Abstract
Neurotrophic factors are critical regulators of the formation and plasticity of neuronal networks. Brain-derived neurotrophic factor (BDNF) is abundant in the brain and periphery, and is found in both human serum and plasma. Animal studies have demonstrated that stress reduces BDNF expression or activity in the hippocampus and that this reduction can be prevented by treatment with antidepressant drugs. A similar change in BDNF activity occurs in the brain of patients with major depression disorder (MDD). Recently, clinical studies have indicated that serum or plasma BDNF levels are decreased in untreated MDD patients. Antidepressant treatment for at least four weeks can restore the decreased BDNF function up to the normal value. Therefore, MDD is associated with impaired neuronal plasticity. Suicidal behavior can be a consequence of severe impaired neuronal plasticity in the brain. Antidepressant treatment promotes increased BDNF activity as well as several forms of neuronal plasticity, including neurogenesis, synaptogenesis and neuronal maturation. BDNF could also play an important role in the modulation of neuronal networks. Such a neuronal plastic change can positively influence mood or recover depressed mood. These alterations of BDNF levels or neuronal plasticity in MDD patients before and after antidepressant treatment can be measured through the examination of serum or plasma BDNF concentrations. BDNF levels can therefore be useful markers for clinical response or improvement of depressive symptoms, but they are not diagnostic markers of major depression.
Keywords: Brain-derived neurotrophic factor, Neuroplasticity, Depression, Antidepressant
INTRODUCTION
Major depressive disorder (MDD) is a common psychiatric mood disorder; mood disorders are episodic illnesses. MDDs consist of a single episode or several instances of recurrent or relapsed episodes of major depression. The pathophysiology of major depression can involve reversible changes. Recent reports have proposed that major depressive episodes and any subsequen recovery are associated with neuronal plasticity. Preclinical and clinical studies demonstrate that reductions of the total volume of neurons and neuronal loss occur in stress and depression in the adult hippocampus.1 These hippocampal alterations can be reversed by chronic antidepressant treatment.1 The associated neuronal plasticity involves actions of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF).
BDNF is an important neurotrophic factor. Its function is mediated by its binding to specific receptors, such as the TrkB receptor among the tropomycin receptor kinase (Trk) family of tyrosine kinase receptors and the pan75 neurotrophin receptor (p75NTR). Neurotrophic factors act in the activity-dependent manner of a neuronal network. BDNF expression is closely regulated by neuronal activity.2 Localization of the TrkB receptor also increases at synaptic sites after neuronal activity.3 p75NTR is a low-affinity receptor of BDNF, and it can mediate neuronal apoptosis only when the Trk receptor is less active or not active.4
The neurotrophic hypothesis of depression proposes that depression is associated with reduced brain BDNF levels and that antidepressant treatments alleviate depressive behavior and increase BDNF levels.5 This alteration was recently explained as activity-dependent neuronal plasticity.6 In this study, we focused on recent findings regarding the role of BDNF in the occurrence and improvement of MDD and suicide, and we summarized findings of blood cell studies of BDNF.
BDNF AND STRESS IN ANIMAL STUDIES
Animal studies have shown that BDNF expression is dysregulated by stress. Several types of stressors, including immobilization stress, foot shocks, social defeat, and early maternal deprivation, significantly decrease BDNF expression in the hippocampus, especially in the dentate gyrus.7-11 In particular, stressors such as forced swimming reduced BDNF messenger RNA (mRNA) in the hippocampus, and the physical activity-antidepressant treatment combinations enhanced swimming time and increased BDNF mRNA in an animal model.12
Several studies have found that exogenous corticosterone treatment reduces hippocampal BDNF expression.13,14 In contrast, adrenalectomy increases the level of BDNF in the hippocampus.15 These findings indicate that hippocampal BDNF expression is regulated via glucocorticoids. Moreover, antidepressant treatments can prevent stress-induced reduction of BDNF,16 and they can restore the corticosterone-mediated decrease in BDNF expression.14 Therefore, stressors stimulate the activity of the hypothalamic-pituitary-adrenal axis, and then glucocorticoids increase, which can reduce the activity of BDNF.
