Abstract
Unbiased methods to assess the firing activity of individual neurons in the neocortex have revealed that a large proportion of cells fire at extremely low rates (<0.1 Hz), both in their spontaneous and evoked activity. Thus, firing in neocortical networks appears to be dominated by a small population of highly active neurons. Here we use a fosGFP transgenic mouse to examine the properties of cells with a recent history of elevated activity. FosGFP-expressing layer 2/3 pyramidal cells fired at higher rates compared to fosGFP− neurons, both in vivo and in vitro. Elevated activity could be attributed to increased excitatory and decreased inhibitory drive to fosGFP+ neurons. Paired-cell recordings indicated that fosGFP+ neurons had a greater likelihood of being connected to each other. These findings indicate that highly active, interconnected neuronal ensembles are present in the neocortex and suggest these cells may play a role in the encoding of sensory information.
Introduction
Neocortical neurons are differentially recruited by network activation. Individual neurons show more than ten-fold variation in stimulus-driven and spontaneous firing output with most cells exhibiting extremely low or no firing activity (Margrie et al., 2002; Brecht et al., 2003; Petersen et al., 2003; de Kock et al., 2007; Hromadka et al., 2008) but see (Vijayan et al., 2010). The reasons underlying the disparity in neocortical firing rates are unclear. It may be that over minutes to hours, mean firing rates across different neocortical neurons become similar, or the disparity in firing rates might be a stable feature of neurons within the network. In this case, the underlying explanation for a more active neural subset might be higher intrinsic excitability or stronger synaptic connectivity. Regardless, the existence of a highly active subset of neurons has important implications for the processing and encoding of sensory or motor information. Detailed analysis of the cellular and network properties of this more active neuronal subset has been hampered by an inability to reliably identify and record from these cells.
It has long been noted that a subset of neocortical neurons express the immediate-early gene (IEG) c-fos under basal conditions, a property that has been ascribed to the recent, experience-dependent activation of these cells. Indeed, fos expression is induced by elevated neuronal firing (Sagar et al., 1988), where expression levels peak 30–60 minutes after stimulation and decline to baseline 2–4 hrs later. Thus, fos has been widely used as an indicator of neuronal activity (reviewed by (Gall et al., 1998). To facilitate the identification and analysis of neurons exhibiting expression of this activity-dependent transcription factor under basal, unstimulated conditions, we employed a transgenic mouse that expresses GFP under the control of the c-fos promoter (Barth et al., 2004). Because fosGFP requires several hours following its induction to become fluorescent, it serves as a marker for neurons that have undergone a prior period of elevated activity in vivo. Thus, analysis of fosGFP-expressing neurons may help elucidate the principles by which active neural subsets are established and maintained.
Targeted recordings in vivo reveal that expression of the IEG c-fos in fosGFP transgenic mice is associated with elevated spontaneous firing activity compared to neighboring, fosGFP− neurons in layer 2/3 of primary sensory cortex. In vitro analysis confirmed this and demonstrated that higher firing rates could not be attributed to elevated intrinsic excitability but rather to increased excitatory and reduced inhibitory drive, specifically in the context of network activity. In addition, we find that fosGFP+ neurons are highly interconnected within the layer 2/3 network.
Thus, IEG expression marks a highly active and interconnected subnetwork of neurons that is stable over time periods of at least many hours. These findings suggest that the preferential activation of specific neuronal ensembles in vivo is not stochastically generated at any instant in time, but is determined by synaptic interconnectivity of a specific cell subset, and that an identifiable subset of highly active cells is likely to play an important role in the representation of information in the neocortex.
Results
fosGFP+ cells show high spontaneous firing activity in vivo
To determine whether fosGFP expression was correlated with elevated spontaneous firing activity in vivo, targeted-juxtacellular recordings were carried out in fosGFP+ and fosGFP− cells pairs within layer 2/3 of primary somatosensory (barrel) cortex of anesthetized animals. Under basal, unstimulated conditions, the percentage of both fos-immunoreactive neurons in wildtype (Figure 1A) and fosGFP+ neurons in transgenic animals (Figure 1B) was similar across different neocortical areas, (~15% of layer 2/3 cells; Figure S1).
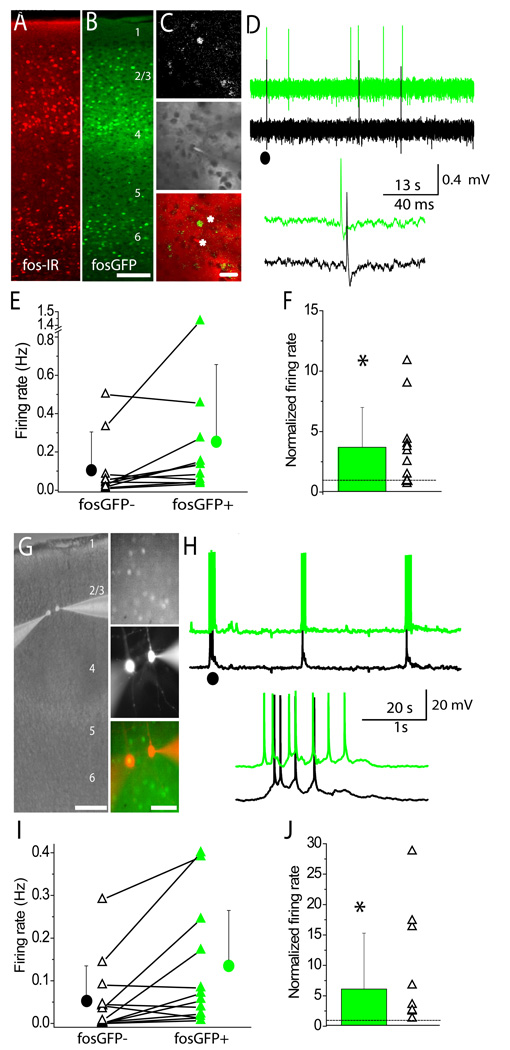
Figure 1. Fos-expressing pyramidal neurons in layer 2/3 exhibit higher spontaneous firing rates in vivo.
