Abstract
We show a method to produce biocompatible polymer-coated silicon (Si) nanocrystals for medical imaging. Silica-embedded Si nanocrystals are formed by HSQ thermolysis. The nanocrystals are then liberated from the oxide and terminated with Si-H bonds by HF etching, followed by alkyl monolayer passivation by thermal hydrosilylation. The Si nanocrystals have an average diameter of 2.1 ± 0.6 nm and photoluminesce (PL) with a peak emission wavelength of 650 nm, which lies within the transmission window of 650–900 nm that is useful for biological imaging. The hydrophobic Si nanocrystals are then coated with an amphiphilic polymer for dispersion in aqueous media with pH ranging between 7 and 10 and ionic strength between 30 mM and 2 M, while maintaining a bright and stable PL and a hydrodynamic radius of only 20 nm. Fluorescence imaging of polymer-coated Si nanocrystals in a biological tissue host is demonstrated, showing the potential for in vivo imaging.
Keywords: Silicon nanocrystals, diagnostic imaging, surface passivation, amphiphilic polymer, water dispersible
1. Introduction
Silicon (Si) nanocrystals are being explored as imaging contrast agents for non-invasive disease detection. They are small, luminescent, more resistant to photobleaching than molecular dyes, and layers of biomolecules can be grafted to their surfaces.[1,2] For biological applications, nanocrystals should be chemically stable, non-toxic, non-immunogenic, and small enough to circulate the blood stream for extended time.[3] Si nanocrystals can be produced with diameter below 5 nm and tunable emission in the transparency window for biological tissue (650 – 900 nm).[4–6] Si is also biocompatible and biodegradable, which is an advantage over cadmium-containing quantum dots (QDs) that are potentially toxic due to Cd2+ leaching.[7–11] These nanocrystals are small enough to circulate in the blood stream, but must be encapsulated in molecular layers such as lipids, polyethylene glycol (PEG) or amphiphilic polymers that “disguise” them from being recognized as foreign bodies and prolong their half-life in the blood stream.[12–15] The additional coating around nanocrystals can significantly increase their hydrodynamic diameter and consequently decrease their circulation time in the blood. Particles larger than 100 nm are quickly removed from the blood by cells of the reticuloendothelial system (RES), primarily in the liver and spleen.[16] Nanocrystals smaller than 5.5 nm in diameter are rapidly filtered by the kidneys.[17] Nanocrystals larger than 5.5 nm can escape rapid renal filtration, but are then prone to capture by RES cells at a rate that varies directly with particle size—the optimal size for crossing cell membranes via receptor-mediated endocytosis is between 25 and 50 nm.[17–20] Therefore, this is the targeted hydrodynamic size after encapsulation of the nanocrystals with non-immunogenic ligands for in vivo targeted tissue labeling.
Recent reports using Si as a contrast agent have coated luminescent Si particles with bulky surface ligands to facilitate biocompatibility, at the expense of significantly increasing the nanocrystal size. Sailor and coworkers used fragments of porous Si coated in Dextran polymer to image tumors in a mouse,[10] and Swihart and coworkers coated aggregates of Si nanocrystals in phospholipid micelles and imaged pancreatic cancer cells in vitro.[2] In both cases, the contrast agents are relatively large, with diameters of more than 100 nm, making them susceptible to rapid RES clearance. By encapsulating alkyl-passivated Si nanocrystals within amphiphilic polymer micelles, biocompatibility can be achieved without compromising a small hydrodynamic diameter.
Biocompatible polymers that have previously been used to coat Si nanocrystals have been synthesized with complicated UV reactors, or with polymer-coupling agents like ethyl-3-dimethyl amino propyl carbodiimide (EDC) that are removed with a slow post synthesis dialysis procedure.[21] Recent advances in polymer chemistry have eliminated the need for coupling agents and complicated polymerization and clean-up processes.[22,23] For instance, Lin et al. demonstrated a facile amphiphilic polymer synthesis using a poly(maleic anhydride) polymer backbone with a high specific reactivity for hydrophobic alkylamine side chains.[24] A two-step synthesis yields a low molecular weight amphiphilic polymer that spontaneously assembles into micelles around hydrophobic nanocrystals, enabling their dispersibility in water. The polymer backbone also has exposed carboxyl groups that provide a platform for further functionalization with cell-targeting biomolecules.[25,26]
Our strategy to biocompatible Si nanocrystals relies on the synthesis of hydrophobic Si nanocrystals with the desired size and bright luminescence in the optical window needed for biological imaging, followed by encapsulation in a low molecular weight amphiphilic polymer. Several methods are available for producing bright, luminescent, hydrophobic Si nanocrystals.[27] Here, we employ the method developed by Hessel and Veinot that generates Si nanocrystals by thermal decomposition of hydrogen silsesquioxane (H8Si8O12) which reliably provides crystalline Si nanocrystals with well-defined surfaces, diameters of approximately 3 nm, and bright red/NIR PL that is well-suited for in vivo imaging.[28,29] The Si nanocrystals remain small after polymer-encapsulation, with a hydrodynamic diameter of about 20 nm, allowing for greater range of in vivo cell targeting applications compared to much larger (> 100 nm) clusters of Si nanocrystals or porous Si fragments.[2,10] These nanocrystals were highly dispersible in aqueous media, without settling for over 2 months at a wide range of pH and ionic strength, and when embedded in biological tissue, were clearly visible by fluorescence imaging; thus, providing a proof-of-principle demonstration of the potential to use these materials as imaging contrast agents for in vivo medical applications.
