Abstract
Shape memory polymer stent prototypes were fabricated from thermoplastic polyurethane. Commercial stents are generally made of stainless steel or other alloys. These alloys are too stiff and prevent most stent designs from being able to navigate small and tortuous vessels to reach intracranial lesions. A solid tubular model and a high flexibility laser etched model are presented. The stents were tested for collapse in a pressure chamber. At 37°C, the full collapse pressure was comparable to that of commercially available stents, and higher than the estimated maximum pressure exerted by intracranial arteries. However, there is a potential for onset of collapse, which needs further study. The stents were crimped and expanded, the laser-etched stent showed full recovery with an expansion ratio of 2.7 and a 1% axial shortening.
Keywords: vascular stents, polyurethane(s), in vitro, mechanical properties
INTRODUCTION
Shape memory polymers (SMPs) have been of particular interest because of their ability to recover a predetermined shape on controlled heat activation.1 Molded into a primary shape, they can be deformed into a secondary shape when heated above their glass transition temperature and remain in that shape after cooling. Reheating the material above the glass transition temperature results in the recovery of the primary shape. Because SMP research in the last decade has resulted in better control of the polymer’s transition temperatures, moduli, and recovery ratios these materials have found a variety of applications ranging from textiles to ergonomic utensils.2 In the medical field, they are currently investigated for applications as clot extraction devices, 3–6 in aneurysm embolization,7 and as vascular stents.8,9
Stents are now widely used after coronary,10 carotid,11 and iliac12 angioplasty to prevent acute vessel occlusion and late restenosis. The vast majorities of stents are currently made of stainless steel and shape memory alloys (SMAs). However, these materials remain too stiff to be delivered to the small and highly tortuous vessels of the neurovasculature, and there is a need for such devices to treat intracranial stenosis.13,14
Our previous investigations on SMP polyurethanes have led us to believe that stents made of this material would offer the longitudinal flexibility and the high shape recovery ratios needed for access and delivery into the neurovasculature. 15 SMP stents can be molded into a primary shape of the size of the target artery; they can then be crimped into a smaller secondary shape and mounted on a delivery system, inserted into an artery, and navigated to the lesion site, where they can finally be thermally actuated to recover their primary shape. SMP materials possess large recovery ratios that would allow high radial expansion ratios; they can be engineered such that they show minimal axial shortening on actuation. In addition, because of their lower modulus, polymer stents might be more conformable and exhibit less compliance mismatch between the arterial wall and the stent than their metallic counterparts. Compliance mismatch has been linked to adverse effects, including restenosis (renarrowing of the vessel).16,17 However, improved compliance matching between stent and vessel should not sacrifice intimate apposition of the stent to the intimal wall. Finally, in addition to their ease and low cost of fabrication in 3D batches, SMP stents could potentially be used as drug delivery platforms by directly embedding bioactive agents during their fabrication, as opposed to metallic stents, which require coating by drug releasing agents.18
In this study, we propose a stent prototype made of thermoplastic Mitsubishi SMP polyurethane material with a glass transition temperature of 75°C. Previous studies have reported the use of light exposure,19,20 electric current,21 or magnetic fields22 as heat sources to deploy SMP materials. The biocompatibility of polyurethanes has already been demonstrated for use as catheters23 and heart valves,24 and their surface can readily be modified for drug elution.25,26 In vitro and in vivo studies by Metcalfe et al.7 of the same Mitsubishi polyurethane materials used as foams for endovascular interventions have recently shown the material to be poorly thrombogenic and noncytotoxic. Based on our in vitro studies, biocompatibility as defined by inflammation, thrombogenesis, and the activation of either platelets and neutrophils, the thermoset- and thermoplastic-SMP materials are unlikely to stimulate a negative response in vivo.27 Animal studies with implanted stents are also under way.
In this article, we report relevant thermomechanical properties of the material toward the development of a neurovascular stent. Tubular stents were prepared: a solid model, and a laser etched model with high longitudinal flexibility. Thermal transitions were characterized by differential scanning calorimetry (DSC), and dynamic mechanical properties were analyzed by dynamic mechanical thermal analysis (DMTA). Because the main function of stents is to support the arterial walls of the vessel in which they are placed, the external pressure at which the stent collapses is of importance, especially with flexible materials such as SMPs. Since Agrawal et al.’s PLLA stent,28 several other authors have reported collapse pressures of commercially available or prototype stents.29–35 Here, because the material’s mechanical properties vary as a function of temperature, collapse pressure of the stent models was measured between 32 and 78°C, using an in-house built pressure chamber. Commercially available stents were also collapsed for comparison purposes. Finally, stent crimping and expansion were evaluated.
MATERIALS AND METHODS
Materials
SMPs
The SMP used in this study was MM7520 polyurethane obtained from DiAPLEX Company. (a subsidiary of Mitsubishi Heavy Industries) as thermoplastic resin in the form of pellets. The nominal first glass transition Tg of this material, as indicated by the manufacturer, is 75°C. Although the compositions are proprietary, it is known that the material is a segmented polyurethane, which has a microphase separated morphology.36 The primary shape is formed at a temperature above the highest glass (Tgh) or crystalline (Tm) transition and the polymer is cooled to fix the shape. Alternatively, the polymer can be dissolved in an appropriate solvent and cast into a mold or coated onto a surface. The secondary shape is obtained by heating the material above the glass transition temperature of the soft phase (Tgs) or the soft phase crystalline melting temperature (Tms), applying a strain to the material, and cooling it down below the same soft phase temperature to fix the shape. The deformation is stored elastically as macromolecular chain orientation within the soft phase, while hard phase segments are relatively unperturbed by this stress. Thus, there is an entropic potential for shape recovery. By heating the material above Tgs or Tms, soft phase segments spring back to their lower entropic state, resulting in recovery of the primary shape.
SMP Neat Material Sample Preparation
Test specimens were prepared from the as received thermoplastic SMP MM7520 pellets for DSC and DMTA testing as follows. The pellets were first vacuum dried (40 mTorr, 50°C) for 120 h. They were then compression molded into plaques with dimensions 3.2 mm by 100 mm by 100 mm using a Carver hot press (200°C, 15,000 pounds, 10 min). The pressed plaques were quickly cooled by placement on an aluminum block, demolded, stored in plastic bags, and cut into 1-cm strips for testing, according to ASTM D3418 for DSC, and ASTM D4065 for DMTA. DSC data from the stents were obtained directly by sacrificing several stent prototypes.
