Abstract
Auditory processing in the cerebral cortex is comprised of an interconnected network of auditory and auditory-related areas distributed throughout the forebrain. The nexus of auditory activity is located in temporal cortex among several specialized areas, or fields, that receive dense inputs from the medial geniculate complex. These areas are collectively referred to as auditory cortex. Auditory activity is extended beyond auditory cortex via connections with auditory-related areas elsewhere in the cortex. Within this network, information flows between areas to and from countless targets, but in a manner that is characterized by orderly regional, areal and laminar patterns. These patterns reflect some of the structural constraints that passively govern the flow of information at all levels of the network. In addition, the exchange of information within these circuits is dynamically regulated by intrinsic neurochemical properties of projecting neurons and their targets. This article begins with an overview of the principal circuits and how each is related to information flow along major axes of the network. The discussion then turns to a description of neurochemical gradients along these axes, highlighting recent work on glutamate transporters in the thalamocortical projections to auditory cortex. The article concludes with a brief discussion of relevant neurophysiological findings as they relate to structural gradients in the network.
Auditory and auditory-related areas in cortex
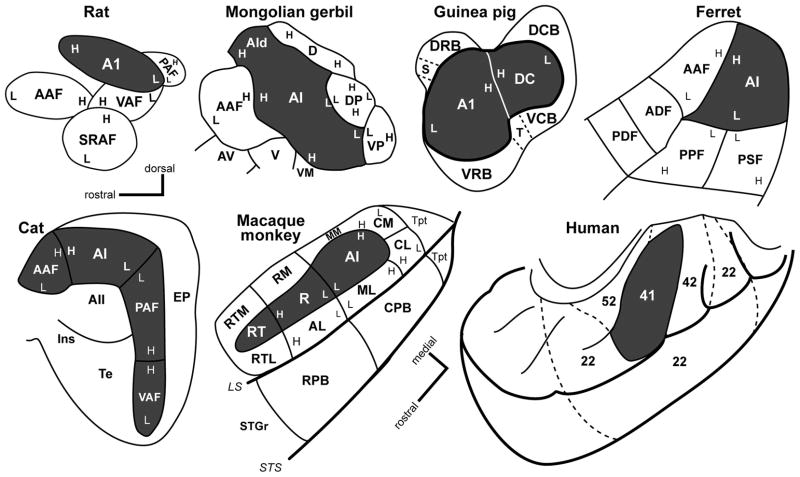
Areas that process sound have been discovered in every lobe of the brain, but are all of these areas part of auditory cortex? The answer depends on how one defines auditory cortex. From an anatomical perspective, auditory cortex can be defined as those areas of the cerebral cortex that receive significant thalamic input from one or more divisions of the medial geniculate complex (MGC). By this definition, the auditory cortex of mammals is confined to a group of adjoining areas in the temporal region, as shown for several mammalian species in Fig. 1. The precise number of areas identified, their arrangement and numerous other features varies by species, but all are considered auditory. If one applies the same definition to nonhuman primates, auditory cortex occupies the caudal two-thirds of the superior temporal lobe. In humans, the identity of auditory cortex is less certain, because the projections of the MGC are not well defined. Therefore, we cannot define auditory cortex in the same way as in other animals. Instead, we rely on comparisons of the neuronal architecture and neurochemistry to identify areas with common features, and then look for support in imaging and electrophysiological studies. At this point, it is rather certain that human auditory cortex occupies the posterior portion of the superior temporal cortex, including Heschl’s gyrus, the planum temporale, and some portion of the posterior superior temporal gyrus. There is no consensus on the number of areas present or their arrangement. The application of advanced structural neuroimaging tools, such as diffusion tensor imaging, may present opportunities to better define the human auditory cortex by revealing key patterns of thalamocortical and corticocortical connections of superior temporal areas.
Fig. 1.
Schematics of the auditory cortex in selected mammals. Primary (core) auditory areas are darkly shaded. Belt and parabelt areas are unshaded. Tonotopic gradients are indicated by H (high) and L (low) frequency. See text for abbreviations. Redrawn from (Polley et al., 2007) (rat); (Budinger et al., 2000) (Mongolian gerbil); (Bizley et al., 2005)(ferret); (Wallace et al., 2000)(guinea pig); (Lee et al., 2004b)(cat); (Hackett et al., 1998a) (macaque monkey); (Brodmann, 1909) (human). Dorsal-rostral axis marker applies to all panels except macaque and human.
The network of areas that process auditory information in cortex also includes numerous auditory-related areas distributed throughout the forebrain. These areas receive inputs from auditory cortex and often other sensory systems, but lack significant inputs from the MGC. This arrangement implies that auditory activity in auditory-related areas depends on inputs from auditory cortex, or perhaps other auditory-related areas. As a whole, then, auditory information in the cerebral cortex is processed by an interconnected network in which auditory areas rely on inputs from the MGC and auditory-related areas largely depend on inputs from the auditory areas. The flow of information within this network is the subject of this article, which draws from past and present studies in several species. For practical reasons, the discussion focuses on cats and primates, but it is acknowledged that such patterns are likely to characterize most if not all mammalian species.
Information flow in the auditory cortical network
As one reviews the myriad studies of auditory cortex over the last 40 years, a number of common themes stand out. These are based on the structural and functional properties that tend to be the most robust, and are referred to herein as principles of auditory cortical organization (Table 1). A thorough treatment of these principles is not provided here, although brief descriptions are included for context as each is related in some way to the flow of information into, within and out of auditory cortex. Perhaps the most fundamental organization feature is that, in nearly all studied mammals, auditory cortex contains more than one area (Fig. 1). In some models, these areas have been grouped into regions based on a set of common features. In cats and primates, where more than ten areas have been identified, the primary areas are grouped into a centrally located “core” region, and the secondary areas are assigned to “belt” or “parabelt” regions, which tend to be located around the core. Inputs to these areas arise from multiple sources in the forebrain. Subcortical inputs include auditory and other sensory nuclei in the thalamus. Cortical inputs come from intra- and interhemispheric connections within auditory cortex, and reciprocal connections with auditory-related areas. The outputs of auditory cortex include myriad cortical and subcortical structures at all levels of the auditory pathways. Individually and collectively, these patterns frame and constrain the processing of auditory and non-auditory information within the entire auditory cortical network.
Table 1.
Principles of auditory cortical organization. Brief descriptions of each principle are provided.
| Regional and areal subdivisions | AC is divided into regions with multiple subdivisions or areas. |
| Architectonic profiles and gradients | Each area has an unique architectonic profile. Gradients in architectonic features occur within and across regions. |
| Specificity of thalamocortical connections | Multiple thalamic inputs converge in all AC areas. These projections vary systematically by thalamic nucleus and cortical target. |
| Specificity of corticocortical connections | Inputs and outputs of each AC area are unique. |
| Serial and parallel connections | Thalamic and cortical connections reveal both serial and parallel patterns of information flow. |
| Connections with auditory-related areas | AC areas have connections with multiple auditory-related areas. These are topographically organized along the major axes of information flow. |
| Hemispheric asymmetry | Evidence of structural or functional asymmetry varies by species. More clear for human AC. |
| Subcortical projections of auditory areas | An elaborate system of descending projections from the AC targets the striatum, amygdala, thalamus and brainstem. |
| Specificity of laminar connections | Thalamic and cortical projections to AC areas are laminar specific and vary by area and region. |
| Neurochemical profiles and gradients | Neurochemical properties of neurons (gene, protein, enzyme expression) vary systematically by region, area, lamina, and thalamic nucleus. |
| Multisensory connections and interactions | Auditory and non-auditory inputs converge in AC with some evidence of topopgraphic specificity. |
| Physiological profiles of regions and areas | Neuronal response properties vary systematically between AC areas. Studies are ongoing and incomplete for most areas. |
| Spatial organization of response properties | Stimulus frequency is topographically organized with AC areas. Also evidence of gradients in the distribution of other response properties (e.g., threshold, tuning bandwidth, monotonicity) |
Information flow into auditory cortex: thalamocortical projections
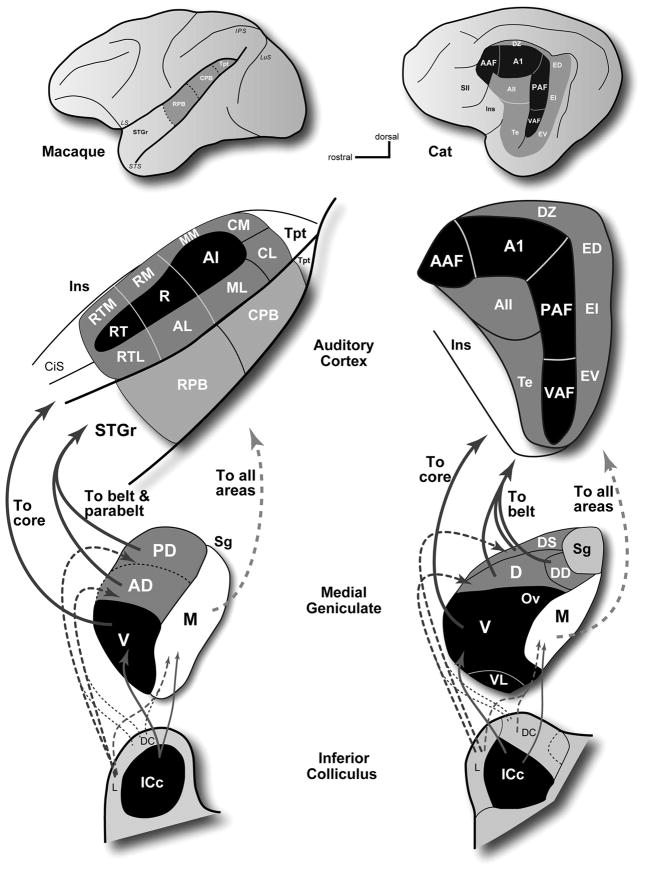
Thalamic inputs to auditory cortex are dominated by projections from the MGC (Jones, 2007; Lee et al., 2008a). In most species, three major MGC divisions are commonly recognized (v, ventral; d, dorsal; m, medial or magnocellular), each of which can be further subdivided. An important distinction between divisions is that each receives a different blend of inputs from nuclei in the brainstem (Aitkin, 1986; Calford et al., 1983) and ultimately targets the auditory areas in a specific way (Fig. 2). In cats and monkeys, as in all studied mammals, the principal source of ascending inputs to the ventral division (MGv) is the central nucleus of the tonotopically organized inferior colliculus (ICc), which is part of the primary (lemniscal) ascending pathway. The dorsal divisions (MGd) mainly receive inputs from the dorsal cortex (DC) and lateral (L) nuclei of the inferior colliculus, although the rostral MGd divisions also receive inputs from the ICc. These divisions of the IC are part of the non-tonotopic or diffuse ascending pathway, and do not appear to be tonotopically organized. The magnocellular division (MGm) receives inputs from all three of these IC divisions, and is generally thought to receive additional inputs from vestibular, somatosensory, and possibly visual systems, as well, although some of these findings have been disputed (see Jones, 2007). In cats, the MGv mainly targets the tonotopically organized core areas (dark shading; AAF, A1, P, VP), the dorsal divisions target the surrounding non-tonotopic belt (lighter shading; AII, EP, Te) areas, and the medial division projects to all areas. In primates, a similar pattern is apparent. The MGv projects mainly to areas in the core region, the dorsal divisions project broadly to the belt and parabelt regions, and the MGm projects broadly to all three regions. Inputs to auditory cortex also include adjoining nuclei in the posterior thalamus that have auditory and multisensory properties. These include the suprageniculate, posterior, peripeduncular, and pulvinar nuclei. Their projections are concentrated in areas outside of the core region, especially the ectosylvian areas in cats, the parabelt region in primates, and several auditory-related areas in all species.
