Abstract
Background and purpose
We previously showed that epidermal growth factor receptor tyrosine kinase (EGFRtk) is essential in the development of myogenic tone. GRB2-SOS, protein kinase B (Akt), Janus kinase (JAK), and Signal Transducer and Activator of Transcription 3 (STAT3) are activated by stretch. Thus, we hypothesized that GRB2-SOS, Akt, JAK and STAT3 are downstream signaling of the EGFR and play role in myogenic tone.
Experimental approach
Myogenic tone was determined in freshly isolated coronary arterioles from C57/BL6 mice with and without inhibitors. Pressurized coronary arterioles under 25 and 75 mm Hg were subjected to Western blot analysis to determine signaling phosphorylation. Smooth muscle cells (SMC) stimulated with EGF were used to determine the interaction between signaling.
Key results
Coronary arteriole myogenic tone was significantly reduced under EGFRtk, GRB2-SOS, JAK, and STAT3 inhibition (53.6±2 vs. 83.4±1.3; 82.8±1; 83.6±1; 86.1±1 % of passive diameter at 75 mm Hg, p<0.05, respectively). However, Akt inhibition had no effect on coronary arteriole myogenic tone. Western blot analysis showed increased EGFRtk, STAT3, JAK, and Akt phosphorylation at 75mm Hg, which was significantly inhibited under EGFRtk inhibition. Interestingly, immunoprecipitation/Western blot analysis showed two intracellular complexes (ERK1/2-JAK-STAT3) involved in myogenic tone and (Akt-JAK-STAT3) not involved in myogenic tone.
Conclusion and implications
These findings demonstrate that ERK1/2-JAK-STAT3 complex and GRB2-SOS, down stream signaling of the EGFRtk, are critical in the development of myogenic tone, thereby highlighting these signaling events as potential therapeutic targets in cardiovascular disease states associated with altered myogenic tone.
Keywords: Coronary arterioles, myogenic tone, signaling, EGFR tyrosine kinase, GRB2-SOS, JAK, STAT3, Akt
INTRODUCTION
Pressure-induced myogenic constriction represents a fundamental and unique property of microvessels and arterioles, and significantly contributes to autoregulation of local blood flow. This constriction is an essential component of basal microvascular tone, which allows resistance arteries to constrict or dilate in response to vasoactive mediators, and to elevations or reductions in intraluminal pressure, respectively. The myogenic response occurs independently of the endothelium and perivascular nerves and is therefore an inherent property of vascular smooth muscle cells.(Hwa and Bevan, 1986; Matrougui et al., 1997) Myogenic tone has been extensively investigated, but the molecular mechanisms are still not fully determined. Studies showed that increased intraluminal pressure leads to depolarization of smooth muscle cells and the opening of calcium channels responsible for increased intracellular calcium leading to the contraction of actin-myosin.(Murphy et al., 2002) It has been shown that myogenic tone is dependent on calcium, PKC,(Osol et al., 1991) ERK1/2, (Khan et al., 2003; Palen et al., 2005) cSrc-type tyrosine kinase(Murphy et al., 2002) and integrins.(Davis et al., 2001; Martinez-Lemus et al., 2005; Martinez-Lemus et al., 2003; Yip and Marsh, 1997) In a previous study, we showed that microvascular myogenic tone is dependent on epidermal growth factor receptor tyrosine kinase (EGFRtk).(Belmadani et al., 2008a; Lucchesi et al., 2004) Thus, pressure-induced myogenic tone involves the activation of metalloproteinases 2/9 responsible for the heparin binding-epidermal growth factor like (HB-EGF like) shedding and subsequently EGFRtk transactivation.(Lucchesi et al., 2004) The Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway is an essential intracellular mechanism of cytokines and other growth factors that regulates cellular function, proliferation, and differentiation.(Yoshimura et al., 2007) Akt is the focal point for survival signals transduced via the PI3-kinase pathway. PI3-kinase/Akt pathway was studied mostly in flow-induced vasodilation.(LeBlanc et al., 2008) GRB2/SOS is ubiquitously expressed and entirely composed of one SH2 domain and two SH3 domains. It has been reported that GRB2/SOS interacts with different growth receptors including EGFR and regulate cellular function.(Li et al., 1994) Thus, the goal of the present study was to determine the signaling pathway and interaction between intracellular downstream signaling (JAK-STAT-Akt-GRb2/SOS-ERK1/2 MAP-Kinase) of the EGFRtk involved in myogenic tone in coronary arterioles.
METHODS
Materials
EGFR tyrosine kinase inhibitors (AG1478 “Sigma” and Erlotinib hydrochloride “Selleck Chemicals Co” 1 µM), JAK inhibitors (JAK inhibitor I, and JAK inhibitor II “Calbiochem” 1 µM), STAT3 inhibitors (STAT3 inhibitor V and STAT3 inhibitor III-WP1066 “Calbiochem”, 1 µM), PI3-kinase inhibitor (LY-294002, 10 µM) were purchased from Sigma. MEK inhibitor (U0126, 10 µM) was obtained from Calbiochem. GRB2/SOS inhibitor (SOS-SH3 domain inhibitor, 1 µM) was obtained from Santa Cruz Biotechnology, Inc. All antibodies were obtained from Cell Signaling and Promega. Vessels were incubated with drugs for 15 – 30 min. All drugs and molecular targets are conform to the British Journal Pharmacol’s Guide to receptor.(Alexander et al., 2008)
Myogenic tone of mice coronary arterioles
These studies conformed to the principles of the National Institutes of Health "Guide for the Care and Use of Laboratory Animals" and were approved by the Tulane University Institutional Animal Care and Use Committee. Coronary preparation, which should be quickly and carefully isolated, was performed as previously described.(Belmadani et al., 2008a; Lucchesi et al., 2004) Cannulated arterial segments were submerged in 10 mL of a physiological salt solution (pH 7.4) gasified with 10% O2, 5% CO2, and 85% N2.(Belmadani et al., 2008a; Palen et al., 2005; Palen et al., 2006)
Coronary arterioles were dissected, mounted onto 2 glass micropipettes in a vessel chamber, and slowly pressurized to 100 mm Hg by use of a pressure-servo-control perfusion (Living Systems Instruments, www.livingsys.com) to stretch the artery and set a constant artery length. Vessel diameter was continuously monitored by a video image analyzer. Cannulated arterial segments were submerged in 10 mL of physiological salt solution (pH 7.4) gasified with 10% O2, 5% CO2, and 85% N2. The functional integrity of the endothelial cell layer was assessed by testing the endothelium-dependent vasodilating effect of acetylcholine after precontraction with thromboxane analogue (U46619). Vessels not responding by full contraction and relaxation to thromboxane analogue and acetylcholine, respectively, were discarded. After a 45-minute equilibration period, diameter changes were measured when intraluminal pressure was increased from 25 to 125 mm Hg with and without inhibitors. Under these conditions, myogenic tone was observed within 1 minute. At the end of each experiment, arteries were perfused and superfused with a calcium-free physiological salt solution containing 2 mmol/L EGTA and 100 µmol/L sodium nitroprusside, and pressure steps were repeated to determine the passive diameter of the coronary arterioles. The experiments with potassium were performed on coronary arterioles under 50 mm Hg of intraluminal pressure.