(A) Fos-IR from wild-type animals. (B) Intrinsic fluorescence from fosGFP transgenic animals. Scale=100µm. (C) (Top) In vivo fosGFP expression in layer 2/3 barrel cortex. (Middle) Dual electrode recording set-up in vivo, where cell bodies are visualized by “shadows” created by the red fluorescent dye Alexa-568. (Bottom) Overlay of images. Asterisks indicate electrode positions. Scale= 20 µM. (D) Example trace from a simultaneously-recorded juxtacellular fosGFP− (black) and fosGFP+ (green) pair. Top traces were filtered and offset for clarity; inset below shows a detail from (●) above. (E) Scatter plot showing firing rates for each recorded pair, mean firing rates are also plotted. (F) Firing rates of fosGFP− cells normalized to paired fosGFP+ firing rate as mean (bar) and scatter (points). Dotted line represents ratio of one. (G) (Left) Recording set-up for dual whole-cell recordings in layer 2/3 pyramidal neurons in acute slices. Scale=200 µM. (Right-Top) FosGFP expression in vitro. (Middle) Alexa-568 filled fosGFP− and fosGFP+ neurons after recording. (Bottom) Overlay of images. Scale=50 µM. (H) Example trace from fosGFP− (black) and fosGFP+ (green) pair. Conventions in (I) and (J) as in (E) and (F)
Two-photon imaging of GFP expression combined with local illumination of cell bodies using a red fluorescent dye (shadow-patching; (Kitamura et al., 2008) enabled identification of fosGFP+ and fosGFP− neurons (Figure 1C). A great deal is known about neurons in this layer with respect to local network properties (Feldmeyer et al., 2006; Wang et al., 2006; Kapfer et al., 2007; Adesnik and Scanziani, 2010), their activity during perception and ability to drive behavior (Kerr et al., 2007; Houweling and Brecht, 2008; Huber et al., 2008; Poulet and Petersen, 2008; Gentet et al., 2010) and their capacity for experience-dependent plasticity (Glazewski and Fox, 1996; Allen et al., 2003; Celikel et al., 2004; Clem et al., 2008); as such they offer a strong entry point for analyses of neocortical networks.
Targeted neurons were 185±46 µM from the pial surface (n=12 pairs), and were located 38.6±19 µM apart (n=7 pairs; not all pairs measured). As in previous studies, firing rates across simultaneously-recorded cell pairs varied substantially (range 0.017–1.43 Hz). However, expression of the immediate-early gene fosGFP was a strong predictor of a cell having a higher overall firing rate compared to neighboring, unlabeled cells (Figure 1D,E; firing rate for simultaneously recorded fosGFP− cells 0.099±0.2 Hz versus fosGFP+ cells 0.25±0.4 Hz, n=12, p=0.03). On average, fosGFP+ cells fired at ~4-fold higher rates compared to fosGFP− cells (Figure 1F).
Thus, fosGFP expression is predictive of neurons with elevated firing activity in vivo. Because of the well-characterized delay between induction of fos expression and intrinsic GFP fluorescence, these data suggest that highly active neuronal subsets may be stable for hours in vivo.
fosGFP+ neurons fire more ex vivo
To carry out a detailed mechanistic analysis of the cellular and synaptic basis of this increased firing within fosGFP+ neurons, we examined whether fosGFP expression was correlated with elevated spontaneous firing activity for layer 2/3 pyramidal neurons in acute brain slices, using paired-cell recordings.
Spontaneous network activity at levels comparable to what was observed in vivo was facilitated by bathing slices in a low-divalent ACSF solution (Sanchez-Vives and McCormick, 2000; Maffei et al., 2004; Shruti et al., 2008). Targeted cells were in mid-layer 2/3 (232±41 µm depth from pia, n=26 cells; 45.5±17 µm apart; n=13 cell pairs).
Because fosGFP expression is not induced by slice preparation (Barth et al., 2004), the stimulus responsible for induction of fosGFP expression was likely to have occurred at least several hours prior to tissue preparation. Ex vivo, fosGFP+ cells maintained significantly higher rates of overall firing activity compared to neighboring fosGFP− cells (Figure 1G–H; firing rate for simultaneously recorded fosGFP− cells 0.050±0.08 Hz versus fosGFP+ cells, 0.12±0.14 Hz, n=13, p=0.01) . Elevated firing rates in fosGFP+ cells could be observed for many hours (3+) after slice preparation and did not decline over the recording session.
In wild-type animals, mean firing rates of individually recorded neurons were similar to those of fosGFP− neurons (Figure S1; 0.103±0.023 Hz, n=30, p=0.9). However, a subset of wild-type neurons exhibited high firing rates comparable to those observed in fosGFP+ cells, suggesting that this subset is present in wild-type animals but can be uniquely visualized in fosGFP transgenic mice.
FosGFP+ neurons fire earlier and more often during network activity
In both our experiments and others’ (Steriade et al., 1993; Sanchez-Vives and McCormick, 2000; MacLean et al., 2005), spontaneous firing in neocortical neurons ex vivo tends to occur during epochs of depolarization, similar to what has been termed Upstates in vivo. Although the precise trigger for these events is unknown, epochs are observed in both neurons that fire frequently and those that do not fire at all, where they appear as prolonged subthreshold events (Figure 2). Are fosGFP+ neurons differentially recruited during these epochs of network activity?