Stent Preparation
Pellets of MM7520 materials were first dissolved into THF resulting in a composition of 17.5% by weight of polymer in solvent. The stents were then prepared by dip coating multiple layers of the solution onto 4-mm stainless steel pins precoated with Fluoropel PFC601FA. Four layers were applied, allowing drying at 50°C for 30 min and cooling for 10 min after each layer. After application of the final layer, the coated pins were dried for 24 h at 50°C under vacuum. After cooling, some of the stents were laser etched to create a mesh pattern. In this design, rings are connected by S-shaped struts, offering a high longitudinal flexibility. Figure 1 shows the prototype tubular stent and details of the laser-etched stent. The surface area of the laser-etched stent was 50% of the solid tube stent. All stents were removed from the pins and stored at room temperature in sealed containers before testing. Final dimensions of the stents tested in the collapse chamber were ~4.0 mm outer diameter, 250 µm thickness, and 18 mm length. The stent’s diameters decreased slightly after removal from the pin due to residual stress in the SMP.
Figure 1.
(a) Prototype 4-mm diameter tubular stent (b) Details of the pattern of the 4-mm diameter high flexibility laser etched stent.
PDMS Tubes
PDMS tubes were used to hold the stent in place in the collapsing chamber. These cylindrical tubes were prepared by dip coating pins in Sylguard 184 preparation; each layer was dried for 1 h by spinning the pins in a 50°C oven equipped with a rotisserie system. Final dimensions of the PDMS tubes were 4 mm ID and 150 µm thickness.
Collapsing Chamber
A pressure chamber, shown in Figure 2, was built based on the model developed by Agrawal et al.28 to perform the collapsing experiments. It consists of an outer Plexiglas cylindrical chamber containing two stainless steel tubes connected by the PDMS tube described above. The stent fits inside the PDMS tube, which can be compressed by increased pressure in the outside chamber. Isolating material can be fit around the outer chamber. Water from a water bath can be pumped into the outer chamber and into the PDMS tube independently. Two pressure transducers read the pressure in the PDMS tube and in the outer chamber. A thermocouple probe allows reading of the temperature in the outer chamber. The PDMS tube is filled with water and connected to a straight tube opened to atmospheric pressure on top, while the outer chamber is pressurized by injecting water at a desired temperature using a peristaltic pump or an automatic syringe pump. The T type thermocouple probe is connected to a thermocouple reader and the pressure transducers are connected to a data acquisition system which allows continuous recording of pressure and time during the experiments.
Figure 2.
Schematic of the pressure chamber used in the collapsing experiments. SS, stainless steel supporting tube; OC, outer chamber filled with water (enveloping isolating material not shown); S, stent inside PDMS tube; PDMS, PDMS tube; P, pressure transducers; T, thermocouple; DAS, data acquisition system; ASP, automatic syringe pump; PP, peristaltic pump; WB, water bath.
Experimental Procedures
Thermomechanical Characterization of SMPs
DSC measurements were made using a Perkin Elmer Diamond DSC equipped with an Intracooler over a temperature range of −20 to 200°C using a heating rate of 20°C/min, with 5 ± 1 mg samples, consistent with ASTM D3418. Two heating/cooling cycles were used and the Tg’s from both heating cycles are reported as the average of 2 repeats.
DMTA measurements were made in an ARES LS2 under dynamic oscillatory mode at a frequency of 1 Hz and initial strain of 0.01%. Testing was done under dry air, from 25 to 120°C at a linear ramp rate of 1°C/min, consistent with standard D4065. Torsion rectangles of 3.2 mm by 12 mm by 50 mm were used as testing samples. The storage shear modulus (G′), the loss modulus (G″), and the ratio of loss to storage modulus (tanδ) were plotted as a function of temperature; Tg was determined from the tanδ peak, and the glassy and rubbery moduli were read from the G′ curve (two repeats).
Collapse Pressure of SMP Stents
Stents were mounted into the PDMS tube held in place by the two stainless steel cylinders. Water at an independently controlled temperature was continuously run into the PDMS tube and the outer chamber until a desired steady state temperature was reached. The outer chamber was then closed off, and the PDMS tube opened to atmospheric pressure. Water at the testing temperature was injected into the outer chamber. The time of onset and full collapse were recorded when visualized. Curves of outer chamber pressure and temperature versus time were generated, and the collapse points were read according to the PDMS tube pressure, which increased at the beginning of collapse, and reached a plateau at full collapse. The time of onset and full collapse recorded manually were used to retrieve the pressure when the plateau transition was difficult to observe, especially at lower temperatures where collapse was more gradual. Collapse pressures were recorded at temperatures ranging from 32 to 78°C and collapse pressure versus time curves were generated (five samples).
Stent Crimping and Deployment
Stents were crimped using a Balloon Wrapping Fixture model W8FH from Interface Associates (Laguna Niguel, CA). The machine consists of eight blades radially arranged with a built in electric heater connected to an eight station temperature and diameter controller. The fixture is shown in Figure 3. The expanded stents were inserted into the machine, the temperature was increased to 200°F (93°C) and the stents were left at this temperature for 2 min before crimping. After crimping, the stents were left 2 min at high temperature, then cooled down to 70°F (21°C) and left at this temperature for 2 more minutes before being removed from the machine. The stents dimensions were then measured with a caliper. Expansion was done in a temperature controlled hot bath and dimensions were measured again with a caliper.
Figure 3.
Picture of the crimper showing the eight blades used to radially crimp the stents.
RESULTS
Glass Transition and Dynamic Measurements
Neat material, tubular stents, and laser etched stents were made of MM7520 (manufacturer’s Tg of 75°C) and their thermal transitions and moduli were measured. DMTA measurements of the stents were not obtained because the samples’ geometry was not suited for the instrument. Results are shown in Table I. The neat material exhibits glass transition temperatures close to the manufacturer’s reported Tg as measured by both DSC and DMTA. The solid and the laser etched stents show similar but lower Tgs than expected, especially in the first heat, where a 25°C decrease compared to manufacturer’s Tg is observed. The Tg obtained during the second heat approaches the nominal Tg, being ~5°C lower. The glassy plateau modulus and the rubbery plateau modulus for the neat material were respectively 7.7E8 Pa (G′ at Tg − 25°C) and 1.8E6 Pa (G′ at Tg + 25°C), with an elastic ratio (G′ (Tg − 25°C)/G′ (Tg + 25°C)) of 428. It has been reported that a high glassy modulus correlates with high shape fixity during simultaneous cooling and unloading, and high elasticity ratios above 100 allow for great resistance to deformation and high shape recovery.25
TABLE I.