Western blot analysis
Western blot analysis was performed as previously described.(Lucchesi et al., 2004) These coronary arterioles were pooled (3–5 arteries, 10 µg protein) for each condition so that Western blot analysis could be performed. Sample loading was assessed with total EGFR tyrosine kinase, total JAK, total STAT3 or total Akt. Western blot analysis was quantified using Fuji system (FujiFilm, MA, USA).
Cultured SMC isolated from coronary arterioles
We used cultured smooth muscle cells isolated from the same vascular bed (coronary arterioles) to further determine the interaction between the proposed signaling. For all experiments, VSMC at passages 3 to 8 were grown to 80% confluency in 10% FBS-DMEM with low glucose (5 mM), and then the growth was arrested for 48 hr in serum-free DMEM before the VSMC were stimulated with exogenous EGF (10 ng/ml) ± inhibitors. The primary cultured SMC isolated from coronary arterioles used in this study are not contractile. As alternative strategy to determine the contractile response, we determined the level of myosin light chain phosphorylation as a marker of contraction.
Immunoprecipitation
The immunoprecipitation was performed as previously published (Belmadani et al., 2008b; Palen et al., 2005). As an internal control, cell lysates were immunoprecipitated with non-specific antibody IgG and then immunoblotted with specific antibodies.
Statistical analysis
Results are expressed as mean±SEM, where n is the number of mice used. The significance of the differences between samples was determined by 1-repeated or 2-factor ANOVA, where appropriate. Differences were considered significant at P < .05.
RESULTS
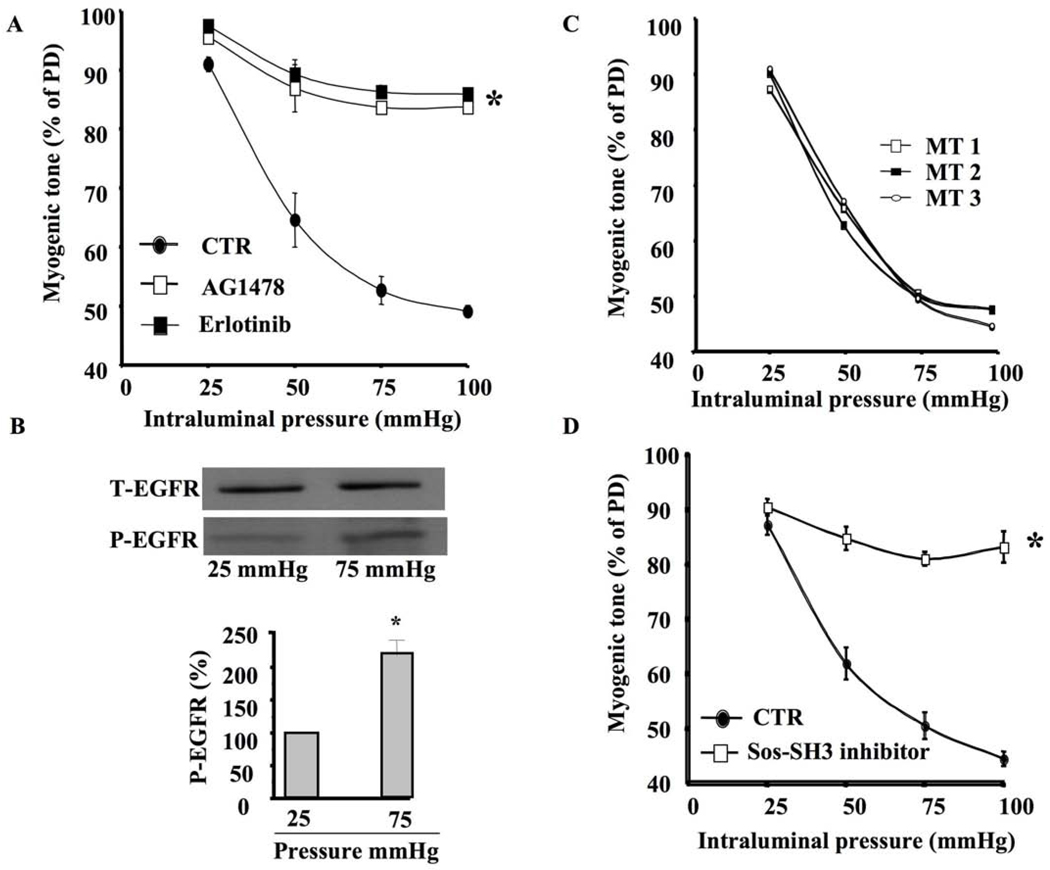
In freshly isolated and mounted coronary arterioles in arteriograph, stepwise-increased intraluminal pressure induced myogenic tone development, which was significantly inhibited when the coronary arterioles were pretreated with EGFR tyrosine kinase inhibitor (AG1478, 1 µM) (Figure 1A). Similar results were obtained using another EGFR tyrosine kinase inhibitor (Erlotininb, 1 µM) (Figure 1A). Western blot analysis indicated that coronary arterioles stimulated with 75 mm Hg of pressure displayed significant increase in EGFR tyrosine kinase phosphorylation compared to those stimulated with 25 mm Hg of pressure (Figure 1B). Equal loading was determined using total EGFR expression (Figure 1B). We performed time control experiments and data showed that myogenic tone is reproducible and stable (Figure 1C).
Figure 1. Myogenic tone of coronary arteriole, EGFR tyrosine kinase and GRB2/SOS.