BDNF AND ANTIDEPRESSANT TREATMENT IN ANIMAL STUDIES
Several classes of antidepressants, including monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), tricyclic agents (TCAs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and noradrenergic and specific serotonergic antidepressants, increase BDNF expression in the brain when given to healthy rodents.17 Electroconvulsive shock (ECS) and transcranial magnetic stimulation also increase the expression of BDNF in the rodent brain.18,19 Another experiment investigated the effects of acute and chronic treatment with different antidepressant agents and ECS on protein levels of BDNF in several brain regions of the rat.20 Chronic (21 days) but not acute (1 day) antidepressant treatment with TCAs, SSRIs and MAOIs increased BDNF levels in the frontal cortex by 10-30%. Chronic MAOI use increased BDNF to a greater extent than treatment with the other classes of agents. Repeated daily treatments (10 days) of ECS enhanced BDNF levels in the hippocampus and frontal cortex by 40-100%, but one ECS treatment (1 day) was not as effective.20 However, the effect of ECS was higher in magnitude compared to the effects of several antidepressant drugs. Various factors that influence this antidepressant effect on BDNF include length of administration, route of administration, class of antidepressant, and doses of the drugs. In general, increases in BDNF expression appear only after long-term treatment with antidepressants.21-23
Chronic antidepressant administration may induce plastic changes in the forebrains of rodents.17 Antidepressant treatments can also increase neurogenesis and synaptogenesis in the hippocampus.1,24 Chronic antidepressant treatment additionally increases the expression of plasticity-related proteins, including phosphorylated the cyclic adenosine monophosphate response element binding protein (CREB) and polysialylated neural cell adhesion molecules, in the hippocampus and prefrontal cortex.25 These findings indicate that antidepressant treatment can increase neuronal plasticity in the brain.
Antidepressant treatment can also modulate chromatin remodeling, which regulates the activity of gene transcription. When histone subunits surround chromosomal DNA, methylation of histone subunits reduces gene transcription, whereas their acetylation enhances transcription. Antidepressant treatment can induce acetylation of histone subunits around the BDNF gene promoter region and lead to increased BDNF gene transcription and increased BDNF production.16
Wild-type mice that were given antidepressant agents experienced increased phosphorylation of CREB as well as phosphorylation of TrkB in the brain.26 When antidepressants were given to BDNF-deficient mice or Trk-defective mice, activations of CREB and Trk were reduced in BDNF-deficient or Trk-defective mice. In addition, the behavioral effects of antidepressants did not occur in BDNF-deficient mice or Trk-defective mice.26,27 BDNF-deficient mice have 50% lower forebrain BDNF mRNA and protein levels than the wild type mice.28 However, BDNF-deficient mice do not have reduced activity or response to the forced swim test compared to the wild-type mice.29 Trk-defective mice also do not exhibit depression-like behaviors.30 Therefore, a deficiency or dysfunction of BDNF or the Trk receptor cannot induce depressed mood or behavior; BDNF itself does not control mood. However, the antidepressant response clearly requires an increase of BDNF activity and recovery of the neuronal network. A neuronal plastic change could positively influence mood or recover depressed mood. BDNF could also play an important role in the modulation of neuronal networks.
BDNF AND MAJOR DEPRESSION IN CLINICAL STUDIES
Previous human postmortem studies showed decreased BDNF expression or CREB immunoreactivity in the brains of participants with major depression who had not been treated with antidepressants, but those who were treated with antidepressant drugs had increased BDNF expression and CREB in the brain.31,32 These data are consistent with those of the aforementioned animal studies.
