Abstract
AIM: To assess the efficacy and toxicity of conformal radiotherapy (CRT) and compare with intensity-modulated radiotherapy (IMRT) in the treatment of gallbladder cancer.
METHODS: Between November 2003 and January 2010, 20 patients with gallbladder cancer were treated with CRT with or without chemotherapy after surgical resection. Preliminary survival data were collected and examined using both Kaplan-Meier and actuarial analysis. Demographic and treatment parameters were collected. All patients were planned to receive 46-56 Gy in 1.8 or 2.0 Gy per fraction. CRT planning was compared with IMRT.
RESULTS: The most common reported acute toxicities requiring medication (Radiation Therapy Oncology Group, Radiation Therapy Oncology Group Grade 2) were nausea (10/20 patients) and diarrhea (3/20). There were no treatment-related deaths. Compared with CRT planning, IMRT significantly reduced the volume of right kidney receiving > 20 Gy and the volume of liver receiving > 30 Gy. IMRT has a negligible impact on the volume of left kidney receiving > 20 Gy. The 95% of prescribed dose for a planning tumor volume using either 3D CRT or IMRT planning were 84.0% ± 6.7%, 82.9% ± 6.1%, respectively (P > 0.05).
CONCLUSION: IMRT achieves similar excellent target coverage as compared with CRT planning, while reducing the mean liver dose and volume above threshold dose. IMRT offers better sparing of the right kidney compared with CRT planning, with a significantly lower mean dose and volume above threshold dose.
Keywords: Gallbladder cancers, Adjuvant treatment, Surgery, Radiation therapy
INTRODUCTION
Gallbladder cancer is the fifth most common malignancy of the gastrointestinal tract[1]. In general, the prognosis of patients with gallbladder cancers is poor, with an overall 5-year survival rate of less than 10%[2]
Gallbladder cancer was associated with a uniformly poor prognosis due to its highly aggressive behavior, only 10%-30% of the patients had resectable tumors at presentation[3]. Better understanding of the biological behavior of the disease, its pattern of dissemination, better diagnostic tools, and more aggressive therapy has resulted in some improvement in survival in the last decade[4]. However, most long-term survivors are patients with incidentally diagnosed carcinomas confined to the mucosa of the gallbladder.
Surgical therapy is the standard treatment for patients presenting with resectable disease. Unfortunately, a large number of patients develop recurrent diseases after undergoing curative resection. The long-term outcome of the patients with recurrent gallbladder cancer is very poor. And local-regional failure is common and is a major cause of mortality[5]. Because of this, adjuvant therapy has been used to improve loco-regional control and survival rate. Several studies have reported improvement in survival for patients treated with adjuvant chemoradiation[6,7].
Actually, the definite role of adjuvant therapy after curative resection is still uncertain. Studies to improve the loco-regional control rates with adjuvant radiation with/without chemotherapy or chemotherapy alone, have been conducted. Although gallbladder cancer is considered to be radiation resistant, radiation has been administered in the form of external beam radiotherapy, intra-operative radiation therapy and brachytherapy[8-11]. However, the relative rarity of this malignant disease made it difficult to conduct large phase III studies to guide management in both the adjuvant and comprehensive treatment. This study reports a single-institution series that used adjuvant radiation therapy with or without chemotherapy after resection in patients with locally advanced gallbladder carcinoma. The aim of the study was to evaluate conformal radiotherapy (CRT) and intensity-modulated radiotherapy (IMRT) planning parameters in the treatment of this malignancy.