1A: Pressure-induced myogenic tone in coronary arterioles with and without EGFR tyrosine kinase inhibitors (AG1478 and Erlotinib, 1 µM) n=6, *P<0.05 CTR vs. EGFR inhibitor; 1B: Western blot analysis showing phosphorylated (P)-EGFR tyrosine kinase and Total (T)-EGFR in coronary arterioles stimulated with intraluminal pressure 25 and 75 mm Hg n=4; 1C: time control experiments showing that coronary arteriole myogenic tone is reproducible and stable 1D: Pressure-induced myogenic tone in coronary arterioles with and without GRB2/SOS inhibitor (SOS-SH3 domain inhibitor, 1 µM) n=6, *P<0.05 CTR vs. GRB2/SOS inhibitor
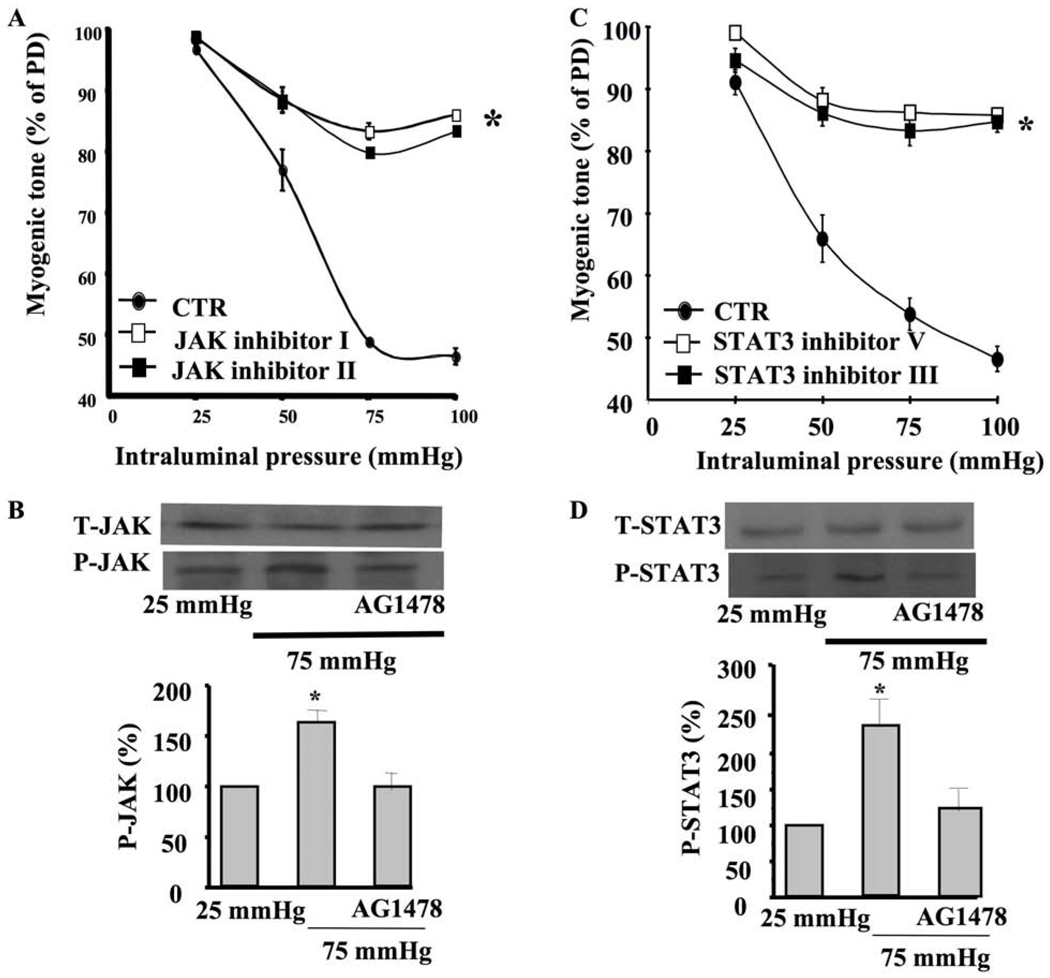
Myogenic tone of coronary arteriole was significantly inhibited when the artery was pretreated with GRB2/SOS, JAK, or STAT inhibitors (Figures 1D, 2A, 2C). Western blot analysis indicated that coronary arterioles under 75 mm Hg showed significant increases in JAK and STAT phosphorylation compared to those under 25 mm Hg (Figure 2B, 2D). Interestingly, the coronary arterioles pretreated with EGFR tyrosine kinase inhibitor significantly reduced JAK and STAT phosphorylation at 75 mm Hg (Figure 2B, 2D).
Figure 2. Coronary arteriolar myogenic tone, JAK and STAT3.
2A: Pressure-induced myogenic tone in coronary arterioles with and without JAK inhibitor (JAK inhibitor I and JAK inhibitor II, 1 µM) n=6, *P<0.05 CTR vs. JAK inhibitor; 2B: Western blot analysis and cumulative data showing phosphorylated (P)-JAK and Total (T)-JAK in coronary arterioles stimulated with intraluminal pressure 25 and 75 mm Hg ± EGFR tyrosine kinase inhibitor (AG1478, 1 µM) n=4; 2C: Pressure-induced myogenic tone in coronary arterioles with and without STAT3 inhibitor (STAT3 inhibitor V and STAT3 inhibitor III, 1 µM) n=6, *P<0.05 CTR vs. STAT3 inhibitor; 2D: Western blot analysis and cumulative data showing phosphorylated (P)-STAT3 and Total (T)-STAT3 in coronary arterioles stimulated with intraluminal pressure 25 and 75 mm Hg ± EGFR tyrosine kinase inhibitor (AG1478, 1 µM) n=4.