2. Results and Discussion
2.1. Si nanocrystal synthesis, etching and hydrosilylation
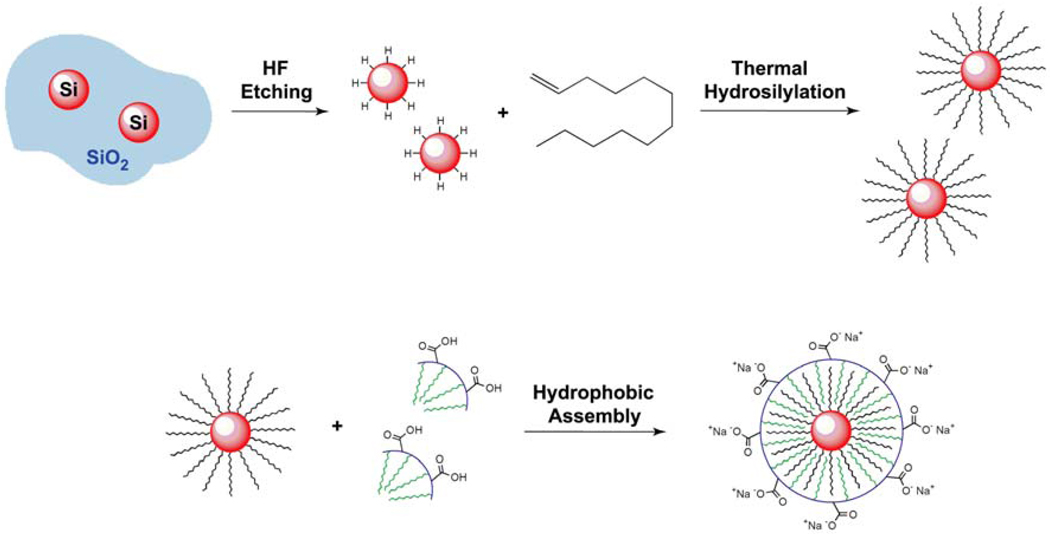
Figure 1 outlines the synthetic approach to polymer-coated Si nanocrystals. Hydrophobic, alkyl-passivated Si nanocrystals are first obtained by a series of steps: (1) Hydrogen silsesquioxane (HSQ) decomposition to form oxide-embedded Si nanocrystals, (2) Hydrofluoric acid (HF) etching and (3) thermal hydrosilylation in dodecene. The hydrophobic nanocrystals are then coated with amphiphilic polymer to render them hydrophilic and dispersible in biologically relevant media.
Figure 1.
Illustration of the Si nanocrystal passivation procedure. Si nanocrystals are liberated from a SiO2 matrix by HF etching. Hydride-terminated Si nanocrystals are refluxed in 1-dodecene to attach a hydrophobic, covalently-bonded alkyl monolayer via thermal hydrosilylation. The hydrophobic dodecene-passivated nanocrystals are finally coated with a poly(maleic anhydride)-based amphiphilic polymer.
2.1.1. Si nanocrystal synthesis
HSQ decomposition at 1100°C in a reducing atmosphere (5% H2/95% N2) yields Si nanocrystals approximately 3 nm in diameter embedded in silica. HSQ is a silicon-rich oxide that disproportionates into SiO2 and Si when heated, and at sufficient temperature, Si nanoparticles nucleate, crystallize and grow within the SiO2 matrix. This chemical transformation and the resulting material has been described in detail elsewhere.[28,29]
The Si nanocrystals are then liberated from the SiO2 matrix using a hydrofluoric acid (HF) etch. For uniform etching throughout the sample, the oxide-embedded Si nanocrystal material was mechanically ground into a fine brown powder with an approximate grain size of 200 nm. A 16% HF etching solution provides good control of the process, and over the course of 3 hr, the solution progresses from a dark brown to light yellow color as the nanocrystal size decreases during the oxide removal process. HF etches both Si and SiO2, but SiO2 etching is much faster due to the polarity difference between Si-Si and Si-O bonds.[30] Nonetheless, there is a slight decrease in nanocrystal diameter from 3 to 2 nm during the oxide removal process. H-terminated Si nanocrystals are obtained.
The liberated Si nanocrystals are isolated by centrifugation and then heated in neat 1-dodecene at 190°C for 3 hr to promote hydrosilylation. The final Si nanocrystals are passivated with a covalently-bonded dodecane monolayer. The alkyl-coated nanocrystals are observably more dispersible in organic solvents after hydrosilylation.
