Abstract
Objective
Differences in antiretroviral (ARV) distribution into the central nervous system (CNS) may impact neurocognitive status. We assessed the relationship between estimates of ARV therapy penetration into the CNS, using a published ranking system, and neurocognitive status in HIV-positive subjects with plasma HIV-1 RNA (vRNA) suppression.
Design
Subjects with ≥6 weeks ongoing ARV use and vRNA<50 copies/mL (N=2,636; 83% male, median baseline CD4 T-cells: 244 cells/uL) had ≥1 neuroscreen assessment (Trailmaking A,B, WAIS-R Digit Symbol) at 10,413 neurovisits. Neuroscreen test scores were demographically adjusted and converted to Z-scores (NPZ3: lower scores imply more impairment). CNS penetration-effectiveness (CPE) ranks of 0.0(low), 0.5(medium) or 1.0(high) were assigned to ARVs and summed per regimen, per neurovisit.
Methods
Multivariate linear regression models using generalized estimating equations assessed NPZ3 scores with respect to ARV regimen. Covariates were retained if p≤0.1.
Results
A final model demonstrated that better NPZ3 scores were associated with higher CPE among subjects taking >3 ARVs (+0.07 per one unit increase in CPE score; p=0.004) but not among subjects with ≤3 ARVs in the regimen (+0.01; p=0.5). Results were adjusted for demographics, injection drug use, HCV serostatus, CD4 count (current and nadir), baseline vRNA, ARV experience and years since first ARV use.
Conclusions
Use of ARVs with better estimated CNS penetration may be associated with better neurocognitive functioning; some people may require >3 ARVs to treat HIV in the CNS. Clinically this means ARV regimens could be designed to optimize estimated CNS penetration without sacrificing virologic and immunologic benefits.
Keywords: HIV, Antiretroviral Agents, Neurologic Impairment, Central Nervous System, HIV-1 RNA suppression
INTRODUCTION
Human immunodeficiency virus (HIV) enters the central nervous system (CNS) within days of initial infection and is present within the CNS throughout the course of disease, frequently leading to HIV-associated neurocognitive disorders (HAND) [1]. Treatment with antiretrovirals (ARVs) can result in significant neurocognitive improvement in many individuals with HAND [2]. Nevertheless, improvement with antiretroviral treatment (ART) varies greatly between individuals [3] and several cohort studies have demonstrated that HAND can persist despite virologic suppression and immune recovery on ART [4],[5]. One hypothesized explanation for persisting HAND is inadequate treatment of CNS HIV infection due to relatively poor penetration of many antiretrovirals across the blood-brain-barrier [6]. With limited CNS penetration, it is possible that subtherapeutic levels of ARVs could lead to the development of resistant virus in the nervous system and might not reverse neurological deficits as well as ARVs with better CNS penetration. [7],[8]. In order to draw conclusions regarding the significance of drug penetration into the CNS, it is important to examine persons who have plasma HIV-1 RNA (vRNA) viral suppression. In this group there is an opportunity to assess whether ARV regimens that lead to vRNA suppression have differing effects on neurocognitive impairment.
In this analysis, we use data from the AIDS Clinical Trials Group (ACTG) Longitudinal Linked Randomized Trials (ALLRT) cohort. Subjects from this prospective study provide a unique opportunity to assess estimated CNS penetration and neurocognitive function; the study consists of a large sample of subjects randomized to a wide variety of ARV regimens in ACTG clinical trials, who completed neurocognitive assessments at pre-determined visits.
METHODS
Study Population
Subjects in the ALLRT cohort study enrolled from 26 United States-based ACTG clinical trials [9]. In the parent clinical trials, subjects were prospectively randomized to receive ART, immune-based therapies or to participate in a new treatment strategy; subjects were either ARV-naïve or ARV-experienced when entering their parent clinical trial (baseline). Long-term observational follow-up continued based on the ALLRT protocol during and after subjects completed their parent clinical trial. ALLRT visits were every 16 weeks. Subjects from two treatment interruption studies and two studies in which ARV medications are still blinded were not eligible for this analysis. Enrollment into parent clinical trials began in 1997 and analysis was performed on data collected through June 30, 2008. Institutional Review Boards at ACTG sites approved the ALLRT protocol; all subjects provided signed written informed consent.
Data Collection
Demographics (sex, race/ethnicity, age) and injection drug use status were collected once at baseline; years of education were collected when the first neuroscreen assessment was completed. Hepatitis C antibody testing was performed at baseline or ALLRT entry (requirements for parent clinical trials varied); subjects were re-screened on ALLRT every 96 weeks. CD4+ T-cell count and HIV-1 RNA plasma viral load were measured at 16-week visits using routine clinical laboratory methods.
Neuroscreen Assessments and Antiretroviral Regimens
Neuroscreen assessments were conducted by trained and certified personnel every 48 weeks in ALLRT and consisted of three tests: Trail Making Test, Part A and B (Trail A, B) [10] and the Wechsler Adult Intelligence Scale-Revised (WAIS-R) Digit Symbol Test [11]. Trail making test scores were reported as the time it took to complete the test, while the score for the WAIS-R test was reported as the number of symbols correctly completed within 90 seconds. These exams were designed to be uncomplicated; trained non-neuropsychologist personnel can reliably administer them in 10 to 15 minutes. A web-based tool, developed to facilitate training and certification, included forms, instructions for their use, as well as training videos for the Trail A, B and WAIS-R. Certification to administer the neuropsychological exams was granted when study site personnel completed an online test. A published validation study [12] assessed the relationship between neurologic screening test performance and clinical diagnoses as classified according to modern nomenclature [1], supporting our ability to interpret the screening data as it relates to clinical diagnoses.