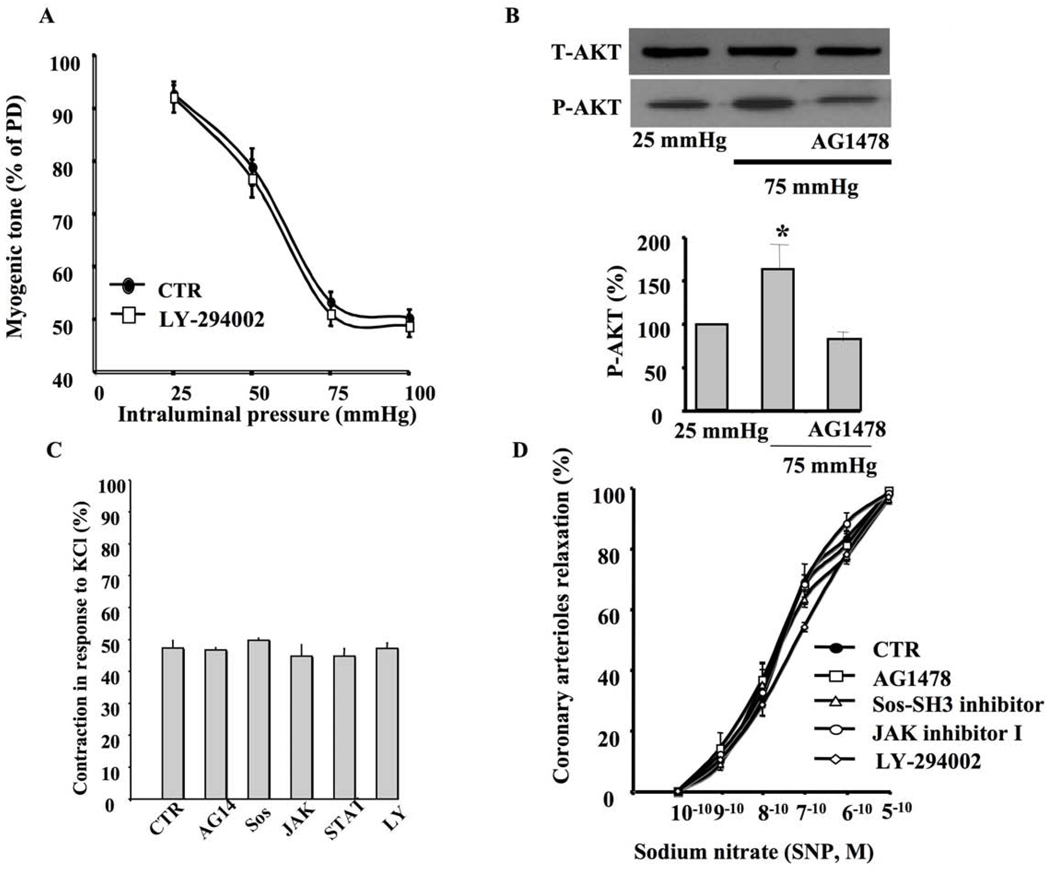
Inhibition of PI3Kinase did not affect myogenic tone of coronary arteriole in response to intraluminal pressure changes (Figure 3A). On the other hand, the coronary arterioles submitted to 75 mm Hg of intraluminal pressure displayed an increase in Akt phosphorylation (Figure 3B). Surprisingly, the coronary arterioles pretreated with EGFR tyrosine kinase inhibitor significantly reduced Akt phosphorylation in response to 75 mm Hg of intraluminal pressure (Figure 3B). Coronary arterioles were also subjected to KCl-induced contraction. The specificity of AG1478, Erlotininb, SOS -SH3 domain inhibitor, JAK inhibitor I, JAK inhibitor II, STAT3 inhibitor V, STAT3 inhibitor III and LY-294002 was tested using KCl contraction. Data revealed that these inhibitors did not affect KCl contraction (Figure 3C). The drugs used in this study did not affect the endothelium-independent relaxation of coronary arteriole to dose-response of exogenous nitric oxide donor sodium nitroprusside (Figure 3D).
Figure 3. Myogenic tone and Akt.
3A: Pressure-induced myogenic tone in coronary arterioles with and without PI3-kinase inhibitor (LY-294002, 10 µM) n=6, *P<0.05 CTR vs. PI3-kinase inhibitor; 3B: Western blot analysis and cumulative data showing phosphorylated (P)-Akt and Total (T)-Akt in coronary arterioles stimulated with intraluminal pressure (25 and 75 mm Hg) with and without EGFR tyrosine kinase inhibitor (AG1478, 1 µM) n=4; 3C: Coronary arteriole contraction in response to KCl with and without inhibitors (AG1478 “AG14”, SOS-SH3 inhibitor “SOS ”, JAK inhibitor I “JAK”, STAT inhibitor V “STAT” PI3-Kinase inhibitor “LY”) n=4; 3D: Relaxation of coronary arterioles in response to dose-response of sodium nitroprusside (SNP) after vessels were subjected to inhibitors n=3.
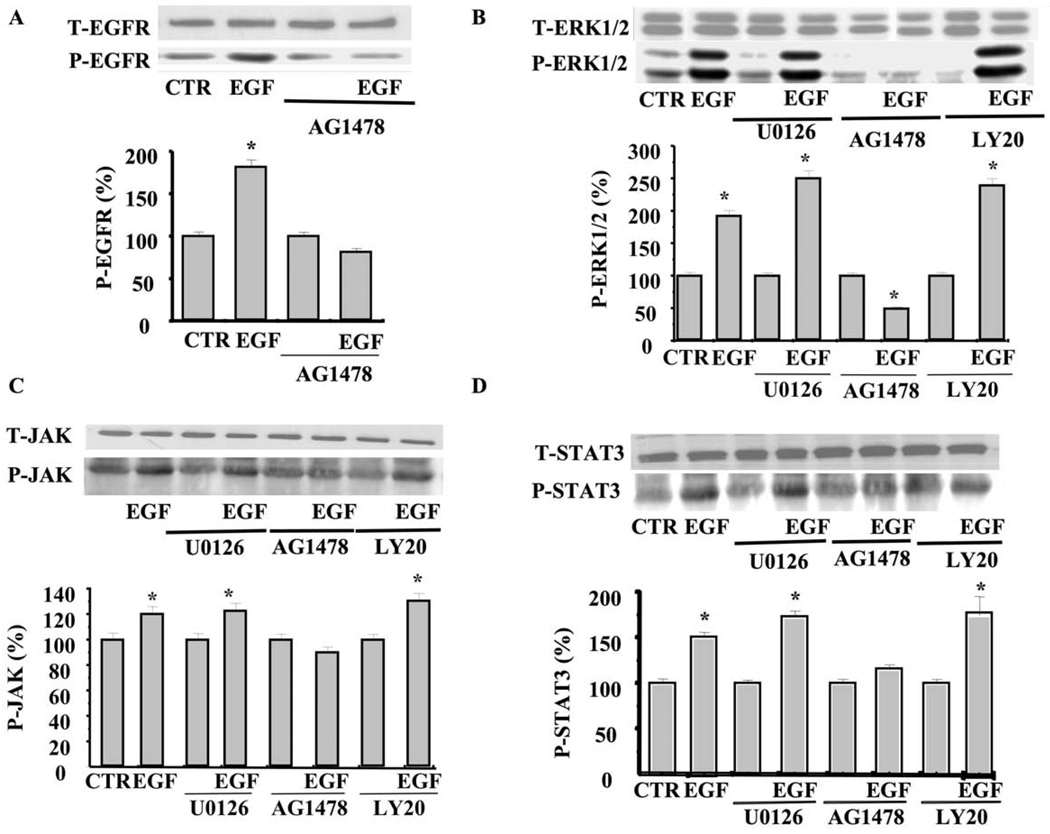
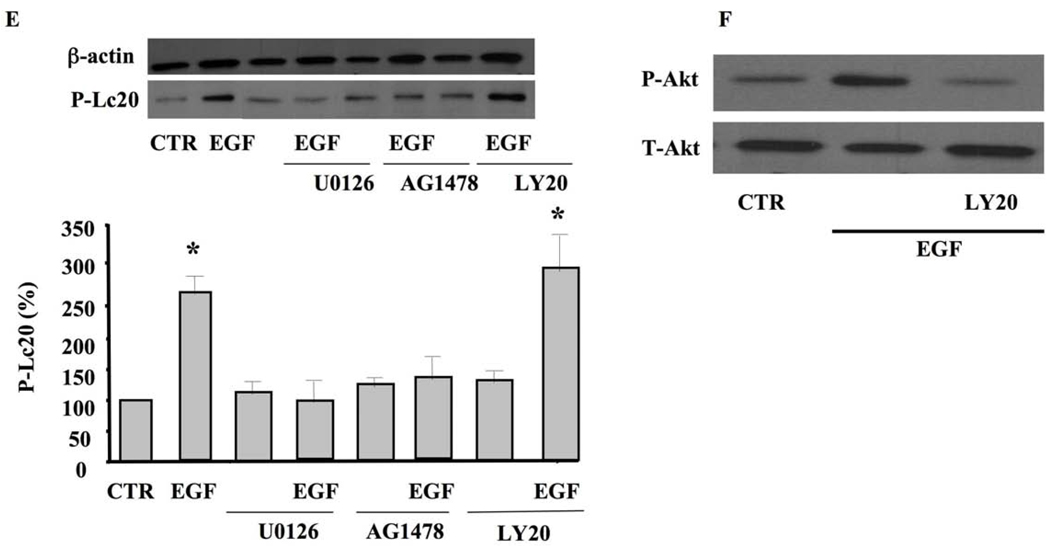
To determine the pathway and interaction between all these signaling events, we used cultured smooth muscle cells (SMC) isolated from mice coronary arterioles stimulated with exogenous EGF with and without inhibitors. Cultured SMC stimulated with exogenous EGF (10 ng/ml) for 5 min significantly increased EGFR tyrosine kinase phosphorylation, which was inhibited with AG1478 (Figure 4A). SMC stimulated with exogenous EGF significantly increased ERK1/2 MAP-kinase phosphorylation, which was independent of MEK and PI3-kinase (Figure 4B). On the other hand, ERK1/2 phosphorylation was significantly reduced when the SMC were pretreated with the EGFR tyrosine kinase inhibitor (AG1478, 1 µM) (Figure 4B). The application of EGF significantly increased JAK phosphorylation, which was not inhibited under the ERK1/2 MAP-kinase inhibitor (U0126, 1 µM) and the PI3-kinase inhibitor (LY-294002, 10 µM) (Figure 4C). STAT3 phosphorylation was significantly increased in response to EGF, which was independent of ERK1/2 and PI3-kinase inhibitors (Figure 4D). We also determined the effect of EGF ± inhibitors on myosin light chain phosphorylation (Lc20, marker of contraction). Figure 4E illustrates that exogenous EGF increases Lc20 phosphorylation, which was inhibited when SMC were pretreated with U0126, AG1478 but not LY-294002. SMC pretreated with PI3-kinase inhibitor (LY-294002, 10 µM) significantly reduced Akt phosphorylation level, but not total Akt, in response to 5 min stimulation with EGF (Figure 4F).
