Abstract
The problem as to whether iodohistidines are normally biosynthetized in thyroglobulin and thyralbumin has been examined both in man and the rat. Evidence has been obtained for the first time that diiodohistidine (DIH) is present in both species in these two iodoproteins. The biosynthesis of monoiodohistidine (MIH) in the thyroglobulin of the normal rat has been confirmed and extended to rat thyralbumin and to human thyroid iodoproteins.
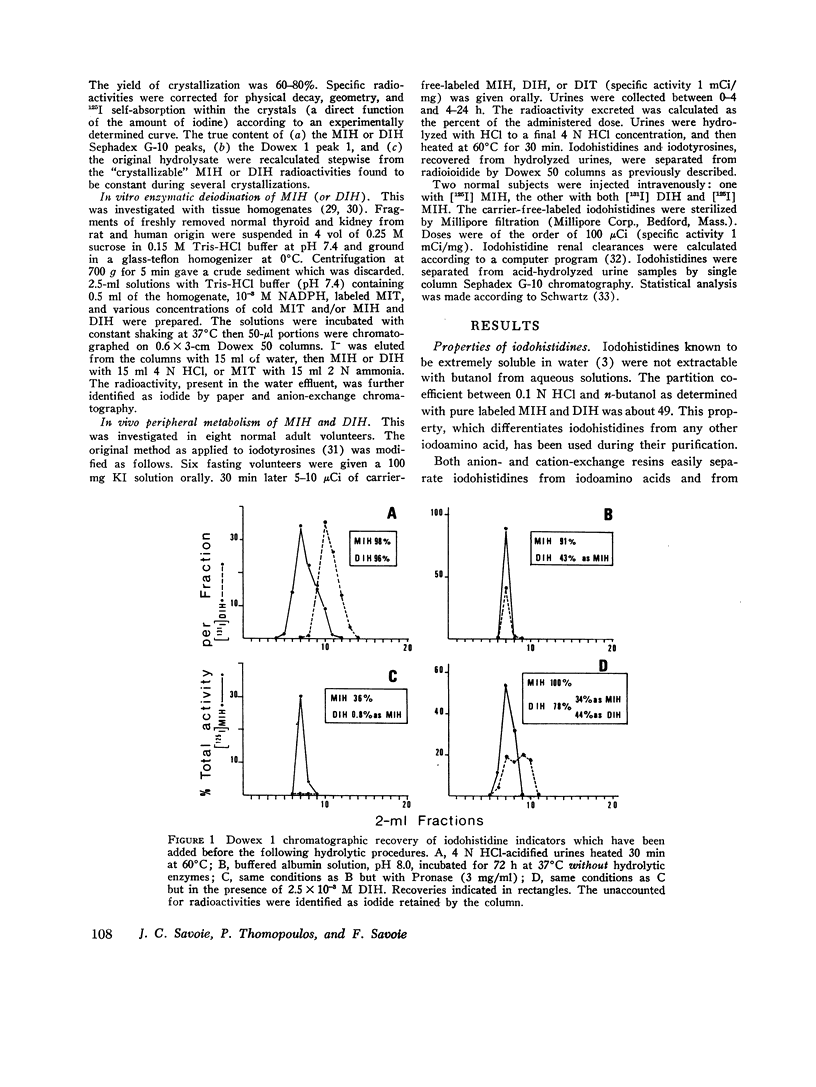
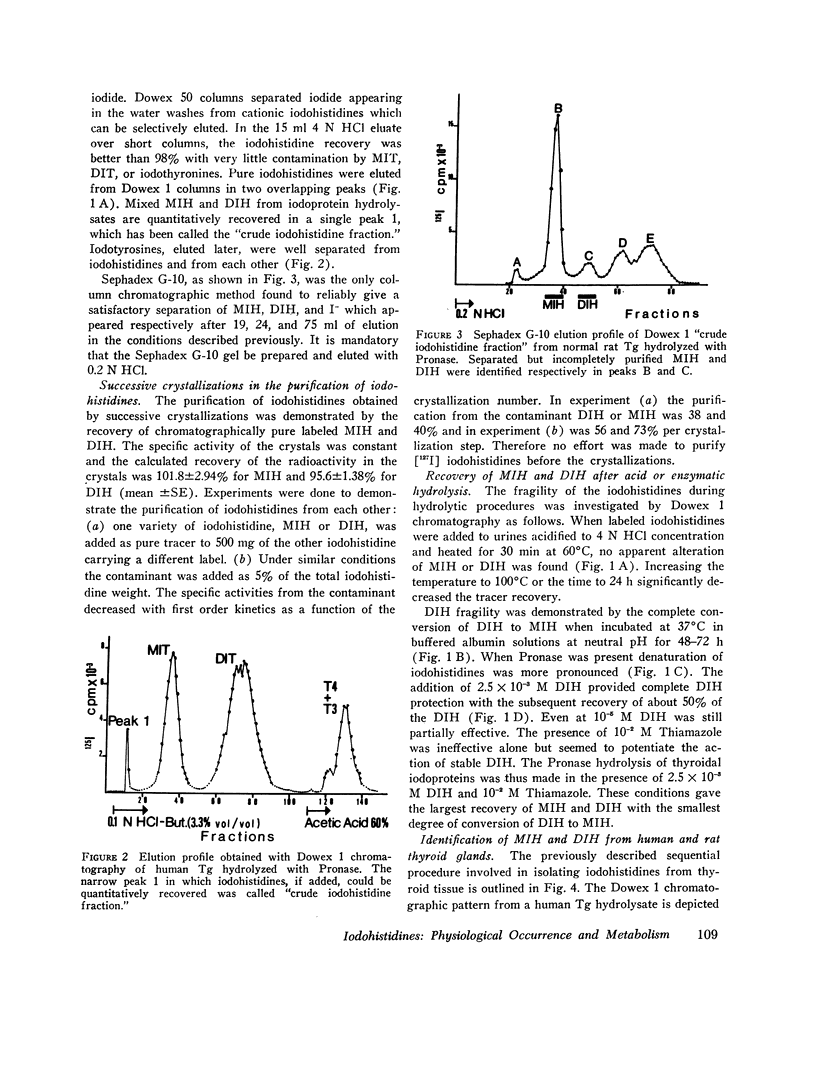