DSC and DMTA Results Showing Tg, Glassy, and Rubbery Modulus
| DSC Data | DMTA Data | ||||
|---|---|---|---|---|---|
| Material/Stent Type |
Tg 1st heat (°C) |
Tg 2nd heat (°C) |
Tg from tan δ (°C) |
Glassy Plateau Modulus (Pa) |
Rubbery Plateau Modulus (Pa) |
| Neat Material | 74.2 | 74.4 | 74.6 | 7.7E + 08 | 1.8E + 06 |
| Solid tube | 49.3 | 70.9 | |||
| Laser-etched stent | 51.8 | 67.7 | |||
DMTA data are not available for the stents as the geometry was not suited for the instrument.
Collapse Pressure
While in place in the artery, the stent exerts what is generally referred to as a radial resistive force.29 It is the force developed within the material resisting the external pressure of the arterial wall. Radial force has been expressed as %compression strain/compressive stress,37 but publications reporting such data are rare. Besides, perhaps a more important property in terms of engineering design and efficacy is the maximum compressive force that the stent can withstand before collapsing, referred to as the collapse pressure. Several authors report experimental measurements of this pressure for the purpose of comparing coronary stents,29–33 and for biodegradable stents.28,34,35 Most of these authors use a value for the maximum compressive force generated by an artery proposed by Agrawal et al.,28 who used the maximal transmural pressure of epinephrine stimulated canine arteries measured by Cox,38 that is 200 to 275 mmHg. Assuming a normal average intraluminal pressure in the order of 100 mmHg, they found a pressure difference between outside and inside the artery of 175 mmHg. Using a “factor of safety,” they concluded that 300 mmHg (5.8 psi) was the minimum acceptable collapse pressure for coronary stents.
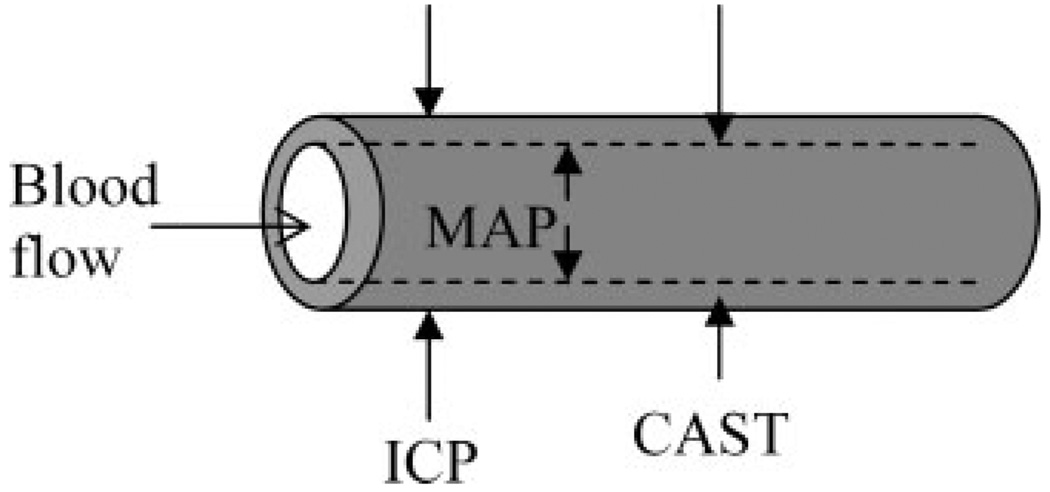
To our knowledge, there is no reference value for the maximum compressive force exerted by intracranial arteries, which is a more relevant number in our study. To evaluate the collapsing pressure in cerebral arteries, the simplified model of an intracranial artery, as shown in Figure 4, can be useful. The intraluminal pressure is approximated by the mean arterial pressure (MAP); external pressures include intracranial pressure (ICP) and cerebral artery smooth muscle cell tone (CAST). Note that this is a simplistic model that does not simulate the exact in-vivo environment of the stent. Intraluminal arterial pressure and the pressure due to smooth muscle cell tone vary between systole and diastole. In addition, vessels are nonsymmetric and nonuniform in their dimensions and mechanical properties, thus, stent expansion creates further local variations in stress distribution; this likely creates torsional stresses which are not accounted for in this model. However, it is a good first approximation for these bench side experiments, providing a reference number for comparing maximum collapse pressures between different stents.
Figure 4.
Forces exerted on an intracranial artery. The intraluminal force is created by mean arterial pressure (MAP); extraluminal forces are generated by intracranial pressure (ICP) and cerebral artery smooth muscle cell tone (CAST).
It turns out that the highest compressive forces on brain vessels are generated during cerebral vasospasm, a constriction of intracranial arteries following subarachnoid hemorrhage (SAH).39 Nagasawa et al. have studied the contractility of spastic basilar arteries following SAH in dogs.40 They harvested arterial segments post SAH and measured the external diameter when increasing intraluminal pressure in a serotonin solution. They found that the highest intraluminal pressure below which the arterial wall exhibited little distention was 220 mmHg at 7 days, and they attributed this phenomenon to smooth muscle cell tone. Beyond this flexion point, the vessel diameter rapidly increased with increasing intraluminal pressure, indicating that the smooth muscle cell tone could no longer provide enough constriction to keep the diameter constant. Thus, we can approximate CAST during vasospasm to 220 mmHg.
The sum of the external pressures CAST and ICP has been called critical closing pressure (CCP),41 and much work has been done to evaluate it. Soehle et al. have measured CCP in vivo in SAH patients and have concluded that nonlinear hemodynamics seem to lead to lower numbers than expected. However, they were able to measure intracranial pressure during vasospasm in a certain number of patients, and found an ICP of 15.5 ± 5.9 mmHg.39 We can approximate the highest ICP during cerebral vasospasm to 21.4 mmHg. Thus, the overall external pressure, consisting of ICP and CAST during cerebral vasospasm is about 242 mmHg, or 4.7 psi.