Recent clinical studies have explored serum or plasma BDNF levels in patients with major depression. Though the source of the circulating BDNF is not clear, BDNF is found in both human serum and plasma, and a large amount of the circulating BDNF is stored in human platelets as well.33 BDNF can cross the blood-brain barrier in both directions, and the circulating BDNF could originate from neurons of the brain.34,35 Some clinical studies that measured serum BDNF levels in drug-free MDD patients have shown that serum BDNF levels are significantly lower in MDD patients than in healthy participants.36-39 Other studies reported that plasma BDNF levels are lower in drug-free MDD patients.38,40 However, there have been inconsistent findings of the relationship between BDNF levels and the severity of depression from these data. Some studies suggested a more significant decrease of BDNF in more severe depression. Though our data did not demonstrate any relationship between BDNF and depression severity, we found that relapsed or recurrent-episode MDD patients had much lower plasma BDNF levels than first-onset ones.40
Many clinical studies have evaluated the changes of plasma or serum BDNF levels before and after antidepressant treatments among MDD patients. Most studies reported increases of BDNF levels after antidepressant treatment.41 Two studies observed that both SSRI and SNRI treatments for eight weeks increased serum BDNF levels in MDD patients.36,42 However, another study reported that SSRI agents increased serum BDNF levels after six months, but that the SNRI agent did not change the level of BDNF even after six months of treatment.43 Additional clinical reports found that plasma and serum BDNF levels in MDD patients increased after six or eight weeks of antidepressant drug treatments.42,44 These studies suggest that the antidepressant-induced increase in BDNF level is more prominent in the responders to treatment rather than the non-responders.42,44 A meta-analysis indicated that post-treatment BDNF levels may be useful since four to eight weeks of antidepressant treatment are recommended to evaluate a change of BDNF or a treatment effect.41 Another recent study investigated changes of serum and plasma from MDD patients at baseline and following the first, third, sixth, and twelfth month of antidepressant treatment.45 MDD patients had lower serum and plasma BDNF levels before treatment. Plasma BDNF increased in parallel with the clinical improvement from the one-month evaluation, while serum BDNF had no change after treatments.
These data of clinical studies of antidepressant treatment are consistent with those of animal studies on the effects of antidepressants on BDNF changes. Therefore, it is possible that serum or plasma levels of BDNF reflect the state of the neuronal network in patients with major depression. MDD patients can have decreased BDNF levels before treatment, which can be restored to the normal level through antidepressant treatment or by improving neuronal plasticity.
BDNF AND SUICIDAL BEHAVIOR
Postmortem studies show that mRNA expression and protein levels of BDNF are reduced in the brains of patients with major depression who commit suicide.46,47 The expression of full-length TrkB or truncated TrkB (TrkB.T1) is significantly decreased in the brains of suicide patients in postmortem studies.47,48 A recent study observed decreased phosphorylation of Trk receptors and increased expression ratios of p75NTR to Trks in the suicide patient's brain.49 These changes were particularly apparent in the prefrontal cortex and the hippocampus.
Clinical studies have examined BDNF levels in serum or plasma of MDD patients who have or have not attempted suicide. MDD patients who attempted suicide had lower serum BDNF levels than healthy controls.50 Our study found that plasma BDNF was significantly lower in suicidal MDD patients than in non-suicidal ones.40,51 Moreover, suicidal patients had the lowest levels of BDNF of all participants.40 We failed to find any correlation between BDNF level and the lethality of suicide.51 Dawood et al.52 took direct blood samples from the internal jugular vein and the brachial artery and then defined the veno-arterial BDNF plasma concentration gradient as an index of brain BDNF production. Their data showed a significantly decreased veno-arterial BDNF concentration gradient in patients at higher suicide risk among MDD participants.
These changes of serum or plasma BDNF levels in suicide patients are consistent with BDNF changes of the brain in postmortem studies on patients who died following suicide. Therefore, the phenomenon of suicidal behavior could be a consequence of a severe dysfunction of the neuronal network or of severely impaired neuronal plasticity in the brain compared to MDD patients.