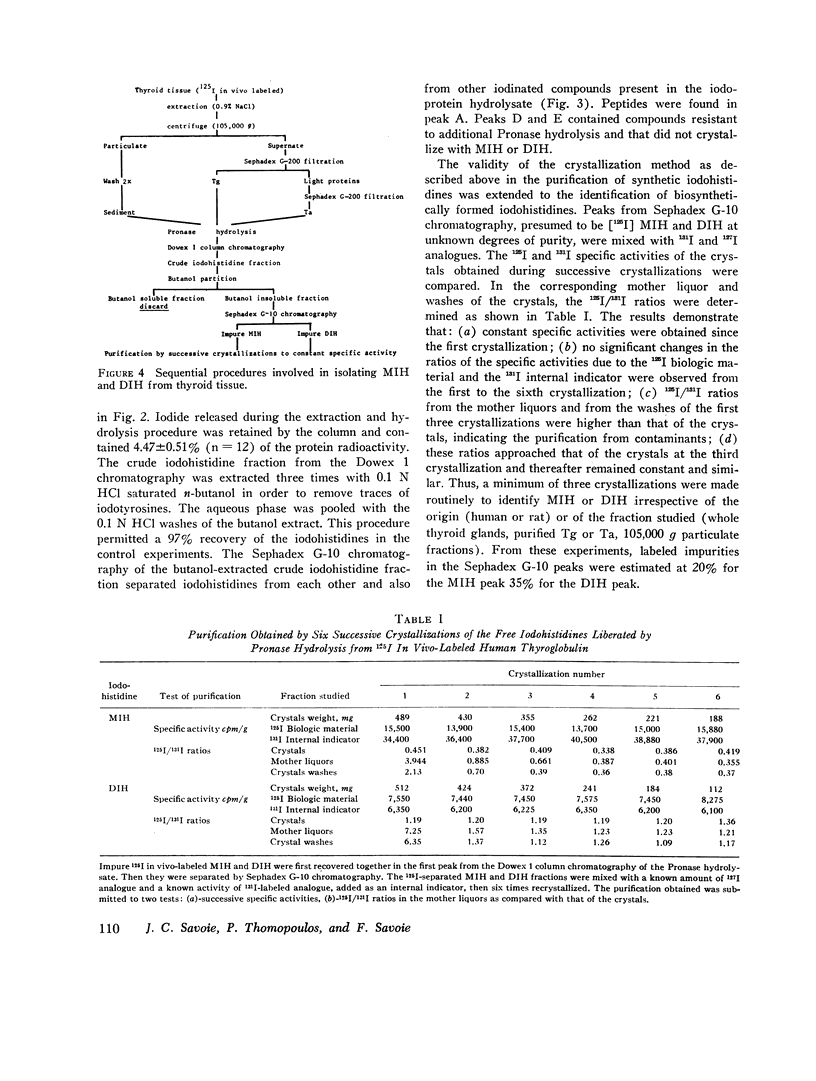
The iodohistidine identification is based on five original methods including: (a) the preparation of stable and radioiodine-labeled iodohistidines; (b) the protection of the labile iodohistidines during the iodoprotein enzymatic hydrolysis; (c) the isolation of iodohistidines by ion-exchange resin chromatography; (d) their separation from each other and from iodinated cationic butanol-insoluble compounds by Sephadex G-10 chromatography; and (e) their purification by successive crystallizations to a constant specific activity.