Raw scores from Trail Making A, B were standardized, adjusting for sex, age, educational status, and black versus non-black race, while Digit Symbol Scores were prorated to WAIS-III scaled scores and standardized adjusting for sex, age, educational status, and race/ethnicity (white, black and Hispanic) [13], [14]. Standardized scores (T-scores) have a normal distribution with a mean of 50 and standard deviation of 10. T-scores were converted to Z-scores (Z-score = (T-score-50)/10), which were used to create a composite neuropsychological score (NPZ3 score) that was the average of the three individual Z-scores; subjects with lower scores are more neurologically impaired than subjects with higher scores. Subjects who did not take Trail A, B and WAIS-R tests due to HIV-associated neurological disease were assigned a corresponding Z-score of −2 (n=11). In the event more than one neuroscreen assessment was conducted within a 48-week interval, the evaluation closest to the center of the interval was used.
For each visit an ARV regimen was determined. Start and stop dates of all ARVs taken by a subject were self-reported and recorded at each 16-week study visit; treatment interruptions of less than 21 days, per the ALLRT protocol, were not documented. A subject’s current ARV regimen, associated with an NPZ3 score, was defined as the ARV regimen taken consistently for at least 6 weeks prior to and through the date the neuroscreen assessment was completed. Neurologic visits were excluded from analysis if a subject was off ARVs for more than 21 days or switched their ARV regimen during the 6 weeks prior to the visit. All visits included in the analysis for previously ARV-naïve subjects are post-baseline, since none of these subjects had 6 weeks of ARV therapy at the time they entered their parent clinical trial. Subjects could switch from one regimen to another over the course of a study (i.e., be taking a 3-drug regimen and then switch to a 4-drug regimen); we used analysis techniques for repeated measures to account for changes among subjects, over time.
Antiretroviral Central Nervous System Penetration Effectiveness (CPE) Ranking System
The method for ranking the antiviral effectiveness of ARVs in the CNS has been previously described [15,16,17,18,19]. Briefly, this hierarchical and adaptive approach to ranking the effectiveness of ARVs in the protected CNS environment uses physicochemical, pharmacokinetic, and pharmacodynamic data to categorically rank ARVs. Physicochemical characteristics considered in constructing the rank included molecular weight, lipophilicity (octanol-water partition coefficients), charge at physiologic pH (dissociation constants) and protein binding. Pharmacokinetic data are considered more influential than physicochemical characteristics and compare drug concentrations in the CNS (typically CSF) to drug concentrations in blood and inhibitory concentrations for wild-type HIV-1. Pharmacodynamic data are considered the most important but few drugs have usable pharmacodynamic data since the criteria for data consideration require the administration of the ARV in a manner that allows determination of its independent effect on nervous system-relevant outcomes. These data were compiled and compared between drugs, which were then categorized into one of 3 categories: 0.0 (low: relatively poor estimated CNS penetration), 0.5 (medium: intermediate estimated CNS penetration), or 1.0 (high: relatively good estimated CNS penetration). The ranks are summarized in Table 1. In this approach, ritonavir used in subtherapeutic ‘boosting’ doses is not counted as an individual drug; instead, different ranks are used for ‘boosted’ and ‘unboosted’ protease inhibitors. Estimated ARV CNS penetration at each neurologic visit was calculated as the sum of the individual CPE ranks for each drug contained in the current ARV regimen, resulting in one CPE rank per ARV regimen, per neuroscreen assessment. Using this ranking system, higher CPE ranks identify regimens that have greater estimated distribution into, and therefore effectiveness in, the CNS.
Table 1.