CONCLUSIONS
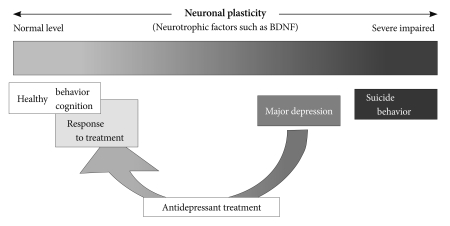
BDNF is involved in activity-dependent neuronal plasticity, such as learning and memory.53 Although animal studies clearly demonstrate that a decline of BDNF does not produce depressed mood or behavior, evidence from clinical studies tells us that decreased activity of BDNF or a neuronal dysfunction occurs in the brain of patients with major depression. Major depression is associated with impaired neuronal plasticity. Suicidal behavior can be a consequence of severely impaired neuronal plasticity in the brain. Antidepressant treatments promote several forms of neuronal plasticity, including neurogenesis, synaptogenesis and neuronal maturation and also increase BDNF activity, which can develop the antidepressant response. Figure 1 represents these effects of neuronal plasticity or BDNF on major depression, antidepressant treatment, and suicide behavior. BDNF could play an important role in the modulation of neuronal networks. Such neuronal plastic change can positively influence mood or recover depressed mood. These alterations of BDNF levels or neuronal plasticity in MDD patients before and after antidepressant treatment can be measured through serum or plasma BDNF concentrations. BDNF levels in serum or plasma will be useful markers for clinical response or improvement of depressive symptoms rather than a diagnostic marker of major depression.
Figure 1.
The neuronal plasticity in major depression, antidepressant treatment, and suicide behavior. Major depression is associated with impaired neuronal plasticity in the brain. Suicide behavior can be a consequence of very severe impaired neuronal plasticity. Antidepressant treatments promote several forms of neuronal plasticity, including neurogenesis, synaptogenesis and neuronal maturation together with increasing brain-derived neurotrophic factor activity, which can develop the antidepressant response. The neuronal plastic change can influence mood or recover depressed mood.
References
- 1.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 2.Mellstrom B, Torres B, Link WA, Naranjo JR. The BDNF gene: exemplifying complexity in Ca2+-dependent gene expression. Crit Rev Neurobiol. 2004;16:43–49. doi: 10.1615/critrevneurobiol.v16.i12.40. [DOI] [PubMed] [Google Scholar]
- 3.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 4.Miller FD, Kaplan DR. Neurotrophin signaling pathways regulating neuronal apoptosis. Cell Mol Life Sci. 2001;58:1045–1053. doi: 10.1007/PL00000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duman RS. Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry. 2002;17(Suppl 3):306–310. doi: 10.1016/s0924-9338(02)00654-5. [DOI] [PubMed] [Google Scholar]
- 6.Castrén E, Võikar V, Rantamäki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueyama T, Kawai Y, Nemoto K, Sekimoto M, Toné S, Senba E. Immobilization stress reduced the expression of neurotrophins and their receptors in the rat brain. Neurosci Res. 1997;28:103–110. doi: 10.1016/s0168-0102(97)00030-8. [DOI] [PubMed] [Google Scholar]
- 9.Rasmusson AM, Shi L, Duman R. Downregulation of BDNF mRNA in the hippocampal dentate gyrus after re-exposure to cues previously associated with footshock. Neuropsychopharmacology. 2002;27:133–142. doi: 10.1016/S0893-133X(02)00286-5. [DOI] [PubMed] [Google Scholar]
- 10.Pizarro JM, Lumley LA, Medina W, Robison CL, Chang WE, Alagappan A, et al. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Res. 2004;1025:10–20. doi: 10.1016/j.brainres.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 11.Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Mol Psychiatry. 2002;7:609–616. doi: 10.1038/sj.mp.4001036. [DOI] [PubMed] [Google Scholar]
- 12.Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP. Physical activity-antidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behav Brain Res. 2001;120:87–95. doi: 10.1016/s0166-4328(00)00364-8. [DOI] [PubMed] [Google Scholar]
- 13.Schaaf MJ, de Jong J, de Kloet ER, Vreugdenhil E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813:112–120. doi: 10.1016/s0006-8993(98)01010-5. [DOI] [PubMed] [Google Scholar]