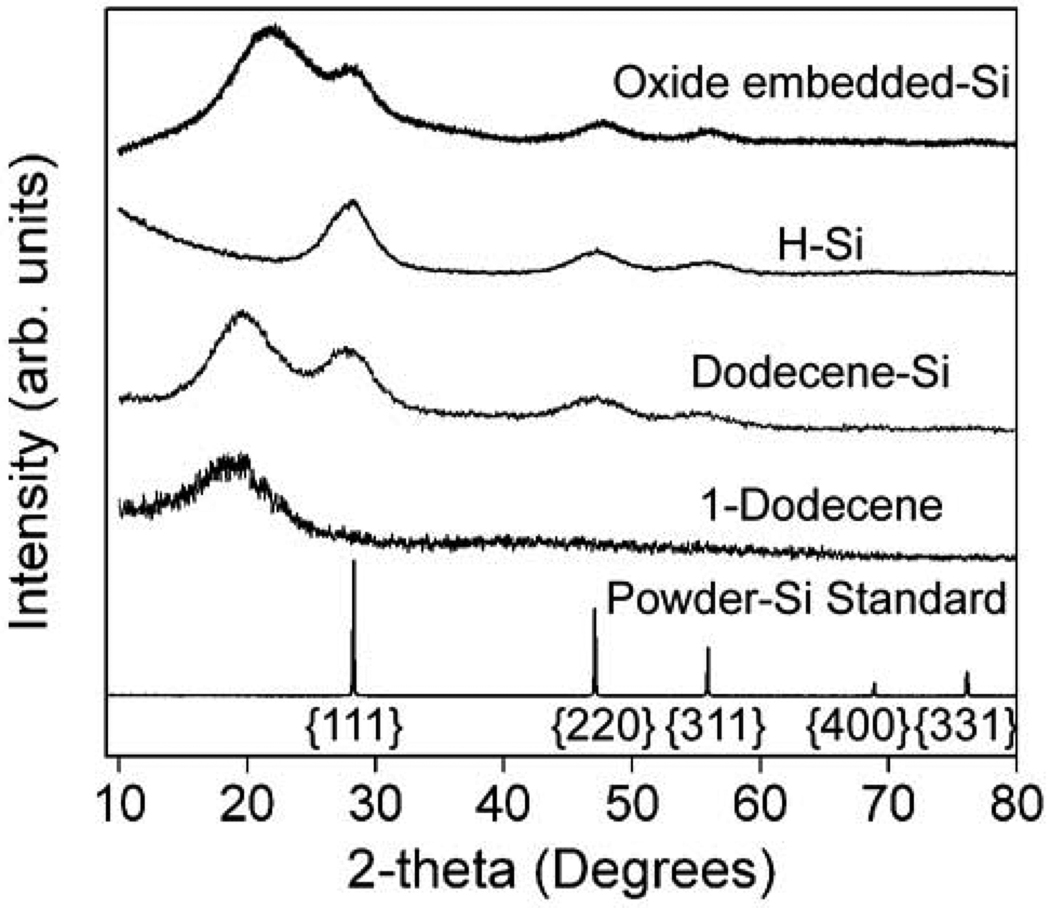
Figure 2 shows XRD data for Si nanocrystals at different stages in the synthesis process. Oxide-embedded Si nanocrystals show broad peaks at 28°, 48°, and 56°, corresponding to reflections from the (111), (220), and (311) crystallographic planes of diamond cubic Si. The prominent peak at ~22° is from the SiO2 matrix. After silica is removed by HF etching, the broad SiO2 reflection at 22° is no longer present, but the Si diffraction peaks remain. A Scherrer analysis of the peak width gives an average nanocrystal size of 2 nm. After refluxing in dodecene, the diamond cubic Si peaks are still observed by XRD, along with a broad peak at 18° from dodecene, confirming that the crystal structure and particle size does not significantly change during the chemical processing of the oxide-embedded nanocrystals.
Figure 2.
X-ray diffraction of oxide-embedded, hydride-terminated, and dodecene-passivated Si nanocrystals. Dodecene and Si powder references show the expected peak positions of the surface alkyl layer and diamond cubic Si (PDF # 027-1402, a = b = c = 5.43088 Å), respectively. Data are normalized to the (111) peak intensity and shifted vertically for clarity.
2.1.2. Hydrophobic Si nanocrystal surface characterization
The Si nanocrystals were also characterized by X-ray photoelectron spectroscopy (XPS) (Figure 3) and attenuated total reflectance FTIR (ATR-FTIR) spectroscopy (Figure 4) after each chemical treatment to monitor changes in the surface chemistry. XPS of the as-prepared oxide-embedded nanocrystals exhibits the characteristic peaks of elemental Si and SiO2 at ~99.4 eV and ~103.5 eV, respectively.[28,29] There is minimal suboxide (Si+1 and Si+2) present, indicating that there is an abrupt interface between Si nanocrystals and the SiO2 matrix.[31,32]
Figure 3.
XPS of (A) oxide-embedded, (B) freshly HF-etched, and (C) dodecene-passivated Si nanocrystals. A Shirley background subtraction was used for the data (○) and the best fit (solid line). Quantification of each Si oxidation state was obtained using a Voigt profile with a 30% Gaussian character. The freshly etched sample was exposed to ambient atmosphere for ~ 10 min prior to acquiring XPS data, while the spectra of oxide embedded and dodecene passivated Si nanocrystals were taken 1 week after synthesis. For reference, vertical dashed lines represent the oxidation states of Si. For clarity, only the Si 2p3/2 spin-orbit couple partner lines are shown.