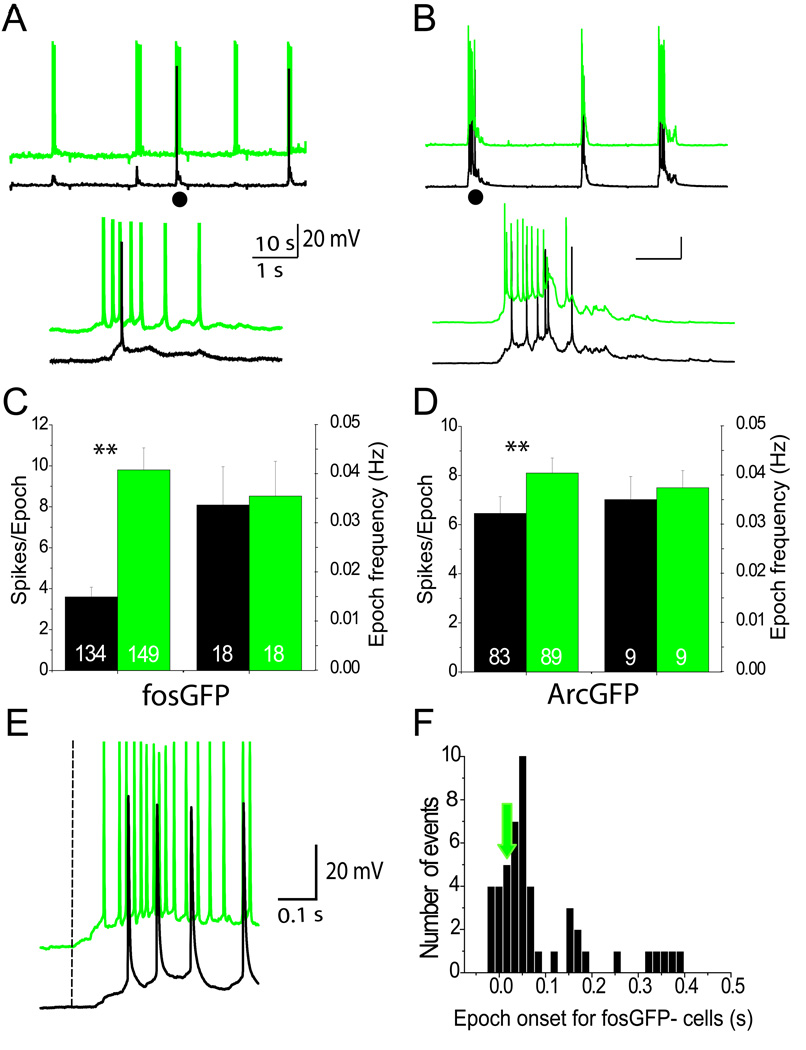
Figure 2. IEG-expressing neurons fire at higher frequencies during network activity.
(A–B) Spontaneous firing activity from a pair of simultaneously-recorded fosGFP− and fosGFP+ neurons (A) and ArcGFP− and ArcGFP+ neurons (B). Traces have been offset for clarity. Inset below (●) shows higher temporal resolution of firing. (C) The number of spikes per epoch was significantly different for fosGFP− (black) and fosGFP+ (green) cells (n=number of epochs for each group). The frequency of depolarizing epochs for simultaneously recorded fosGFP+ and fosGFP− cells is similar (p=0.14). (D) Same as in (C) but for ArcGFP − (black) and ArcGFP+ (green) cells. (E) Example traces showing depolarizing epochs begin earlier for fosGFP+ cells (dotted line) compared to simultaneously-recorded fosGFP− cells. (F) Histogram of the timing of epoch onset for a representative subset of fosGFP− cells relative to fosGFP+ cells. Epoch onset times for fosGFP− cells are shifted later (0=time of epoch onset for fosGFP+ cells; green arrow).
FosGFP+ cells fired more spikes during a depolarizing epoch compared to fosGFP− cells, although epoch duration was identical (fosGFP− 2.7±0.17, n=134 epochs over 18 cells, versus fosGFP+ 2.7±0.14, n=149 epochs over 18 cells, p=0.8). FosGFP+ cells showed significantly more spikes per epoch (s/e) than simultaneously recorded, neighboring fosGFP− cells (Figure 2C; fosGFP− 3.61±0.47 s/e; fosGFP+ 9.8±1.1 s/e, p<0.01).
Epoch frequency (including subthreshold depolarizations) was not significantly increased in fosGFP+ cells (Figure 2C; fosGFP− cells 0.035±0.007 Hz; fosGFP+ cells 0.034±0.007 Hz, p=0.26), indicating that network activity can engage both cell populations.
Identification of high-firing neurons using expression of an alternate IEG
To verify that the elevated spontaneous firing activity observed in fosGFP+ neurons was not due to expression of the fosGFP transgene, a second strain of transgenic mice expressing GFP under the control of the Arc/Arg3.1 promoter was analyzed (GENSAT BAC transgenic resource, Rockefeller University; (Gong et al., 2003)). Similar to fosGFP+ neurons, ArcGFP+ neurons tended to fire more than ArcGFP− neurons within a cell pair (Figure 2B and data not shown; mean overall firing rate, ArcGFP− 0.23±0.21 Hz versus ArcGFP+ 0.32±0.14 Hz; n=9 pairs, p=0.07). Like fosGFP+/− cell pairs, the frequency of depolarizing epochs was identical, and ArcGFP+ neurons showed significantly more spikes/epoch than ArcGFP− cells (Figure 2D; ArcGFP− 6.4±0.7 s/e, n=83 epochs over 9 cells versus ArcGFP+ 8.1±0.6 s/e, n=89 epochs over 9 cells, p=0.003). On average, ArcGFP+ cells fired 2.5-fold more than ArcGFP−cells, a significant difference (p=0.04). Although values from ArcGFP+ neurons were more variable compared to fosGFP+ neurons, it is remarkable that the basic observations made in both transgenic mice are so similar. Thus, it is unlikely that the increased firing activity characterized in fosGFP+ neurons is due to expression of the fosGFP transgene.