Figure 4. Western blot analysis in cultured SMC isolated from coronary arterioles.
4A: Western blot analysis and cumulative data showing phosphorylated (P)-EGFR tyrosine kinase and Total (T)-EGFR in SMC isolated from coronary arterioles stimulated with exogenous EGF ± EGFR tyrosine kinase inhibitor (AG1478, 1 µM) n=4; 4B: Western blot analysis and cumulative data showing phosphorylated (P)-ERK1/2 MAP-Kinase and Total (T)-ERK1/2 MAP-Kinase in SMC isolated from coronary arterioles stimulated with exogenous EGF ± EGFR tyrosine kinase inhibitor (AG1478, 1 µM), MEK inhibitor (U0126, 10 µM), PI3-kinase inhibitor (LY-294002, 10 µM), n=4–5 for each condition; 4C: Western blot analysis and cumulative data showing phosphorylated (P)-JAK and Total (T)-JAK in SMC isolated from coronary arterioles stimulated with exogenous EGF ± EGFR tyrosine kinase inhibitor (AG1478, 1 µM), MEK inhibitor (U0126, 10 µM), and PI3-kinase inhibitor (LY-294002, 10 µM) n=4–5 for each condition; 4D: Western blot analysis and cumulative data showing phosphorylated (P)-STAT3 and Total (T)-STAT3 in SMC isolated from coronary arterioles stimulated with exogenous EGF ± EGFR tyrosine kinase inhibitor (AG1478, 1 µM), MEK inhibitor (U0126, 10 µM), and PI3-kinase inhibitor (LY-294002, 10 µM) n=4–5 for each condition; 4E: Western blot analysis and cumulative data showing the Lc20 phosphorylation in SMC stimulated with exogenous EGF ± EGFR tyrosine kinase inhibitor (AG1478, 1 µM), MEK inhibitor (U0126, 10 µM), and PI3-kinase inhibitor (LY-294002, 10 µM) n=4 for each condition; 4F: Western blot analysis showing the total Akt (T-Akt) and phosphorylated Akt (P-Akt) in SMC stimulated with exogenous EGF ± PI3-kinase inhibitor (LY-294002, 10 µM) n=3 for each condition.
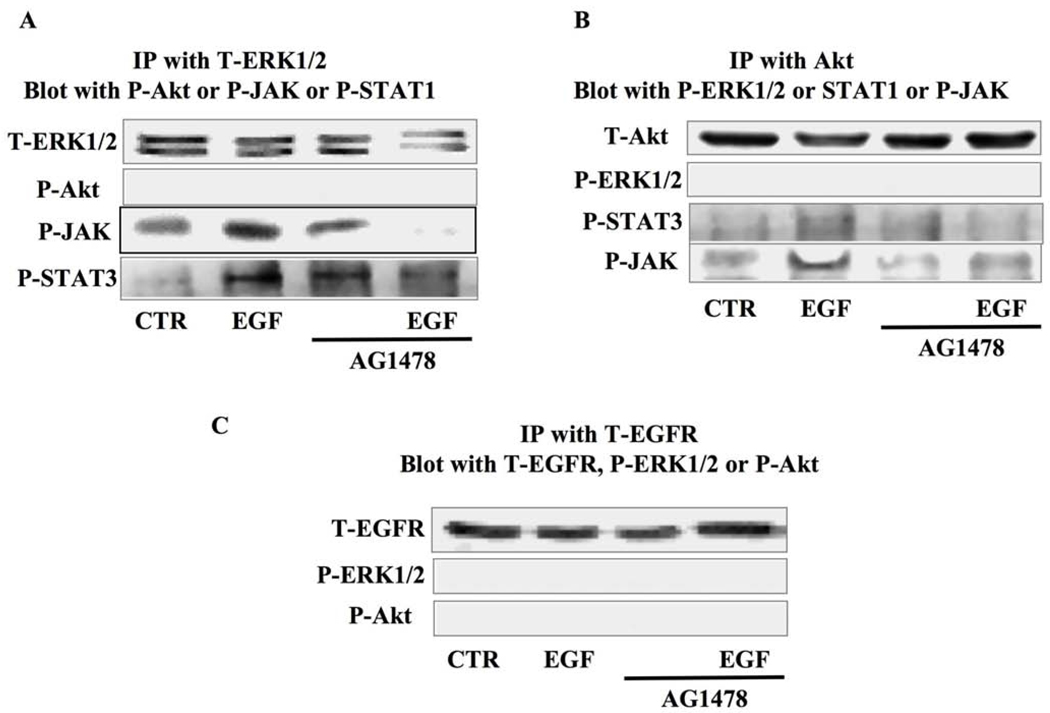
SMC stimulated with exogenous EGF with and without the EGFRtk inhibitor (AG1478) were subjected to immunoprecipitation (IP) and Western blot analysis. SMC were immunoprecipitated with the total ERK1/2 antibody and then blotted with phosphorylated Akt, JAK, or STAT antibodies. The data revealed the formation of a complex between ERK1/2-JAK-STAT3 under EGF stimulation, but not under EGFRtk inhibition (Figure 5A). When SMC were immunoprecipitated with total Akt and blotted with phosphorylated ERK1/2, JAK, or STAT3 antibodies, the data revealed the formation of a complex between Akt-JAK-STAT3 only under EGF stimulation (Figure 5B). The immunoprecipitation with the total EGFR antibody did not show any interaction between EGFRtk, ERK1/2, or Akt (Figure 5C). IgG was used as a negative control for immunoprecipitation and revealed no band (data not shown).