Antiretroviral Central Nervous System Penetration Scoring System: CNS penetration effectiveness score
| Drug Class | CPE Score | ||
|---|---|---|---|
| 1 | 0.5 | 0 | |
| Nucleoside Reverse Transcriptase Inhibitor (NRTI) | Abacavir | Emtricitabine | Adefovir |
| Zidovudine | Lamivudine | Zalcitabine | |
| Stavudine | Didanosine | ||
| Tenofovir | |||
| Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI) | Delavirdine | Efavirenz | |
| Nevirapine | |||
| Protease Inhibitor (PI)* | Amprenavir-r | Amprenavir | Nelfinavir |
| Darunavir | Atazanavir | Ritonavir | |
| Fosamprenavir-r | Atazanavir-r | Saquinavir | |
| Lopinavir-r | Fosamprenavir | Saquinavir-r | |
| Indinavir-r | Indinavir | Tipranavir-r | |
| Integrase Inhibitors | Elvitegravir | ||
| Raltegravir | |||
| Entry Inhibitors | Vicriviroc | Enfuvirtide | |
| Maraviroc | T-1249 | ||
Antiretroviral Central Nervous System (ARV CNS); CNS penetration effectiveness (CPE); Amprenavir-r: Ritonavir boosted Amprenavir; Fosamprenavir-r: Ritonavir boosted Fosamprenavir; Lopinavir-r: Ritonavir boosted Lopinavir; Indinavir-r: Ritonavir boosted Indinavir; Atazanavir-r: Ritonavir boosted Atazanavir; Saquinavir-r: Ritonavir boosted Saquinavir; Tipranavir-r: Ritonavir boosted Tipranavir
Data Analysis
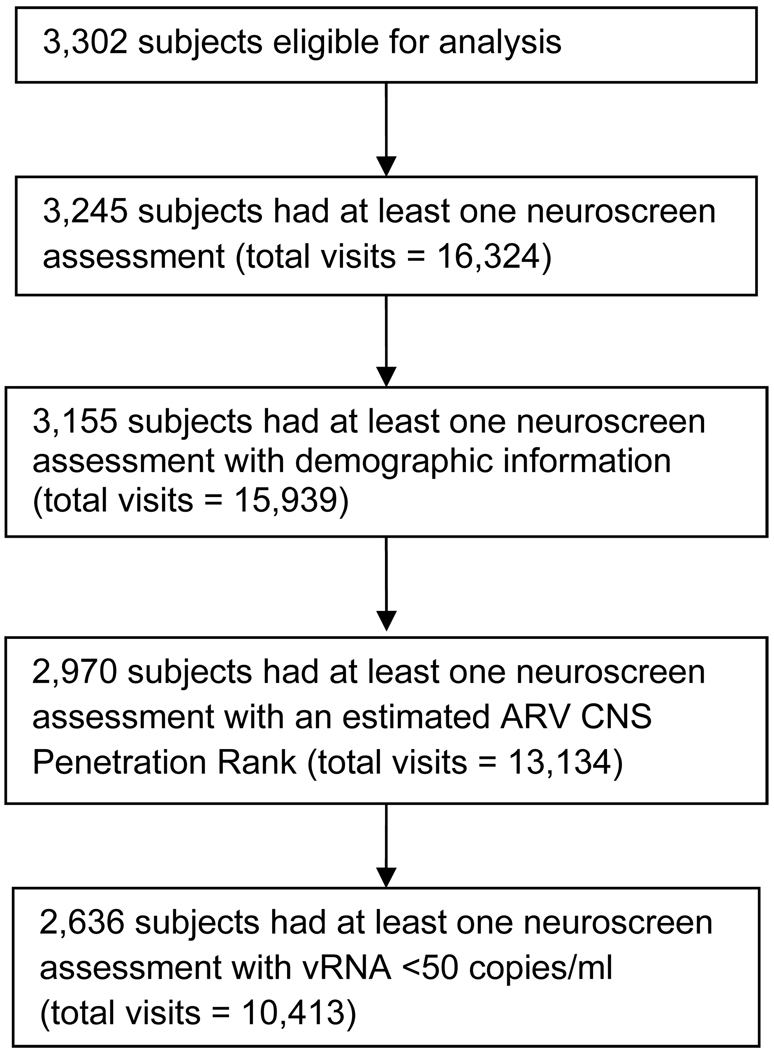
Of the 3,302 ALLRT subjects eligible for analysis, 3,245 subjects (98%) had at least one neuroscreen assessment. (Figure 1) Demographic information necessary for adjusting NPZ3 scores was incomplete for 90 subjects. For 17% of the neurologic visits, available ARV data did not meet criteria required to assess the CNS penetration rank (11%: subjects were off ARVs the entire 6 weeks prior to the visit; 4%: subjects were off ARVs for >21 days, but less than 6 weeks, prior to the visit; 2%: subjects switched ARV regimens). A total of 2,970 subjects had at least one NPZ3 score with an estimated CPE rank (total visits: 13,134). In this analysis, only subjects with a vRNA <50 copies/mL at the time of a neuroscreen assessment were included. In total, 2,636 subjects had 10,413 visits with a neuroscreen assessment at which plasma vRNA was <50 copies/mL; these visits are the time-points included in this analysis.
Figure 1.
Flow diagram for subjects in the analysis
Linear regression models, using generalized estimating equations (GEE) to account for repeated measures, were fit to assess the relationship between estimates of ART penetration in the CNS (CPE ranks) and neurocognitive outcomes (NPZ3). Univariate regressions were used to examine unadjusted relationships between covariates and NPZ3 scores. Covariates included baseline demographics, injection drug use, and baseline and time-updated concurrent CD4+ T-cell count, ARV status at parent entry, years since first ARV use and total number of ARVs in the current regimen. Multiple linear regression models, constructed using backwards elimination, were used to assess the relationship between the CPE rank and NPZ3 score, while adjusting for potential confounders; potential confounders were retained if the p-value was ≤0.1. We assessed collinearity; none was identified.
Additional evaluation of intermediate models was conducted. Examination of the addition of one potential confounder at a time to the simple CPE rank and NPZ score model and construction of the final multivariate model using forward selection were used to initially identify covariates that altered the CPE effect estimate. Number of ARVs was a key covariate that, when added to the model, caused a change in the CPE score estimate; further exploration showed evidence of an interaction. The cut-point for the number of ARVs was based on changes in the point estimates when the interaction was examined in modeling. The final, adjusted model includes the interaction between CPE score and number of ARVs; the two main effects reflect every one score increase in the CPE among observations where there are less than or equal to three ARVs in the regimen and where there are four or more ARVs in the regimen. Results from the model are estimates of the change in NPZ3 score, per unit of each covariate. Data were analyzed using SAS version 9.13 (SAS Institute Inc, Cary, NC).