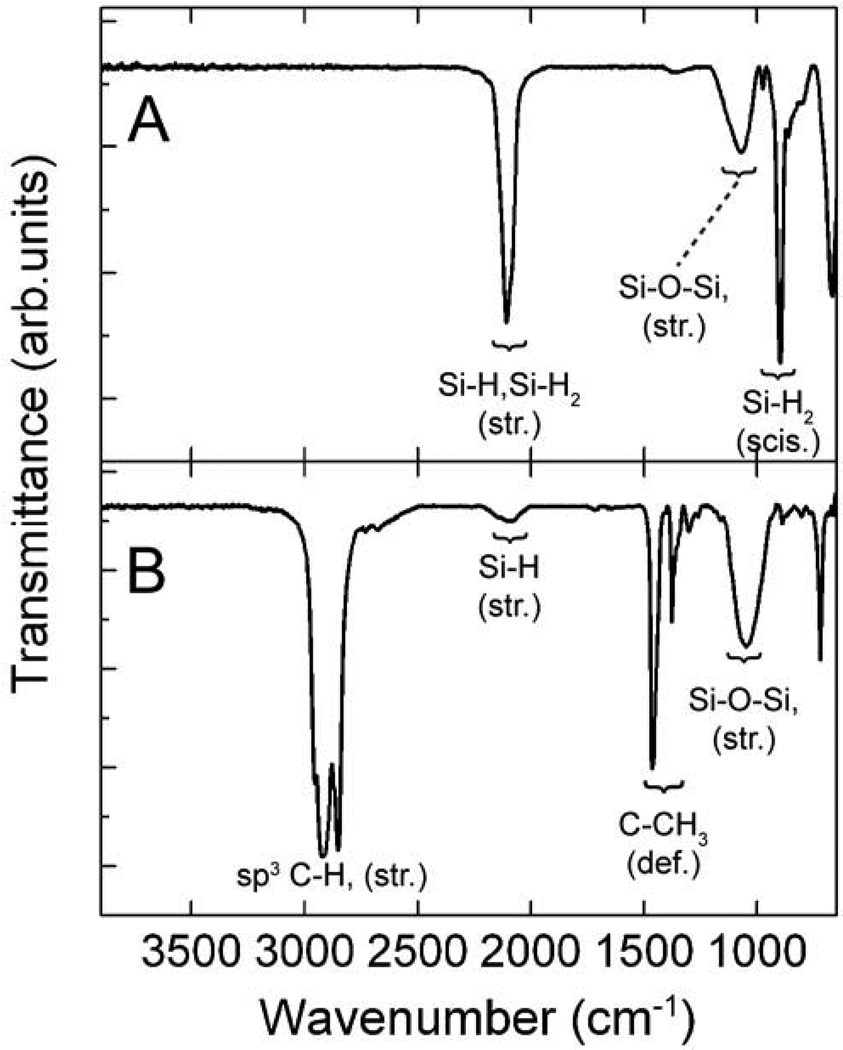
Figure 4.
ATR-FTIR spectra of (A) HF-etched and (B) dodecene-passivated Si nanocrystals.
After HF-etching, there is only a single XPS peak at 99.2 eV attributed to elemental Si, indicating that the entire SiO2 matrix has been cleanly removed. The FTIR spectrum exhibits intense bands corresponding to SiHx at 2108 cm−1 (Si-Hx stretch; x = 1, 2) and 898 cm−1 (Si-H2 scissor),[33,34] indicating the nanocrystals are hydride-coated. There is also a broad absorption centered at 1070 cm−1 in the FTIR spectra, which is assigned to Si-O-Si species that most likely form during the 20 min ambient atmosphere clean up.
After heating in dodecene, the Si-H1 and Si-H2 peaks at 2108 cm−1 and Si-H2 (scissoring absorption) peak at 898 cm−1 have disappeared, and strong aliphatic C-Hx stretching absorptions at 2957 cm−1, 2925 cm−1, 2853 cm−1, and C-CH3 deformations at 1464 cm−1 and 1378 cm−1 have appeared, indicating that the hydrosilylation reaction on the nanocrystal surfaces progressed as expected. XPS exhibits two dominant peaks at 99.2 eV and 101.9 eV, and a small shoulder at 103.5 eV. The peak at 101.9 eV is assigned to Si-C bonding at the nanocrystal surface, consistent with prior XPS studies of silicon carbide (SiC) nanocrystals and thin films, indicating covalent bonding of the alkyl chains to the nanocrystal surface.[35,36] The small shoulder at 103.5 eV corresponds to an oxide species. The FTIR spectra also show evidence of surface oxidation during hydrosilylation, as there was an intensity increase in the absorption centered at 1051 cm−1.