Figure 5. Immunoprecipitation and Western blot analysis in cultured SMC isolated from coronary arterioles.
5A: SMC lysate were immunoprecipitated with total ERK1/2 MAP-Kinase antibody and then blotted with total ERK1/2 MAP-Kinase, phosphorylated Akt, JAK, or STAT3 antibodies under control and EGF ± AG1478 (n=4–5); 5B: SMC lysate were immunoprecipitated with total Akt antibody and then blotted with total Akt, phosphorylated ERK1/2 MAP-Kinase, STAT3 or JAK antibodies under control and EGF ± AG1478 (n=4–5); 5C: SMC lysate were immunoprecipitated with total EGFR antibody and then blotted with total EGFR, phosphorylated ERK1/2 MAP-Kinase or Akt antibodies under control and EGF ± AG1478 (n=4–5).
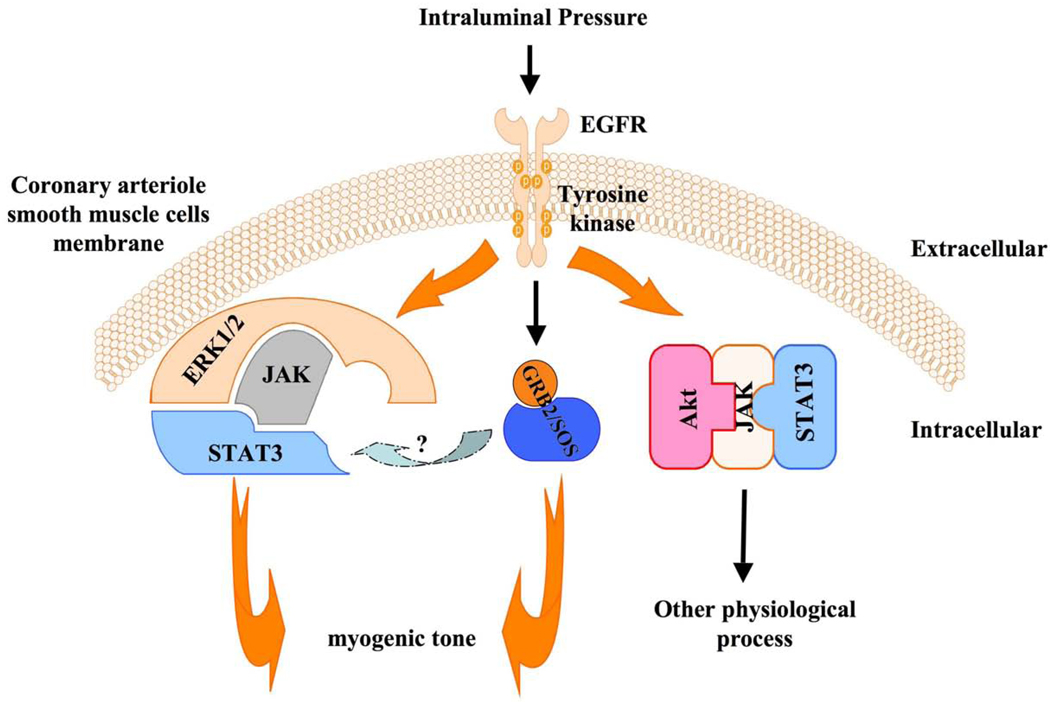
Figure 6 shows a model of the signaling involved in myogenic tone of coronary arteriole involving the EGFRtk, GRB2/SOS, and the complex ERK1/2-JAK-STAT3. Interestingly, the complex Akt-JAK-STAT3 complex formed under EGFRtk activation is not involved in myogenic tone of coronary arteriole (Figure 6).
Figure 6.
Figure 6 shows a model of intracellular signaling involved in myogenic tone of coronary arteriole including EGFR tyrosine kinase, GRB2/SOS and the complex ERK1/2-JAK-STAT3. Interestingly, the complex Akt-JAK-STAT3 formation under EGFR tyrosine kinase activation is not involved in coronary arteriolar myogenic tone. These data were obtained from pressurized coronary arterioles and cultured SMC isolated from coronary arterioles.
DISCUSSION
The main finding in the present study is that myogenic tone development of coronary arteriole in response to intraluminal pressure changes involves EGFR tyrosine kinase (EGFRtk), and down stream signaling GrB2/SOS, and the complex ERK1/2-JAK-STAT3. The formation of the complex Akt-JAK-STAT3, even downstream signaling to EGFRtk activation, is not involved in myogenic tone of coronary arteriole.
Myogenic tone occurs independently of the endothelium and perivascular nerves and is therefore an inherent property of vascular smooth cells.(Hill and Meininger, 1994; Liu et al., 1994; Matrougui et al., 1997) The intracellular signaling pathways dictating the myogenic constriction are not fully understood; however, the role of increases in intracellular calcium and myosin light chain phosphorylation has been largely demonstrated. In the present study, we investigated downstream intracellular signaling events (GRB2/SOS, Akt, JAK, STAT) of the EGFRtk and their interactions in myogenic tone of coronary arteriole.
In a previous study, we showed that mesenteric resistance artery myogenic tone is dependent on EGFRtk.(Lucchesi et al., 2004) Thus, pressure activates metalloproteinases 2/9 leading to heparin binding EGF-like shedding and subsequently transactivation of EGFRtk and development of myogenic tone (Lucchesi et al., 2004). We also reported an exacerbation of EGFRtk in type 2 diabetes responsible for endothelial and myogenic tone dysfunction of coronary arteriole.(Belmadani et al., 2008a) These data emphasize the importance of EGFRtk in the physio-pathology of microvascular tone. Since we established an important role for EGFRtk in microvascular tone in physiology and pathology, it is very critical to determine the intracellular signaling downstream of the EGFRtk involved in myogenic tone of coronary arteriole.