RESULTS
Of the 2,636 ALLRT subjects analyzed, the majority were male (83%), white, non-Hispanic (53%) with a baseline median age of 40 and 14 years of education; only 8% reported past or current injection drug use. [Table 2] Nadir CD4+ T-cell counts were lower than the CD4 counts at baseline (median: 182 cells/µL vs. 244 cells/µL). Subjects had a median follow-up time of 4.7 years, which was defined as time from parent clinical trial entry (baseline) to last neuroscreen assessment; median number of NPZ3 scores per subject was 4. The relationship between CD4 T-cell count and CPE was examined; there was no correlation between a subject’s CD4 count and their CPE rank (Spearman correlation coefficient: 1st neurologic visit, −0.05; 2nd neurologic visit, −0.04).
Table 2.
Baseline demographics, behavioral, clinical, virologic and neurologic data (N=2,636)
| Characteristic | Total | |
|---|---|---|
| Sex | Male | 2,195 (83%) |
| Female | 441 (17%) | |
| Race | White Non-Hispanic | 1,384 (53%) |
| Black Non-Hispanic | 730 (28%) | |
| Hispanic (Regardless of Race) | 522 (20%) | |
| Age (years) | Median (Q1, Q3) | 40 (34, 47) |
| CD4 cell count (cells/ul) at baseline | Median | 243.5 |
| 0–50 | 435 (17%) | |
| 51–200 | 670 (25%) | |
| >200 | 1,529 (58%) | |
| Missing | 2 (0%) | |
| Nadir CD4 (cells/ul) on or before baseline | Median | 182 |
| 0–50 | 589 (22%) | |
| 51–200 | 834 (32%) | |
| >200 | 1,213 (46%) | |
| Viral load (copies/ml) at baseline | Median | 43,036 |
| 0–10,000 | 685 (26%) | |
| >10,000–100,000 | 1,035 (39%) | |
| >100,000 | 916 (35%) | |
| Injection drug use at baseline | None | 2,415 (92%) |
| Reported (currently or previously) | 221 (8%) | |
| Hepatitis C Ab status | Negative | 2,228 (85%) |
| Positive ever | 278 (11%) | |
| Years of education | Median (Q1, Q3) | 14 (12, 16) |
| # neurology visits | Median (Q1, Q3) | 4 (2, 6) |
| Years from baseline to the first neurology visit | Median (Q1, Q3) | 1.4 (0.9, 2.6) |
| Years from baseline to the last neurology visit | Median (Q1, Q3) | 4.7 (3.0, 7.5) |
| ARV- status baseline | Naive | 69% |
| Years from first neuro visit to last neuro visit | Median (Q1, Q3) | 3.1 (1.0, 5.5) |
The overall median CPE rank score was 2.0 (first quartile, third quartile): 1.5, 2.5; 73% ≤2.0)). (Table 3) Median CPE rank score was 1.5 (1.0, 2.0) among observations where there were ≤3 ARVs in the regimen and was 2.5 (2.0, 3.0) when there were >3 ARVs in the regimen. None of the subjects taking ≤3 ARVs had a CPE score higher than 2.5 while 43% of the observations among subjects taking >3 ARVS had CPE scores of 3 or higher. The majority of observations (63%) were among subjects who were on 3 ARV medications when the CPE rank was calculated; approximately 30% were on 4 ARVS, and <8% were on fewer than 3 ARVs (median: 3 ARVs; Q1, Q3: 3, 4). Fifty-one percent of subjects were on the same ARV regimen throughout the analysis period and hence had the same CPE rank; 36% had only one change in their ARV regimen. A majority (61%; n=1618) had the same CPE rank at each neuroscreen measurement; fewer than 5% of our study population had more than 2 different CPE ranks. A higher percentage of subjects with 4 ARVs had a PI in their regimen as compared to subjects with 3 ARVs in the regimen (59% vs. 38%). There was little variation in median NPZ3 scores by CPE rank, when evaluating this relationship at the first neurovisit; median time to the first neuroscreen assessment was 1.4 years from baseline. Additionally, the mean (−0.3) and median (−0.3) NPZ3 scores were the same among observations where there were 3 or 4 ARVs in the regimen.
Table 3.
Distribution of the CNS penetration effectiveness (CPE) score by number of ARVs in the regimen, total visits=10,413
| Number of ARVs in Regimen | |||
|---|---|---|---|
| CPE Score | Overall N=10,413 | ARVs ≤3 N=7367 | ARVs >3 N=3046 |
| 0 – 0.5 | 323 (3%) | 319 (4%) | 4 (<1%) |
| 1 | 1,862 (18%) | 1,629 (22%) | 233 (8%) |
| 1.5 | 2,048 (20%) | 1,787 (24%) | 261 (9%) |
| 2 | 3,412 (33%) | 2,753 (37%) | 659 (22%) |
| 2.5 | 1,458 (14%) | 864 (12%) | 594 (20%) |
| 3 | 1,052 (10%) | 15 (0%) | 1,037 (34%) |
| 3.5 | 241 (2%) | 0 (0%) | 241 (8%) |
| 4 – 4.5 | 17 (<1%) | 0 (0%) | 17 (1%) |
| Median | 2 | 1.5 | 2.5 |
| Q1, Q3 | 1.5, 2.5 | 1, 2 | 2, 3 |
In univariate regression analyses, there was no association between CPE rank and NPZ3 scores [estimate of the change in NPZ3 score per one unit in CPE score: −0.0049, 95% CI (−0.0318, 0.0219), p-value 0.7]. However, there was effect measure modification between the CPE score and the total number of ARVs in the regimen. An association was seen in the observations where there were >3 ARVs in the regimen [estimate: 0.07, 95% CI (0.02, 0.11), p-value 0.005] but not where there were ≤3 ARVs in the regimen [estimate: −0.02, 95% CI (−0.05, 0.02), p-value 0.3]. Injection drug use [estimate: −0.12, p-value 0.03] and a positive HCV serostatus [estimate: −0.18, p-value <0.001] were independently associated with worse performance on the neuroscreen assessment, while a nadir CD4 count above 200 (versus ≤200) was associated with better performance. All baseline demographic characteristics were associated with changes in the NPZ3 score (p<0.05). (Table 4)
Table 4.