2.1.3. Si nanocrystal size determination
The diameter of the hydrophobic Si nanocrystals after hydrosilylation was determined by small angle X-ray scattering (SAXS). Imaging Si nanocrystals in this size range by TEM is very difficult because there is a very low electron density difference between silicon and carbon, the typical TEM support. Not only is it difficult to resolve the nanocrystals themselves, it is even more difficult to determine an accurate diameter from the edge of the nanocrystal, as needed to obtain a statistically meaningful measure of the nanocrystal size distribution in the sample. Grazing incidence small angle X-ray scattering (GISAXS) and transmission SAXS have been used to determine the size distribution of Si nanocrystals embedded in porous Si,[37] SiO2,[38] and amorphous silicon matrices,[39] however, SAXS has not been used before to determine the size distribution of sterically-stabilized silicon nanocrystals in a solvent dispersion.
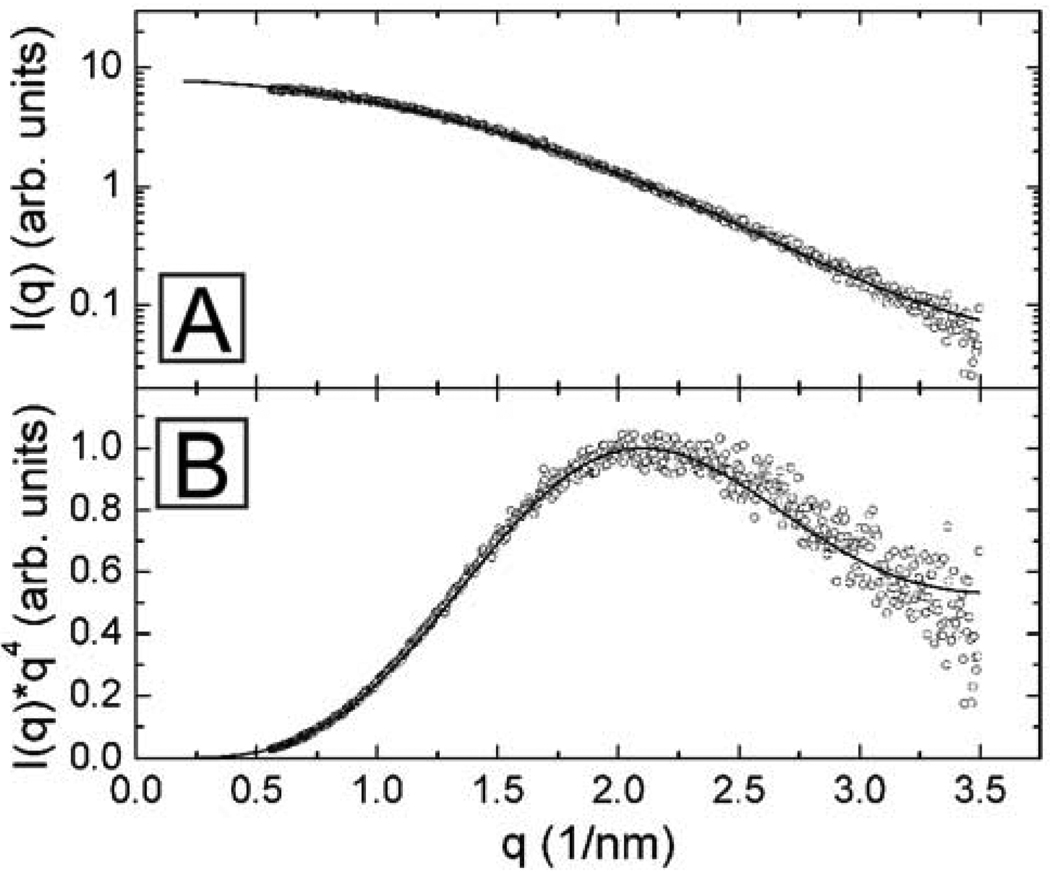
Figure 5 shows SAXS data for dodecene-passivated Si nanocrystals dispersed in toluene. The scattering intensity I(q), from a dilute dispersion of scatterers, is
| (1) |
where P(qR) is the form factor. For solid homogeneous spheres, P(qR) = [3(sin(qR)−qR cos(qR))/(qR)3]2. q is the scattering wave vector, which is related to the X-ray wavelength λ, and the scattering angle θ : q = (4π / λ)sin(θ/2).[40–43] N(R) is the number fraction of nanocrystals of radius R, in the sample, which is typically assumed to have a Gaussian distribution: R̅ is the average nanocrystal radius and σ is the standard deviation of the size distribution. Fitting the SAXS data in Figure 5 to Equation (1) gave an average nanocrystal diameter of 2.1 nm ± 0.6 nm, corresponding well with the size determined by a Scherrer analysis (d = 2 nm) of peak broadening in the XRD spectrum.
Figure 5.
SAXS of dodecene-passivated Si nanocrystals dispersed in toluene: plotted as log(I(q)) vs. q in (A) and as a Porod plot with I(q)×q4 vs. q in (B). The scattering intensity is normalized to the intensity of the first maximum in the Porod plot in (B) at q=2.14 nm−1. The average nanocrystal diameter determined from a best fit (solid line) of Eqn (1) to the (○) data, assuming a Gaussian size distribution, is 2.1 nm ± 0.6 nm.