Previous work has shown that myogenic tone in mesenteric resistance arteries depends upon intracellular calcium and the activation of phospholipase C and protein kinase C.(Davis and Hill, 1999; Osol et al., 1991) We also showed that ERK1/2 MAP-kinase is involved in myogenic tone of resistance artery.(Palen et al., 2005) Our data are in agreement with previous reports showing that ERK1/2 MAP-kinase is critical in coronary myogenic tone. (Khan et al., 2003) On the other hand, The group of Dr. Hill reported temporal dissociation of MAP-Kinase phosphorylation from myogenic contractile response in intramuscular arteriole isolated from rat.(Spurrell et al., 2003) Other authors showed that microtubules and Rhokinase are important in resistance artery myogenic tone.(Matrougui et al., 2001; Platts et al., 2002) An important finding showed that actin polymerization in vascular smooth muscle cells is a requirement in the development of myogenic tone.(Cipolla et al., 2002) Integrins have also been proposed to play a role in resistance artery myogenic tone.(Davis et al., 2001) All these studies indicate the complexity of myogenic tone mechanisms. The intermediates signaling linking EGFRtk phosphorylated-mediated pathways to others signaling events (such calcium, myosin light chain phosphorylation…etc) are not yet clear.
In the present study, we also showed that myogenic tone of coronary arteriole is in fact dependent on EGFRtk activation, indicating that EGFRtk is a key element in microvascular myogenic tone development. Myogenic tone of coronary arteriole was significantly inhibited when the artery was pretreated with Grb2/SOS, JAK, or STAT3 inhibitors. Western blot analysis indicated that the coronary arterioles under 75 mm Hg of intraluminal pressure significantly increased JAK and STAT3 phosphorylation compared to the basal level. Interestingly, coronary arterioles pretreated with EGFRtk inhibitor significantly reduced JAK and STAT3 phosphorylation at 75 mm Hg of pressure. These data indicate that GRB2/SOS, JAK, and STAT are downstream signaling events of the EGFRtk and are critical in myogenic tone of coronary arteriole development.
Inhibition of PI3Kinase did not affect myogenic tone of coronary arteriole. On the other hand, coronary arterioles submitted to 75 mm Hg of pressure displayed an increase in Akt phosphorylation. Surprisingly, coronary arterioles pretreated with EGFRtk inhibitor significantly reduced Akt phosphorylation in response to 75 mm Hg of pressure. These data indicate that PI3Kinase-Akt pathway is also a downstream signaling event of the EGFRtk but not involved in myogenic tone of coronary arteriole.
To determine the interaction between these signaling events, we used cultured SMC isolated from coronary arterioles stimulated with exogenous EGF with and without different inhibitors. Cultured SMC stimulated with exogenous EGF displayed a significant increase in EGFRtk phosphorylation, which was blocked in the presence of the EGFRtk inhibitor. EGF was also able to increase ERK1/2 MAP-kinase phosphorylation, which was blocked with the EGFRtk inhibitor, indicating that ERK1/2 MAP-kinase is a downstream signaling of the EGFRtk. Surprisingly, ERK1/2 phosphorylation in response to EGF was independent of MEK1/2, the upstream activator of ERK1/2, since U0126 had no effect. Similar data were obtained illustrating that alpha-v-beta-3 integrin phosphorylates ERK1/2 MAP-kinase independently of the MEK1/2 inhibitor.(Rucci et al., 2005) In addition, inhibition of PI3-kinase did not affect ERK1/2 MAP-kinase phosphorylation in response to EGF, indicating that PI3-kinase is not upstream signaling of the ERK1/2. These data reveal the complexity of intracellular signaling involved in myogenic tone of coronary arteriole.
EGF significantly increased JAK phosphorylation, which was independent of ERK1/2 and PI3-Kinase-Akt pathway, but completely inhibited when SMC were pretreated with the EGFRtk inhibitor. These data indicate that JAK is downstream signaling of the EGFRtk but not of the ERK1/2 MAP-kinase and Akt. A similar pattern was observed for STAT3 signaling. Since primary cultured SMC from coronary arterioles do not display contractile phenotype, we determined the phosphorylation level of Lc20 as a marker of contraction. Lc20 phosphorylation was significantly increase in response to exogenous EGF indicating that activation of EGFRtk is involved in microvascular contraction similar to our previous study. (Belmadani et al., 2008a) The increased Lc20 phosphorylation in response to EGF was significantly reduced when SMC were pretreated with ERK1/2 MAP-Kinase and EGFR tyrosine kinase inhibitor. On the other hand, the inhibition of PI3-Kinase/Akt pathway did not affect the Lc20 phosphorylation indication that Akt is not involved in contraction of coronary arteriole in response to EGFRtk activation.
We also used immunoprecipitation and Western blot analysis to determine the interaction between these intracellular signaling pathways. Thus, SMC lysate was immunoprecipitated with a total ERK1/2 antibody and blotted with total ERK1/2, phosphorylated Akt, phosphorylated JAK, or phosphorylated STAT3. The data revealed that SMC stimulated with EGF show formation of a complex between ERK1/2-JAK-STAT3, and not Akt. This complex formation is absent under EGFRtk inhibition. SMC lysate was immunoprecipitated with the total Akt antibody and blotted with total Akt, phosphorylated ERK1/2, phosphorylated STAT3, or phosphorylated JAK antibodies. The data revealed the formation of a complex between Akt-STAT3-JAK but not ERK1/2 MAP-kinase under EGF stimulation. This interaction was absent when SMC were pretreated with the EGFRtk inhibitor. Since ERK1/2 MAP-kinase is critical in myogenic tone,(Khan et al., 2003; Palen et al., 2005) we strongly believe that myogenic tone of coronary arteriole involves the complex ERK1/2-JAK-STAT3 and not the complex Akt-JAK-STAT3. In another experiment, SMC lysate was immunoprecipitated with total EGFRtk and blotted with phosphorylated ERK1/2 or phosphorylated Akt. Data revealed no interaction between EGFRtk, Akt, and ERK1/2 suggesting that unknown intermediates signaling link EGFRtk to the complex ERK1/2-JAK-STAT3 and GRB2/SOS.
Figure 6 shows a model of intracellular signaling (GRB2/SOS, and the complex ERK1/2-JAK-STAT3 downstream of the EGFRtk) involved in myogenic tone of coronary arteriole. Interestingly, the formation of the complex Akt-JAK-STAT under EGFRtk activation is not involved in myogenic tone of coronary arteriole (Figure 6), and could be involved in other physiological response to pressure changes, which indicate the complexity and the presence of intracellular signaling compartmentation. The link between these signaling (EGFRtk, ERK1/2 MAP-Kinase, STAT, JAK and GRB2/SOS) and for instance intracellular calcium, acidosis, calcium channels, stretch activated channels, integrins and protein kinase C is not known. Therefore, it would be interesting to determine the intracellular interactions to further understand the mechanisms dictating coronary myogenic tone.