Fig. 2.
Summary of medial geniculate projections to auditory cortex of the macaque monkey (left) and cat (right). In both species, a chain of projections links the core region with the central nucleus of the inferior colliculus (ICc) through the MGv. The belt and parabelt areas are linked to the dorsal cortex (DC) and lateral (L) nuclei of the IC through the dorsal divisions of the medial geniculate. All areas of auditory cortex receive inputs from the MGm, which is connected with the ICc, DC, and L divisions of the IC.
Projections to the core and belt regions from the MGC tend to be topographically distributed. The most conspicuous gradient is organized along the rostro-caudal axis of the MGC. In cats, for example, the AAF receives inputs from the most rostral portion of the MGv and the adjacent rostral pole (RP), whereas progressively more ventral areas in auditory cortex receive inputs from increasingly caudal locations in the MGv (Huang et al., 2000; Imig et al., 1983; Imig et al., 1984; Lee et al., 2008a; Rodrigues-Dagaeff et al., 1989; Rouiller et al., 1989; Winer et al., 2001). In primates, the most caudal areas, including MM, CM, and A1, receive inputs from the rostral pole and rostral MGv, whereas projections to rostral core and especially belt/parabelt areas tend to arise from increasingly caudal sites in the MGv and MGd (Burton et al., 1976; de la Mothe et al., 2006b; Hackett et al., 1998b; Molinari et al., 1995; Morel et al., 1992). Comparable topography has also been observed in projections to core areas in rats, where inputs to A1 and the ventral auditory field (VAF) arose from rostral and caudal parts of the MGv, respectively (Storace et al., 2009). Thalamocortical topography is therefore likely to be a general feature of auditory forebrain organization in mammals, and forms the basis for many of the topographic relationships in cortex. These relationships provide insights and allow predictions about information flow in cortex, and may also support the identification of corresponding or homologous areas between species.
In addition to gross topography, the unique set of connections and neurochemical properties associated with each of the MGC nuclei suggest that they could be regarded as sources of separate information streams that project in parallel to their respective auditory and auditory-related targets (Jones, 2003; Jones, 2007; Lee et al., 2008a; Rodrigues-Dagaeff et al., 1989; Rouiller et al., 1991). Each cortical area tends to receive a unique blend of inputs from several thalamic nuclei, which in turn receive a unique set of inputs from other sources. Thus, it can be assumed that each thalamic nucleus provides a distinct variety of information to its cortical targets. This way of thinking about the thalamic projections was amplified by Jones and colleagues who associated patterns of calcium binding protein expression with specific thalamic nuclei (Hashikawa et al., 1991; Hashikawa et al., 1995; Jones, 2003; Molinari et al., 1995). In these studies it was reported that nearly all neurons in the MGv were immunoreactive (-ir) for parvalbumin, whereas about half of the neurons in the MGpd were parvalbumin-ir and the other half were calbindin-ir. These and other neurochemical gradients are overlaid on the topographic connection patterns, indicating that information flow is regulated by additional, perhaps numerous, factors embedded in the network. As discussed in greater detail below, recent studies indicate that there is much to be learned about the areal, laminar and neurochemical specificity of these thalamic projection systems that will yield additional insights into the nature of information processing in this portion of the network.
Information flow within AC: connections within auditory cortex
The local connections of each auditory area are unique, with links to several others. Although the full complement of connections of a single area is extremely complex upon detailed examination, the following general properties are typically observed: 1) a single area typically has reciprocal connections with several others; 2) adjacent areas tend to be more densely interconnected than non-adjacent areas; 3) the densest connections link neurons within a single area; and 4) laminar and sublaminar patterns of connections vary systematically (Bizley et al., 2005; Budinger et al., 2000; de la Mothe et al., 2006a; Fitzpatrick et al., 1980; Kaas et al., 1998; Lee et al., 2008b; Lee et al., 2004a; Lee et al., 2004b; Read et al., 2001; Winer et al., 2007). To some extent, the flow of information between areas can be inferred from consideration of these anatomical relationships. Feedforward projections, where the outputs of one area target layer IV of another, suggest that information is flowing in that direction, and may be ascending from a lower hierarchical level to a higher level. Feedback projections, which arise from the infragranular layers of one area, but avoid layer IV of another, are interpreted as moving against the feedforward flow of information (i.e., descending), especially when the infragranular projections dominate and thought to be modulatory instead of driving. Lateral connections involve all layers and are typical of adjacent areas, which tend to be densely interconnected. For such areas it is more difficult to resolve feedforward and feedback projections, therefore hierarchical relationships are less certain. In the remainder of this section, information flow is examined in the cat and monkey along two major anatomical axes, where the most robust patterns have been identified. Lesser pathways are not considered.
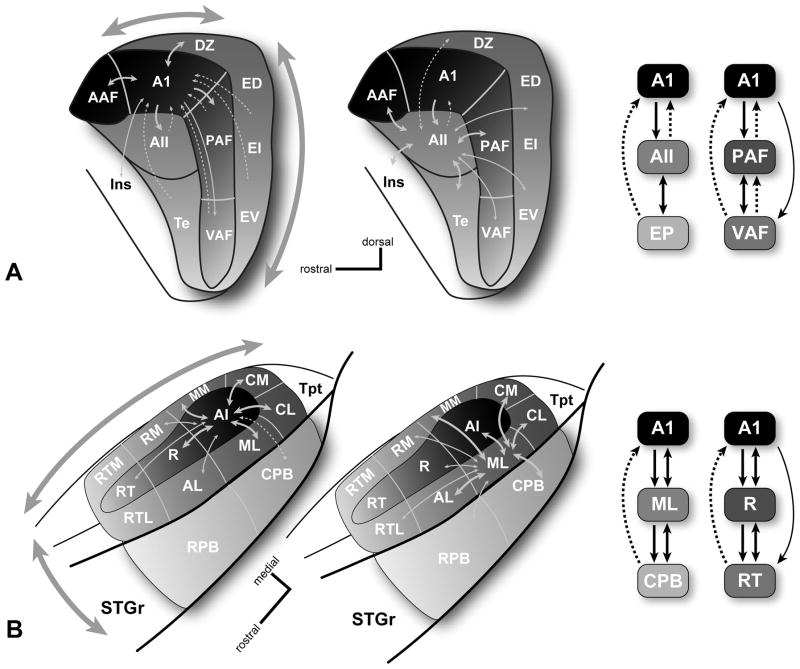
In the cat, where auditory cortical connectivity has been the most intensively studied, certain hierarchical relationships have been proposed from examination of thalamic and cortical connection patterns (Fig. 3a) (Imig et al., 1984; Lee et al., 2004a; Lee et al., 2004b; Rouiller et al., 1991). Generally, areas with similar thalamic inputs and cortical (usually lateral) connections have been placed at the same hierarchical level, and areas with different thalamic input sources and conspicuous feedforward or feedback connections are assigned to different levels. For example, A1 and AAF are adjacent core areas with comparable thalamic inputs, cortical connections, and neurophysiological properties (Eggermont, 1998; Imig et al., 1985a; Imig et al., 1985b; Knight, 1977; Kowalski et al., 1995; Lee et al., 2004a; Lee et al., 2004b; Tian et al., 1994). In contrast, A1 and AII have distinct thalamic inputs and AII receives feedforward projections from A1, placing them at different hierarchical levels. From consideration of these relationships, there is rather compelling evidence of at least one processing hierarchy in cat auditory cortex, comprising some three different levels (Imig et al., 1980; Lee et al., 2005; Lee et al., 2008b; Lee et al., 2004a; Rouiller et al., 1991; Winer et al., 2007). In the example shown in Fig. 3a, A1 sends feedforward projections to AII and receive mainly feedback inputs from AII. Thus, A1 is positioned at the first tier and AII occupies a second tier. At a possible third level, the posterior ectosylvian areas (e.g., ED, EI, EV) receive feedforward inputs from AII, but not from A1, which receives mainly feedback projections from this region. These hierarchical relationships have been compared to the core-belt-parabelt construction in the primate (discussed below) (Winer et al., 2007). In addition, a second gradient is evident in the cat auditory cortex among the tonotopically organized areas of the core region (AAF, A1, PAF, and VAF). This band of areas is oriented along an irregular axis that is roughly antero-dorsal to ventral. As shown in Fig. 3a, laminar connection patterns indicate that feedforward projections are directed ventrally away from AAF and A1. For example, AAF and A1 send feedforward inputs to P and VP, but the projections of P and VP are primarily feedback (Lee et al., 2008b; Rouiller et al., 1991). This resembles the caudal-rostral pattern of information flow within the core region of primates. Thus, information in the cat auditory cortex appears to move along at least two conspicuous gradients.
Fig. 3.
Local connections of selected core and belt areas. (A) Left, connections of the core area, A1 (left), and belt area, AII (middle), in the cat. Right, schematics of information flow along the caudal-rostral axis (A1-AII-EP)(core-belt-parabelt?) and dorsal-ventral axis in the core (A1-PAF-VAF). (B) Left, connections of the core area, A1 (left), and lateral belt area, ML (middle) in the primate. Right, schematics of information flow along the medial-lateral axis (A1-ML-CPB)(core-belt-parabelt) and caudal-rostral axis in the core (A1-R-RT). Line thickness denotes the relative density of each projection. Dashed lines indicate feedback projections. Shading intensity (all panels) and large arrows (left panels) denote anatomical and physiological gradients along two major axes of information flow in both species. See text for details.
In primates, where the orientation of the temporal lobe is more closely aligned to the horizontal plane than in cats, information also moves along at least two, perhaps comparable, anatomical axes (Fig. 3b). Along the medial-lateral axis, it has been observed that the belt region is densely interconnected with the core and parabelt regions, whereas only sparse connections link the core and parabelt (Hackett et al., 1998a). Neurons in the core region have feedforward projections to the surrounding belt region, but almost none to the parabelt. Conversely, a subpopulation of neurons in layer V of the parabelt projects back to the core region, presumably reflecting a feedback circuit (de la Mothe et al., 2006a). These laminar connection patterns suggest that the flow of information along the medial-lateral axis progresses serially between regions; that is, from core to belt to parabelt. This has been interpreted as evidence of a processing hierarchy along this axis (Kaas et al., 1998; Kaas et al., 1999b; Rauschecker, 1998a). Along the caudal-rostral axis, the patterns are not as well defined, but indicate that information flows rostrally toward auditory and auditory-related areas in the rostral third of the temporal lobe and caudally toward the temporal-parietal region (Fig. 3b). The flow of information rostrally was the first to be identified and has been observed in the patterns of lateral, feedforward, and feedback projections within in the core, belt, and parabelt regions (de la Mothe et al., 2006a; Fitzpatrick et al., 1980; Galaburda et al., 1983). In addition to dense lateral connections between adjacent areas along this axis, the defining laminar pattern consists of feedforward projections from caudal areas to layer IV of rostral areas, and feedback projections to caudal areas from infragranular layers of rostral areas. With increasing distance between rostral and caudal areas, feedforward inputs to rostral areas weaken and the feedback projections to caudal areas become increasingly dominant over all other types of connections. These patterns are illustrated in Fig. 3b for connections within the core. Information also flows caudally along this same axis, but mainly in the lateral connections between adjacent areas, as the feedforward and feedback relationships between nonadjacent areas are weaker, absent, or not yet defined. The caudal flow is most obvious in projections from A1 to the caudal belt areas (CM, CL), which then pass information on to temporal parietal areas (e.g., Tpt, Ri) (de la Mothe et al., 2006a; Smiley et al., 2007). Although, since CM and CL are considered belt areas, this route should probably be considered part of the core-belt-parabelt hierarchy.