Network activity rapidly engages fosGFP+ neurons
Simultaneous recordings of fosGFP+ and fosGFP− cells enabled a direct comparison of cell engagement during an epoch of network activity. We found that fosGFP+ neurons were recruited into a depolarizing epoch significantly earlier than fosGFP− neurons (Figure 2E–F; mean onset timing for fosGFP− was 67.3±27 ms after onset in fosGFP+ cells; n=31 epochs over 9 cell pairs; p<0.001). Thus, although spontaneous network activity engages both cell types, fosGFP+ cells are activated earlier and are more likely to fire during a depolarizing epoch.
FosGFP+ neurons show reduced intrinsic excitability
Why do fosGFP-expressing neurons display elevated spontaneous firing activity? One explanation is that these neurons show greater intrinsic excitability (i.e., depolarized resting membrane potential, action potential (AP) threshold, or input resistance). However, comparison between fosGFP+ and fosGFP− cells showed that these properties were identical between groups (Table S1).
To evaluate intrinsic excitability, input-output curves were constructed, using constant current injection to elicit firing (Figure S2). FosGFP+ cells required more current to generate a single spike (mean rheobase current fosGFP− 37.12±1.6 pA versus fosGFP+ 45.6±2.99 pA, n=16 for both; p=0.02) and exhibited fewer spikes at all levels of current injection compared to fosGFP− cells (Figure S2). AP threshold for fosGFP+ and fosGFP− cells was similar, despite the finding that fosGFP+ cells required more current to spike. This suggests that other voltage-dependent, spike-delaying conductances are differentially regulated in fosGFP+ neurons. However, increased spontaneous activity in fosGFP+ neurons is unlikely to result from cell-intrinsic electrophysiological properties.
FosGFP+ cells receive increased excitatory and reduced inhibitory drive
Under our recording conditions, spontaneous activity in layer 2/3 neurons requires synaptic input since it is abolished in the presence of GABA- and glutamate receptor antagonists (Shruti et al., 2008). To examine whether fosGFP+ neurons receive differential synaptic drive, the frequency and amplitude of post-synaptic currents (PSCs) during spontaneous network activity was analyzed.
Although the amplitude of spontaneous excitatory PSCs (sEPSCs) was similar between fosGFP+ and fosGFP− neurons, fosGFP+ neurons showed a significantly greater sEPSCs frequency (Figure 3A–C; sEPSC frequency fosGFP− 1.3±0.2 Hz versus fosGFP+ 1.7±0.2 Hz; n=13 cells for both; p=0.001), a finding further confirmed by paired-cell recordings (Figure 3D).
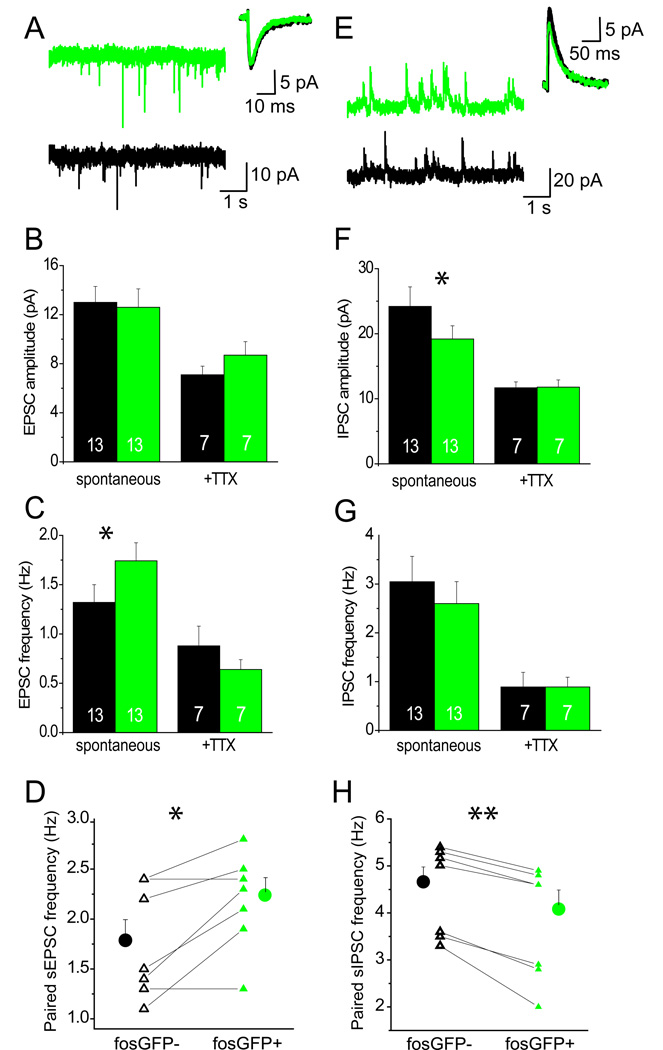
Figure 3. FosGFP+ neurons receive increased excitatory and decreased inhibitory synaptic drive during network activity.
(A) Sample traces of sEPSCs for fosGFP− (black) and fosGFP+ (green) individually-recorded neurons. Inset shows mean sEPSC from representative fosGFP− and fosGFP+ cells. (B) Mean sEPSC (spontaneous) and mEPSC (+TTX) amplitude in fosGFP− (black) and fosGFP+ (green) neurons. Number of cells indicated in bar. (C) As in (B) but for sEPSC and mEPSC frequency. (D) Scatter plot for sEPSC frequency from simultaneously-recorded fosGFP− and fosGFP+ neurons. Group mean±sem are plotted as solid circles. n=7 pairs. (E) As in (A) but for sIPSCs in fosGFP− and fosGFP+ individually-recorded neurons. Inset shows mean sIPSC from representative fosGFP− and fosGFP+ cells. (F) As in (B) but for sIPSC and mIPSC amplitude. (G) As in (B) but for sIPSC and mIPSC frequency. (H) As in (D), but for sIPSC frequency from simultaneously-recorded fosGFP− and fosGFP+ neurons. n=7 pairs.