- 14.Dwivedi Y, Rizavi HS, Pandey GN. Antidepressants reverse corticosterone-mediated decrease in BDNF expression: dissociation in regulation of specific exons by antidepressants and corticosterone. Neuroscience. 2006;139:1017–1029. doi: 10.1016/j.neuroscience.2005.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao HM, Sakai RR, Ma LY, McEwen BS. Adrenal steroid regulation of neurotrophic factor expression in the rat hippocampus. Endocrinology. 1998;139:3112–3118. doi: 10.1210/endo.139.7.6114. [DOI] [PubMed] [Google Scholar]
- 16.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 17.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Müller MB, Toschi N, Kresse AE, Post A, Keck ME. Long-term repetitive transcranial magnetic stimulation increases the expression of brain-derived neurotrophic factor and cholecystokinin mRNA, but not neuropeptide tyrosine mRNA in specific areas of rat brain. Neuropsychopharmacology. 2000;23:205–215. doi: 10.1016/S0893-133X(00)00099-3. [DOI] [PubMed] [Google Scholar]
- 19.Altar CA, Laeng P, Jurata LW, Brockman JA, Lemire A, Bullard J, et al. Electroconvulsive seizures regulate gene expression of distinct neurotrophic signaling pathways. J Neurosci. 2004;24:2667–2677. doi: 10.1523/JNEUROSCI.5377-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balu DT, Hoshaw BA, Malberg JE, Rosenzweig-Lipson S, Schechter LE, Lucki I. Differential regulation of central BDNF protein levels by antidepressant and non-antidepressant drug treatments. Brain Res. 2008;1211:37–43. doi: 10.1016/j.brainres.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coppell AL, Pei Q, Zetterström TS. Bi-phasic change in BDNF gene expression following antidepressant drug treatment. Neuropharmacology. 2003;44:903–910. doi: 10.1016/s0028-3908(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 22.De Foubert G, Carney SL, Robinson CS, Destexhe EJ, Tomlinson R, Hicks CA, et al. Fluoxetine-induced change in rat brain expression of brain-derived neurotrophic factor varies depending on length of treatment. Neuroscience. 2004;128:597–604. doi: 10.1016/j.neuroscience.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 23.Altieri M, Marini F, Arban R, Vitulli G, Jansson BO. Expression analysis of brain-derived neurotrophic factor (BDNF) mRNA isoforms after chronic and acute antidepressant treatment. Brain Res. 2004;1000:148–155. doi: 10.1016/j.brainres.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 24.Hajszan T, MacLusky NJ, Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur J Neurosci. 2005;21:1299–1303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- 25.Sairanen M, O'Leary OF, Knuuttila JE, Castrén E. Chronic antidepressant treatment selectively increases expression of plasticity-related proteins in the hippocampus and medial prefrontal cortex of the rat. Neuroscience. 2007;144:368–374. doi: 10.1016/j.neuroscience.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 26.Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altar CA, Whitehead RE, Chen R, Wörtwein G, Madsen TM. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry. 2003;54:703–709. doi: 10.1016/s0006-3223(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 29.MacQueen GM, Ramakrishnan K, Croll SD, Siuciak JA, Yu G, Young LT, et al. Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav Neurosci. 2001;115:1145–1153. doi: 10.1037//0735-7044.115.5.1145. [DOI] [PubMed] [Google Scholar]
- 30.Zörner B, Wolfer DP, Brandis D, Kretz O, Zacher C, Madani R, et al. Forebrain-specific trkB-receptor knockout mice: behaviorally more hyperactive than "depressive". Biol Psychiatry. 2003;54:972–982. doi: 10.1016/s0006-3223(03)00418-9. [DOI] [PubMed] [Google Scholar]
- 31.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 32.Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased temporal cortex CREB concentrations and antidepressant treatment in major depression. Lancet. 1998;352:1754–1755. doi: 10.1016/S0140-6736(05)79827-5. [DOI] [PubMed] [Google Scholar]