The overall impression from these patterns, then, is that information tends to move along two major axes in the cat and primate. The patterns are generally consistent with classical descriptions of processing hierarchies in auditory and other sensory systems. While these are by no means the only patterns that can be identified in the connections between areas in the cat, these are the most robust. In both species, greater specificity could be achieved by systematic studies of laminar interconnectivity combining retrograde and anterograde tracing techniques. Further, these anatomical patterns suggest that physiological gradients should also be observed along the axes of information flow. This article concludes with a discussion of those features.
Information flow beyond auditory cortex: connections with auditory-related areas
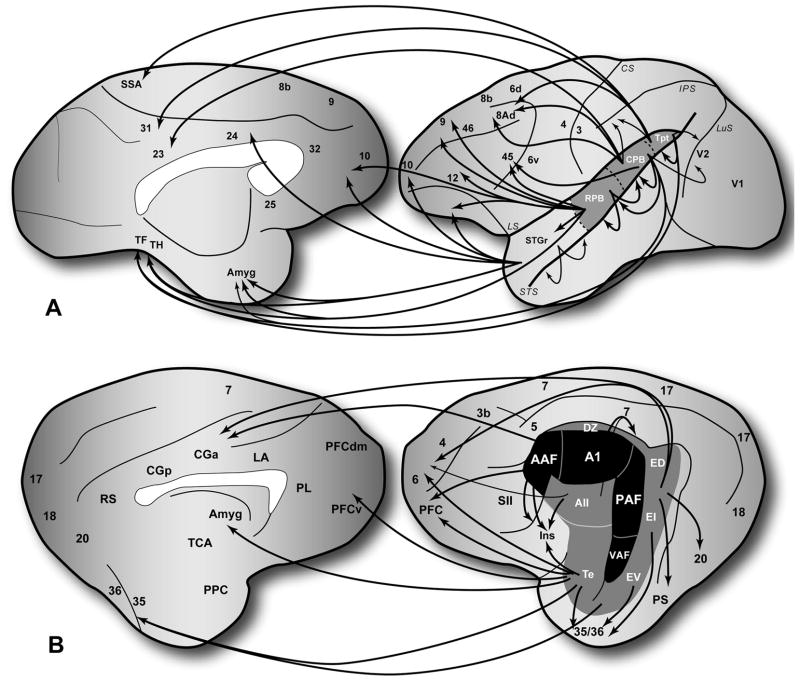
The projections of the auditory cortex to auditory-related areas elsewhere in the forebrain are mainly derived from the belt and parabelt areas. In cats and primates, the projections of the core beyond auditory cortex are rather sparse. In rodents such as the Mongolian gerbil, however, this does not appear to be the case, as A1 is broadly connected with frontal, parietal, and occipital areas (Budinger et al., 2009). In any event, the flow of information out of auditory cortex leads in many directions, but as illustrated in Fig. 4, some directional trends are evident. In many respects, directional biases in the auditory-related pathways are extensions of the topographic gradients found within auditory cortex (discussed above). Thus, the flow of information in the auditory cortical network reflects the topographic patterns of connectivity among the auditory and auditory-related areas. The dominant pathways in the network are commonly referred to as processing streams (Kaas et al., 1999a; Kaas et al., 2000; Rauschecker et al., 2000; Rauschecker et al., 2009), a term also used to describe analogous pathways identified in the somatosensory and visual systems (Mishkin, 1979; Ungerleider et al., 1982; Ungerleider et al., 1994). This has been most intensively studied in the visual cortex, where the topographic connections between areas revealed two largely segregated pathways known as the dorsal and ventral streams. Functionally, these streams are associated with the processing of information related to ‘where’ and ‘what’, respectively. To some extent, projections to auditory-related areas overlap and interact with these streams.
Fig. 4.
Summary of auditory-related projections in the monkey and cat. (A) Projections from auditory cortex to auditory-related areas in frontal, orbital, parietal, occipital, temporal and cingulate cortex, as well as amygdala and striatum (not shown). (B) Projections from auditory cortex to auditory-related areas in comparable areas of the cat left hemisphere. In both species, connections with auditory related areas of cortex mainly involve the belt and parabelt areas, whereas connections with the core are sparse. Topography is evident in the connection patterns between auditory and auditory-related areas, especially along the caudal-rostral axis in monkeys dorsal-ventral axis in cats. Connections between most cortical areas are reciprocal. Projections to the amydala and striatum are not.
From the auditory cortex of primates, information moves in four principal directions, or axes: rostral, caudal, medial, and lateral (Fig. 4a) (Barbas, 2007; Cavada et al., 2000; Falchier et al., 2002; Falchier et al., 2009; Ghashghaei et al., 2002; Hackett et al., 1999; Kosmal et al., 1997; Lavenex et al., 2002; Lewis et al., 2000; Petrides et al., 2002; Rockland et al., 2003; Romanski et al., 1999a; Romanski et al., 1999b; Saleem et al., 2008; Smiley et al., 2007; Tranel et al., 1988). The rostrally-directed stream has auditory-related targets in the temporal pole, ventral, rostral and medial prefrontal areas, rostral cingulate, parahippocampal areas and the amygdala. A caudally-directed stream flows from the caudal belt and parabelt areas into the temporoparietal junction, posterior parietal and occipital regions (such as secondary visual cortex), caudal and dorsal prefrontal areas, dorsal cingulate and parahippocampal areas. Thus, the rostral and caudal areas of auditory cortex project to auditory-related targets that are largely segregated, many of which are located in regions of the brain associated with the ventral and dorsal networks of the extrastriate visual system (Cohen et al., 2004; Cohen et al., 2005; Cohen et al., 2007; Falchier et al., 2009; Romanski, 2007; Romanski et al., 2002; Romanski et al., 2005; Smiley et al., 2009). As such, these features provide anatomical support for the existence of dual streams of processing within the auditory cortical network (Kaas et al., 1999a; Kaas et al., 2000; Rauschecker et al., 2000; Rauschecker et al., 2009). The other two ‘streams’ flow laterally from the belt and parabelt regions to the upper bank of the superior temporal sulcus and medially into the insula and retroinsular areas within the lateral sulcus (de la Mothe et al., 2006a; Galaburda et al., 1983; Hackett et al., 1998a; Smiley et al., 2007). Neither system of projections has been intensively studied or functionally classified, but the targets of both include several areas distributed along the caudal-rostral axis of the temporal lobe. Most of these areas exhibit anatomical and physiological multisensory properties (Hackett, 2007b; Seltzer et al., 1994; Seltzer et al., 1996). Compared to the rostral and caudal streams, the lateral and medial projections do not extend significantly beyond the superior temporal cortex and insula. There are almost no connections between auditory cortex and the visual areas of the inferior temporal gyrus, and connections with somatosensory areas in the parietal region are relatively sparse (Campbell, 1905; Cappe et al., 2005).
In cats, the projections to auditory-related targets are also extensive. Although the available maps are probably incomplete, known targets and topographic connection patterns bear considerable similarity to primates (Clasca et al., 2000; Lee et al., 2008b; Scannell et al., 1995) (Fig. 4b). The major targets include the insula, anterior ectosylvian sulcus, orbital and medial frontal cortex, cingulate cortex, and the parahippocampal region. Within the projections to those targets, some directional topography is apparent. For example, the ventral auditory areas nearest the temporal pole, Te and EV, have connections with orbital and ventral prefrontal areas, as well as amygdala and the parahippocampal region. The dorsal auditory areas, including ED and even AAF, have projections to dorsal frontal and cingulate areas rostrally, and areas PS and 20 caudally. Thus, there is notable topographic segregation in the projections from the dorsal and ventral auditory temporal areas. These two gradients and associated targets resemble those of the caudal and rostral streams in primates, respectively, and suggest that such streams are a common organizational feature in the auditory cortical network of mammals.
Laminar aspects of information flow
In addition to the establishment of a simple ‘connection’ between two areas, the laminar patterns of retrogradely-labeled cells and anterogradely-labeled axonal terminations represent an additional level of refinement that is needed to achieve a more accurate understanding of information flow within these circuits. In the discussion of information flow presented above, some of these details were factored into the classification of connection type (e.g., feedforward, feedback, lateral) and estimation of directionality (information flow). Laminar patterns are more for the auditory cortical areas of cats, but much less so for primates where experiments have tended to emphasize the discovery of connections over other details. In this section and the one that follows, the discussion focuses on laminar and neurochemical specificity in the thalamocortical and corticocortical connections of auditory cortex. Of these, the thalamic input patterns are the best characterized to date and are the focus of the discussion. In contrast, the corticocortical patterns remain to be established for many areas, and so it is not yet possible to identify functional subtypes or link classes of laminar projections to their neurophysiological profiles (Atencio et al., 2009; Lomber et al., 2007). Elaboration of these details will require intensive and sustained efforts.