FosGFP+ neurons showed a significantly reduced amplitude of spontaneous inhibitory PSCs (sIPSCs; Figure 3E,F; fosGFP− 24±3 pA versus fosGFP+ 19±2 pA; p=0.02), although frequency was not significantly different (Figure 3G; frequency fosGFP− 3.1±0.5 Hz versus fosGFP+ 2.6±0.5 Hz; p=0.3). Paired-cell recordings show a significant reduction in sIPSC frequency for fosGFP+ cells (Figure 3H). These data indicate that fosGFP+ neurons receive both more excitation and less inhibition during spontaneous network firing.
To determine whether this difference in excitatory and inhibitory input would be maintained in the absence of spiking, miniature EPSC and IPSCs (mEPSCs and mIPSCs) were assessed in the presence of the Na+ channel blocker tetrodotoxin (TTX). In TTX, no significant differences in mEPSC or mIPSC frequency or amplitude were detected (Figure 3). This result is intriguing, since it indicates that differences in synaptic drive require network firing to be manifested. During unconstrained network activity, presynaptic excitatory neurons may fire more onto fosGFP+ neurons, inputs that may provide a basis for increased spontaneous activity of this cell subset.
Direct and indirect connectivity between fosGFP+ neurons
Are fosGFP+ neurons are non-randomly wired into the neocortical network, receiving presynaptic input from excitatory neurons that are themselves more active? We speculated that fosGFP+ neurons might show a high degree of either direct or indirect interconnectivity.
Connectivity between pairs of layer 2/3 pyramidal neurons was assessed by a second series of dual-cell recording experiments. In total, 214 paired recordings were performed representing all combinations of fosGFP+ and fosGFP− cells as well as cells from wild-type animals (Figure 4). Using the criterion of a short latency EPSP (1–5 ms) with high (>50%) trial-to-trial reliability, six cells (all fosGFP+/fosGFP+ pairs) out of the group (162 pairs), exhibited a direct, unidirectional synaptic input (Figure 4). In no other case were examples of direct synaptic connectivity observed. In connected cells, mean EPSP amplitude was 0.99+0.21 mV (n=6 cells; 10 responses per cell averaged), similar to previously described unitary EPSP amplitudes (Thomson et al., 2002; Feldmeyer et al., 2006).
Figure 4. FosGFP+ cells show greater interconnectivity within the cortical network.
(A) Example pair of fosGFP+ neurons with a direct, unidirectional synaptic connection. Top green trace shows 10 APs evoked in the trigger cell. Red trace shows the average (10 traces) EPSP evoked in the follower cell. Lower traces are individual stimulation trials. (B) Example pair of fosGFP− cells. Traces are as in (A). (C) Percent of connected cell pairs for each group of wildtype and all combinations of fosGFP+ and fosGFP− neurons. In some cases, potential connections could only be evaluated in one direction. (D) Example of a pair of fosGFP+ cells that were not directly connected (note absence of stimulus-locked EPSPs in averaged red trace). Shadowed area shows time window for EPSP event (*) identification. Black bars at bottom represent stimulus APs. (E) EPSP frequencies in the 50 ms period following each AP in the trigger cell for each group, normalized to EPSP frequency in the prestimulus window. Connected pairs not included.
Distance between connected pairs was similar to that of all cells overall (68.8+16 µm; n=6 pairs; p=0.2 versus group mean) and showed a similar distance from the pia surface (222.6+30 µm; n=6; p=0.7 versus group mean). Thus, the location of the cell soma was not a critical variable in predicting cell connectivity.
Although the frequency of directly connected cells was low, we noted that current injection into the trigger cell often led to an increase in PSP frequency in the second cell (Figure 4D,E). These putative EPSPs (since recordings were carried out at the experimentally determined reversal potential for Cl−) were of variable latency (~5–50 ms) with respect to the presynaptic AP, suggesting that they were of polysynaptic origin. APs triggered in a fosGFP− cell led to a ~1.2-fold, not significant, increase in EPSP frequency in the “follower” cell compared to the prestimulus window (Figure 4E; fold-change in EPSP frequency from baseline: fosGFP− trigger to fosGFP− follower: 1.18±0.05, n=45 cells; fosGFP− trigger to fosGFP+ follower: 1.17±0.07, n=23 cells; fosGFP+ trigger to fosGFP− follower: 1.13±0.1, n=19, p>0.5).
However, depolarization of fosGFP+ cells often led to a significant increase in EPSP frequency in unconnected fosGFP+ cells. Stimulation of the trigger cell led to a significant, 1.5-fold increase in EPSP frequency compared to that in the prestimulus window (fold-change in EPSP frequency from baseline: fosGFP+ trigger to fosGFP+ follower: 1.53±0.1, n=45 cells; p=0.004; Figure 4E). Because EPSPs were not evoked at a consistent time interval following each presynaptic AP but were distributed between 10–50 ms after each stimulus, it is likely that they were polysynaptic in origin.
Discussion
We identified a subset of neurons within superficial layers of the neocortex that show activity-dependent gene expression in vivo and sustained, elevated spontaneous firing activity both in vivo and in acute brain slices. Elevated firing is not due to increased intrinsic excitability, since fosGFP+ neurons show no difference in spike threshold and a suppressed F:I curve. Instead, in the context of network activity, fosGFP+ neurons receive greater excitation and less inhibition compared to neighboring, fosGFP− cells. Dual-cell recordings reveal that fosGFP+ neurons are more effective at driving recurrent activity in the neocortical network than fosGFP− neurons, suggesting that fos-expressing cells show a higher frequency of both direct and indirect connections to each other than to fosGFP− cells. Compared to neighboring cells, these neurons are preferentially activated during epochs of network depolarization. Based upon the delay between induction of IEG expression, GFP fluorescence, and targeted recordings, fosGFP+ neurons appear to be maintained as a coactive ensemble over the course of many hours.