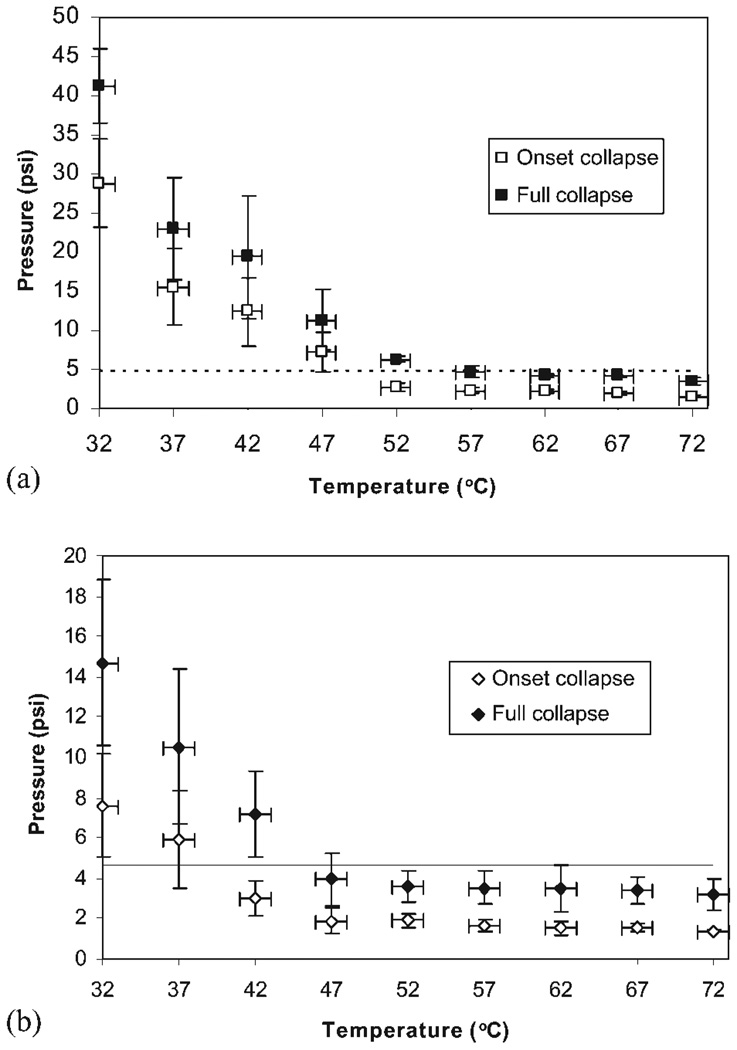
Collapse pressure versus temperature curves were generated from the average of three data points at each temperature, with increments of 5°C, starting at 32°C. Figure 5 shows collapse of the solid tubular stent (a) and of the laser etched stent (b). The stents were seen to collapse into flat rectangles, with collapse starting in the center or on one end. As expected, collapse pressure of the solid tubular stent is higher than that of the laser-etched stent. In addition, collapse pressure rapidly decreases with increasing temperature between 32 and 47°C, reaching a plateau above 47°C. At 37°C, the solid tubular stent starts to collapse at 15.5 psi and reaches full collapse at 23 psi. The laser-etched stent exhibits lower collapse pressures, beginning at 5.9 psi and ending at 10.5 psi. Note the large error (standard deviation of three points) on the pressure at lower temperatures, due to the variation in visually determining collapse as it occurred very slowly.
Figure 5.
Collapse pressure versus temperature for the solid tubular stent (DSC first heat Tg = 49.3°C) (a) and the laser etched stent (DSC first heat Tg = 51.8°C) (b). The dashed line at 4.7 psi represents the maximum pressure exerted by a vasospastic artery.
For comparison purposes, we collapsed two 4-mm diameter commercially available coronary stents at 37°C; the 15-mm long cobalt chromium multi-link vision (Guidant Corporation, Santa Clara, CA) started collapsing at 10.8 psi, and finished at 13.6 psi. The 13-mm long 316L stainless steel BxVelocity (Cordis, Warren, New Jersey) exhibited a collapse onset at 26.3 psi, and full collapse at 36.4 psi. The solid tubular SMP stent showed higher full collapse pressure than the Multi-link vision stent, while the BxVelocity’s radial force is unequivocally higher. We also gathered published data on collapse pressure of commercially available stents. A summary of stents’ dimensions, when available, and their collapse pressures is shown in Table II.
TABLE II.
Collected Data on Collapse Pressure of Commercially Available and Prototype Stents
| Stent Type | Manufacturer | Material | Diameter (mm) |
Length (mm) |
Thickness (mm) |
Collapse Pressure (psi) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Onset | Full | |||||||
| Solid tubular | LLNL | Polyurethane | 4 | 18 | 0.25 | 15.5 | 23 | |
| Laser etched | LLNL | Polyurethane | 4 | 18 | 0.25 | 5.9 | 10.5 | |
| Multi-link Vision | Guidant | Cobalt Chromium | 4 | 15 | 0.1 | 10.8 | 13.6 | |
| BxVelocity | Cordis | Steel 316L | 4 | 13 | 0.22 | 26.3 | 36.4 | |
| Wiktor | Medtronic | Tantalum | 3.5 | 16 | 10.1 | 30 | ||
| Tenax Complete | Biotronik | Steel 316L | 3.5 | 15 | 7.7 | 30 | ||
| NIR Primo | Scimed | Steel 316L | 3.5 | 16 | >21.8 | 30 | ||
| Crossflex | Cordis | Stainless steel | 3.5 | 15 | 8.7 | 31 | ||
| BeStent Brava | Medtronic | Stainless steel | 3.5 | 15 | 14.5 | 31 | ||
| Tenax XR | Biotronik | Steel 316L | 3.5 | 15 | 8.7 | 31 | ||
| Wiktor | Medtronic | Tantalum | 3 | 15 | <4.3a | 11.6 | 29 | |
| Crossflex | Cordis | Stainless steel | 3 | 15 | 15.9a | 31.9 | 29 | |
| GFX stent | AVE | Steel 316L | 3 | 18 | <10.1a | 29 | 29 | |
| PLLA 2.4 helical | PLLA | 3 | 32?b | 26.1–36.2 | 35 | |||
| Multi-link Tetra | Guidant | Stainless steel | 3? | ? | 29–31.9 | 35 | ||
| PLLA mesh | PLLA* | PLLA | 4 | ? | 23.8–39.7 | 28 | ||
Pressure taken at 2% change in diameter.