Iodohistidine levels (in percent of protein radioactivity from iodide given in vivo) were found comparable in man and the rat. However, the values (mean ±SE) for thyroglobulin (MIH, 0.61±0.10%; DIH, 0.050±0.015%) and for thyralbumin (MIH, 2.61±0.57%; DIH, 0.28±0.09%) differ significantly (P < 0.05).
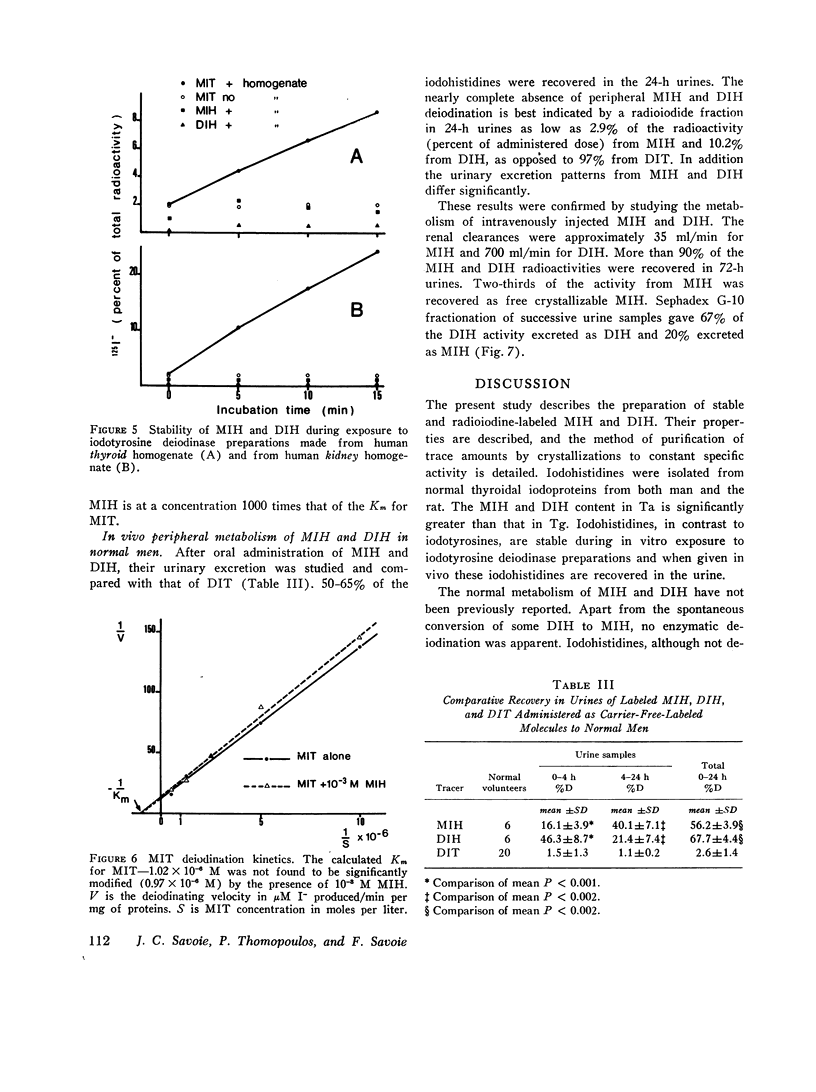
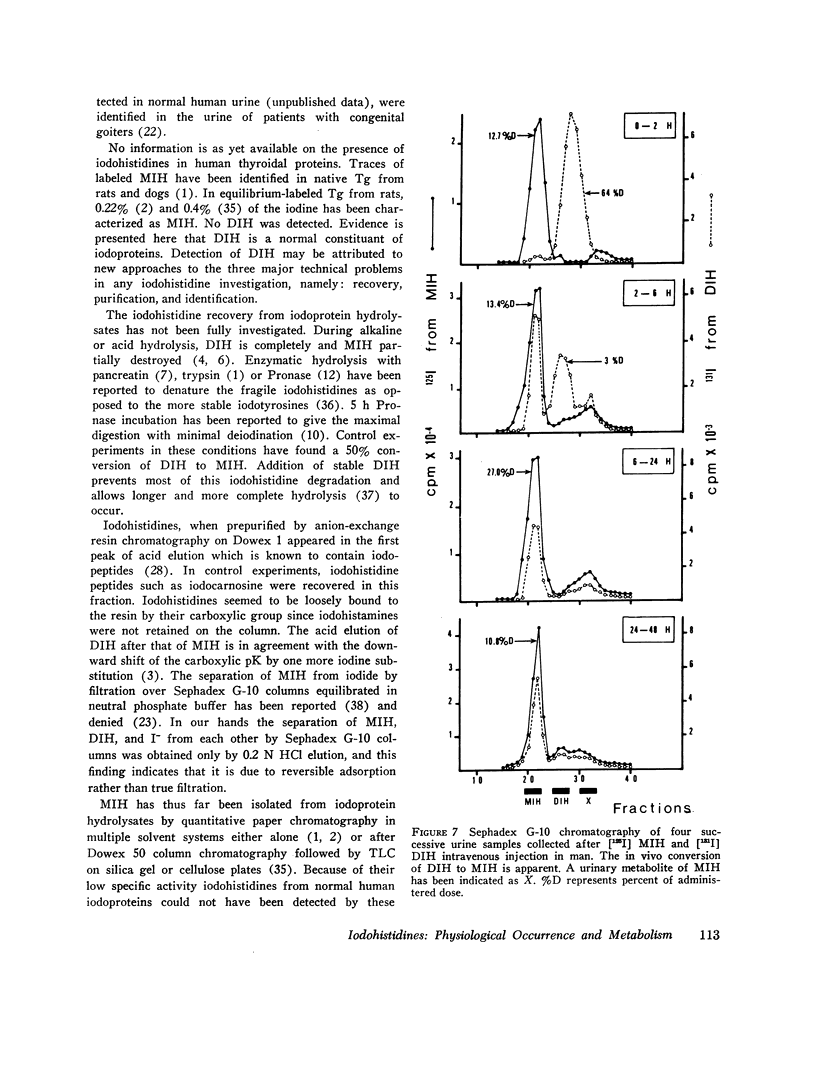
Iodohistidines are stable during in vitro exposure to iodotyrosine dehalogenase preparations. In contrast to iodotyrosines the iodohistidines when given in vivo to man either orally or intravenously were in large part recovered in 24-h urines.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensusan H. B., Naidu M. S. The identification of 4(5)-iodohistidine as the product of the limited iodination of histidine. Biochemistry. 1967 Jan;6(1):12–15. doi: 10.1021/bi00853a003. [DOI] [PubMed] [Google Scholar]
- Bensusan H. B. Protein crosslinkages. I. Effect of iodination on the time-dependent solubility changes of reconstituted collagen fibrils. Arch Biochem Biophys. 1966 Jul;115(1):77–83. doi: 10.1016/s0003-9861(66)81040-8. [DOI] [PubMed] [Google Scholar]
- Covelli I., Wolff J. Iodination of the normal and buried tyrosyl residues of lysozyme. I. Chromatographic analysis. Biochemistry. 1966 Mar;5(3):860–866. doi: 10.1021/bi00867a008. [DOI] [PubMed] [Google Scholar]
- Covelli I., Wolff J. Iodohistidine formation in ribonuclease A. J Biol Chem. 1966 Oct 10;241(19):4444–4451. [PubMed] [Google Scholar]
- Covelli I., Wolff J. The histidyl residues of insulin. I. Reactivity toward iodine. J Biol Chem. 1967 Mar 10;242(5):881–886. [PubMed] [Google Scholar]
- DEGROOT L. J., POSTEL S., LITVAK J., STANBURY J. B. Peptide-linked iodotyrosines and iodothyronines in the blood of a patient with congenital goiter. J Clin Endocrinol Metab. 1958 Feb;18(2):158–166. doi: 10.1210/jcem-18-2-158. [DOI] [PubMed] [Google Scholar]
- DEGROOT L. J., STANBURY J. B. The syndrome of congenital goiter with butanol-insoluble serum iodine. Am J Med. 1959 Oct;27:586–595. doi: 10.1016/0002-9343(59)90044-0. [DOI] [PubMed] [Google Scholar]