Activity as a parameter to define cell function
Despite the fact that layer 2/3 pyramidal neurons have been considered to be a relatively homogeneous cell population, it is well-established that in vivo firing rates amongst these cells can vary more than ten-fold. Similarly, it has long been noted that a subset of neurons in the neocortex exhibit expression of the activity-dependent gene c-fos even under basal conditions. Here we show that a history of immediate-early gene expression is an indicator of elevated spontaneous firing activity in a subpopulation of layer 2/3 pyramidal neurons. Whether elevated activity is a cause or an effect of activated IEG expression, these data indicate that some neurons disproportionately contribute to the propagation of neocortical activity. It has recently been proposed that a single extra spike within a neocortical neuron might be capable of driving dozens of spikes in its synaptically connected partners (London et al., 2010), and indeed, under some conditions stimulation of a single neurons can alter global network activity (Li et al., 2009) and perception (Houweling and Brecht, 2008). Based upon this finding, we propose that fosGFP+ neurons may drive or propagate network activity and information transfer across brain areas.
What drives firing and IEG expression in the active subnetwork?
Is sensory input required to drive fosGFP expression and indirectly, the increased spontaneous firing activity of these cells? Our preliminary analysis suggests this is not the case. Bilateral removal of all large facial vibrissae for 24 hrs did not eliminate or even noticeably reduce fosGFP expression in layer 2/3. In addition, paired-cell recordings showed that fosGFP+ cells maintained elevated firing activity in sensory deprived tissue (data not shown). Although it is possible that whisker removal is not sufficient to get rid of all afferent activity, these data suggest that sensory input is not required for fosGFP expression or elevated spontaneous firing. The question of whether these cell assemblies are generated from internal neocortical dynamics or are constructed by information from the periphery (Kenet et al., 2003; MacLean et al., 2005; Golshani et al., 2009) is of great interest.
Non-random distribution of activity across neocortical networks
It has been suggested that the population of neurons exhibiting both high and low levels of activity are unstable and drift over timescales ranging from seconds to minutes (Ikegaya et al., 2004; Kerr et al., 2007; Mokeichev et al., 2007). Our data indicate that the firing output of a cell is much more conserved than previously estimated. Due to the timecourse of fosGFP expression, which requires at least 2–3 hours to become fluorescently visible, we conclude that a subpopulation of neurons exhibits specific network connectivity, driving elevated activity that can be maintained for at least 4–7 hours.
Homeostasis of neuronal firing rates is a powerful concept that has guided current thinking about how neurons respond to perturbations in firing output (Turrigiano and Nelson, 2000). The reduction in intrinsic excitability in fosGFP+ cells may be a homeostatic adjustment to limit participation of these neurons in positive feedback loops that might otherwise lead to epileptic-like activity. This may be an intermediate step in hobbling highly active cells, in order to make way for a new population of cells to step in and take their place; alternatively, the suppressed input-output function may represent a new set-point for a stable subset of highly-active neurons.
Subnetwork of highly active neurons
It has been controversial whether there is structure that repeats itself during episodes of spontaneous activity. The search for recurrent motifs of activity has been evaluated at the levels of patterns of EPSCs received by a single cell, or in the temporal pattern of spikes in neurons across epochs of activity (Ikegaya et al., 2004; Luczak et al., 2007; Mokeichev et al., 2007; Ikegaya et al., 2008). Previous analyses may have inadvertently focused on the specific temporal dynamics of these motifs, when in fact the precise sequence of neuronal activation is less conserved than the particular cells that are recruited over time. In other words, the singers may be more conserved than the song.
Although the absolute number of neurons that exhibited a direct synaptic connection was low, it is notable that in all cases synaptically-connected pairs were fosGFP+ neurons receiving input from other fosGFP+ neurons. Consistent with this, other studies have shown that coactive neurons are more likely to share strong synaptic connections (Yoshimura et al., 2005).
Implications for information transmission
Neurons transform synaptic input into spikes, and it is well-accepted that the information encoded by a cell is determined almost exclusively by its firing output. Neurons that fire more are thus likely to convey more information within a neural circuit. In addition, neurons that fire earlier during a stimulus are thought to convey more information than neurons firing later (Johansson and Birznieks, 2004; VanRullen et al., 2005). Although it has been disputed whether rate codes or timing codes are more important, fosGFP+ cells show both a higher rate of firing and earlier recruitment during network activation and as such their spikes may carry more information than other neurons.
These data do not resolve the questions of whether neural activity leads to the development of a synaptically-connected cell assembly (i.e. neural activity is the independent variable), or whether this population of fos-expressing neurons is developmentally specified (and neural activity might be the dependent variable linking this subpopulation). However, the dynamic network properties of these cells indicate that a subpopulation of highly active neurons may dominate the way information is transmitted across the neocortex. Because these cells may constitute the neural substrate of “sparse coding” in the cerebral cortex (Wolfe et al., 2010), it will be of great interest to evaluate the role of these highly active neurons in the representation of sensory input during normal experience as well as during conditions that evoke plasticity.
Materials and Methods
In vivo recording
Surgical Procedures
Mice P13–23 were urethane-anesthetized (1.2g/kg), and a small (1–2 mm) cranial window was created over the barrel cortex. All in vivo experimental procedures were in accordance with national regulation and institutional guidelines and follow previously described methods (Crochet and Petersen, 2006; Glazewski et al., 2007).