MATERIALS AND METHODS
Between November 2003 and January 2010, a total of 20 patients with pathologically diagnosed primary adenocarcinoma of gallbladder were treated in the Department of Radiation Oncology in our hospital. All patients were diagnosed with adenocarcinoma of gallbladder. All patients had complete resection, with negative microscopic margins. Demographic data were collected regarding patient age, gender, histological classification, tumor staging (Table 1). In addition to radiotherapy, the majority of patients received concurrent fluoropyrimidine-based and oxaliplatin-based chemotherapy. Concurrent and adjuvant chemotherapy regimens are shown in Table 2. Six patients received postoperative radiotherapy alone. No clear pattern of chemotherapy or standardized dosing regimen was evidenced from chart data available for review. All patients were simulated on a computed tomography-scanner (Siemens Definition AS 40) and were imaged using a slice of thickness 3.0 mm. All simulations were performed using a timed bolus of non-ionic intravenous contrast media to acquire images of early arterial/portal venous contrast phase, and a secondary venous contrast phase. Digital imaging and communications in medicine data were transferred to an inverse IMRT treatment planning station (Philips Pinnacle3 7.6C). Gross target volumes and clinical target volumes (CTVs) according to ICRU 62 definitions[12] were delineated on a slice-by-slice basis. External beam radiation therapy fields generally encompassed the tumor bed and regional lymph nodes (porta hepatis, celiac, pancreatico-duodenal) to a dose of 45 Gy in 1.8-2.0 Gy daily fractions. Reduced fields to tumor bed plus a 2-2.5 cm margin received an additional 5.0-10.0 Gy. A variety of multi-beam techniques were used to treat the tumor bed to a median total prescription dose of 52 Gy (range, 46-56 Gy). Organs-at-risk (OAR) delineated included the spinal cord, kidneys, and healthy liver. A planning tumor volume (PTV) was created by volumetric expansion of the CTV by 10-15 mm. Dose volume histograms (DVHs) were utilized to evaluate the plans. Prescribed doses to the initial PTV ranged from 46 to 56 Gy in daily doses of 1.8-2.0 Gy. Relative constraints included left kidneys constrained to a D100 of < 20 Gy and D66 < 18 Gy; right kidneys were specified to achieve D100 < 30 Gy, with D66 < 20 Gy. The total mean liver dose was specified to < 22 Gy, and liver V20 kept under 33%. Treatment was delivered using a 10 MV linear accelerator (Siemens Primus M) with 200 MU/min delivery capability using a multi-leaf collimator. Survival data were collected and examined using the Kaplan-Meier method.
Table 1.
Patient and tumor characteristics (n = 20)
| Characteristics | n (%) |
| Median age (range, yr) | 56 (33-73) |
| Gender | |
| Male | 8 (40) |
| Female | 12 (60) |
| ECOG performance status | |
| 0 | 2 (10) |
| 1 | 16 (80) |
| 2 | 2 (10) |
| Tumor and node status | |
| PT1 | 0 (0) |
| PT2 | 13 (65) |
| PT3 | 6 (30) |
| PT4 | 1 (5) |
| NX | |
| PN0 | 13 (65) |
| PN | 7 (35) |
| Tumor grade | |
| Well | 8 (40) |
| Moderate | 9 (45) |
| Poor | 3 (15) |
ECOG: Eastern Cooperative Oncology Group; NX: Node staging.
Table 2.
Treatment details for the patients
| Treatment | No. of patients |
| CT concurrent RT | 9 |
| 5-fluorouracil | 4 |
| Oxaliplatin | 5 |
| Concurrent RT followed by CT | 7 |
| 5-fluorouracil + oxaliplatin | 4 |
| Gemcitabine + oxaliplatin | 3 |
| RT followed by CT | 3 |
| 5-fluorouracil + oxaliplatin | 1 |
| Gemcitabine + oxaliplatin | 2 |
| Postoperative CT before RT | 2 |
| 5-fluorouracil bolus | |
| RT alone | 6 |
Nine patients received concurrent radiochemotherapy, 7 of them received adjuvant chemotherapy. CT: Chemotherapy; RT: Radiotherapy.