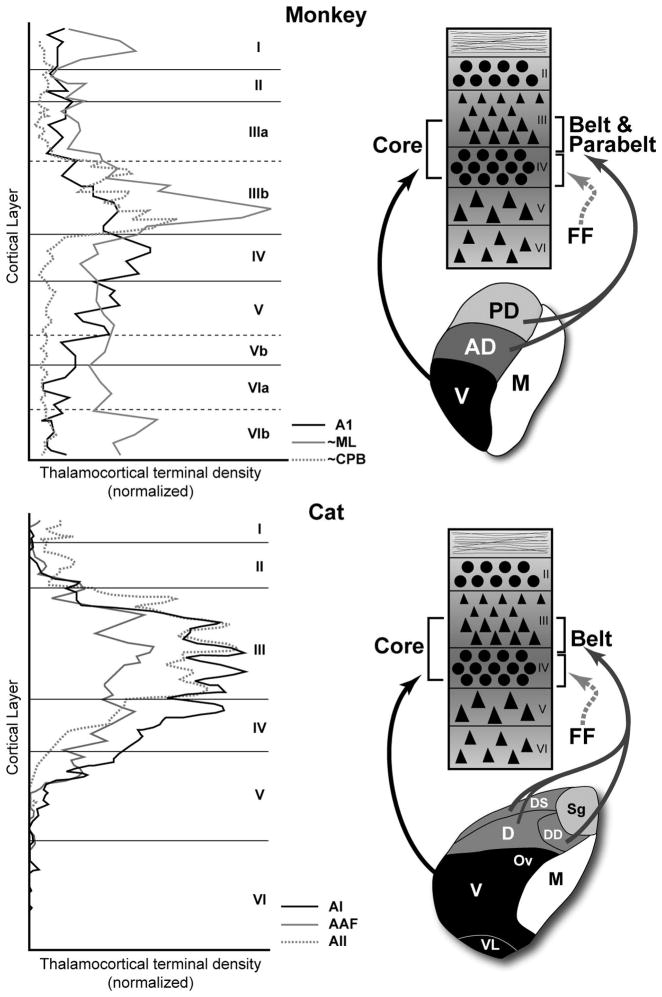
In 1976, Harold Burton and Ted Jones published two foundational papers on the auditory cortex of macaque monkeys and squirrel monkeys (Burton et al., 1976; Jones et al., 1976). Among the many important contributions of those studies were the tracer injections (tritiated proline) placed within various divisions of the MGC and other nuclei in the posterior thalamus. Some of their findings are summarized in Fig. 5a, where axon terminal density is plotted as a function of cortical layer. Labeled terminals projecting to the core region from the MGv are concentrated in layer IIIb, most of layer IV, and even into layer Va. By comparison, terminals in the belt and parabelt regions from the MGad and MGpd were somewhat less dense, concentrated in layer IIIb, and very sparse in layer IV. Comparable patterns have been found in the cat and are illustrated in Fig. 5b (Huang et al., 2000; Mitani et al., 1987). Overall, these data indicate that information from the ventral division flows mainly into layers IIIb and IV of the core, while information from the dorsal divisions flows mainly into IIIb of the belt and parabelt, avoiding layer IV. What, then, is the source of input to layer IV of the belt and parabelt? Although incomplete for many areas, the available data suggest that feedforward projections from hierarchically lower areas of auditory cortex (e.g., core) target layer IV of higher areas (e.g., belt), as illustrated in Fig. 5.
Fig. 5.
Laminar patterns of projections to auditory cortex from the medial geniculate complex (MGC) of the monkey (top) and cat (bottom). The left-hand panels plot the normalized density of MGC terminals by laminae for selected areas of the core, belt, and parabelt regions. The summary diagrams in the right-hand panels illustrate the major MGC projections to auditory cortex and their laminar targets. Feedfoward (FF) projections (dashed arrow) from cortical sources to layer IV are also shown. Redrawn and adapted from Jones and Burton (1976) (monkey) and Huang and Winer (2000)(cats).
These patterns highlight fundamental differences in the systems of projections that target the layer IIIb/IV band in the core and belt/parabelt regions, which certainly contribute to basic physiological differences between areas. As an example, consider A1 and the lateral belt area ML. A1 receives inputs into layer IIIb and IV from the MGv. The belt area, ML, receives MGpd projections concentrated in layer IIIb and feedforward inputs to layer IV from A1. If we assume that MGpd neurons projecting to belt cortex have longer latencies than MGv neurons (Allon et al., 1981), and that layer IV neurons project to layer IIIb within columns (Mitani et al., 1985), it may be that layer IIIb neurons in ML receive convergent inputs from the MGpd (directly) and through the MGv-A1-ML pathway within their effective temporal integration window. This is speculative, but generally in line with the spike timing delays observed in the belt areas of primates, which are typically longer than in the core (Crum et al., 2009; Kusmierek et al., 2009; Recanzone, 2000). Comparable patterns may characterize projections to the parabelt areas. In the caudal parabelt (CPB), for example, layer IIIb in the parabelt receives MGpd and MGad inputs (MGpd/MGad – CPB), and there is some evidence of feedforward inputs to layer IV of the parabelt from the belt (MGad/MGpd – ML – CPB) (de la Mothe et al., 2006a). Because belt and parabelt areas receive inputs from the dorsal divisions of the MGC, latencies in the parabelt may not necessarily be longer, although recent findings in marmosets indicate that latencies do increase systematically from core to belt to parabelt (Crum et al., 2009), consistent with anatomical indications of a processing hierarchy along the core-belt-parabelt axis.
Along the caudal-rostral axis in monkeys and dorsal-ventral axis in cats, there is no indication that the laminar patterns of thalamocortical inputs change within the core or belt and parabelt regions (Huang et al., 2000; Jones et al., 1976). That is, MGC inputs target layers IIIb and IV throughout the core, and layer IIIb in the belt and parabelt. Two patterns are worth noting, however. First, terminal density in the IIIb/IV band of the core is greater in A1 than the other core areas in both cats and monkeys. Although the data are insufficient to determine whether this holds for the belt region, at present, this pattern suggests that the impact of the thalamocortical projection could vary within regions along these axes. Second, as described earlier, rostral areas of auditory cortex in monkeys tend to receive inputs from the caudal MGC, and vice versa. In cats, a similar trend is evident as ventral areas receive stronger projections from the caudal MGC. Note that these gradients are not to be confused with those related to tonotopic organization (Imig et al., 1984; Morel et al., 1992). Since single cells in thalamus rarely project to more than one area of auditory cortex (Lee et al., 2008a; Lee et al., 2004a), the connection patterns indicate that different cell populations within the MGad, MGpd and MGv project topographically within regions along these cortical axes. The resulting gradients provide a structural framework for the distribution of physiologically distinct information to areas within a region from the same nuclei in the thalamus.
For example, there is evidence that the rostral pole of the MGC in cats, which corresponds to the MGad in primates, is tonotopically organized and its neurons have short latencies (Imig et al., 1984; Imig et al., 1985a; Imig et al., 1985b). The primary target of the rostral pole in cats is the AAF (Andersen et al., 1980; Lee et al., 2005; Lee et al., 2004b), where temporal precision and average spike latencies are comparable to that of A1. In core and belt areas ventral to AAF and A1, which receive inputs from more caudal portions of the MGC, neuronal responses are significantly more sluggish. Thus, the latency gradient along the dorsal-ventral axis is correlated with the rostrocaudal topography of MGC projections. A similar relationship is present in monkeys. The principal targets of the MGad are the caudomedial (CM) and middle medial (MM) belt areas (de la Mothe et al., 2006b; Hackett, 2007a; Hackett et al., 2007), where average latencies are at least as fast as in A1 and temporal precision is very high (Bieser et al., 1996; Cheung et al., 2001; Kajikawa et al., 2005a; Kajikawa et al., 2005b; Kajikawa et al., 2008; Kusmierek et al., 2009; Lakatos et al., 2005). By comparison, the rostral areas of the core and belt have relatively longer latencies and reduced temporal precision (Bendor et al., 2008; Kusmierek et al., 2009; Recanzone, 2000), but receive inputs from the caudal MGC. Detailed studies of neuronal activity in the MGC are needed, especially in primates, to determine whether physiological gradients in auditory cortex and thalamus are correlated,, as suggested by the anatomical relationships.
Neurochemical influences on information flow
In consideration of the thalamocortical, corticocortical, and laminar patterns of connections involving auditory cortex reviewed so far, there is rather clear evidence of anatomical gradients along at least two axes in cats and monkeys. Connectivity is only part of a much more elaborate picture, however. Differences between areas are also related to the neurochemical properties of their intrinsic and extrinsic circuitry, but we know relatively little about the neurochemistry of the networks that link subcortical nuclei with cortical areas, and even less about how these properties affect the processing of information. This section explores recent work in this area, mainly in primates, with an emphasis on the thalamocortical portion of the network.
As discussed above, the division of monkey auditory cortex into areas and regions is primarily based on connectivity. Supporting the conclusions reached from analyses of connections are various architectonic features, including cytoarchitecture, myeloarchitecture and chemoarchitecture. The latter refers to neurochemical expression patterns obtained from various histochemical or immuoncytochemical treatments of tissue. In Fig. 6 (Hackett et al., 2009), adjacent sections of the macaque auditory cortex were processed to reveal the distribution of 6 different compounds: Nissl substance (N), localized to the rough endoplasmic reticulum of the cytoplasm; myelinated axons (MF); cytochrome oxidase (CO), a metabolic enzyme found in mitochondria; acetylcholinesterase (AChE), an enzyme that lyses acetyolcholine after release; parvalbumin (PV), a calcium binding protein found in cortex and thalamus; and the vesicular glutamate transporter 2 (VGluT2), one of three known glutamate transporters that regulates the storage and release of glutamate in synaptic vesicles.
Fig. 6.
Series of adjacent coronal sections through caudal areas of auditory cortex stained for several chemoarchitectonic markers: Parvalbumin (PV), vesicular glutamate transporter 2 (VGluT2); cytochrome oxidase (CO); acetylcholinesterease (AChE); myelinated fibers (MF); Nissl (N). Note that the dense band of staining in layer IIIb/IV is highest in the core area, A1, and weaker in the belt and parabelt areas. Arrowheads denote borders between areas. Scale bars, 2 mm. From Hackett and de la Mothe (2009).
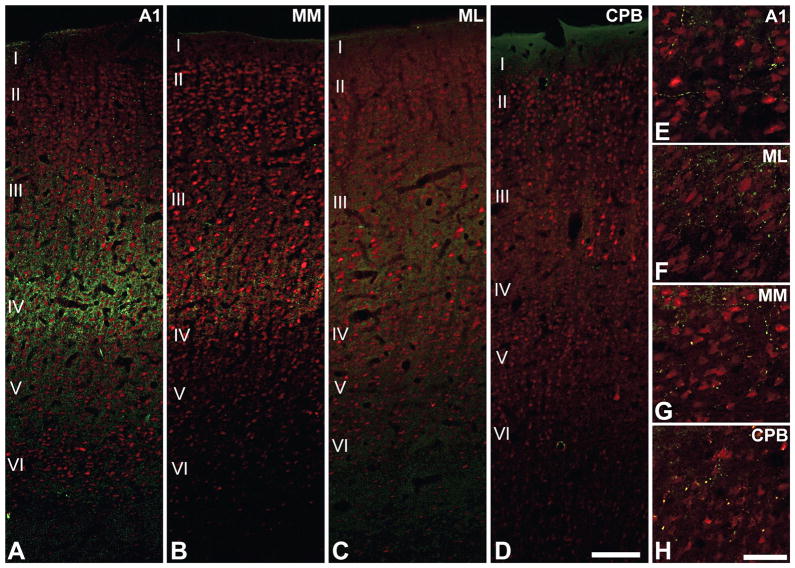
One of the most obvious features at low magnification (Fig. 6) is that the expression of the neurochemical markers (CO, AChE, PV, VGluT2) is concentrated in a horizontal band roughly corresponding to the thalamorecipient layers. Secondly, marker expression is very dense in the core, intermediate in the medial and lateral belt, and weak in the parabelt, indicating a diminishing gradient along the core-belt-parabelt axis. Thus, there is a high degree of overlap in the distribution of these markers in layers IIIb and IV. Additionally, PV and VGluT2 protein expression is localized, and perhaps co-localized, in the thalamocortical axon terminals concentrated in these laminae (Graziano et al., 2008; Hackett et al., 2009; Hashikawa et al., 1991; Hashikawa et al., 1995; Jones, 2003). Accordingly, their sublaminar distribution would be expected to match thalamocortical projection patterns. This is illustrated in Fig. 7 where sections of the macaque auditory cortex were double-labeled for VGluT2 (green) and the neuronal marker NeuN (red)(Hackett et al., 2009). Note that in A1, VGluT2-ir spans layers IIIb and IV. In the medial belt (MM), VGluT2-ir is less dense, and confined to layer IIIb. In the lateral belt (ML), VGluT2-ir is a bit weaker, and also confined to layer IIIb. In the parabelt (CPB), VGluT2-ir is barely detectable. There is some weak labeling of terminals in layer VI of all areas.