- DUBE S. K., ROHOLT O. A., PRESSMAN D. THE REQUIRED INTEGRITY OF TYROSINE IN CHYMOTRYPSIN FOR THE PRESERVATION OF THE CATALYTIC FUNCTION. J Biol Chem. 1964 Jun;239:1809–1812. [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H. The essential groups of lysozyme, with particular reference to its reaction with iodine. Arch Biochem. 1950 Jun;27(1):109–124. [PubMed] [Google Scholar]
- Glazer A. N., Sanger F. The iodination of chymotrypsinogen. Biochem J. 1964 Jan;90(1):92–98. doi: 10.1042/bj0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway C. T., Bond R. P., Knight I. G., Beechey R. B. The alleged presence and role of monoiodohistidine in mitochondrial oxidative phosphorylation. Biochemistry. 1967 Jan;6(1):19–25. doi: 10.1021/bi00853a005. [DOI] [PubMed] [Google Scholar]
- Jonckheer M. H., Karcher D. M. Thyroid albumin. I. Isolation and purification. J Clin Endocrinol Metab. 1971 Jan;32(1):7–17. doi: 10.1210/jcem-32-1-7. [DOI] [PubMed] [Google Scholar]
- KOSHLAND M. E., ENGLBERGER F. M., ERWIN M. J., GADDONE S. M. Modification of amino acid residues in anti-p-azobenzenearsonic acid antibody during extensive iodination. J Biol Chem. 1963 Apr;238:1343–1348. [PubMed] [Google Scholar]
- LISSITZKY S., CODACCIONI J. L., CARTOUZOU G., MANTE S. EUMETABOLIC GOITROUS ADULT WITH IODOPREALBUMIN IN THYROID TISSUE AND BLOOD. J Clin Endocrinol Metab. 1964 Apr;24:305–312. doi: 10.1210/jcem-24-4-305. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lissitzky S., Bismuth J., Codaccioni J. L., Cartouzou G. Congenital goiter with iodoalbumin replacing thyroglobulin and defect of deiodination of iodotyrosines. Serum origin of the thyroid iodoalbumin. J Clin Endocrinol Metab. 1968 Dec;28(12):1797–1806. doi: 10.1210/jcem-28-12-1797. [DOI] [PubMed] [Google Scholar]
- Lissitzky S., Codaccioni J. L., Bismuth J., Depieds R. Congenital goiter with hypothyroidism and iodo-serum albumin replacing thyroglobulin. J Clin Endocrinol Metab. 1967 Feb;27(2):185–196. doi: 10.1210/jcem-27-2-185. [DOI] [PubMed] [Google Scholar]
- MEYNIEL G., BLANQUET P., BERGER J. A., CROIZET M., GAILLARD G. S'EPARATION DES COMPOS'ES IOD'ES MIN'ERAUX ET ORGANIQUES DE LA GLANDE THYROUIDE SUR R'ESINE 'ECHANGEUSE D'ANIONS DOWEX 1 X 2. Bull Soc Chim Biol (Paris) 1965;47:99–106. [PubMed] [Google Scholar]