By a comparison of CRT and IMRT, all fields of the patients were coplanar. DVHs were obtained for the PTV, kidneys, liver and spinal cord. Acute toxicity was scored using the Radiation Therapy Oncology Group (RTOG) morbidity scoring criteria[13]. Dosimetric endpoints for the target and critical structures were compared using the two tailed paired t test.
RESULTS
Survival analysis
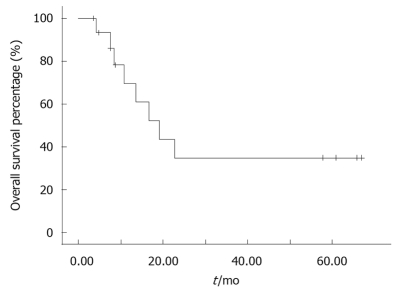
The median follow-up for patients alive at analysis was 14.0 mo (range, 3.0-66.9 mo). Nine patients were alive. The median preliminary survival from diagnosis in the 20 patients was 19.2 mo (range, 4.2-66.9 mo, 95% confidence interval: 10.1-28.3). Kaplan-Meier analysis revealed an estimated one-year survival rate of 40.48% (Figure 1).
Figure 1.
Overall survival for all the patients.
Toxicity analysis
Twenty patients completed their planned course of treatment without breaks and no reduction of planned chemotherapy. For gastrointestinal (GI) toxicity analysis, radiotherapy or chemotherapy with CRT was well tolerated without > grade 3 acute GI toxicity occurring during radiotherapy, and 11/20 and 4/20 patients reported grade 1 upper and lower acute RTOG GI toxicity scores. The most common reported acute toxicities requiring medication (RTOG Grade 2) were nausea (10/20 patients) and diarrhea (3/20). There were no treatment-related deaths.
Fourteen patients received chemotherapy (including concurrent chemotherapy), the major grade 3-4 adverse events were leukopenia (21%), neutropenia (29%) and anemia (14%). Compared with pre-treatment values, no abnormalities were detected in the laboratory test of kidney function for any of the 20 patients, either during treatment or at follow-up. Three patients had elevated liver enzymes about 6 mo after the completion of radiotherapy. No late toxicity was seen in this series.
Dosimetric comparison between 3D CRT and IMRT plans
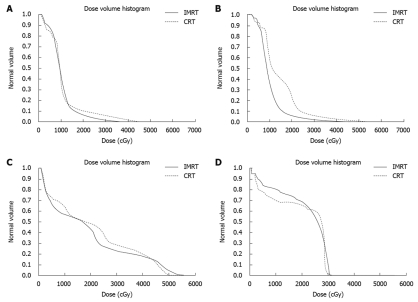
To demonstrate the differences in dose distribution, Figure 2 shows isodose curves on an axial slice for one representative patient for IMRT and CRT. Figure 3 shows the DVH curves for the kidneys and liver at risk for one representative patient. Table 3 summarizes the mean doses to the PTV, kidneys and liver for CRT and IMRT plans. Compared with the CRT plan, IMRT significantly reduced the mean dose to the right kidney and liver, while the improvement in the dose to the left kidney was not significant. Both of CRT and IMRT limited the dose to spinal cord under 40 Gy. Table 4 summarizes the volume of critical structures receiving greater than the threshold dose[14]. Compared with CRT planning, IMRT significantly reduced the volume of right kidney receiving > 20 Gy and the volume of liver receiving > 30 Gy. IMRT has a negligible impact on the volume of left kidney receiving > 20 Gy. The range of the 95% of prescribed dose for PTV using either 3DCRT or IMRT planning was 84.0% ± 6.7% and 82.9% ± 6.1%, respectively (P > 0.05).
Figure 2.