Evaluated from picture.
Stent Crimping and Deployment
The stents were crimped to their minimum diameter in the crimping machine. They were then placed at 37°C for 10 min and observed for expansion; actuation did not occur with either stents. Full expansion was reached at 80°C. Measurements are shown in Table III, where axial shortening and radial expansion ratios are calculated. Axial shortening is defined as:
TABLE III.
Measured Crimped and Expanded Values Obtained with the Solid Tubular and the Laser Etched Stents
| Stent Type | |||
|---|---|---|---|
| Solid Tubular | Laser Etched | ||
| Crimped | Diameter (mm) | 1.85 ± 0.04 | 1.40 ± 0.03 |
| Length (mm) | 18.5 ± 0.20 | 17.3 ± 0.50 | |
| Expanded | Diameter (mm) | 3.96 ± 0.03 | 3.88 ± 0.08 |
| Thickness (mm) | 0.25 ± 0.02 | 0.25 ± 0.02 | |
| Length (mm) | 17.87 ± 0.03 | 17.12 ± 0.74 | |
| Axial shortening (%) | 3.41 ± 0.90 | 1.14 ± 1.60 | |
| Radial expansion ratio | 2.14 ± 0.08 | 2.72 ± 0.11 | |
It quantifies the variation in length between crimped and expanded states as stents generally shorten on actuation. The larger this number is, the longer the crimped stent has to be for delivery to a specific artery lesion’s length. This not only reduces the flexibility of the device during navigation, but it also impedes precision during positioning and increases potential vessel damage during deployment if the stent is in contact with the arterial wall during shortening. An axial shortening of 1.14 ± 1.60% was obtained with the laser etched stent. The radial expansion ratio, defined as the diameter of the stent in the expanded state over that in the crimped state, was 2.72 ± 0.11 for the laser etched stent. The radial expansion ratio of the 4 mm Multi-link vision stent (Guidant (crimped length − expanded length) × 100 crimped length Corporation, Santa Clara, CA) was 3.48 ± 0.06 (nominal expanded diameter over measured diameter when crimped over the balloon), and that of the 4 mm BxVelocity (Cordis, Warren, New Jersey) was 3.17 ± 0.09.
DISCUSSION
DSC and DMTA results are shown in Table I. As reported in our previous studies on thermomechanical properties of this material,15 the Tg is highly dependent on the processing conditions of the material. The neat material was prepared by compression molding at high temperature. Its measured Tg was close to the nominal Tg. However, the stents were manufactured by dissolving the material into a solvent before dip-coating pins. They were also used in the collapse pressure chamber and thus exposed to water before being subjected to DSC measurements. Their first heat Tg was lower than manufacturer’s Tg by about 25°C. This is likely due to the presence of residual solvent and/or moisture. Evidence for this comes from the much higher Tg measured in the 2nd heating (after the sample is heated to 200°C and cooled) as well as experiments on the effect of exposure to different solvents on the SMP Tg, which shows that a larger depression in Tg is observed as the goodness of the solvent (i.e. matching of solubility parameters) increases. Humidity has been reported to lower the Tg of polyurethane42 and could be partially responsible for Tg depression here. However, the fact that high temperature heating causes the SMP to nearly recover it’s nominal Tg is evidence that laser etching does not significantly degrade the SMP and is not responsible. Also, the SMP is well dried prior to laser etching which makes degradation by hydrolysis unlikely. Thus, the most likely explanation is that some of the solvent and/or water have evaporated during the first heating process. A new type of SMP is currently being developed in our lab, with a high degree of crosslinking which makes it less susceptible to thermal history effects and less prone to moisture or solvent induced depression of Tg. In addition, these new materials are less hydrophilic and do not contain either ester or ether linkages, which are prone to hydrolysis and oxidation in vivo, respectively.43 Finally, further studies will need to include the effect of sterilization on the material. A variety of sterilization techniques for polyurethanes are currently used; they include gamma-irradiation, steam autoclave, ethylene oxide, or hydrogen peroxide plasma. Gamma-irradiation seems to impart less changes in materials properties and cytotoxic effect but these aspects will need to be investigated in the device presented here.44,45
As seen in Figure 5(a,b), onset and full collapse of both stents occurs at higher pressures than the estimated 4.7 psi maximum value of collapsing pressure exerted by vasospastic arteries. However, there is a large error on the pressure, especially at these lower temperatures, due to the variability in visually determining when collapse occurred, as the process was much slower than at higher pressures. Thus, the laser etched stent could potentially start to collapse at pressures lower than 4.7 psi. In addition, collapse pressures below 4.7 psi are reached above 50°C for the onset, and 55°C for the full collapse of the solid tubular stent, and around 39°C for the onset, and 46°C for full collapse of the laser etched stent. These two critical temperatures for the solid tubular stents are outside the range of possible temperatures in a living body, but the laser-etched stent could potentially start to collapse at low grade fevers, and fully collapse at high grade fevers.