Fig. 7.
Dual fluorescence immunoreactivity (-ir) for VGluT2 (green puncta) and NeuN (red somata) in areas A1, MM, ML and CPB of the macaque monkey. (A) In A1, VGluT2-ir is most densely concentrated in the layers IIIb and IV, where it forms vertical bands around NeuN-ir cell columns. (B) In MM, VGluT2-ir is most dense in layer IIIb with some extension into layer IV. (C) In ML, VGluT2-ir is reduced compared to MM and mainly concentrated in IIIb. (D) VGluT2-ir is absent in the IIIb/IV band of the CPB. (E – H) In all areas, sparse VGluT2-ir is contained in boutons en passant positioned along local axon branches in layers Vb and VI. Scale bars: 250 μm (A-D), 25 μm (E-H). Adapted from Hackett and de la Mothe (2009).
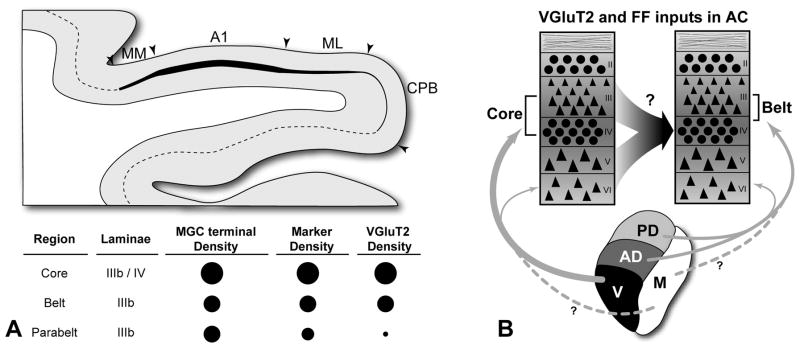
So, as indexed by VGluT2 expression, it appears that the impact of this class of excitatory thalamic inputs to auditory cortex varies along the core, belt and parabelt axis by cortical layer in a manner that matches known projection patterns. In some ways the convergence of anatomical indices is satisfying, as gradients in the distribution of VGluT2 and the other markers seem to be consistent with neurophysiological changes observed along this axis. Yet, the patterns observed also raise some questions (Fig. 8). First, note that VGluT2-ir density decreased to almost nothing in the lateral belt and parabelt regions, whereas the density of thalamic inputs to these areas observed by Jones and Burton is only slightly weaker compared to the core. Second, note that VGluT2-ir is largely absent from layer IV of the belt and parabelt regions – the laminar target of feedforward projections. While informative with respect to the distribution of the VGluT2 protein, the patterns also reveal that the neurochemical properties of a substantial portion of the thalamocortical and corticocortical projections are unknown. On the assumption that these projections are glutamatergic, it is possible that they may utilize different glutamate transporters, such as VGluT1 or perhaps one that has not been identified. Such distinctions may be functionally significant. As an example, VGluT1 and VGluT2 have been associated with lower and higher release probabilities, respectively, in some brain areas (Kaneko et al., 2002; Varoqui et al., 2002). Such details suggest that a better understanding of glutamate trafficking in these circuits will be needed to account for differences between areas (Atzori et al., 2004; Jones, 2009). Of course, glutamate is just one of many molecules that serve as neurotransmitters and neuromodulators in the auditory cortical system. GABA (Razak et al., 2009; Wang et al., 2002; Zhang et al., 2003), acetylcholine (Hsieh et al., 2000; Kilgard et al., 1998; Metherate, 2004; Metherate et al., 1991), dopamine (Atzori et al., 2005), norepinephrine (Dinh et al., 2009) and serotonin (Hegerl et al., 1993; Wutzler et al., 2008) also impact the processing of information in auditory cortex. Although such details complicate our models, their characterization is necessary to achieve a more complete understanding of information processing in auditory cortex, in general, and the changes that occur along the major axes of information flow, in particular. As discussed in the next section, these features certainly contribute to the physiological differences observed along these axes.
Fig. 8.
Summary and interpretations of results. (A) Graphical and tabular representation of relative densities of MGC axon terminals, chemoarchitectonic markers (CO, AChE, PV), and VGluT2 in layers IIIb and IV of the core, belt, and parabelt. Larger circles denote highest density; (B) Projections (arrows) to laminae of the auditory core and belt regions from major divisions of the MGC expressing VGluT2 (parabelt not shown). Question mark indicates that the glutamate transported utilized by feedforward (FF) projections to layer IV has not been determined. Line thickness denotes relative density of each projection. Dashed lines with question marks from MGm indicate that the proportion of inputs from neurons in this division that are VGluT2-ir is unknown. MGC divisions: V, ventral; AD, anterodorsal; PD, posterodorsal; M, magnocellular. See text for details.
Neurophysiological correlates of information flow
A wide range of neurophysiological findings are consistent with the various anatomical schema described above. Briefly highlighted here are results from studies in which systematic changes in neuronal response properties were observed along the major axes of information flow in auditory cortex.
Core-belt-parabelt axis
A simple prediction of the core-belt-parabelt model is that neuron response properties would change in a systematic way along this axis. Specifically, one would predict that response latencies would increase, spectral integration (tuning bandwidth) would increase, and temporal precision (entrainment to periodic temporal events) would decrease (Rauschecker, 1998b). Typically, this is what has been found in studies of both anesthetized and awake animals. With respect to frequency tuning, studies in all species have reported greater tuning bandwidth in belt areas, as compared to the core. Less is known about the parabelt of primates, but in a recent study of awake marmosets (Crum et al., 2009), the first to compare activity across all three regions, frequency tuning was found to increase systematically from core to belt to parabelt.
With the exception of the medial belt areas, MM and CM, response latencies also increase along this axis. As discussed above, latencies in MM and CM are atypical of other belt areas in that the distribution of spike latencies are highly overlapping with those of A1, and often shorter in MM and CM, despite broader spectral tuning (Crum et al., 2009; Kusmierek et al., 2009; Recanzone, 2000). The other belt areas studied to date, however, have longer latencies compared to neurons in the core, and latencies in the parabelt region are the longest (Crum et al., 2009).
Finally, temporal precision, which is related to response latencies, tends to decrease along the core-belt-parabelt axis, although it has not been as well studied as other response properties. The clearest evidence was found by Crum et al (2009) who found that entrainment to amplitude modulated stimuli systematically decreased from A1 to ML to CPB, consistent with the model. Once again, MM and CM appear to be an exception, as neurons in this area entrain to periodic stimuli at rates comparable to A1 (Bieser et al., 1996; Kajikawa et al., 2008).
Thus, with the exception of the caudal medial belt areas, the available data indicate that neurons become more broadly tuned and increasingly sluggish along this axis, consistent with the notion that information is being transformed in the transition from core to belt to parabelt. As suggested by Rauschecker (1998), it is likely that these changes reflect increasing stimulus specificity as information ascends the processing hierarchy. Similar trends are evident between processing levels in the cat, such as A1 and AII (Carrasco et al., 2009a; Eggermont, 1998; Schreiner et al., 1984; Schreiner et al., 1986; Schreiner et al., 1988).
Caudal-rostral axis
Based on the structural patterns described above, one would expect to find physiological gradients along the caudal-rostral axis, as well. Comparative studies of areas along this axis are unfortunately limited, but some trends are evident. Generally, it appears that there is a decrease in the magnitude of auditory activation from caudal to rostral along the superior temporal gyrus, especially rostral to auditory cortex. In recent PET studies, a decreasing gradient in auditory- related activity was observed along this axis, reaching a minimum at the temporal pole (Poremba et al., 2007; Poremba et al., 2004). These differences likely reflect changes in both cortical and thalamic inputs, and may be related to an increase in response specificity rostrally (Petkov et al., 2008).
In single unit studies of primates, response latencies have been observed to increase from caudal to rostral, although not all areas have been studied to date. As discussed above, the shortest latencies anywhere in primate auditory cortex have been recorded from neurons in the belt areas caudal and medial to A1 (e.g., MM and CM) (Bieser et al., 1996; Cheung et al., 2001; Kajikawa et al., 2005a; Kajikawa et al., 2005b; Kajikawa et al., 2008; Kusmierek et al., 2009; Lakatos et al., 2005). Within the core response latencies also increase rostrally. In macaques, Recanzone et al (2000a) found that latencies in R were significantly longer than in A1. This finding was amplified by Kusmierek et al (2009) in their study of the medial belt areas RM and MM, and the core areas R and A1. Within regions, they found that latencies in A1 were shorter than R, and MM latencies were shorter than RM. On average, the longest latencies across all four areas were in R. In marmosets, Bendor and Wang (2008) reported a systematic increase in response latencies from A1 to R to RT along this axis. The long latencies in R and RT were not expected, given that neurons in the core areas receive their principal driving inputs in parallel from the MGv. Instead, the findings are more in line with the rostrally-directed flow of feedforward projections from A1 to R to RT, as described above. However, Rauschecker et al (1997) found that activity in R was unaffected by ablation of A1, suggesting that activity in R is not dependent on A1 inputs. Could the differences in latencies between A1 and R reflect properties of the neuronal subpopulations in the MGv that project to these areas? This is not known, and underscores the tremendous need for detailed studies of the thalamic and cortical inputs to the core areas in relation to the physiological properties of their neurons.
Within the core region of cats, there is evidence of physiological gradients have been observed within areas, such as A1 (Schreiner et al., 2000), and between areas along the dorsal-ventral axis. Functional gradients, such as sensitivity and importance for sound localization have been the focus of several recent studies comparing areas within the core (Harrington et al., 2008; Malhotra et al., 2007; Malhotra et al., 2004). Other gradients concern differences in basic response properties. For example, response latencies are shortest in AAF, slightly longer in A1, and longest in PAF, which has fewer monotonic units than either A1 or AAF (Eggermont, 1998; Harrington et al., 2008; Imaizumi et al., 2004; Phillips et al., 1984). In part these reflect differences in latencies of their thalamic input sources (Calford, 1983; Imig et al., 1985a; Imig et al., 1985b; Rodrigues-Dagaeff et al., 1989), but it may also be related to pathway length and other structural features that affect conduction velocity, such as axonal diameter and myelination (Aggelopoulos et al., 1995; Salami et al., 2003; Steriade, 1995; Sugihara et al., 1993).
Additional support for gradients along this axis can be found in the recent work by Lomber and colleagues (this volume) in which areas of the core were selectively deactivated by cooling. Carrasco and Lomber (2009a) found that cooling A1 had minor effects on activity in AAF, while cooling of area AAF was associated with reduced response strength, increased threshold, and sharpened spectral tuning in A1 (Carrasco et al., 2009c). This result suggests that activity in AAF is relatively independent of A1, whereas activity in A1 is significantly modulated by inputs from AAF. This is interesting, since areas AAF and A1 are densely interconnected and receive independent parallel inputs from the MGC. In a second study of the core (Carrasco et al., 2009b), cooling of A1 was followed by a decrease in response strength and sharpened spectral tuning in PAF. Activity in A1 was not found to be altered by cooling of PAF, however. While this result contrasts with an earlier study in which activity in PAF was not substantially affected by ablation of A1 (Kitzes et al., 1996), it may be that these methods of deactivation involve different processes. Overall, however, the combined data are consistent with the hypothesis that a processing hierarchy exists along the dorsal-ventral axis of the core region in cats.