Electrophysiology and imaging
Juxtacellular, loose patch recordings were performed with glass microelectrodes. Internal solution contained 50µM Alexa 594 for shadow patching (Kitamura et al., 2008). For imaging, the laser was tuned to 930nm for GFP visualization or 820nm for Alexa594 emission during electrode positioning.
Analysis
To verify that electrodes could detect APs from a target, cells were only included in the analysis if they fired at least once during the recording period. Firing rates were calculated over ~300+ sec.
In vitro recording
Brain slice preparation
Coronal or thalamocortical brain slices (350 µm thick) from mice P12–P15 (wild-type C57Bl6, fosGFP heterozygotes, or arcGFP heterozygotes) were prepared. Slices were recovered in regular ACSF at 35°C for 30 minutes and maintained at room temperature in low-divalent ACSF (0.5 mM MgSO4, 1 mM CaCl2, 3.5 mM KCl).
Whole-cell recording of evoked and spontaneous activity
Spiny, pyramidal layer 2/3 neurons in primary somatosensory cortex were targeted for recording based upon fosGFP+/− expression. Internal pipette solution contained a K-gluconate internal solution (Supplemental Methods). Because the whole-cell recording configuration unambiguously indicated that a single neuron was targeted for recording, pairs in which one cell did not exhibit any firing (0 Hz) were included in analysis of whole-cell recordings.
Evoked firing
APs were elicited by injection of minimal current (using 20, 60, 80 pA steps), and trials that yielded a single AP were used for analysis. AP threshold, peak, and half-width (threshold to peak) were determined using custom-written Igor Pro macros.
Spontaneous firing
Spontaneous firing activity was collected where Vrest was maintained at −50 mV to normalize differences in resting potential between cells, a technique that did not appreciably alter firing rates (data not shown). Onset and offset of synchronized network activity were determined using membrane potential mean and standard deviation over 500 ms, with a 10 ms sliding window (Gerkin et al, in press).
Connectivity analysis
Cells were maintained at their normal resting potential, which was approximately −60 mV, the experimentally determined reversal potential for Cl−. One cell was assigned as the trigger cell and a series of 10 pulses (500 pA, 5 ms duration) at 20 Hz were delivered across 20 separate trials. Bidirectional connectivity was assessed sequentially for each pair. Spontaneous EPSP frequency was calculated in the 500 ms time window preceding the stimulus, and evoked EPSP frequency was calculated across the 10-pulse series including 50 ms after the last pulse (500 ms total).
m- and sPSC analysis
Cells were held at −60 mV for EPSC isolation (the Cl− reversal potential.) Cells were held at +10 mV to isolate IPSCs.
Statistical analysis
All values are mean±s.e.m. unless otherwise indicated. Where data were normally distributed, t-tests were used. For non-normal distributions, a two-tailed Mann-Whitney test for individual comparisons was used.
Supplementary Material
Acknowledgements
Work was supported by the NIH DA0171-88 (A.L.B.), the Alexander von Humboldt Foundation (A.L.B.), NeuroCure (A.L.B. and J.F.A.P.), the Deutsch Forschung Gemeinshaft (Exc 257; J.F.A.P.), the Swiss-German Research Unit “Barrel Cortex Function” (SNF-DFG FOR 1341; J.F.A.P.) and the Max-Delbruck Center-Berlin (J.F.A.P.). Thanks to Kazuo Kitamura for help with fosGFP imaging in vivo and Rick Gerkin for custom Igor Pro macros. Thanks also to Jesse Sheehan, Jill Guy, and Joanne Steinmiller for animal care, and Michael Brecht and members of the Barth, Urban and Crowley laboratories for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adesnik H, Scanziani M. Lateral competition for cortical space by layer-specific horizontal circuits. Nature. 2010;464:1155–1160. doi: 10.1038/nature08935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CB, Celikel T, Feldman DE. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci. 2003;6:291–299. doi: 10.1038/nn1012. [DOI] [PubMed] [Google Scholar]
- Barth AL, Gerkin RC, Dean KL. Alteration of neuronal firing properties after in vivo experience in a FosGFP transgenic mouse. J Neurosci. 2004;24:6466–6475. doi: 10.1523/JNEUROSCI.4737-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M, Roth A, Sakmann B. Dynamic receptive fields of reconstructed pyramidal cells in layers 3 and 2 of rat somatosensory barrel cortex. J Physiol. 2003;553:243–265. doi: 10.1113/jphysiol.2003.044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celikel T, Szostak VA, Feldman DE. Modulation of spike timing by sensory deprivation during induction of cortical map plasticity. Nat Neurosci. 2004;7:534–541. doi: 10.1038/nn1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Barth A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron. 2006;49:663–670. doi: 10.1016/j.neuron.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Clem RL, Celikel T, Barth AL. Ongoing in vivo experience triggers synaptic metaplasticity in the neocortex. Science. 2008;319:101–104. doi: 10.1126/science.1143808. [DOI] [PubMed] [Google Scholar]
- Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat Neurosci. 2006;9:608–610. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- de Kock CP, Bruno RM, Spors H, Sakmann B. Layer- and cell-type-specific suprathreshold stimulus representation in rat primary somatosensory cortex. J Physiol. 2007;581:139–154. doi: 10.1113/jphysiol.2006.124321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D, Lubke J, Sakmann B. Efficacy and connectivity of intracolumnar pairs of layer 2/3 pyramidal cells in the barrel cortex of juvenile rats. J Physiol. 2006;575:583–602. doi: 10.1113/jphysiol.2006.105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall CM, Hess US, Lynch G. Mapping brain networks engaged by, and changed by, learning. Neurobiol Learn Mem. 1998;70:14–36. doi: 10.1006/nlme.1998.3835. [DOI] [PubMed] [Google Scholar]