Table II is a summary of results obtained with the stents tested in this study (the solid tubular, the laser-etched SMP stents, and two commercially available coronary stents), and of published data on collapse pressure of other stents which are currently available or under investigation; collapse pressure was recorded in similar apparatuses as ours. Several remarks can be made. First, there is a wide range of full collapse pressures, from 7.7 to 31.9 psi, amongst reported commercially available stents. Second, onset of collapse can occur at pressures lower than 4.3 psi, as shown by Rieu et al.’s results.29 Note that because in their set up, collapse was measured by an optical device, the beginning of collapse occurred earlier than the naked eye can detect, which is the measurement method in our study. Thus, we reported the pressures read at a 2% decrease in diameter. Third, not all recorded values are in good agreement: the Medtronic Wiktor was shown to fully collapse at 10.1 psi30 and 11.6 psi29; however, the Cordis Crossflex had a full collapse pressure of 8.7 psi31 and 31.9 psi.29 The PLLA stents developed by Venkatraman et al.35) exhibited higher collapse pressures than the SMP stents; however, the PLLA stent had a surface area of 67%, compared to 50% for the SMP stent, and a thickness of 1.5 to 1.8 mm, compared to 25 mm for the SMP stent. In addition, only the collapse pressure onset of the PLLA stents was provided; it is unknown if complete collapse occurred at pressures close to the onset or much higher. Thus, there is a wide variation in collapse pressures of coronary stents, and the neurovascular SMP stents proposed in this study are within the range of the “low pressure” stents. This challenging aspect of polymeric stents is discussed in a review by Waksman in the context of biodegradable stents.46
This first SMP laser etched prototype was designed for high flexibility in both expanded and collapsed states. It may need to be optimized for higher radial strength. On the one end, the potential onset of collapse at 37°C might not be a problem as SMP stents do not permanently crush like their metallic counterparts do from large acute strains. As the temperature approaches Tg, the stent is easily deformed due to its lower modulus, but it also has an increased ability to recover its primary shape. If the stent is well anchored to the arterial wall and if it maintains a high recovery stress, it could remain in place and adjust its shape to the different conformations of the artery during and after vasospasm. We are currently developing SMP material with a similar glass transition temperature and higher recovery stress.6 On the other end, there are several ways to increase the stent’s collapse pressure. The first one would be to increase the Tg of the material. As discussed earlier, the actual Tg of the stent is about 25°C below the manufacturer’s Tg of 75°C, and this is likely due to residual solvent and/or moisture. Drying the stent at higher temperatures could evaporate the solvent and result in a higher Tg. Alternatively, collapse pressure could be increased by optimizing the design pattern, the thickness, and the surface area of the stent to provide a stronger scaffold while maintaining adequate lumen pattency and biocompatibility. Finally, the material could be strengthened with a shape memory alloy because SMAs have higher moduli at comparable temperatures.
Stent expansion results are shown in Table III. Neither the solid tubular nor the laser-etched stend started to visually expand when placed for 10 min at 37°C. This shows that the device will not start to self-expand when navigated into the artery, so that actuation can be controllably achieved by an external heat source. Axial shortening, typically between 1 and 10% in most coronary stents,47 was 1.14 ± 1.60% for the laser etched stent. Thus, the stents’ length could be chosen so that it closely matches that of the target lesion, ensuring a minimal crimped length during navigation. Finally, radial expansion ratios were measured. They generally range from 2.3 to 4.4 (nominal expanded diameter over profile when crimped over the balloon for 4 mm outer diameter stents).47 The radial expansion ratio of the laser etched stent was 2.72 ± 0.11, which appears to be in the lower range. However, in an actual clinical device, the stent would be mounted over a delivery vehicle, such as a laser light diffuser,19 which would allow navigation and self-expansion of the stent. Higher expansion ratios could be obtained by further expanding the stent, using the shape memory property of the polymer: the stent could be stretched to larger diameters with a combination of laser illumination and balloon dilation at an appropriate temperature, followed by cooling to fix the new shape.
Heat actuation and temperature changes introduce the potential thermal damage to tissue and blood in the vicinity of the device. Maitland et al. have discussed heat consideration with laser activated SMPs. The temperature around the stent depends on the cooling capacity of the blood and tissue, the laser power, its duration, and blood flow. Blood flow provides an efficient convection cooling system, and the overall short actuation time might result in minimal blood and tissue damage.19,48 This aspect is currently being studied in our lab.
CONCLUSION
The neurovascular shape memory polymer prototype stents used in this study, fabricated from MM7520 thermoplastic polyurethane, exhibited lower Tg s than expected. This was attributed to remaining solvent and/or moisture trapped into the polymer chains. The stents showed decreasing collapse pressures with increasing temperature from 32 to 47°C, and reached a plateau at higher temperatures. At 37°C, both stents exhibited full collapse pressures higher than 4.7 psi, the estimated maximum vasospastic pressure that could collapse an intracranial stent. Full collapse pressure of the laser-etched stent was similar to low collapse pressure stents reported in the literature. However, this stent could start collapsing and further studies are required to determine precise collapse and recovery behavior of the material at 37°C. A material with higher recovery stress and/or higher Tg might be a solution to allow quick recovery when compressed at body temperature, or to ensure a higher collapse pressure. The stents showed full recovery after crimping, with a radial expansion ratio up to 2.7, an axial shortening of 1.1%, but higher recovery ratios could be obtained by further dilation. This work shows that SMP is a promising material for the development of neurovascular stents. Future work should focus on optimizing the device for higher collapse pressure at body temperature by using higher Tg or higher recovery stress material, and expansion should be studied in the context of a delivery system. Finally, scaling down this prototype will be necessary to treat intracranial arteries, which are less than 2.5 mm inner diameter. In developing smaller diameter stents, thickness tradeoff between increased hoop stress (thicker wall) and reduced flow impedance (thinner walls) will be considered. Constitutive models for predicting SMP device thermomechanical properties are currently being developed by other groups, they will be important in engineering the final device.49,50
Acknowledgments
The authors thank Jeffrey Loge for his help with building the pressure chamber.