Conclusions and future directions
In this article, a broad range of anatomical and physiological data were compiled in an effort to track information flow into, within, and out of auditory cortex. The patterns and trends observed indicate that while information processing in auditory cortex is strongly parallel at all levels, information tends to move along axes that are hierarchically ordered. They also form the basis of the processing streams that impact numerous auditory-related areas downstream. At present, our models of auditory cortical organization depend heavily on the patterns of connections established over several decades. In general, the results of neurophysiological studies have been consistent with the basic tenets of these models, but some exceptions have been noted. These will require further study and ultimately lead to revisions of the models. Looking ahead, there is still a desperate need for neurophysiological and neuroimaging studies of basic and advanced response properties across all areas of auditory cortex, especially areas beyond A1. On the anatomical side, the availability of probes to study gene and protein expression has opened the door for detailed studies of the neurochemistry of the circuits that comprise the auditory cortical network. Recent studies in this area indicate that a comprehensive understanding of information processing in auditory cortex must incorporate information on these circuits at a laminar and sublaminar scale.
Acknowledgments
The author gratefully acknowledges the support of NIH/NIDCD grant RO1 DC04318 to T.A. Hackett and T32 MH075883 to Vanderbilt Kennedy Center for support of the confocal microscope. We also thank Lisa de la Mothe, Corrie Camalier and the reviewers for their helpful and insightful comments.
List of Abbreviations
- AAF
Anterior auditory field
- AD
Anterodorsal division (medial geniculate)
- A1
Auditory area 1
- AII
Second auditory field
- AChE
Acetylcholinesterase
- AL
Anterolateral area
- CB
Calbindin
- CL
Caudolateral area
- CM
Caudomedial area
- CO
Cytochrome oxidase
- CPB
Caudal parabelt area
- CS
Central sulcus
- D
Dorsal nucleus (medial geniculate)
- DC
Dorsal cortex (inferior colliculus)
- DD
Deep dorsal nucleus (medial geniculate)
- DS
Superficial dorsal nucleus (medial geniculate)
- DZ
Dorsal zone
- EP
Posterior ectosylvian region
- ED
Dorsal ectosylvian region
- EI
Intermediate ectosylvian region
- EV
Ventral ectosylvian region
- FF
Feedforward projection
- IC
Inferior colliculus
- ICc
Inferior colliculus, central nucleus
- Ins
Insula
- IPS
Intraparietal sulcus
- L
Lateral nucleus (inferior colliculus)
- LGN
Lateral geniculate nucleus
- LS
Lateral sulcus
- LuS
Lunate sulcus
- M
Magnocellular division (medial geniculate)
- MF
Myelinated fibers (axons)
- MGad
Medial geniculate complex, anterodorsal division
- MGC
Medial geniculate complex
- MGd
Medial geniculate complex, dorsal division
- MGm
Medial geniculate complex, magnocellular division
- MGpd
Medial geniculate complex, posterodorsal division
- MGv
Medial geniculate complex, ventral division
- ML
Middle lateral area
- MM
Middle medial area
- Ov
Pars ovoidea (of medial geniculate)
- PAF
Posterior auditory field
- PD
Posterodorsal division (medial geniculate)
- Pro
Proisocortical area
- proA
Prokoniocortex area
- PS
Principal sulcus
- PV
Parvalbumin
- R
Rostral area
- Ri
Retroinsular area
- RM
Rostromedial area
- RPB
Rostral parabelt area
- RT
Rostrotemporal area
- RTL
Rostrotemporal lateral area
- RTM
Rostrotemporal medial area
- Sg
Suprageniculate nucleus
- STG
Supeior temporal gyrus
- Te
Temporal auditory field
- Tpt
Temporal parietotemporal area
- VGluT
Vesicular glutamate transporter
- V
Ventral division (medial geniculate)
- VAF
Ventral posterior auditory field (same as VPAF)
- VGluT2
Vesicular glutamate transporter 2
- VL
Ventrolateral nucleus (medial geniculate)
- VP
Ventroposterior nucleus
- VPAF
Ventral posterior auditory field
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggelopoulos NC, Duke C, Edgley SA. Non-uniform conduction time in the olivocerebellar pathway in the anaesthetized cat. J Physiol. 1995;486 ( Pt 3):763–8. doi: 10.1113/jphysiol.1995.sp020851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitkin L. The Auditory Midbrain: Structure and Function in the Central Auditory Pathway. Humana Press; Clifton, N.J: 1986. [Google Scholar]
- Allon N, Yeshurun Y, Wollberg Z. Responses of single cells in the medial geniculate body of awake squirrel monkeys. Exp Brain Res. 1981;41:222–32. doi: 10.1007/BF00238879. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Knight PL, Merzenich MM. The thalamocortical and corticothalamic connections of AI, AII, and the anterior auditory field (AAF) in the cat: evidence for two largely segregated systems of connections. J Comp Neurol. 1980;194:663–701. doi: 10.1002/cne.901940312. [DOI] [PubMed] [Google Scholar]
- Atencio CA, Schreiner CE. Laminar diversity of dynamic sound processing in cat primary auditory cortex. J Neurophysiol. 2009 doi: 10.1152/jn.00624.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzori M, Flores Hernandez J, Pineda JC. Interlaminar differences of spike activation threshold in the auditory cortex of the rat. Hear Res. 2004;189:101–6. doi: 10.1016/S0378-5955(03)00301-0. [DOI] [PubMed] [Google Scholar]
- Atzori M, Kanold PO, Pineda JC, Flores-Hernandez J, Paz RD. Dopamine prevents muscarinic-induced decrease of glutamate release in the auditory cortex. Neuroscience. 2005;134:1153–65. doi: 10.1016/j.neuroscience.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Barbas H. Specialized elements of orbitofrontal cortex in primates. Ann N Y Acad Sci. 2007;1121:10–32. doi: 10.1196/annals.1401.015. [DOI] [PubMed] [Google Scholar]
- Bendor D, Wang X. Neural response properties of core fields AI, R, and RT in the auditory cortex of marmoset monkeys. J Neurophysiol. 2008 doi: 10.1152/jn.00884.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieser A, Muller-Preuss P. Auditory responsive cortex in the squirrel monkey: neural responses to amplitude-modulated sounds. Exp Brain Res. 1996;108:273–84. doi: 10.1007/BF00228100. [DOI] [PubMed] [Google Scholar]
- Bizley JK, Nodal FR, Nelken I, King AJ. Functional Organization of Ferret Auditory Cortex. Cereb Cortex. 2005;15:1637–1653. doi: 10.1093/cercor/bhi042. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde. Barth; Leipzig: 1909. [Google Scholar]
- Budinger E, Scheich H. Anatomical connections suitable for the direct processing of neuronal information of different modalities via the rodent primary auditory cortex. Hear Res. 2009;258:16–27. doi: 10.1016/j.heares.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Budinger E, Heil P, Scheich H. Functional organization of auditory cortex in the Mongolian gerbil (Meriones unguiculatus). III. Anatomical subdivisions and corticocortical connections. Eur J Neurosci. 2000;12:2425–51. doi: 10.1046/j.1460-9568.2000.00142.x. [DOI] [PubMed] [Google Scholar]
- Burton H, Jones EG. The posterior thalamic region and its cortical projection in New World and Old World monkeys. J Comp Neurol. 1976;168:249–301. doi: 10.1002/cne.901680204. [DOI] [PubMed] [Google Scholar]
- Calford MB. The parcellation of the medial geniculate body of the cat defined by the auditory response properties of single units. J Neurosci. 1983;3:2350–64. doi: 10.1523/JNEUROSCI.03-11-02350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford MB, Aitkin LM. Ascending projections to the medial geniculate body of the cat: evidence for multiple, parallel auditory pathways through thalamus. J Neurosci. 1983;3:2365–80. doi: 10.1523/JNEUROSCI.03-11-02365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell HW. Histological Studies on the Localization of Cerebral Function. Cambridge University Press; Cambridge, UK: 1905. [Google Scholar]
- Cappe C, Barone P. Heteromodal connections supporting multisensory integration at low levels of cortical processing in the monkey. Eur J Neurosci. 2005;22:2886–902. doi: 10.1111/j.1460-9568.2005.04462.x. [DOI] [PubMed] [Google Scholar]
- Carrasco A, Lomber SG. Reciprocal modulatory influencesbetween tonotopic and non-tonotopic cortical fields in the cat. J Neurosci. 2009a doi: 10.1523/JNEUROSCI.5708-09.2009. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco A, Lomber SG. Evidence for hierarchical processing in cat auditory cortex: nonreciprocal influence of primary auditory cortex on the posterior auditory field. J Neurosci. 2009b:29. doi: 10.1523/JNEUROSCI.2905-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco A, Lomber SG. Differential modulatory influences between primary auditory cortex and the anterior auditory field. J Neurosci. 2009c;29:8350–62. doi: 10.1523/JNEUROSCI.6001-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–42. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Cheung SW, Bedenbaugh PH, Nagarajan SS, Schreiner CE. Functional organization of squirrel monkey primary auditory cortex: responses to pure tones. J Neurophysiol. 2001;85:1732–49. doi: 10.1152/jn.2001.85.4.1732. [DOI] [PubMed] [Google Scholar]
- Clasca F, Llamas A, Reinoso-Suarez F. Cortical connections of the insular and adjacent parieto-temporal fields in the cat. Cereb Cortex. 2000;10:371–99. doi: 10.1093/cercor/10.4.371. [DOI] [PubMed] [Google Scholar]
- Cohen YE, Cohen IS, Gifford GW., 3rd Modulation of LIP activity by predictive auditory and visual cues. Cereb Cortex. 2004;14:1287–301. doi: 10.1093/cercor/bhh090. [DOI] [PubMed] [Google Scholar]
- Cohen YE, Russ BE, Gifford GW., 3rd Auditory processing in the posterior parietal cortex. Behav Cogn Neurosci Rev. 2005;4:218–31. doi: 10.1177/1534582305285861. [DOI] [PubMed] [Google Scholar]
- Cohen YE, Theunissen F, Russ BE, Gill P. Acoustic features of rhesus vocalizations and their representation in the ventrolateral prefrontal cortex. J Neurophysiol. 2007;97:1470–84. doi: 10.1152/jn.00769.2006. [DOI] [PubMed] [Google Scholar]
- Crum PAC, Issa E, Hackett TA, Wang X. Hierarchical processing in awake primate auditory cortex. 2009. Submitted. [Google Scholar]
- de la Mothe LA, Blumell S, Kajikawa Y, Hackett TA. Cortical connections of auditory cortex in marmoset monkeys: core and medial belt regions. J Comp Neurol. 2006a;496:27–71. doi: 10.1002/cne.20923. [DOI] [PubMed] [Google Scholar]
- de la Mothe LA, Blumell S, Kajikawa Y, Hackett TA. Thalamic connections of auditory cortex in marmoset monkeys: core and medial belt regions. J Comp Neurol. 2006b;496:72–96. doi: 10.1002/cne.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh L, Nguyen T, Salgado H, Atzori M. Norepinephrine homogeneously inhibits alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate- (AMPAR-) mediated currents in all layers of the temporal cortex of the rat. Neurochem Res. 2009;34:1896–906. doi: 10.1007/s11064-009-9966-z. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Representation of spectral and temporal sound features in three cortical fields of the cat. Similarities outweigh differences. J Neurophysiol. 1998;80:2743–64. doi: 10.1152/jn.1998.80.5.2743. [DOI] [PubMed] [Google Scholar]