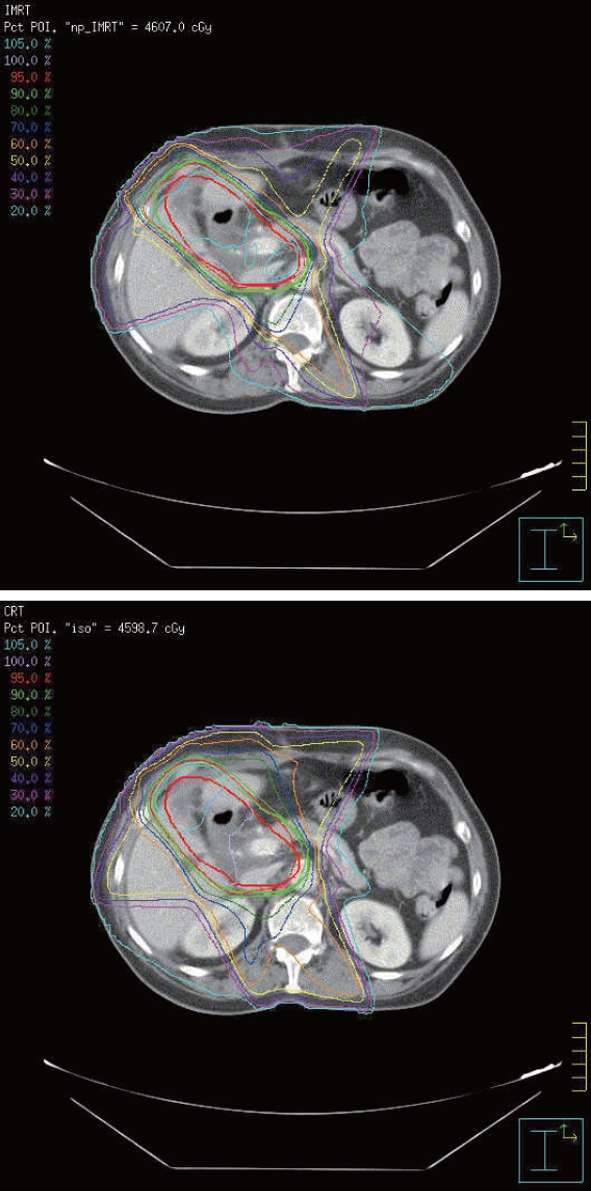
Isodose curves on an axial slice for a representative case. Intensity-modulated radiotherapy plan (upper picture) and conformal radiotherapy plan (lower picture).
Figure 3.
Dose volume histogram curves for the organs at risk in a representative case. Left kidney (A), right kidney (B), liver (C), and spinal cord (D). IMRT: Intensity-modulated radiation therapy; CRT: Conformal radiation therapy.
Table 3.
Mean dose to the targeted structures/organs
| Structure/organs |
Mean dose (Gy) |
P value1 | |
| CRT | IMRT | ||
| PTV | 50.1 ± 2.8 | 51.5 ± 2.7 | 0.00015 |
| Right kidney | 10.6 ± 4.4 | 8.6 ± 2.4 | 0.032 |
| Left kidney | 8.1 ± 3.4 | 9.4 ± 4.6 | NS |
| Liver | 22.4 ± 3.9 | 21.0 ± 2.9 | 0.027 |
Two-tailed paired t test. Values are expressed as mean ± SD. CRT: Three dimensional conformal radiation therapy; IMRT: Intensity-modulated radiation therapy; PTV: Planning tumor volume; NS: Not significant (P > 0.05).
Table 4.
Volume of structures/organs receiving greater than the threshold dose
| Structure/organs | Dose (Gy) |
Volume above threshold dose (%) |
P value1 | |
| CRT | IMRT | |||
| PTV | 95% of prescribed dose | 84.0 ± 6.7 | 82.9 ± 6.1 | NS |
| Organs at risk | ||||
| Right kidney | 20.0 | 12.5 ± 11.7 | 5.4 ± 2.7 | 0.031 |
| Left kidney | 20.0 | 5.3 ± 6.0 | 3.7 ± 2.3 | NS |
| Liver | 30.0 | 34.5 ± 8.0 | 30.1 ± 6.3 | 0.0065 |
Two-tailed paired t test. Values are expressed as mean ± SD. CRT: Three dimensional conformal radiation therapy; IMRT: Intensity-modulated radiation therapy; PTV: Planning tumor volume; NS: Not significant (P > 0.05).