- Gentet LJ, Avermann M, Matyas F, Staiger JF, Petersen CC. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron. 2010;65:422–435. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Fox K. Time course of experience-dependent synaptic potentiation and depression in barrel cortex of adolescent rats. J Neurophysiol. 1996;75:1714–1729. doi: 10.1152/jn.1996.75.4.1714. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Benedetti BL, Barth AL. Ipsilateral whiskers suppress experience-dependent plasticity in the barrel cortex. J Neurosci. 2007;27:3910–3920. doi: 10.1523/JNEUROSCI.0181-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golshani P, Goncalves JT, Khoshkhoo S, Mostany R, Smirnakis S, Portera-Cailliau C. Internally mediated developmental desynchronization of neocortical network activity. J Neurosci. 2009;29:10890–10899. doi: 10.1523/JNEUROSCI.2012-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Houweling AR, Brecht M. Behavioural report of single neuron stimulation in somatosensory cortex. Nature. 2008;451:65–68. doi: 10.1038/nature06447. [DOI] [PubMed] [Google Scholar]
- Hromadka T, Deweese MR, Zador AM. Sparse representation of sounds in the unanesthetized auditory cortex. PLoS Biol. 2008;6:e16. doi: 10.1371/journal.pbio.0060016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Petreanu L, Ghitani N, Ranade S, Hromadka T, Mainen Z, Svoboda K. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2008;451:61–64. doi: 10.1038/nature06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Matsumoto W, Chiou HY, Yuste R, Aaron G. Statistical significance of precisely repeated intracellular synaptic patterns. PLoS One. 2008;3:e3983. doi: 10.1371/journal.pone.0003983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Aaron G, Cossart R, Aronov D, Lampl I, Ferster D, Yuste R. Synfire chains and cortical songs: temporal modules of cortical activity. Science. 2004;304:559–564. doi: 10.1126/science.1093173. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Birznieks I. First spikes in ensembles of human tactile afferents code complex spatial fingertip events. Nat Neurosci. 2004;7:170–177. doi: 10.1038/nn1177. [DOI] [PubMed] [Google Scholar]
- Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat Neurosci. 2007;10:743–753. doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A. Spontaneously emerging cortical representations of visual attributes. Nature. 2003;425:954–956. doi: 10.1038/nature02078. [DOI] [PubMed] [Google Scholar]
- Kerr JN, de Kock CP, Greenberg DS, Bruno RM, Sakmann B, Helmchen F. Spatial organization of neuronal population responses in layer 2/3 of rat barrel cortex. J Neurosci. 2007;27:13316–13328. doi: 10.1523/JNEUROSCI.2210-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K, Judkewitz B, Kano M, Denk W, Hausser M. Targeted patch-clamp recordings and single-cell electroporation of unlabeled neurons in vivo. Nat Methods. 2008;5:61–67. doi: 10.1038/nmeth1150. [DOI] [PubMed] [Google Scholar]
- Li CY, Poo MM, Dan Y. Burst spiking of a single cortical neuron modifies global brain state. Science. 2009;324:643–646. doi: 10.1126/science.1169957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London M, Roth A, Beeren L, Hausser M, Latham PE. Sensitivity to perturbations in vivo implies high noise and suggests rate coding in cortex. Nature. 2010;466:123–127. doi: 10.1038/nature09086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak A, Bartho P, Marguet SL, Buzsaki G, Harris KD. Sequential structure of neocortical spontaneous activity in vivo. Proc Natl Acad Sci U S A. 2007;104:347–352. doi: 10.1073/pnas.0605643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JN, Watson BO, Aaron GB, Yuste R. Internal dynamics determine the cortical response to thalamic stimulation. Neuron. 2005;48:811–823. doi: 10.1016/j.neuron.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Margrie TW, Brecht M, Sakmann B. In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflugers Arch. 2002;444:491–498. doi: 10.1007/s00424-002-0831-z. [DOI] [PubMed] [Google Scholar]
- Mokeichev A, Okun M, Barak O, Katz Y, Ben-Shahar O, Lampl I. Stochastic emergence of repeating cortical motifs in spontaneous membrane potential fluctuations in vivo. Neuron. 2007;53:413–425. doi: 10.1016/j.neuron.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci U S A. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Shruti S, Clem RL, Barth AL. A seizure-induced gain-of-function in BK channels is associated with elevated firing activity in neocortical pyramidal neurons. Neurobiol Dis. 2008 doi: 10.1016/j.nbd.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, West DC, Wang Y, Bannister AP. Synaptic connections and small circuits involving excitatory and inhibitory neurons in layers 2–5 of adult rat and cat neocortex: triple intracellular recordings and biocytin labelling in vitro. Cereb Cortex. 2002;12:936–953. doi: 10.1093/cercor/12.9.936. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000;10:358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- VanRullen R, Guyonneau R, Thorpe SJ. Spike times make sense. Trends Neurosci. 2005;28:1–4. doi: 10.1016/j.tins.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Vijayan S, Hale GJ, Moore CI, Brown EN, Wilson MA. Activity in the Barrel Cortex During Active Behavior and Sleep. J Neurophysiol. 2010 doi: 10.1152/jn.00474.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KH, Majewska A, Schummers J, Farley B, Hu C, Sur M, Tonegawa S. In vivo two-photon imaging reveals a role of arc in enhancing orientation specificity in visual cortex. Cell. 2006;126:389–402. doi: 10.1016/j.cell.2006.06.038. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Houweling AR, Brecht M. Sparse and powerful cortical spikes. Curr Opin Neurobiol. 2010;20:306–312. doi: 10.1016/j.conb.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Dantzker JL, Callaway EM. Excitatory cortical neurons form fine-scale functional networks. Nature. 2005;433:868–873. doi: 10.1038/nature03252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.