- Falchier A, Clavagnier S, Barone P, Kennedy H. Anatomical evidence of multimodal integration in primate striate cortex. J Neurosci. 2002;22:5749–59. doi: 10.1523/JNEUROSCI.22-13-05749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchier A, Schroeder CE, Hackett TA, Lakatos P, Nascimento-Silva S, Ulbert I, Karmos G, Smiley JF. Projection from Visual Areas V2 and Prostriata to Caudal Auditory Cortex in the Monkey. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick KA, Imig TJ. Auditory cortico-cortical connections in the owl monkey. J Comp Neurol. 1980;192:589–610. doi: 10.1002/cne.901920314. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Pandya DN. The intrinsic architectonic and connectional organization of the superior temporal region of the rhesus monkey. J Comp Neurol. 1983;221:169–84. doi: 10.1002/cne.902210206. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–79. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Graziano A, Liu XB, Murray KD, Jones EG. Vesicular glutamate transporters define two sets of glutamatergic afferents to the somatosensory thalamus and two thalamocortical projections in the mouse. J Comp Neurol. 2008;507:1258–76. doi: 10.1002/cne.21592. [DOI] [PubMed] [Google Scholar]
- Hackett TA. Organization of the thalamocortical auditory pathways in primates. In: Burkard RF, Don M, Eggermont JJ, editors. Auditory Evoked Potentials: Basic Principles and Clinical Application. Lippincott Williams & Wilkins; Baltimore: 2007a. pp. 428–440. [Google Scholar]
- Hackett TA, de la Mothe LA. Regional and laminar distribution of the vesicular glutamate transporter, VGluT2, in the macaque monkey auditory cortex. J Chem Neuroanat. 2009;38:106–16. doi: 10.1016/j.jchemneu.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, Stepniewska I, Kaas JH. Subdivisions of auditory cortex and ipsilateral cortical connections of the parabelt auditory cortex in macaque monkeys. J Comp Neurol. 1998a;394:475–95. doi: 10.1002/(sici)1096-9861(19980518)394:4<475::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Stepniewska I, Kaas JH. Thalamocortical connections of the parabelt auditory cortex in macaque monkeys. J Comp Neurol. 1998b;400:271–86. doi: 10.1002/(sici)1096-9861(19981019)400:2<271::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Stepniewska I, Kaas JH. Prefrontal connections of the parabelt auditory cortex in macaque monkeys. Brain Res. 1999;817:45–58. doi: 10.1016/s0006-8993(98)01182-2. [DOI] [PubMed] [Google Scholar]
- Hackett TA, De La Mothe LA, Ulbert I, Karmos G, Smiley J, Schroeder CE. Multisensory convergence in auditory cortex, II. Thalamocortical connections of the caudal superior temporal plane. J Comp Neurol. 2007;502:924–52. doi: 10.1002/cne.21326. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Smiley JF, Ulbert I, Karmos G, Lakatos P, de la Mothe LA, Schroeder CE. Sources of somatosensory input to the caudal belt areas of auditory cortex. Perception. 2007b;36:1419–1430. doi: 10.1068/p5841. [DOI] [PubMed] [Google Scholar]
- Harrington IA, Stecker GC, Macpherson EA, Middlebrooks JC. Spatial sensitivity of neurons in the anterior, posterior, and primary fields of cat auditory cortex. Hear Res. 2008;240:22–41. doi: 10.1016/j.heares.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashikawa T, Rausell E, Molinari M, Jones EG. Parvalbumin- and calbindin-containing neurons in the monkey medial geniculate complex: differential distribution and cortical layer specific projections. Brain Res. 1991;544:335–41. doi: 10.1016/0006-8993(91)90076-8. [DOI] [PubMed] [Google Scholar]
- Hashikawa T, Molinari M, Rausell E, Jones EG. Patchy and laminar terminations of medial geniculate axons in monkey auditory cortex. J Comp Neurol. 1995;362:195–208. doi: 10.1002/cne.903620204. [DOI] [PubMed] [Google Scholar]
- Hegerl U, Juckel G. Intensity dependence of auditory evoked potentials as an indicator of central serotonergic neurotransmission: a new hypothesis. Biol Psychiatry. 1993;33:173–87. doi: 10.1016/0006-3223(93)90137-3. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Cruikshank SJ, Metherate R. Differential modulation of auditory thalamocortical and intracortical synaptic transmission by cholinergic agonist. Brain Res. 2000;880:51–64. doi: 10.1016/s0006-8993(00)02766-9. [DOI] [PubMed] [Google Scholar]
- Huang CL, Winer JA. Auditory thalamocortical projections in the cat: laminar and areal patterns of input. J Comp Neurol. 2000;427:302–31. doi: 10.1002/1096-9861(20001113)427:2<302::aid-cne10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Imaizumi K, Priebe NJ, Crum PA, Bedenbaugh PH, Cheung SW, Schreiner CE. Modular functional organization of cat anterior auditory field. J Neurophysiol. 2004;92:444–57. doi: 10.1152/jn.01173.2003. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Reale RA. Patterns of cortico-cortical connections related to tonotopic maps in cat auditory cortex. J Comp Neurol. 1980;192:293–332. doi: 10.1002/cne.901920208. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Morel A. Organization of the thalamocortical auditory system in the cat. Annu Rev Neurosci. 1983;6:95–120. doi: 10.1146/annurev.ne.06.030183.000523. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Morel A. Topographic and cytoarchitectonic organization of thalamic neurons related to their targets in low-, middle-, and high-frequency representations in cat auditory cortex. J Comp Neurol. 1984;227:511–39. doi: 10.1002/cne.902270405. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Morel A. Tonotopic organization in lateral part of posterior group of thalamic nuclei in the cat. J Neurophysiol. 1985a;53:836–51. doi: 10.1152/jn.1985.53.3.836. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Morel A. Tonotopic organization in ventral nucleus of medial geniculate body in the cat. J Neurophysiol. 1985b;53:309–40. doi: 10.1152/jn.1985.53.1.309. [DOI] [PubMed] [Google Scholar]
- Jones EG. Chemically defined parallel pathways in the monkey auditory system. Ann N Y Acad Sci. 2003;999:218–33. doi: 10.1196/annals.1284.033. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. 2. Cambridge University Press; Cambridge: 2007. [Google Scholar]
- Jones EG. Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Ann N Y Acad Sci. 2009;1157:10–23. doi: 10.1111/j.1749-6632.2009.04534.x. [DOI] [PubMed] [Google Scholar]
- Jones EG, Burton H. Areal differences in the laminar distribution of thalamic afferents in cortical fields of the insular, parietal and temporal regions of primates. J Comp Neurol. 1976;168:197–247. doi: 10.1002/cne.901680203. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and levels of processing in primates. Audiol Neurootol. 1998;3:73–85. doi: 10.1159/000013783. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. ‘What’ and ‘where’ processing in auditory cortex. Nat Neurosci. 1999a;2:1045–7. doi: 10.1038/15967. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci U S A. 2000;97:11793–9. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA, Tramo MJ. Auditory processing in primate cerebral cortex. Curr Opin Neurobiol. 1999b;9:164–70. doi: 10.1016/s0959-4388(99)80022-1. [DOI] [PubMed] [Google Scholar]
- Kajikawa Y, Hackett TA. Entropy analysis of neuronal spike train synchrony. J Neurosci Methods. 2005a;149:90–3. doi: 10.1016/j.jneumeth.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Kajikawa Y, de la Mothe LA, Blumell S, Hackett TA. A comparison of neuron response properties in areas A1 and CM of the marmoset monkey auditory cortex: tones and broad band noise. J Neurophysiol. 2005b;93:22–34. doi: 10.1152/jn.00248.2004. [DOI] [PubMed] [Google Scholar]
- Kajikawa Y, de la Mothe LA, Blumell S, Sterbing SJ, d’Angelo WR, Camalier CR, Hackett TA. Coding of FM sweep trains and twitter calls in area CM of marmoset auditory cortex. Hear Res. 2008;239:107–125. doi: 10.1016/j.heares.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res. 2002;42:243–50. doi: 10.1016/s0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–8. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kitzes LM, Hollrigel GS. Response properties of units in the posterior auditory field deprived of input from the ipsilateral primary auditory cortex. Hear Res. 1996;100:120–30. doi: 10.1016/0378-5955(96)00103-7. [DOI] [PubMed] [Google Scholar]
- Knight PL. Representation of the cochlea within the anterior auditory field (AAF) of the cat. Brain Res. 1977;130:447–67. doi: 10.1016/0006-8993(77)90108-1. [DOI] [PubMed] [Google Scholar]
- Kosmal A, Malinowska M, Kowalska DM. Thalamic and amygdaloid connections of the auditory association cortex of the superior temporal gyrus in rhesus monkey (Macaca mulatta) Acta Neurobiol Exp (Wars) 1997;57:165–88. doi: 10.55782/ane-1997-1224. [DOI] [PubMed] [Google Scholar]
- Kowalski N, Versnel H, Shamma SA. Comparison of responses in the anterior and primary auditory fields of the ferret cortex. J Neurophysiol. 1995;73:1513–23. doi: 10.1152/jn.1995.73.4.1513. [DOI] [PubMed] [Google Scholar]
- Kusmierek P, Rauschecker JP. Functional specialization of medial auditory belt cortex in the alert rhesus monkey. J Neurophysiol. 2009;102:1606–22. doi: 10.1152/jn.00167.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Pincze Z, Fu KG, Javitt DC, Karmos G, Schroeder CE. Timing of pure tone and noise-evoked responses in macaque auditory cortex. Neuroreport. 2005;16:933–937. doi: 10.1097/00001756-200506210-00011. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: projections to the neocortex. J Comp Neurol. 2002;447:394–420. doi: 10.1002/cne.10243. [DOI] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Principles governing auditory cortex connections. Cereb Cortex. 2005;15:1804–14. doi: 10.1093/cercor/bhi057. [DOI] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Connections of cat auditory cortex: I. Thalamocortical system. J Comp Neurol. 2008a;507:1879–900. doi: 10.1002/cne.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Connections of cat auditory cortex: III. Corticocortical system. J Comp Neurol. 2008b;507:1920–43. doi: 10.1002/cne.21613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Imaizumi K, Schreiner CE, Winer JA. Concurrent tonotopic processing streams in auditory cortex. Cereb Cortex. 2004a;14:441–51. doi: 10.1093/cercor/bhh006. [DOI] [PubMed] [Google Scholar]
- Lee CC, Schreiner CE, Imaizumi K, Winer JA. Tonotopic and heterotopic projection systems in physiologically defined auditory cortex. Neuroscience. 2004b;128:871–87. doi: 10.1016/j.neuroscience.2004.06.062. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000;428:112–37. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Malhotra S, Hall AJ. Functional specialization in non-primary auditory cortex of the cat: areal and laminar contributions to sound localization. Hear Res. 2007;229:31–45. doi: 10.1016/j.heares.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Lomber SG. Sound localization during homotopic and heterotopic bilateral cooling deactivation of primary and nonprimary auditory cortical areas in the cat. J Neurophysiol. 2007;97:26–43. doi: 10.1152/jn.00720.2006. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Hall AJ, Lomber SG. Cortical Control of Sound Localization in the Cat: Unilateral Cooling Deactivation of 19 Cerebral Areas. J Neurophysiol. 2004;92:1625–1643. doi: 10.1152/jn.01205.2003. [DOI] [PubMed] [Google Scholar]