Contract grant sponsor: U.S. Department of Energy by Lawrence Livermore National Laboratory (LLNL); contract grant number: W-7405-ENG-48
Contract grant sponsor: National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering; contract grant number: R01EB000462
Contract grant sponsor: LLNL (Directed Research and Development grant); contract grant number: 04-ERD-093
Contract grant sponsor: National Science Foundation Center for Biophotonics, Science34 and Technology Center; contract grant number: PHY 0120999
REFERENCES
- 1.Lendlein A, Kelch S. Shape-memory polymers. Angew Chem Int Ed. 2002;41:2034–2057. [PubMed] [Google Scholar]
- 2.Tobushi H, Hara H, Yamada E, Hayashi S. Thermomechanical properties in a thin film of shape memory polymer of polyurethane series. Smart Mater Struct. 1996;5:483–491. [Google Scholar]
- 3.Metzger M, Wilson TS, Schumann DL, Matthews DL, Maitland DJ. Mechanical properties of mechanical actuator for treating ischemic stroke. Biomed Microdevices. 2002;4:89–96. [Google Scholar]
- 4.Small W, Wilson T, Benett W, Loge J, Maitland DJ. Laser-activated shape memory polymer intravascular thrombectomy device. Opt Express. 2005;13:8204–8213. doi: 10.1364/opex.13.008204. [DOI] [PubMed] [Google Scholar]
- 5.Small W, Metzger M, Wilson T, Maitland DJ. Laser-activated shape memory polymer microactuator for thrombus removal following ischemic stroke: Preliminary in vitro analysis. IEEE J Sel Top Quantum Electron. 2005;11:892–901. [Google Scholar]
- 6.Wilson TS, Small W, Benett WJ, Bearinger JP, Maitland DJ. Shape memory polymer therapeutic devices for stroke. Proc SPIE. 2005;6007 60070R. [Google Scholar]
- 7.Metcalfe A, Desfaits A-C, Salazkin I, Yahia LH, Sokolowski WM, Raymond J. Cold hibernated elastic memory foams for endovascular interventions. Biomaterials. 2003;24:491–498. doi: 10.1016/s0142-9612(02)00362-9. [DOI] [PubMed] [Google Scholar]
- 8.Wache HM, Tartakowska DJ, Hentrich A, Wagner MH. Development of a polymer stent with shape memory effect as a drug delivery system. J Mater Sci Mater Med. 2003;14:109–112. doi: 10.1023/a:1022007510352. [DOI] [PubMed] [Google Scholar]
- 9.Gall K, Yakacki CM, Liu Y, Shandas R, Willett N, Anseth KS. Thermomechanics of the shape memory effect in polymers for biomedical applications. J Biomed Mater Res A. 2005;73:339–348. doi: 10.1002/jbm.a.30296. [DOI] [PubMed] [Google Scholar]
- 10.Holmes DR., Jr State of the art in coronary intervention. Am J Cardiol. 2003;91:50A–53A. doi: 10.1016/s0002-9149(02)03150-8. [DOI] [PubMed] [Google Scholar]
- 11.Rabe K, Sievert HJ. Carotid artery stenting state of the art. J Interv Cardiol. 2004;17:417–426. doi: 10.1111/j.1540-8183.2004.04085.x. [DOI] [PubMed] [Google Scholar]
- 12.Kudo T, Chandra FA, Ahn SS. Long-term outcomes and predictors of iliac angioplasty with selective stenting. J Vasc Surg. 2005;42:466–475. doi: 10.1016/j.jvs.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Higashida R, Meyers PM. Intracranial angioplasty and stenting for cerebral atherosclerosis: New treatments for stroke are needed! Neuroradiology. 2006;48:367–372. doi: 10.1007/s00234-006-0071-6. [DOI] [PubMed] [Google Scholar]
- 14.Hartmann M, Jansen O. Angioplasty and stenting of intracranial stenosis. Curr Opin Neurol. 2005;8:39–45. doi: 10.1097/00019052-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Baer G, Wilson T, Maitland DJ, Matthews DL. Shape-memory behavior of thermally stimulated polyurethanes for medical applications. J Appl Polym Sci. 2007;103:3882–3892. [Google Scholar]
- 16.Yazdani SK, Moore JE, Jr, Berry JL, Vlachos PP. DPIV measurements of flow disturbances in stented artery models: Adverse effects of compliance mismatch. J Biomech Eng. 2004;126:559–555. doi: 10.1115/1.1797904. [DOI] [PubMed] [Google Scholar]
- 17.Rolland PH, Mekkaoui C, Vidal V, Berry JL, Moore JE, Moreno M, AMabile P, Bartoli JM. Compliance matching stent placement in the carotid artery of the swine promotes optimal blood flow and attenuates restenosis. Eur J Vasc Endovasc Surg. 2004;28:431–438. doi: 10.1016/j.ejvs.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Commandeur S, Van Beusekom HM, Van der Giessen WJ. Polymers, drug release, and drug-eluting stents. J Interv Cardiol. 2006;19:500–506. doi: 10.1111/j.1540-8183.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- 19.Maitland DJ, Metzger M, Schumann DL, Lee A, Wilson TS. Photothermal properties of shape memory polymer microactuators for treating stroke. Lasers Surg Med. 2002;30:1–11. doi: 10.1002/lsm.10007. [DOI] [PubMed] [Google Scholar]
- 20.Lendlein A, Jiang H, Junger O, Langer R. Light-induced shape-memory polymers. Nature. 2005;434:879–882. doi: 10.1038/nature03496. [DOI] [PubMed] [Google Scholar]
- 21.Hilmar K, Price G, Pearce N, Alexander M, Vai RA. Remotely actuated polymer nanocomposites - stress recovery of carbon-nanotube-filled thermoplastic elastomers. Nat Mater. 2004;3:115–120. doi: 10.1038/nmat1059. [DOI] [PubMed] [Google Scholar]
- 22.Buckley PR, McKinley GH, Wilson TS, Small W, Bearinger JP, McElfresh MW, Benett W, Maitland DJ. Inductively heated shape memory polymer for the magnetic actuation of medical devices. IEEE Trans Biomed Eng. 2006;53:2075–2083. doi: 10.1109/TBME.2006.877113. [DOI] [PubMed] [Google Scholar]
- 23.Lamba NMK, Woodhouse KA, Cooper SL. Polyurethanes in Biomedical Applications. New York: CRC Press; 1997. [Google Scholar]
- 24.Butterfield M, Wheatley DJ, Williams DF, Fisher J. A new design for polyurethane heart valves. J Heart Valve Dis. 2001;10:105–110. [PubMed] [Google Scholar]