DISCUSSION
Gallbladder cancer has a dismal prognosis and loco-regional recurrence has been described as the most frequent site of relapse, and death most commonly occurred due to complications and sequelae of loco-regional recurrence[15]. Hepatic infiltration has been reported in 60%-70% and nodal involvement in 20%-40% in some series[16,17]. The loco-regional pattern of recurrence after surgery provides a rationale for the use of radiotherapy as a component of gallbladder cancer treatment. The radiosensitive nature of gallbladder cancer is evidenced by numerous clinical studies reporting tumor size reduction after radiotherapy for unresectable diseases[18,19].
Our one-year survival rates and median preliminary survival time are similar to those reported in the limited radiotherapy literature and are generally better than those reported with surgery alone in patients with local advanced disease[20,21].
IMRT and automated optimization have the ability to shape isodose curves, avoiding the dose to the OARs. The use of direct machine parameter optimization IMRT improves the PTV coverage compared with CRT for most patients, although dose escalation was only possible in a minority of patients. It is suspected that the dosimetric improvement with IMRT was less in these plans compared with CRT plans. However, these complex CRT plans are challenging to develop without automated optimization, and IMRT might have planning efficiency and time-saving advantages for these cases.
Our data demonstrated that IMRT offers better sparing of the right kidney compared with CRT planning, with a significantly lower mean dose and volume above threshold dose. IMRT achieves similar excellent target coverage as compared with CRT, while reducing the mean liver dose and volume above threshold dose. In summary, IMRT offers improved sparing of normal structures, however, it warrants further studies in the treatment of gallbladder carcinoma.
In conclusion, gallbladder carcinoma is an aggressive disease with a dismal prognosis. More effective adjuvant therapy is needed to improve overall survival. There was a clear association between adjuvant therapy use and improved survival in patients with loco-regional disease. The real benefit of adjuvant radiotherapy in gallbladder carcinoma remains unclear. A retrospective analysis[21] was done about the surveillance, epidemiological, and end results survey by the American National Cancer Institute. The results showed that adjuvant radiotherapy is associated with improved survival in patients with locally advanced gallbladder cancer or gallbladder cancer with regional disease. Gallbladder cancer remains an aggressive disease that requires multimodality approach to individualize and optimize therapy. Prospective randomized trials of adjuvant therapy are needed in this disease. However, the low incidence of gallbladder cancer may make it difficult to successfully complete such trials, unless they are designed as inter-group studies within China or as international studies. In the future, methods of achieving earlier diagnoses may help improve the outcomes of the treatment. IMRT for dose escalation to improve tumor control and spare surrounding structure/organs from receiving radiation tolerance doses should be further studied.
COMMENTS
Background
Surgical therapy is the standard treatment for patients with resectable gallbladder cancer. Unfortunately, a large number of the patients develop recurrent disease despite curative resection. And local-regional failure is common and is a major cause of mortality. Because of this, adjuvant therapy has been used to improve loco-regional control and survival rate. Several studies have reported improvement for patients treated with adjuvant chemoradiation.
Research frontiers
Although gallbladder cancer is considered to be radiation resistant, radiation has been tried in the form of external beam radiotherapy, intra-operative radiation therapy and brachytherapy.
Innovations and breakthroughs
Intensity-modulated radiotherapy (IMRT) achieves similar excellent target coverage as compared with conformal radiotherapy (CRT) planning, while reducing the mean liver dose and volume above threshold dose. IMRT offers better sparing of the right kidney compared with CRT planning, with a significantly lower mean dose and volume above threshold dose.