2.1.4. Optical properties of hydrophobic Si nanocrystals
The optical properties of the Si nanocrystals were measured before and after HF etching and dodecene passivation. Figure 6 shows the UV-visible absorbance, photoluminescence (PL), and photoluminescence excitation (PLE) spectra of the nanocrystals dispersed in chloroform. The as-prepared oxide-embedded Si nanocrystals exhibit a PL peak at 780 nm, as previously reported.[28,29] HF etching shrinks the nanocrystals slightly and shifts the PL to slightly lower wavelength at 590 nm. The absorbance increases monotonically from the optical edge with decreasing wavelength and overlaps with the PLE spectrum, which exhibits a peak at 320 nm. The very large Stokes shift of almost 300 nm between the PLE and PL spectra is characteristic of most Si nanocrystal samples examined in the literature.[4,5,44] Similar size-dependence of the PL emission energy has been observed by the groups of Kortshagen, Swihart, and Veinot, who manipulated Si nanocrystal size by etching with HF,[4,5,29] or CF4 plasma.[45]
Figure 6.
Room temperature UV-visible photoluminescence emission (PL, dotted line), photoluminescence excitation (PLE, solid line), and absorbance spectra (dashed line) for (A) oxide-embedded, (B) freshly HF-etched, and (C) dodecene-passivated Si nanocrystals dispersed in chloroform. PL was collected with excitation wavelengths of (A) 350 nm, (B) 320 nm, and (C) 380 nm. Spectra are shifted vertically for clarity. Absorbance and PLE spectra are not reported for oxide-embedded Si nanocrystals due to their limited colloidal stability in organic solvents.
Dodecene passivation then led to nanocrystals with a quantum yield of 8%, relative to Rhodamine 101, and a slight red-shift in the PL to 648 nm (Figure 6C). The PLE peak also shifted slightly to the red to 380 nm. Similar changes in PL from green/yellow to orange after hydrosilylation were also observed when the HF-etched nanocrystals were heated in octene or decene. Regardless of alkyl chain length, alkene passivation was observed to red-shift the PL to wavelengths between 605 nm and 650 nm. Similar post-hydrosilylation red-shifts in PL have also been observed by other groups,[4,44,46] and has been attributed to a small amount of surface oxidation during the passivation process.[47]
2.2. Coating Si nanocrystals with amphiphilic polymer
2.2.1. Amphilic polymer chemistry
In vivo imaging requires nanocrystals that emit within the transparency window of biological tissue (650–900 nm) and disperse in aqueous solutions under physiological conditions (i.e., pH of ~7.4 and ionic strength of ~150 mM).[6] The hydrophobic, dodecene-passivated Si nanocrystals were coated with an amphiphilic polymer with a maleic anhydride backbone made using the approach described by Lin et al.[22–24] A commercially available poly(maleic anhydride) serves as a hydrophilic backbone that is grafted with hydrophobic alkylamine sidechains through spontaneous amide bond formation. The hydrophilic-lipophilic balance can be tailored during synthesis by choosing the ratio of dodecylamine sidechains to the poly(maleic anhydride) backbone.
The amphiphilic polymer was made by adjusting the stoichiometry to react 75% of the anhydride rings with dodecylamine. The remaining 25% of unreacted anhydride rings were then opened by exposure to a reducing aqueous environment (pH 12), giving two hydrophilic carboxyl groups per ring, which are subsequently available for biomolecule conjugation.[48]
The nanocrystals were encapsulated in amphiphilic polymer micelles by drying a film of nanocrystals and polymer, followed by rehydration with aqueous sodium borate buffer. The polymer-coated nanocrystals were then purified by ultracentrifugation and transferred to aqueous phosphate buffered saline (PBS). The final polymer-coated Si nanocrystals in PBS solution had a hydrodynamic diameter of 17.8 ± 0.4 nm measured by dynamic light scattering (DLS), and a zeta potential of −31 mV. The DLS data shows that passivating nanocrystals with a low molecular weight amphiphilic polymer is an effective way to provide a hydrophilic coating, without compromising a significant increase in overall hydrodynamic diameter. The Si nanocrystals should be large enough to bypass the renal system, yet small enough to remain in the circulatory system for extended periods of time without the possibility of rapid removal by the RES system.