- Nauman J. A., Nauman A., Werner S. C. Total and free triiodothyronine in human serum. J Clin Invest. 1967 Aug;46(8):1346–1355. doi: 10.1172/JCI105627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawara H., Bilstad J. M., Cahnmann H. J. Iodoamino acid distribution in thyroglobulin iodinated in vivo and in vitro. Biochim Biophys Acta. 1972 Feb 29;257(2):339–349. doi: 10.1016/0005-2795(72)90286-3. [DOI] [PubMed] [Google Scholar]
- Perlgut L. E., Wainio W. W. Identification of mitochondrial iodohistidine and phosphoriodohistidine on a Sephadex G-10 column. Biochemistry. 1967 Jan;6(1):15–18. doi: 10.1021/bi00853a004. [DOI] [PubMed] [Google Scholar]
- RAMAGOPAL E., SPIRO M. J., STANBURY J. B. SOME PROPERTIES OF THE SOLUBLE IODOPROTEINS FROM NORMAL AND CERTAIN ABNORMAL THYROID GLANDS. J Clin Endocrinol Metab. 1965 Jun;25:742–752. doi: 10.1210/jcem-25-6-742. [DOI] [PubMed] [Google Scholar]
- ROCHE J., JUTTSZ M., LISSITZKY S., MICHEL R. Chromatographie quantitative des acides aminés iodés radioactifs de la thyroglobuline marquée. Biochim Biophys Acta. 1951 Jul;7(2):257–262. doi: 10.1016/0006-3002(51)90025-x. [DOI] [PubMed] [Google Scholar]
- ROCHE J., LISSITIZKY S., MICHEL R. Caractérisation des iodohistidines dans les protéines iodées (thyroglobuline et iodoglobine). Biochim Biophys Acta. 1952 Mar;8(3):339–345. doi: 10.1016/0006-3002(52)90049-8. [DOI] [PubMed] [Google Scholar]
- Rapoport G., Chambert J. R., Dedonder R. Iodation de la levane-sucrase de Bacillus subtilis. Biochim Biophys Acta. 1967 Mar 15;132(2):330–337. doi: 10.1016/0005-2744(67)90152-0. [DOI] [PubMed] [Google Scholar]
- Rolland M., Aquaron R., Lissitzky S. Thyroglobulin iodoamino acids estimation after digestion with pronase and leucylaminopeptidase. Anal Biochem. 1970 Feb;33(2):307–317. doi: 10.1016/0003-2697(70)90301-5. [DOI] [PubMed] [Google Scholar]
- Rosen S. M., Rosen O. M. The effect of iodination upon the catalytic and regulatory activities of fructose 1,6-diphosphatase. Biochemistry. 1967 Jul;6(7):2094–2097. doi: 10.1021/bi00859a029. [DOI] [PubMed] [Google Scholar]
- Rosenberg I. N. Purification of iodotyrosine deiodinase from bovine thyroid. Metabolism. 1970 Oct;19(10):785–798. doi: 10.1016/0026-0495(70)90076-4. [DOI] [PubMed] [Google Scholar]