Applications
The mainstay of treatment has been surgery and the role of adjuvant therapy in the form of chemotherapy and/or radiation therapy remains to be defined. Some clinical studies suggest that adjuvant radiotherapy dosage was associated with a better local control of the tumor. IMRT may offer better sparing of the right kidney and liver compared with CRT planning, this makes it possible for dose escalation to improve tumor control and spare surrounding structure/organs.
Terminology
IMRT: A type of three-dimensional radiation therapy that uses computer-generated images to match radiation to the size and shape of a tumor, which is used to deliver a higher radiation dose to a tumor with less damage to the nearby healthy tissues.
Peer review
The role of adjuvant radiotherapy in gallbladder cancer is not definite. This paper deals with a methodological comparison of CRT and IMRT for gallbladder cancer. IMRT offered better sparing of right kidney and liver compared with CRT. This result can help clinicians understand the role and toxicity of radiotherapy in gallblader cancer treatment.
Footnotes
Peer reviewer: Kyu Taek Lee, MD, PhD, Professor, Department of Medicine Samsung Medical Center, Sungkyunkwan, University School of Medicine, #50, Irwon-dong, Gangnam-gu, Seoul, 135-710, South Korea
S- Editor Sun H L- Editor Ma JY E- Editor Lin YP
References
- 1.Bartlett DL. Gallbladder cancer. Semin Surg Oncol. 2000;19:145–155. doi: 10.1002/1098-2388(200009)19:2<145::aid-ssu7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Lotze MT, Flickinger JC, Carr BI. Hepatobiliary Neoplasms. In: Devita VT, Helman S, Rosenburg SA, editors. Cancer: Principles and Practice of Oncology. 4th ed. Philadelphia, PA: Lippincott; 1993. pp. 883–914. [Google Scholar]
- 3.Mahe M, Stampfli C, Romestaing P, Salerno N, Gerard JP. Primary carcinoma of the gall-bladder: potential for external radiation therapy. Radiother Oncol. 1994;33:204–208. doi: 10.1016/0167-8140(94)90355-7. [DOI] [PubMed] [Google Scholar]
- 4.Rajagopalan V, Daines WP, Grossbard ML, Kozuch P. Gallbladder and biliary tract carcinoma: A comprehensive update, Part 1. Oncology (Williston Park) 2004;18:889–896. [PubMed] [Google Scholar]
- 5.Maibenco DC, Smith JL, Nava HR, Petrelli NJ, Douglass HO Jr. Carcinoma of the gallbladder. Cancer Invest. 1998;16:33–39. doi: 10.3109/07357909809039751. [DOI] [PubMed] [Google Scholar]
- 6.Kresl JJ, Schild SE, Henning GT, Gunderson LL, Donohue J, Pitot H, Haddock MG, Nagorney D. Adjuvant external beam radiation therapy with concurrent chemotherapy in the management of gallbladder carcinoma. Int J Radiat Oncol Biol Phys. 2002;52:167–175. doi: 10.1016/s0360-3016(01)01764-3. [DOI] [PubMed] [Google Scholar]
- 7.Chou RH, Lee CG, Anscher MS. Radiation therapy for disease of the biliary tree and gallbladder. In: Clavien PA, Baille J, editors. Diseases of the gallbladder and bile ducts: Diagnosis and treatment. Malden, MA: Blackwell Science; 2001. pp. 126–139. [Google Scholar]
- 8.Houry S, Schlienger M, Huguier M, Lacaine F, Penne F, Laugier A. Gallbladder carcinoma: role of radiation therapy. Br J Surg. 1989;76:448–450. doi: 10.1002/bjs.1800760508. [DOI] [PubMed] [Google Scholar]