These results identify a new intracellular signaling pathway involved in the control of microvascular function and therefore may lead to the discovery of therapeutic targets that could have important implications in cardiovascular disease.
Acknowledgments
N/A
Sources of Funding
We acknowledge grant support from National Institutes of Health (1R01HL095566; PI: Dr. Matrougui) (P20RR017659; PI: Dr. Navar).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
REFERENCES
- Alexander SP, et al. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153 Suppl 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmadani S, et al. Elevated Epidermal Growth Factor Receptor Phosphorylation Induces Resistance Artery Dysfunction in Diabetic db/db mice. Diabetes. 2008a doi: 10.2337/db07-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmadani S, et al. Microvessel vascular smooth muscle cells contribute to collagen type I deposition through ERK1/2 MAP kinase, alphavbeta3-integrin, and TGF-beta1 in response to ANG II and high glucose. Am J Physiol Heart Circ Physiol. 2008b;295:H69–H76. doi: 10.1152/ajpheart.00341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ, et al. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. Faseb J. 2002;16:72–76. doi: 10.1096/cj.01-0104hyp. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- Davis MJ, et al. Integrins and mechanotransduction of the vascular myogenic response. Am J Physiol Heart Circ Physiol. 2001;280:H1427–H1433. doi: 10.1152/ajpheart.2001.280.4.H1427. [DOI] [PubMed] [Google Scholar]
- Hill MA, Meininger GA. Calcium entry and myogenic phenomena in skeletal muscle arterioles. Am J Physiol. 1994;267:H1085–H1092. doi: 10.1152/ajpheart.1994.267.3.H1085. [DOI] [PubMed] [Google Scholar]
- Hwa JJ, Bevan JA. Stretch-dependent (myogenic) tone in rabbit ear resistance arteries. Am J Physiol. 1986;250:H87–H95. doi: 10.1152/ajpheart.1986.250.1.H87. [DOI] [PubMed] [Google Scholar]
- Khan TA, et al. Mitogen-activated protein kinase inhibition and cardioplegia-cardiopulmonary bypass reduce coronary myogenic tone. Circulation. 2003;108 Suppl 1:II348–II353. doi: 10.1161/01.cir.0000087652.93751.0e. [DOI] [PubMed] [Google Scholar]
- LeBlanc AJ, et al. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol. 2008;295:H2280–H2288. doi: 10.1152/ajpheart.00541.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, et al. A new function for a phosphotyrosine phosphatase: linking GRB2-Sos to a receptor tyrosine kinase. Mol Cell Biol. 1994;14:509–517. doi: 10.1128/mcb.14.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, et al. Mechanisms of myogenic enhancement by norepinephrine. Am J Physiol. 1994;266:H440–H446. doi: 10.1152/ajpheart.1994.266.2.H440. [DOI] [PubMed] [Google Scholar]
- Lucchesi PA, et al. Involvement of metalloproteinases 2/9 in epidermal growth factor receptor transactivation in pressure-induced myogenic tone in mouse mesenteric resistance arteries. Circulation. 2004;110:3587–3593. doi: 10.1161/01.CIR.0000148780.36121.47. [DOI] [PubMed] [Google Scholar]
- Martinez-Lemus LA, et al. alphavbeta3- and alpha5beta1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol Heart Circ Physiol. 2005;289:H322–H329. doi: 10.1152/ajpheart.00923.2003. [DOI] [PubMed] [Google Scholar]
- Martinez-Lemus LA, et al. Integrins as unique receptors for vascular control. J Vasc Res. 2003;40:211–233. doi: 10.1159/000071886. [DOI] [PubMed] [Google Scholar]
- Matrougui K, et al. Impaired nitric oxide- and prostaglandin-mediated responses to flow in resistance arteries of hypertensive rats. Hypertension. 1997;30:942–947. doi: 10.1161/01.hyp.30.4.942. [DOI] [PubMed] [Google Scholar]
- Matrougui K, et al. Involvement of Rho-kinase and the actin filament network in angiotensin II-induced contraction and extracellular signal-regulated kinase activity in intact rat mesenteric resistance arteries. Arterioscler Thromb Vasc Biol. 2001;21:1288–1293. doi: 10.1161/hq0801.093653. [DOI] [PubMed] [Google Scholar]
- Murphy TV, et al. Cellular signalling in arteriolar myogenic constriction: involvement of tyrosine phosphorylation pathways. Clin Exp Pharmacol Physiol. 2002;29:612–619. doi: 10.1046/j.1440-1681.2002.03698.x. [DOI] [PubMed] [Google Scholar]
- Osol G, et al. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circ Res. 1991;68:359–367. doi: 10.1161/01.res.68.2.359. [DOI] [PubMed] [Google Scholar]
- Palen DI, et al. Role of SHP-1, Kv.1.2, and cGMP in nitric oxide-induced ERK1/2 MAP kinase dephosphorylation in rat vascular smooth muscle cells. Cardiovasc Res. 2005;68:268–277. doi: 10.1016/j.cardiores.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Palen DI, et al. Hydrogen peroxide acts as relaxing factor in human vascular smooth muscle cells independent of map-kinase and nitric oxide. Front Biosci. 2006;11:2526–2534. doi: 10.2741/1987. [DOI] [PubMed] [Google Scholar]
- Platts SH, et al. Microtubule-dependent regulation of vasomotor tone requires Rho-kinase. J Vasc Res. 2002;39:173–182. doi: 10.1159/000057765. [DOI] [PubMed] [Google Scholar]
- Rucci N, et al. A novel protein kinase C alpha-dependent signal to ERK1/2 activated by alphaVbeta3 integrin in osteoclasts and in Chinese hamster ovary (CHO) cells. J Cell Sci. 2005;118:3263–3275. doi: 10.1242/jcs.02436. [DOI] [PubMed] [Google Scholar]
- Spurrell BE, et al. Intraluminal pressure stimulates MAPK phosphorylation in arterioles: temporal dissociation from myogenic contractile response. Am J Physiol Heart Circ Physiol. 2003;285:H1764–H1773. doi: 10.1152/ajpheart.00468.2003. [DOI] [PubMed] [Google Scholar]
- Yip KP, Marsh DJ. An Arg-Gly-Asp peptide stimulates constriction in rat afferent arteriole. Am J Physiol. 1997;273:F768–F776. doi: 10.1152/ajprenal.1997.273.5.F768. [DOI] [PubMed] [Google Scholar]
- Yoshimura A, et al. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]