- STANBURY J. B., KASSENAAR A. A., MEIJER J. W. The metabolism of iodotyrosines. I. The fate of mono- and di-iodotyrosine in normal subjects and in patients with various diseases. J Clin Endocrinol Metab. 1956 Jun;16(6):735–746. doi: 10.1210/jcem-16-6-735. [DOI] [PubMed] [Google Scholar]
- STANBURY J. B. The requirement of monoiodotyrosine deiodinase for triphosphopyridine nucleotide. J Biol Chem. 1957 Oct;228(2):801–811. [PubMed] [Google Scholar]
- Savoie J. C., Massin J. P., Savoie F. Studies on mono- and diiodohistidine. II. Congenital goitrous hypothyroidism with thyroglobulin defect and iodohistidine-rich iodoalbumin production. J Clin Invest. 1973 Jan;52(1):116–125. doi: 10.1172/JCI107154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoie J. C., Vallée G., Massin J. P. Cinétique de l'iodure chez l'homme. Analyses par ordinateur et modèle tricompartimental. I. Application à l'athyroïdie et au goitre simple. Ann Endocrinol (Paris) 1970 Jul-Aug;31(4):688–698. [PubMed] [Google Scholar]
- Shulman S., Mates G., Bronson P. Studies on thyroid proteins. II. Purification of human thyroid proteins by gel filtration and the isolation of 19-S, 7-S, and 4-S components. Biochim Biophys Acta. 1967 Oct 23;147(2):208–221. [PubMed] [Google Scholar]
- Taurog A., Howells E. M. Enzymatic iodination of tyrosine and thyroglobulin with chloroperoxidase. J Biol Chem. 1966 Mar 25;241(6):1329–1339. [PubMed] [Google Scholar]
- Tubiana M., Vignalou J., Nataf B., Thomas C., Parmentier C., Barre C., Monnier J. P. Goitre avec troubles de formation des iodothyronines et présence d'iodoprotéines anormales. Ann Endocrinol (Paris) 1968 May-Jun;29(3):313–329. [PubMed] [Google Scholar]
- Wolff J., Covelli I. Factors in the iodination of histidine in proteins. Eur J Biochem. 1969 Jun;9(3):371–377. doi: 10.1111/j.1432-1033.1969.tb00618.x. [DOI] [PubMed] [Google Scholar]
- Wolter R., Kinthaert J., Jonckheer M. H., Ermans A. M. Etude d'un goitre congénital avec hypothyroidie, associé à la présence d'une iodoprotéine sérique. Rev Fr Etud Clin Biol. 1965 Oct;10(8):825–832. [PubMed] [Google Scholar]