2.2.2. Optical properties of hydrophilic polymer-coated Si nanocrystals
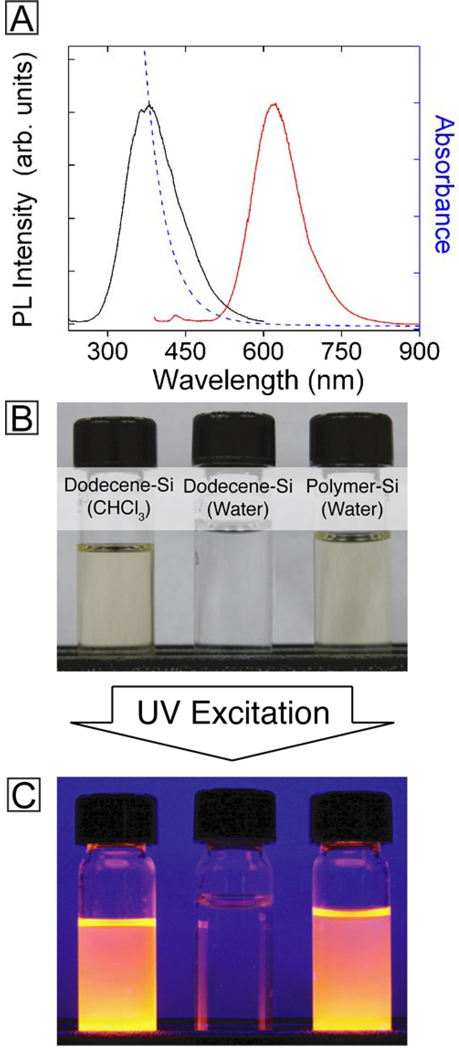
The PL emission wavelength of the polymer-coated Si nanocrystals dispersed in water matched those of the hydrophobic nanocrystals, as shown in Figure 7, with only a slight decrease in quantum yield to 3%. The dodecene-passivated nanocrystals do not disperse in water without the polymer coating and are readily removed by passing through a 200 nm pore size filter (See photograph in Figure 7B). Conversely, polymer-coated Si nanocrystals form optically clear dispersions in PBS buffer that easily pass through a 200 nm pore size filter and maintain their relatively strong PL intensity.
Figure 7.
(A) Room temperature PL (red), PLE (black), and absorbance (blue) of polymer-coated Si nanocrystals dispersed in phosphate buffered saline (PBS) solution. (B) Photographs of vials containing (from left to right) alkyl-passivated Si nanocrystals in chloroform, alkyl-passivated Si nanocrystals in phosphate buffered saline (PBS) at pH 7, and polymer-coated Si nanocrystals in PBS after passing through a 200 nm pore size filter. (C) Photographs of the vials shown in (B) illuminated by UV (350 nm) light.
2.2.3 Dispersion stability of polymer-coated Si nanocrystals
The PL and dispersion stability of polymer-coated Si nanocrystals were tested at various ionic strength and pH. The nanocrystals dispersed without aggregation in aqueous media with ionic strength up to 2 M, and remained emissive up to 4 M. In 250 mM sodium borate buffer, the nanocrystal dispersions were emissive and stable between a pH of 7 and 10. The nanocrystals aggregated when the pH was lowered below 7.0, but could be redispersed by raising the pH back above 7.0. The dispersion stability at low pH is dictated by the protonation/deprotonation of the carboxyl groups in the amphiphilic polymer backbone. Their stability in aqueous media over a wide range of pH and ionic strength makes polymer-coated Si nanocrystals well-suited for use in physiologically-relevant conditions.
2.3. Fluorescence imaging in biological tissue
Polymer-coated Si nanocrystals were injected into a model host and then imaged with a custom-built fluorescence imaging system to determine their potential as contrast agents for in vivo biological imaging.[49] Figure 8 shows a section of chicken tissue injected with Si nanocrystals imaged with a custom built wide field fluorescence imaging system. The fluorescence from the nanocrystals is readily observable in the tissue illuminated with a 337 nm laser and imaged with a 600 nm long pass filter to remove reflected light from the excitation source (Figure 8B).
Figure 8.
Grey-scale CCD images of biological tissue injected with polymer coated Si nanocrystals under room light (A) and under UV illumination with a 337 nm pulsed nitrogen laser (B). Grey-scale fluorescence images were collected with a custom-built wide field fluorescence imaging system equipped with a 600 nm long pass filter to remove the excitation contribution from the image. The location of the injected Si nanocrystals is outlined in image A for clarity. 150 µL of a PBS dispersion of Si nanocrystals at a concentration of 1016 nanocrystals mL−1 was injected 3 mm below the tissue surface.
3. Conclusions
We have demonstrated a chemical route to produce fluorescent Si nanocrystals suitable for biological imaging. Extensive characterization of the nanocrystals using XRD, SAXS, ATR-FTIR and XPS was used to confirm the nanocrystal composition and surface chemistry after each synthetic step. This detailed characterization is important to ensure that the chemical properties of the contrast agents are well understood.
The Si nanocrystals coated with amphiphilic polymer were very dispersible in aqueous media over a wide range of pH and ionic strength and fluorescence imaging in chicken tissue confirmed the ability to image these polymer-coated Si nanocrystals under biologically relevant conditions. This proof-of-concept experiment demonstrates that amphiphilic polymer-coated Si nanocrystals are suitable for in vivo biological imaging applications. The carboxylic acid side chains on the amphiphilic polymer are also available for conjugation to biomolecules, which could make this a smart material to target and label specific antigens. However, surface chemistry resistant to non-specific protein adsorption in serum will probably need to be developed for targeted labeling studies, and attachment of hydrophilic polyethylene glycol or zwitterionic functional groups to the polymer backbone seems like a promising route.[17,50]
4. Experimental Section
Materials
FOx 17® (Dow Corning Corporation, 17% HSQ by weight in methylisobutylketone), methanol (Sigma, 99%), ethanol (Sigma, 99%), anhydrous ethanol (Sigma, ≥ 99%) hydrofluoric acid (Sigma, 48%), 1-dodecene (Acros Organics, 93%), acetonitrile (Acros Organics, 99%) Rhodamine 101 (Sigma), poly(isobutylene-alt-maleic anhydride) (Mw~6000 Da, 39 monomer units per molecule, Sigma), dodecylamine (Sigma, 98 %), anhydrous tetrahydrofuran (Sigma, ≥ 99.9%), anhydrous chloroform (Sigma, ≥ 99%), sodium hydroxide pellets (Sigma, ≥ 98%), boric acid (Sigma, 99%), and phosphate-buffered saline powder (Sigma) were purchased and used as received. All aqueous solutions were prepared using deionized (DI) water having an 18 MΩ resistance.