Univariate and multivariate linear regression estimates of the change in NPZ3 score and 95 percent confidence intervals for the association between CPE score, immunologic, virologic, additional risk factors and neurological function (NPZ3 score), where plasma HIV RNA < 50 (copies/ml) at the neurovisit (N=2636)
| Univariate*^ | Multivariate*^ | |||
|---|---|---|---|---|
| Main Variable | Change in NPZ3 score (95% CI) | P-value | Change in NPZ3 score (95% CI) | P-value |
| CPE score | ||||
| Every 1 score increase | −0.01 (−0.03,0.02) | 0.7 | ||
| CPE score (every 1 score increase) | ||||
| Among observation with ≤ 3 ARVs | −0.02 (−0.05,0.02) | 0.3 | 0.01 (−0.02,0.05) | 0.5 |
| Among observation with >3 ARVs | 0.07 (0.02,0.11) | 0.005 | 0.07 (0.02,0.12) | 0.004 |
| Confounders | ||||
| Sex | ||||
| Female vs male | −0.13 (−0.21, −0.05) | 0.002 | −0.13 (−0.22, −0.05) | 0.001 |
| Race/Ethnicity | ||||
| White vs Black | −0.11 (−0.18, −0.04) | 0.002 | −0.18 (−0.25, −0.1) | <0.001 |
| Hispanic vs Black | −0.48 (−0.56, −0.4) | <0.001 | −0.48 (−0.57, −0.4) | <0.001 |
| Age | ||||
| Every 10 year increase in age | −0.07 (−0.1, −0.03) | <0.001 | −0.09 (−0.12, −0.05) | <0.001 |
| Years of education | ||||
| Every 1 year increase | 0.03 (0.02,0.04) | <0.001 | 0.02 (0.01,0.03) | <0.001 |
| Injection drug use | ||||
| Reported vs Not reported | −0.12 (−0.22, −0.01) | 0.03 | ||
| HCV sero-status | ||||
| Ever positive vs Never or Unknown | −0.18 (−0.28, −0.09) | <0.001 | −0.15 (−0.24, −0.06) | 0.001 |
| Baseline nadir CD4 cell count (cells/ul) | ||||
| >200 vs ≤ 200 | 0.09 (0.03,0.15) | 0.003 | 0.06 (−0.01,0.13) | 0.08 |
| Baseline CD4 cell count (cells/ul) | ||||
| >200 vs ≤ 200 | 0.09 (0.03,0.16) | 0.003 | ||
| Current CD4 cell count (cells/ul) | ||||
| 351–500 vs ≤ 350 | 0.07 (0.03,0.11) | <0.001 | 0.03 (−0.01,0.06) | 0.1 |
| >500 vs ≤ 350 | 0.1 (0.06,0.14) | <0.001 | 0.04 (−0.01,0.08) | 0.09 |
| Baseline plasma HIV RNA (copies/ml) | ||||
| 10,000–100,000 vs <10,000 | −0.06 (−0.13,0.02) | 0.1 | −0.1 (−0.19, −0.01) | 0.02 |
| >100,000 vs <10,000 | −0.06 (−0.14,0.02) | 0.1 | −0.09 (−0.19,0.01) | 0.08 |
| Baseline ARV status | ||||
| ARV experienced vs ARV naive | −0.02 (−0.09,0.04) | 0.5 | −0.38 (−0.48, −0.29) | <0.001 |
| Years since first ARV use | ||||
| Every 1 year increase | 0.03 (0.03,0.04) | <0.001 | 0.05 (0.04,0.06) | <0.001 |
Number of ARVs in the regimen (>3 vs ≤ 3); Estimate: −0.23 (univariate), −0.16 (multivariate). These variables cannot be interpreted alone but are in the model because of the interaction.
Interaction term [#ARVs and CPE score]; p-value: 0.004 (univariate), 0.055 (multivariate).
In the final multivariate linear regression model, when adjusting for potential confounders where p≤0.1, results demonstrated that for every 1 unit increase in the CPE rank there was an associated increase in the NPZ3 score among subjects with >3 ARVs in the regimen [estimate: 0.07, 95% CI (0.02, 0.12), p-value 0.004] but not among subjects taking ≤3 ARVs [estimate: 0.01, 95% CI (−0.02, 0.05), p-value 0.5] (Table 4). Thus, a subject with a CPE rank of 4.0 who was taking >3 ARVs could have an NPZ3 score of 0.20, while a subject with identical covariate characteristics with a CPE rank of 1.0 would have an NPZ3 score of −0.01. Longer duration since initiating ARV use was associated with better performance on neurocognitive assessments [estimate per one year increase since first ARV use: 0.05; 95% CI: 0.04, 0.06)], as were nadir CD4 counts above 200 [estimate 0.06; 95% CI −0.01,0.13] and higher current CD4 counts [estimate for CD4 count 351 to 500 vs. ≤ 350: 0.03; 95% CI −0.01, 0.06; estimate for CD4 count >500 vs. ≤ 350: 0.04; 95% CI −0.01, 0.08]. Positive HCV antibody continued to be associated with a lower NPZ3 score [estimate −0.15; 95% CI −0.24, −0.06].
