. [PubMed] [Google Scholar]
- 31.Warshaw AL. Pancreatic necrosis: to debride or not to debride-that is the question. Ann Surg. 2000;232:627–629. doi: 10.1097/00000658-200011000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carter CR, McKay CJ, Imrie CW. Percutaneous necrosectomy and sinus tract endoscopy in the management of infected pancreatic necrosis: an initial experience. Ann Surg. 2000;232:175–180. doi: 10.1097/00000658-200008000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babu BI, Sheen AJ, Lee SH, O'Shea S, Eddleston JM, Siriwardena AK. Open pancreatic necrosectomy in the multidisciplinary management of postinflammatory necrosis. Ann Surg. 2010;251:783–786. doi: 10.1097/SLA.0b013e3181b59303. [DOI] [PubMed] [Google Scholar]
- 34.Mann DV, Hershman MJ, Hittinger R, Glazer G. Multicentre audit of death from acute pancreatitis. Br J Surg. 1994;81:890–893. doi: 10.1002/bjs.1800810632. [DOI] [PubMed] [Google Scholar]
- 35.Wilson C, Imrie CW, Carter DC. Fatal acute pancreatitis. Gut. 1988;29:782–788. doi: 10.1136/gut.29.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nathens AB, Curtis JR, Beale RJ, Cook DJ, Moreno RP, Romand JA, Skerrett SJ, Stapleton RD, Ware LB, Waldmann CS. Management of the critically ill patient with severe acute pancreatitis. Crit Care Med. 2004;32:2524–2536. doi: 10.1097/01.ccm.0000148222.09869.92. [DOI] [PubMed] [Google Scholar]
- 37.Manes G, Rabitti PG, Menchise A, Riccio E, Balzano A, Uomo G. Prophylaxis with meropenem of septic complications in acute pancreatitis: a randomized, controlled trial versus imipenem. Pancreas. 2003;27:e79–e83. doi: 10.1097/00006676-200311000-00018. [DOI] [PubMed] [Google Scholar]
- 38.Bassi C, Larvin M, Villatoro E. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2003:CD002941. doi: 10.1002/14651858.CD002941. [DOI] [PubMed] [Google Scholar]
- 39.Manes G, Uomo I, Menchise A, Rabitti PG, Ferrara EC, Uomo G. Timing of antibiotic prophylaxis in acute pancreatitis: a controlled randomized study with meropenem. Am J Gastroenterol. 2006;101:1348–1353. doi: 10.1111/j.1572-0241.2006.00567.x. [DOI] [PubMed] [Google Scholar]
- 40.Connor S, Alexakis N, Neal T, Raraty M, Ghaneh P, Evans J, Hughes M, Rowlands P, Garvey CJ, Sutton R, et al. Fungal infection but not type of bacterial infection is associated with a high mortality in primary and secondary infected pancreatic necrosis. Dig Surg. 2004;21:297–304. doi: 10.1159/000080884. [DOI] [PubMed] [Google Scholar]
- 41.Connor S, Alexakis N, Raraty MG, Ghaneh P, Evans J, Hughes M, Garvey CJ, Sutton R, Neoptolemos JP. Early and late complications after pancreatic necrosectomy. Surgery. 2005;137:499–505. doi: 10.1016/j.surg.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Barrreto G, Rodrigues J, Pinto RGW, Rodrigues S, Rodrigues MJ, Mallaya V, Dias A. Pancreatic abscess due to aspergillus fumigatus. J Cytol. 2005;22:191–193. [Google Scholar]
- 43.Kingsnorth A, O'Reilly D. Acute pancreatitis. BMJ. 2006;332:1072–1076. doi: 10.1136/bmj.332.7549.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Reilly DA, Kingsnorth AN. Management of acute pancreatitis. BMJ. 2004;328:968–969. doi: 10.1136/bmj.328.7446.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pezzilli R. Early antibiotic treatment in acute pancreatitis: more news. JOP. 2006;7:435–437. [PubMed] [Google Scholar]
- 46.Grewe M, Tsiotos GG, Luque de-Leon E, Sarr MG. Fungal infection in acute necrotizing pancreatitis. J Am Coll Surg. 1999;188:408–414. doi: 10.1016/s1072-7515(98)00334-2. [DOI] [PubMed] [Google Scholar]
- 47.Howard TJ, Wiebke EA, Mogavero G, Kopecky K, Baer JC, Sherman S, Hawes RH, Lehman GA, Goulet RJ, Madura JA. Classification and treatment of local septic complications in acute pancreatitis. Am J Surg. 1995;170:44–50. doi: 10.1016/s0002-9610(99)80250-5. [DOI] [PubMed] [Google Scholar]
- 48.Farkas G, Márton J, Mándi Y, Leindler L. Surgical management and complex treatment of infected pancreatic necrosis: 18-year experience at a single center. J Gastrointest Surg. 2006;10:278–285. doi: 10.1016/j.gassur.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Götzinger P, Sautner T, Kriwanek S, Beckerhinn P, Barlan M, Armbruster C, Wamser P, Függer R. Surgical treatment for severe acute pancreatitis: extent and surgical control of necrosis determine outcome. World J Surg. 2002;26:474–478. doi: 10.1007/s00268-001-0252-8. [DOI] [PubMed] [Google Scholar]
- 50.Lee VT, Chung AY, Chow PK, Thng CH, Low AS, Ooi LL, Wong WK. Infected pancreatic necrosis--an evaluation of the timing and technique of necrosectomy in a Southeast Asian population. Ann Acad Med Singapore. 2006;35:523–530. [PubMed] [Google Scholar]
- 51.Beger HG, Bittner R, Block S, Büchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986;91:433–438. doi: 10.1016/0016-5085(86)90579-2. [DOI] [PubMed] [Google Scholar]
- 52.Dionigi R, Rovera F, Dionigi G, Diurni M, Cuffari S. Infected pancreatic necrosis. Surg Infect (Larchmt) 2006;7 Suppl 2:S49–S52. doi: 10.1089/sur.2006.7.s2-49. [DOI] [PubMed] [Google Scholar]
- 53.Fernández-Cruz L, Navarro S, Valderrama R, Sáenz A, Guarner L, Aparisi L, Espi A, Jaurietta E, Marruecos L, Gener J. Acute necrotizing pancreatitis: a multicenter study. Hepatogastroenterology. 1994;41:185–189. [PubMed] [Google Scholar]
- 54.Heinrich S, Schäfer M, Rousson V, Clavien PA. Evidence-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg. 2006;243:154–168. doi: 10.1097/01.sla.0000197334.58374.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hungness ES, Robb BW, Seeskin C, Hasselgren PO, Luchette FA. Early debridement for necrotizing pancreatitis: is it worthwhile? J Am Coll Surg. 2002;194:740–744; discussion 744-745. doi: 10.1016/s1072-7515(02)01182-1. [DOI] [PubMed] [Google Scholar]
- 56.Kelly TR, Wagner DS. Gallstone pancreatitis: a prospective randomized trial of the timing of surgery. Surgery. 1988;104:600–605. [PubMed] [Google Scholar]
- 57.Rau B, Uhl W, Buchler MW, Beger HG. Surgical treatment of infected necrosis. World J Surg. 1997;21:155–161. doi: 10.1007/s002689900208. [DOI] [PubMed] [Google Scholar]