- 9.Flickinger JC, Epstein AH, Iwatsuki S, Carr BI, Starzl TE. Radiation therapy for primary carcinoma of the extrahepatic biliary system. An analysis of 63 cases. Cancer. 1991;68:289–294. doi: 10.1002/1097-0142(19910715)68:2<289::aid-cncr2820680213>3.0.co;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin HS, Seong J, Kim WC, Lee HS, Moon SR, Lee IJ, Lee KK, Park KR, Suh CO, Kim GE. Combination of external beam irradiation and high-dose-rate intraluminal brachytherapy for inoperable carcinoma of the extrahepatic bile ducts. Int J Radiat Oncol Biol Phys. 2003;57:105–112. doi: 10.1016/s0360-3016(03)00410-3. [DOI] [PubMed] [Google Scholar]
- 11.Morganti AG, Trodella L, Valentini V, Montemaggi P, Costamagna G, Smaniotto D, Luzi S, Ziccarelli P, Macchia G, Perri V, et al. Combined modality treatment in unresectable extrahepatic biliary carcinoma. Int J Radiat Oncol Biol Phys. 2000;46:913–919. doi: 10.1016/s0360-3016(99)00487-3. [DOI] [PubMed] [Google Scholar]
- 12.Purdy JA. Current ICRU definitions of volumes: limitations and future directions. Semin Radiat Oncol. 2004;14:27–40. doi: 10.1053/j.semradonc.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, Gunderson L, McCormick B, Morrisintegral M, Rich T, et al. Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:13–47. doi: 10.1016/s0360-3016(99)00559-3. [DOI] [PubMed] [Google Scholar]
- 14.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 15.Itoh H, Nishijima K, Kurosaka Y, Takegawa S, Kiriyama M, Dohba S, Kojima Y, Saitoh Y. Magnitude of combination therapy of radical resection and external beam radiotherapy for patients with carcinomas of the extrahepatic bile duct and gallbladder. Dig Dis Sci. 2005;50:2231–2242. doi: 10.1007/s10620-005-3040-8. [DOI] [PubMed] [Google Scholar]
- 16.Czito BG, Hurwitz HI, Clough RW, Tyler DS, Morse MA, Clary BM, Pappas TN, Fernando NH, Willett CG. Adjuvant external-beam radiotherapy with concurrent chemotherapy after resection of primary gallbladder carcinoma: a 23-year experience. Int J Radiat Oncol Biol Phys. 2005;62:1030–1034. doi: 10.1016/j.ijrobp.2004.12.059. [DOI] [PubMed] [Google Scholar]
- 17.Sons HU, Borchard F, Joel BS. Carcinoma of the gallbladder: autopsy findings in 287 cases and review of the literature. J Surg Oncol. 1985;28:199–206. doi: 10.1002/jso.2930280311. [DOI] [PubMed] [Google Scholar]
- 18.Smoron GL. Radiation therapy of carcinoma of gallbladder and biliary tract. Cancer. 1977;40:1422–1424. doi: 10.1002/1097-0142(197710)40:4<1422::aid-cncr2820400410>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Buskirk SJ, Gunderson LL, Adson MA, Martinez A, May GR, McIlrath DC, Nagorney DM, Edmundson GK, Bender CE, Martin JK Jr. Analysis of failure following curative irradiation of gallbladder and extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1984;10:2013–2023. doi: 10.1016/0360-3016(84)90198-6. [DOI] [PubMed] [Google Scholar]
- 20.Wang SJ, Fuller CD, Kim JS, Sittig DF, Thomas CR Jr, Ravdin PM. Prediction model for estimating the survival benefit of adjuvant radiotherapy for gallbladder cancer. J Clin Oncol. 2008;26:2112–2117. doi: 10.1200/JCO.2007.14.7934. [DOI] [PubMed] [Google Scholar]
- 21.Mojica P, Smith D, Ellenhorn J. Adjuvant radiation therapy is associated with improved survival for gallbladder carcinoma with regional metastatic disease. J Surg Oncol. 2007;96:8–13. doi: 10.1002/jso.20831. [DOI] [PubMed] [Google Scholar]