- 33.Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87:728–734. [PubMed] [Google Scholar]
- 34.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 35.Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res Mol Brain Res. 1996;36:280–286. doi: 10.1016/0169-328x(95)00250-v. [DOI] [PubMed] [Google Scholar]
- 36.Gonul AS, Akdeniz F, Taneli F, Donat O, Eker C, Vahip S. Effect of treatment on serum brain-derived neurotrophic factor levels in depressed patients. Eur Arch Psychiatry Clin Neurosci. 2005;255:381–386. doi: 10.1007/s00406-005-0578-6. [DOI] [PubMed] [Google Scholar]
- 37.Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 38.Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry. 2005;57:1068–1072. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 40.Lee BH, Kim H, Park SH, Kim YK. Decreased plasma BDNF level in depressive patients. J Affect Disord. 2007;101:239–244. doi: 10.1016/j.jad.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. 2008;11:1169–1180. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimura R, Mitoma M, Sugita A, Hori H, Okamoto T, Umene W, et al. Effects of paroxetine or milnacipran on serum brain-derived neurotrophic factor in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1034–1037. doi: 10.1016/j.pnpbp.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Matrisciano F, Bonaccorso S, Ricciardi A, Scaccianoce S, Panaccione I, Wang L, et al. Changes in BDNF serum levels in patients with depression disorder (MDD) after 6 months treatment with sertraline, escitalopram, or venlafaxine. J Psychiatr Res. 2009;43:247–254. doi: 10.1016/j.jpsychires.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee HY, Kim YK. Plasma brain-derived neurotrophic factor as a peripheral marker for the action mechanism of antidepressants. Neuropsychobiology. 2008;57:194–199. doi: 10.1159/000149817. [DOI] [PubMed] [Google Scholar]
- 45.Piccinni A, Marazziti D, Catena M, Domenici L, Del Debbio A, Bianchi C, et al. Plasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatments. J Affect Disord. 2008;105:279–283. doi: 10.1016/j.jad.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 47.Pandey GN, Ren X, Rizavi HS, Conley RR, Roberts RC, Dwivedi Y. Brain-derived neurotrophic factor and tyrosine kinase B receptor signalling in post-mortem brain of teenage suicide victims. Int J Neuropsychopharmacol. 2008;11:1047–1061. doi: 10.1017/S1461145708009000. [DOI] [PubMed] [Google Scholar]
- 48.Ernst C, Deleva V, Deng X, Sequeira A, Pomarenski A, Klempan T, et al. Alternative splicing, methylation state, and expression profile of tropomyosin-related kinase B in the frontal cortex of suicide completers. Arch Gen Psychiatry. 2009;66:22–32. doi: 10.1001/archpsyc.66.1.22. [DOI] [PubMed] [Google Scholar]
- 49.Dwivedi Y, Rizavi HS, Zhang H, Mondal AC, Roberts RC, Conley RR, et al. Neurotrophin receptor activation and expression in human postmortem brain: effect of suicide. Biol Psychiatry. 2009;65:319–328. doi: 10.1016/j.biopsych.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deveci A, Aydemir O, Taskin O, Taneli F, Esen-Danaci A. Serum BDNF levels in suicide attempters related to psychosocial stressors: a comparative study with depression. Neuropsychobiology. 2007;56:93–97. doi: 10.1159/000111539. [DOI] [PubMed] [Google Scholar]
- 51.Kim YK, Lee HP, Won SD, Park EY, Lee HY, Lee BH, et al. Low plasma BDNF is associated with suicidal behavior in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:78–85. doi: 10.1016/j.pnpbp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 52.Dawood T, Anderson J, Barton D, Lambert E, Esler M, Hotchkin E, et al. Reduced overflow of BDNF from the brain is linked with suicide risk in depressive illness. Mol Psychiatry. 2007;12:981–983. doi: 10.1038/sj.mp.4002059. [DOI] [PubMed] [Google Scholar]
- 53.Malcangio M, Lessmann V. A common thread for pain and memory synapses? Brain-derived neurotrophic factor and trkB receptors. Trends Pharmacol Sci. 2003;24:116–121. doi: 10.1016/S0165-6147(03)00025-7. [DOI] [PubMed] [Google Scholar]