The same multivariate model (same covariates) was run using a dichotomous rather than a continuous CPE score in order to examine the role of particular CPE thresholds on NPZ3 score. The estimate using a CPE threshold of 2.0 (>2.0 versus ≤2.0) was 0.11 (95% CI: 0.04, 0.18; p=0.002) among subjects with >3 ARVs in the regimen and 0.02 (95% CI: −0.05,0.08; p=0.6) among subjects with ≤3 ARVs.
DISCUSSION
Among our large study population of HIV-positive individuals on ARV treatment who had vRNA<50 copies/mL, those taking regimens with >3 ARVs and with better predicted CNS penetration had better neurocognitive outcomes, after adjusting for other potentially confounding factors, although the magnitude of this effect was not large. The same association was not seen among subjects taking ≤3 ARVs. One explanation for these findings is that the better penetrating >3-drug regimens have more of an effect on neurocognitive functioning than the worse penetrating >3-drug regimens; this same effect may not be as apparent among subjects taking ≤3-drug regimen. An additional explanation is that some people may require more than 3 ARVs to reach sufficiently high drug penetration to treat HIV in the nervous system.
To provide a frame of reference for interpreting the magnitude of the CPE effect on neurocognitive outcome we compare it to the magnitude of the effect of nadir CD4 cell count, an important risk factor for HAND [5], [20], [21]. The impact of a 1-unit increase in CPE on neurocognitive outcome among subjects taking >3 ARVs (estimate: 0.07; p-value 0.004) was similar in magnitude to the difference in outcome between subjects with a nadir CD4 above 200 cells/µL versus ≤200 cells/µL (estimate 0.06; p-value 0.08). Our analyses suggest that careful selection of ARV regimens could increase the CPE by at least one unit for many individuals, and may yield neurocognitive benefits proportionate to the differences seen in neurocognitive impairment between subjects with and without an AIDS diagnosis. Improved neurocognitive function would be expected to have an impact on activities of daily living [22] including medication adherence [23],[24], driving [25], and employment [26]. To put into context the level of neurocognitive impairment in this group of individuals, it should be noted that we recently found that 26% of 1,160 ALLRT subjects analyzed had mild to moderate impairment at their first visit, and 22% of 991 with at least one follow-up visit had sustained impairment [5].
To eliminate potential biases related to differences in ARV regimen potency, indexed by residual plasma viremia, we limited the analysis to subjects who had achieved viral suppression on their ARV regimen (vRNA <50 copies/mL). In addition, we were able to use virologic suppression as a proxy for ARV medication adherence; since subjects were virally suppressed, we can, with reasonable certainty, state that these subjects were consistently taking the medications that they reported as their regimen. In addition, subjects in this analysis had similar CD4 counts regardless of CPE rank. These findings suggest that for subjects experiencing neurologic impairment, a switch to a regimen with higher CNS penetration might assist neurologic recovery while maintaining a similar immunologic and virologic profile.
This analysis has some other distinct advantages over prior analyses. While other analyses used a cross-sectional study design [27] this analysis provides estimates both of antiretroviral regimen CPE and neuropsychological outcome over time, and uses a repeated measures analysis method. Additionally, the number of subjects included is much larger than in prior studies [18], which strengthens the robustness and potential generalizability of the results.
Another clear benefit in this study was that the CPE was estimated using a ranking scale that has been successfully applied in other analyses among a variety of cohorts, worldwide. [16, 17, 19, 28]. Letendre et al. have developed an adaptable approach to estimating the CNS penetration of available antiretroviral agents, including nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside RTIs (NNRTIs), protease inhibitors (PIs), integrase inhibitors, and entry inhibitors; the system also permits scoring of combination regimens [16, 17]. As described in the Methods section, this ranking system uses physicochemical, pharmacokinetic, and pharmacodynamic data to compare ARVs. Although brain parenchymal extracellular concentrations of drugs have been measured by in vivo microdialysis in animals [29], this is not feasible in humans. Instead, cerebrospinal fluid (CSF) drug concentrations serve as a surrogate measure of CNS pharmacokinetics. Pharmacodynamic data considered include a drug’s ability to suppress viral load in CSF and improve neurocognitive performance [15]. The multidimensional, integrative nature of the system yields relative, rather than absolute, rankings. This flexible system is intended to be refined as new drugs and new pharmacokinetic and pharmacodynamic data become available.