- 25.Lendlein A, Langer R. Biodegradable, elastic shape-memory polymers of potential biomedical applications. Science. 2002;296:1673–1676. doi: 10.1126/science.1066102. [DOI] [PubMed] [Google Scholar]
- 26.Bertmer M, Buda A, Blomenkamp-Hofges I, Kelch S, Lendlein A. Biodegradable shape-memory polymer networks: Characterization with solid-state NMR. Macromolecules. 2005;38:3793–3799. [Google Scholar]
- 27.Cabanlit M, Maitland D, Wilson T, Simon S, Wun T, Gershwin E, Van de Water J. Polyurethane shape memory polymers demonstrate functional biocompatibility in vitro. Macromol Biosci. doi: 10.1002/mabi.200600177. (in press) [DOI] [PubMed] [Google Scholar]
- 28.Agrawal CM, Haas KF, Leopold DA, Clark HG. Evaluation of poly(l-lactic acid) as a material for intravascular polymeric stents. Biomaterials. 1992;13:176–182. doi: 10.1016/0142-9612(92)90068-y. [DOI] [PubMed] [Google Scholar]
- 29.Rieu R, Barragan P, Masson C, Fuseri J, Gariety V, Silvestri M, Roquebert P, Sainsous J. Radial force of coronary stents: A comparative analysis. Catheter Cardiovasc Interv. 1999;46:380–391. doi: 10.1002/(SICI)1522-726X(199903)46:3<380::AID-CCD27>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt W, Behrens P, Behrend D, Schmitz K-P. Comparative studies of different stent designs. Prog Biomed Res. 1999;4:52–58. [Google Scholar]
- 31.Schmitz K-P, Schmidt W, Behrens P, Behrend D. In-vitro examination of clinically relevant stent paramenters. Prog Biomed Res. 2000;5:197–203. [Google Scholar]
- 32.Duda SH, Wiskirchen J, Tepe G, Bitzer M, Kaulich TW, Stoeckel D, Claussen CD. Physical properties of endovascular stents: An experimental comparison. SCVIR. 2000;11:645–654. doi: 10.1016/s1051-0443(07)61620-0. [DOI] [PubMed] [Google Scholar]
- 33.Snowhill PB, Nosher JL, Siegel RL, Silver FH. Characterization of radial forces in Z stents. Invest Radiol. 2001;36:521–530. doi: 10.1097/00004424-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Tan LP, Venkatraman SS, Joso JFD, Boey FYC. Collapse pressure of bilayered biodegradable stents. J Biomed Mater Res Part B: Appl Biomater. 2006;75B:102–107. doi: 10.1002/jbm.b.30518. [DOI] [PubMed] [Google Scholar]
- 35.Venkatraman S, Poh TL, Vinalia T, Mak KH, Boey F. Collapse pressure of biodegradable stents. Biomaterials. 2003;24:2105–2111. doi: 10.1016/s0142-9612(02)00640-3. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi S. Properties and applications of polyurethane-series shape memory polymer. In: Ashida K, Kanesoshi Ashida, editors. International Progress in Urethanes. Vol. 6. CRC Pr I Llc; 1993. pp. 90–115. [Google Scholar]
- 37.Tamai H, Igaki K, Kyo E, Kosuga K, Kawashima A, Matsui S, Komori H, Tsuji T, Motohara S, Uehata H. Initial and 6-month results of biodegradable poly-L-lactic acid coronary stents in humans. Circulation. 2000;102:399–404. doi: 10.1161/01.cir.102.4.399. [DOI] [PubMed] [Google Scholar]
- 38.Cox RH. Mechanics of canine iliac artery smooth muscle in vitro. Am J Phys. 1973;225:659–663. doi: 10.1152/ajplegacy.1976.230.2.462. [DOI] [PubMed] [Google Scholar]
- 39.Soehle M, Czosnyka M, Pickard JD, Kirkpatrick PJ. Critical closing pressure in subarachoid hemorrhage: Effect of cerebral vasospasm and liminations of a transcranial Doppler-derived estimation. Stroke. 2004;35:1393–1398. doi: 10.1161/01.STR.0000128411.07036.a9. [DOI] [PubMed] [Google Scholar]
- 40.Nagasawa S, Handa H, Naruo Y, Watanabe H, Moritake K, Hayashi K. Experimental cerebral vasospams. Part II. Contractility of spastic arterial wall. Stroke. 1983;14:579–584. doi: 10.1161/01.str.14.4.579. [DOI] [PubMed] [Google Scholar]
- 41.Burton AC. On the physical equilibrium of small blood vessels. Am J Physiol. 1951;164:319–329. doi: 10.1152/ajplegacy.1951.164.2.319. [DOI] [PubMed] [Google Scholar]
- 42.Yang B, Huang WM, Li C, Lee DM, Li L. On the effects of moisture in a polyurethane shape memory polymer. Smart Mater Struct. 2004;13:191–195. [Google Scholar]
- 43.Wilson TS, Bearinger JP, Herberg JL, Marion JE, Wright WJ, Evans CL, Maitland DJ. Shape memory Polymers based on uniform aliphatic urethane networks. J Appl Polym Sci. 2007;106:540–551. [Google Scholar]
- 44.Haugen HJ, Brunner M, Pellkofer F, Aigner J, Will J, Wintermantel E. Effect of different gamma-irradiation doses on cytotoxicity and material properties of porous polyether-urethane polymer. J Biomed Mater Res B Appl Biomater. 2007;80:415–423. doi: 10.1002/jbm.b.30612. [DOI] [PubMed] [Google Scholar]
- 45.Ma N, Petit A, Huk OL, Yahia L, Tabrizian M. Safety issue of re-sterilization of polyurethane electrophysiology catheters: A cytotoxicity study. J Biomater Sci Polym Ed. 2003;14:213–226. doi: 10.1163/156856203763572671. [DOI] [PubMed] [Google Scholar]
- 46.Waksman R. Update on bioabsorbable stents: From bench to clinical. J Interv Cardiol. 2006;19:414–421. doi: 10.1111/j.1540-8183.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 47.Serruys PW, Jutryk MJB, editors. Handbook or Coronary Stents. 4th ed. London: M Dunitz; 2002. [Google Scholar]
- 48.Ortega JM, Maitland DJ, Wilson TS, Tsai W, Savas O, Saloner D. Vascular dynamics of a shape memory polymer foam aneurysm treatment technique. Ann Biomed Eng. 2007;35:1870–1884. doi: 10.1007/s10439-007-9358-y. DOI 10.1007/s10439-007-9358-y. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Gall K, Dunn ML, Greenberg AR, Diani J. Thermomechanics of shape memory polymers: Uniaxial experiments and constitutive modeling. Int J Plast. 2006;22:279–313. [Google Scholar]
- 50.Diani J, Liu Y, Gall K. Finite strain 3D thermoelastic constitutive model for shape memory polymers. Polym Eng Sci. 2006;46:486–492. [Google Scholar]