- Metherate R. Nicotinic acetylcholine receptors in sensory cortex. Learn Mem. 2004;11:50–9. doi: 10.1101/lm.69904. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Basal forebrain stimulation modifies auditory cortex responsiveness by an action at muscarinic receptors. Brain Res. 1991;559:163–7. doi: 10.1016/0006-8993(91)90301-b. [DOI] [PubMed] [Google Scholar]
- Mishkin M. Analogous neural models for tactual and visual learning. Neuropsychologia. 1979;17:139–51. doi: 10.1016/0028-3932(79)90005-8. [DOI] [PubMed] [Google Scholar]
- Mitani A, Itoh K, Mizuno N. Distribution and size of thalamic neurons projecting to layer I of the auditory cortical fields of the cat compared to those projecting to layer IV. J Comp Neurol. 1987;257:105–21. doi: 10.1002/cne.902570108. [DOI] [PubMed] [Google Scholar]
- Mitani A, Shimokouchi M, Itoh K, Nomura S, Kudo M, Mizuno N. Morphology and laminar organization of electrophysiologically identified neurons in the primary auditory cortex in the cat. J Comp Neurol. 1985;235:430–47. doi: 10.1002/cne.902350403. [DOI] [PubMed] [Google Scholar]
- Molinari M, Dell’Anna ME, Rausell E, Leggio MG, Hashikawa T, Jones EG. Auditory thalamocortical pathways defined in monkeys by calcium-binding protein immunoreactivity. J Comp Neurol. 1995;362:171–94. doi: 10.1002/cne.903620203. [DOI] [PubMed] [Google Scholar]
- Morel A, Kaas JH. Subdivisions and connections of auditory cortex in owl monkeys. J Comp Neurol. 1992;318:27–63. doi: 10.1002/cne.903180104. [DOI] [PubMed] [Google Scholar]
- Petkov CI, Kayser C, Steudel T, Whittingstall K, Augath M, Logothetis NK. A voice region in the monkey brain. Nat Neurosci. 2008;11:367–74. doi: 10.1038/nn2043. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Orman SS. Responses of single neurons in posterior field of cat auditory cortex to tonal stimulation. J Neurophysiol. 1984;51:147–63. doi: 10.1152/jn.1984.51.1.147. [DOI] [PubMed] [Google Scholar]
- Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J Neurophysiol. 2007;97:3621–38. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- Poremba A, Mishkin M. Exploring the extent and function of higher-order auditory cortex in rhesus monkeys. Hear Res. 2007;229:14–23. doi: 10.1016/j.heares.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A, Malloy M, Saunders RC, Carson RE, Herscovitch P, Mishkin M. Species-specific calls evoke asymmetric activity in the monkey’s temporal poles. Nature. 2004;427:448–451. doi: 10.1038/nature02268. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Parallel processing in the auditory cortex of primates. Audiol Neurootol. 1998a;3:86–103. doi: 10.1159/000013784. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Cortical processing of complex sounds. Curr Opin Neurobiol. 1998b;8:516–21. doi: 10.1016/s0959-4388(98)80040-8. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci U S A. 2000;97:11800–6. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12:718–24. doi: 10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM. GABA shapes selectivity for the rate and direction of frequency-modulated sweeps in the auditory cortex. J Neurophysiol. 2009;102:1366–78. doi: 10.1152/jn.00334.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read HL, Winer JA, Schreiner CE. Modular organization of intrinsic connections associated with spectral tuning in cat auditory cortex. Proc Natl Acad Sci U S A. 2001;98:8042–7. doi: 10.1073/pnas.131591898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH. Response profiles of auditory cortical neurons to tones and noise in behaving macaque monkeys. Hear Res. 2000;150:104–18. doi: 10.1016/s0378-5955(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Ojima H. Multisensory convergence in calcarine visual areas in macaque monkey. Int J Psychophysiol. 2003;50:19–26. doi: 10.1016/s0167-8760(03)00121-1. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Dagaeff C, Simm G, De Ribaupierre Y, Villa A, De Ribaupierre F, Rouiller EM. Functional organization of the ventral division of the medial geniculate body of the cat: evidence for a rostro-caudal gradient of response properties and cortical projections. Hear Res. 1989;39:103–25. doi: 10.1016/0378-5955(89)90085-3. [DOI] [PubMed] [Google Scholar]
- Romanski LM. Representation and integration of auditory and visual stimuli in the primate ventral lateral prefrontal cortex. Cereb Cortex. 2007;17(Suppl 1):i61–9. doi: 10.1093/cercor/bhm099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Goldman-Rakic PS. An auditory domain in primate prefrontal cortex. Nat Neurosci. 2002;5:15–6. doi: 10.1038/nn781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Bates JF, Goldman-Rakic PS. Auditory belt and parabelt projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1999a;403:141–57. doi: 10.1002/(sici)1096-9861(19990111)403:2<141::aid-cne1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Averbeck BB, Diltz M. Neural representation of vocalizations in the primate ventrolateral prefrontal cortex. J Neurophysiol. 2005;93:734–47. doi: 10.1152/jn.00675.2004. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci. 1999b;2:1131–6. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller EM, Simm GM, Villa AE, de Ribaupierre Y, de Ribaupierre F. Auditory corticocortical interconnections in the cat: evidence for parallel and hierarchical arrangement of the auditory cortical areas. Exp Brain Res. 1991;86:483–505. doi: 10.1007/BF00230523. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Rodrigues-Dagaeff C, Simm G, De Ribaupierre Y, Villa A, De Ribaupierre F. Functional organization of the medial division of the medial geniculate body of the cat: tonotopic organization, spatial distribution of response properties and cortical connections. Hear Res. 1989;39:127–42. doi: 10.1016/0378-5955(89)90086-5. [DOI] [PubMed] [Google Scholar]
- Salami M, Itami C, Tsumoto T, Kimura F. Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex. Proc Natl Acad Sci U S A. 2003;100:6174–9. doi: 10.1073/pnas.0937380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem KS, Kondo H, Price JL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol. 2008;506:659–93. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- Scannell JW, Blakemore C, Young MP. Analysis of connectivity in the cat cerebral cortex. J Neurosci. 1995;15:1463–83. doi: 10.1523/JNEUROSCI.15-02-01463.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner CE, Cynader MS. Basic functional organization of second auditory cortical field (AII) of the cat. J Neurophysiol. 1984;51:1284–305. doi: 10.1152/jn.1984.51.6.1284. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Urbas JV. Representation of amplitude modulation in the auditory cortex of the cat. I. The anterior auditory field (AAF) Hear Res. 1986;21:227–41. doi: 10.1016/0378-5955(86)90221-2. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Urbas JV. Representation of amplitude modulation in the auditory cortex of the cat. II. Comparison between cortical fields. Hear Res. 1988;32:49–63. doi: 10.1016/0378-5955(88)90146-3. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Read HL, Sutter ML. Modular organization of frequency integration in primary auditory cortex. Annu Rev Neurosci. 2000;23:501–29. doi: 10.1146/annurev.neuro.23.1.501. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Parietal, temporal, and occipital projections to cortex of the superior temporal sulcus in the rhesus monkey: a retrograde tracer study. J Comp Neurol. 1994;343:445–63. doi: 10.1002/cne.903430308. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Cola MG, Gutierrez C, Massee M, Weldon C, Cusick CG. Overlapping and nonoverlapping cortical projections to cortex of the superior temporal sulcus in the rhesus monkey: double anterograde tracer studies. J Comp Neurol. 1996;370:173–90. doi: 10.1002/(SICI)1096-9861(19960624)370:2<173::AID-CNE4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Falchier A. Multisensory connections of monkey auditory cerebral cortex. Hear Res. 2009 doi: 10.1016/j.heares.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Hackett TA, Ulbert I, Karmas G, Lakatos P, Javitt DC, Schroeder CE. Multisensory convergence in auditory cortex, I. Cortical connections of the caudal superior temporal plane in macaque monkeys. J Comp Neurol. 2007;502:894–923. doi: 10.1002/cne.21325. [DOI] [PubMed] [Google Scholar]
- Steriade M. Two channels in the cerebellothalamocortical system. J Comp Neurol. 1995;354:57–70. doi: 10.1002/cne.903540106. [DOI] [PubMed] [Google Scholar]
- Storace CA, Higgins NC, Read HL. Thalamic label patterns suggest primary and ventral auditory fields are distinct core regions. J Comp Neurol. 2009 doi: 10.1002/cne.22345. in press. [DOI] [PubMed] [Google Scholar]
- Sugihara I, Lang EJ, Llinas R. Uniform olivocerebellar conduction time underlies Purkinje cell complex spike synchronicity in the rat cerebellum. J Physiol. 1993;470:243–71. doi: 10.1113/jphysiol.1993.sp019857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Rauschecker JP. Processing of frequency-modulated sounds in the cat’s anterior auditory field. J Neurophysiol. 1994;71:1959–75. doi: 10.1152/jn.1994.71.5.1959. [DOI] [PubMed] [Google Scholar]
- Tranel D, Brady DR, Van Hoesen GW, Damasio AR. Parahippocampal projections to posterior auditory association cortex (area Tpt) in Old-World monkeys. Exp Brain Res. 1988;70:406–16. doi: 10.1007/BF00248365. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. MIT Press; Cambridge: 1982. pp. 549–586. [Google Scholar]
- Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4:157–65. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–55. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MN, Rutkowski RG, Palmer AR. Identification and localisation of auditory areas in guinea pig cortex. Exp Brain Res. 2000;132:445–56. doi: 10.1007/s002210000362. [DOI] [PubMed] [Google Scholar]
- Wang J, McFadden SL, Caspary D, Salvi R. Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res. 2002;944:219–31. doi: 10.1016/s0006-8993(02)02926-8. [DOI] [PubMed] [Google Scholar]
- Winer JA, Lee CC. The distributed auditory cortex. Hear Res. 2007;229:3–13. doi: 10.1016/j.heares.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Diehl JJ, Larue DT. Projections of auditory cortex to the medial geniculate body of the cat. J Comp Neurol. 2001;430:27–55. [PubMed] [Google Scholar]
- Wutzler A, Winter C, Kitzrow W, Uhl I, Wolf RJ, Heinz A, Juckel G. Loudness dependence of auditory evoked potentials as indicator of central serotonergic neurotransmission: simultaneous electrophysiological recordings and in vivo microdialysis in the rat primary auditory cortex. Neuropsychopharmacology. 2008;33:3176–81. doi: 10.1038/npp.2008.42. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Tan AY, Schreiner CE, Merzenich MM. Topography and synaptic shaping of direction selectivity in primary auditory cortex. Nature. 2003;424:201–5. doi: 10.1038/nature01796. [DOI] [PubMed] [Google Scholar]