Our analyses have limitations. Although many of our subjects contributed data from more than one time point during antiretroviral therapy, the analytical technique we used did not assess improvement or decline in cognitive function over time, but rather the concurrent relationship between CNS penetration scores and cognitive function as measured by NPZ3 scores; we did not analyze changes in NPZ3 scores. Also, our data were limited to evaluations during treatment, and did not include pre-treatment neurocognitive assessments which would be needed to evaluate whether the extent of improvement after initiating ARVs is related to the regimen’s CPE. While our findings can be discussed in the context of current regimens and their relationship with neurocognitive performance, they cannot be used to assess changes in regimens and how these directly affect changes in neurocognitive status in a particular individual. In terms of generalizability, our cohort was enrolled at multiple sites in the US and Puerto Rico and is comprised primarily of males, while the HIV epidemic is expanding in women [30].
Conclusion
These findings suggest that optimizing regimens with higher CNS penetration effectiveness scores could yield improved neurocognitive function without sacrificing the benefits of ART use. These neurocognitive gains could be expected to provide longer term productivity and functional ability in our patients living with HIV infection; however, based on the small, yet statistically significant, magnitude of the effect, it is not completely clear what the extent of these changes would be. Such a strategy should be tested in randomized clinical trials in which groups of HIV-infected individuals with a greater likelihood of neurocognitive impairment (such as those with nadir CD4 count <200 cells/µL) might best be able to demonstrate improvement.
Acknowledgements
We would like to thank the study participants; without them we would be unable to present these data. We would also like to thank the ALLRT team and the staff who work at participating ACTG sites and laboratories. In addition, we would like to thank Daniel R. Kuritzkes, M.D. for his helpful suggestions.
Work for this manuscript was supported by the following grants: NIH grants: 5 U01 AI38855 and 1 U01 AI068634, University of Washington AI069434, Washington University in St. Louis ACTU (021) AI069495, Neurologic AIDS Research Consortium NS32228, RO1 MH58076, P30 MH62512. In addition, the project described was supported by Award Number U01AI068636 from the National Institute of Allergy and Infectious Diseases and supported by National Institute of Mental Health (NIMH), National Institute of Dental and Craniofacial Research (NIDCR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Abbreviations in Manuscript
- ACTG
AIDS Clinical Trials Group
- ALLRT ACTG
Longitudinal Linked Randomized Trials
- ART
Antiretroviral treatment
- ARV
Antiretroviral
- CNS
Central nervous system
- CPE
CNS penetration effectiveness
- CSF
Cerebrospinal fluid
- GEE
Generalized estimating equations
- HAND
HIV-associated neurocognitive disorders
- HIV
Human immunodeficiency virus
- NPZ3
Composite neuropsychological score
- Trail A, B
Trail making test A, Trail making test B
- vRNA
Plasma HIV-1 RNA
- WAIS-R
Wechsler Adult Intelligence Scale - Revised
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Role of author:
Marlene Smurzynski: study and conceptual design, assisted with data analysis, interpretation of findings, wrote and edited manuscript, finalized manuscript for publication
Kunling Wu: data analysis, created figures and tables, interpretation of findings, reviewed manuscript
Scott Letendre: study and conceptual design, interpretation of findings, contributed to manuscript development, edited manuscript
Kevin Robertson: study and conceptual design, interpretation of findings, edited manuscript
Ronald J. Bosch: assisted with data analysis, interpretation of findings, edited manuscript
David B. Clifford: interpretation of findings, edited manuscript
Scott Evans: assisted with data analysis, interpretation of findings, reviewed manuscript
Ann C. Collier: interpretation of findings, edited manuscript
Michael Taylor: completed the normalized neurocognitive test scores (NPZ3 scores), reviewed manuscript
Ronald Ellis: study and conceptual design, interpretation of findings, contributed to manuscript development, edited manuscript
References
- 1.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007 Oct 30;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Letendre SL, McCutchan JA, Childers ME, Woods SP, Lazzaretto D, Heaton RK, et al. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurol. 2004 Sep;56(3):416–423. doi: 10.1002/ana.20198. [DOI] [PubMed] [Google Scholar]
- 3.Cysique LA, Vaida F, Letendre S, Gibson S, Cherner M, Woods SP, et al. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009 Aug 4;73(5):342–348. doi: 10.1212/WNL.0b013e3181ab2b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tozzi V, Balestra P, Murri R, Galgani S, Bellagamba R, Narciso P, et al. Neurocognitive impairment influences quality of life in HIV-infected patients receiving HAART. Int J STD AIDS. 2004;15(4):254–259. doi: 10.1258/095646204773557794. [DOI] [PubMed] [Google Scholar]
- 5.Robertson KR, Smurzynski M, Parsons T, Wu K, Bosch R, Wu J, et al. The Prevalence and Incidence of Neurocognitive Impairment in the HAART Era. AIDS. 2007;21(14):1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 6.Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 2009 May;82(2):A99–A109. doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis RJ, Gamst AC, Capparelli E, Spector SA, Hsia K, Wolfson T, et al. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology. 2000;54:927–936. doi: 10.1212/wnl.54.4.927. [DOI] [PubMed] [Google Scholar]
- 8.Price RW, Deeks SG. Antiretroviral drug treatment interruption in human immunodeficiency virus-infected adults: Clinical and pathogenetic implications for the central nervous system. J Neurovirol. 2004;10 Suppl 1:44–51. doi: 10.1080/753312752. [DOI] [PubMed] [Google Scholar]
- 9.Smurzynski M, Collier AC, Koletar SL, Bosch RJ, Wu K, Bastow B, et al. AIDS Clinical Trials Group Longitudinal Linked Randomized Trials (ALLRT): Rationale, Design, and Baseline Characteristics. HIV Clinical Trials. 2008;9(4):268–281. doi: 10.1310/hct0904-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Army. Army Individual Test Battery-manual of directions and scoring. Washington, DC: War Department, Adjutant General’s Office; 1994. [Google Scholar]
- 11.Wechsler D. Wechsler Adult Intelligence Scale-Revised (WAIS-R) New York: The Psychological Corporation; 1981. [Google Scholar]
- 12.Ellis RJ, Evans SR, Clifford DB, Moo LR, McArthur JC, Collier AC, et al. Clinical validation of the NeuroScreen. J Neurovirol. 2005;11(6):503–511. doi: 10.1080/13550280500384966. [DOI] [PubMed] [Google Scholar]
- 13.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- 14.Taylor MJ, Heaton RK. Sensitivity and Specificity of WAIS-III/WMS-III Demographically Corrected Factor Scores in Neuropsychological Assessment. Journal of the International Neuropsychological Society. 2001;7:867–874. [PubMed] [Google Scholar]
- 15.Letendre S, Ellis RJ, Best B, Bhatt A, Marquie-Beck J, LeBlanc S, et al. Penetration and effectiveness of antiretroviral therapy in the central nervous system. Anti-inflammatory & Anti-Allergy Agents in Medicinal Chemistry. 2009 June;8(2):169–183. [Google Scholar]
- 16.Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008 Jan;65(1):65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Letendre S, Ellis RJ, Best B, Bhatt A, Marquie-Beck J, et al. Penetration and Effectiveness of Antiretroviral Therapy in the Central Nervous System. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry. 2009;(8):169–183. [Google Scholar]
- 18.Letendre S, Capparelli E, Best B, Clifford D, Collier A, Gelman B, et al. Better antiretroviral penetration into the central nervous system is associated with lower CSF viral load. 13th Conference on Retroviruses and Opportunistic Infections; Denver, CO. 2006. Abstract 74. [Google Scholar]
- 19.Tozzi V, Balestra P, Salvatori MF, Vlassi C, Liuzzi G, Giancola ML, et al. Changes in cognition during antiretroviral therapy: comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders. J Acquir Immune Defic Syndr. 2009 Sep 1;52(1):56–63. doi: 10.1097/qai.0b013e3181af83d6. [DOI] [PubMed] [Google Scholar]
- 20.Heaton RK. HIV-associated neurocognitive impairment (NCI) remains common in the era of combination antiretroviral therapy (CART): the CHARTER study. Neurology. 2010 doi: 10.1212/WNL.0b013e318200d727. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis R, Heaton R, Letendre S, Badiee J, Munoz-Moreno J, Vaida F, et al. Higher CD4 Nadir Is Associated with Reduced Rates of HIV-Associated Neurocognitive Disorders in the CHARTER Study: Potential Implications for Early Treatment Initiation. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. 2010. Abstract 429. [Google Scholar]
- 22.Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004 May;10(3):317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 23.Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, et al. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004 Jan 1;18 Suppl 1:S19–S25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woods SP, Moran LM, Carey CL, Dawson MS, Iudicello JE, Gibson S, et al. Prospective memory in HIV infection: is "remembering to remember" a unique predictor of self-reported medication management? Arch Clin Neuropsychol. 2008 May;23(3):257–270. doi: 10.1016/j.acn.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcotte TD, Lazzaretto D, Scott JC, Roberts E, Woods SP, Letendre S. Visual attention deficits are associated with driving accidents in cognitively-impaired HIV-infected individuals. J Clin Exp Neuropsychol. 2006 Jan;28(1):13–28. doi: 10.1080/13803390490918048. [DOI] [PubMed] [Google Scholar]
- 26.Heaton RK, Velin RA, McCutchan JA, Gulevich SJ, Atkinson JH, Wallace MR, et al. Neuropsychological impairment in human immunodeficiency virus-infection: implications for employment. HNRC Group. HIV Neurobehavioral Research Center. Psychosom Med. 1994 Jan–Feb;56(1):8–17. doi: 10.1097/00006842-199401000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Cysique LA, Maruff P, Brew BJ. Antiretroviral therapy in HIV infection: are neurologically active drugs important? Arch Neurol. 2004 Nov;61(11):1699–1704. doi: 10.1001/archneur.61.11.1699. [DOI] [PubMed] [Google Scholar]
- 28.Patel K, Ming X, Williams PL, Robertson KR, Oleske JM, Seage GR., 3rd International Maternal Pediatric Adolescent AIDS Clinical Trials 219/219C Study Team. Impact of HAART and CNS-penetrating antiretroviral regimens on HIV encephalopathy among perinatally infected children and adolescents. AIDS. 2009 Sep 10;23(14):1893–1901. doi: 10.1097/QAD.0b013e32832dc041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox E, Bungay PM, Bacher J, McCully CL, Dedrick RL, Balis FM. Zidovudine concentration in brain extracellular fluid measured by microdialysis: steady-state and transient results in rhesus monkey. J Pharmacol Exp Ther. 2002 Jun;301(3):1003–1011. doi: 10.1124/jpet.301.3.1003. [DOI] [PubMed] [Google Scholar]
- 30.Fenton KA. Changing epidemiology of HIV/AIDS in the United States: implications for enhancing and promoting HIV testing strategies. Clin Infect Dis. 2007 Dec 15;45 Suppl 4:S213–S220. doi: 10.1086/522615. [DOI] [PubMed] [Google Scholar]