Oxide-embedded Si nanocrystals
Oxide-embedded Si nanocrystals were generated using the adaptation of a literature protocol.[29] In a typical synthesis, solid white HSQ (0.3 g) was heated to 1100°C at a rate of 18°C min−1 under flowing forming gas (93% N2/7% H2). The temperature was maintained at 1100°C for 1 hour, and then allowed to cool to room temperature. The dark brown/black glassy product was ground for 20 min in an agate mortar and pestle to reduce the grain size to ~2 µm. The resulting light brown powder was then shaken in a wrist-action shaker for 15 hr with 3 mm borosilicate glass beads (15 g) to further reduce the average grain size to ~200 nm.
Oxide-embedded nanocrystal etching and alkene passivation
Si nanocrystals were liberated from the SiO2 matrix by etching with HF. Shaken nanocrystals (0.3 g) were combined with a mixture of 1:1:1; 48% HF:H2O:Acetonitrile (10 mL) and stirred at 250 rpm for 1.5 hr. The addition of the oxide embedded Si nanocrystals to the etching solution resulted in the production of small gas bubbles that persistently evolved throughout the reaction. The gas is SiF4, one of the major products in the reaction of HF and Si or SiO2, and is produced as surfaces of the Si nanocrystals are passivated with hydrides. The (hydride-terminated) Si nanocrystals were isolated from the HF solution by centrifugation at 8000 rpm for 2 min. The light yellow precipitate was rinsed twice with acetonitrile (5 mL) and finally dispersed in neat dodecene (12 mL). The turbid nanocrystal dispersion was transferred to a 3 neck round bottom flask and degassed with 4 freeze-pump-thaw cycles on a greaseless Schlenk line.
Dodecane passivation of the Si nanocrystals was accomplished through thermal hydrosilylation by heating to 190°C for 3 hr. During the first 30 min of heating, the opaque yellow dispersion became optically clear with a bright orange color. The dodecane passivated Si nanocrystals were washed three times by precipitation with a 2:1 ethanol:methanol mixture (15 mL), and finally dispersed in chloroform.
Amphiphilic polymer synthesis
The amphiphilic polymer synthesis was adapted from a published protocol.[24] In a capped, single neck round bottom flask, anhydrous THF (100 mL), dodecylamine (15 mmol, 3.45 mL), and poly(isobutylene-alt-maleic anhydride) (20 mmol of monomer units, 3.084 g) were added sequentially to form a turbid white solution. The flask was sonicated for 1 min to suspend the insoluble polymer, and was heated at 60°C for 3 hr under vigorous stirring. The suspension became clear after 15 minutes of stirring, indicating that the polymer became soluble in THF after covalently coupling to hydrophobic dodecylamine molecules. After 3 hours, the solution was cooled to room temperature and reduced in volume to 20 mL with a rotary evaporator. The clear solution was stirred again at 60°C for 12 hours under vigorous stirring to ensure complete coupling between the polymer and dodecylamine. The solution was cooled to room temperature and the remaining solvent was removed with a rotary evaporator, yielding the pale yellow, solid amphiphilic polymer. The solid polymer was transferred to a nitrogen filled glove box (< 0.1 ppm O2) and dissolved in anhydrous CHCl3 (25 mL) to give a monomer unit concentration of 0.8 M. The amphiphilic polymer solution was stored in a glass vial within the glovebox until use.
Coating the Si nanocrystals with polymer
Si nanocrystals were coated with polymer following a protocol that had been developed for other hydrophobic nanocrystal materials.[24] In a 50 mL round bottom flask, the amphiphilic polymer stock solution (0.8 M monomer units in CHCl3, 53 µL), the alkyl-Si nanocrystals (3.0 mg/mL in anhydrous CHCl3, 0.40 mL), and anhydrous CHCl3 (2.55 mL) were combined and vortexed with magnetic stirring for 15 minutes at room temperature. The solvent was removed by rotary evaporation to yield a yellow Si-polymer film on the inner wall of the flask. Aqueous sodium borate buffer (SBB) (50 mM borate, 2.0 mL, pH 12) was added to the flask and stirred for 15 minutes at room temperature to disperse the Si-polymer. Once suspended, DI water (13.0 mL) was added to the flask to dilute the nanocrystals. The aqueous nanocrystal solution was passed through a 0.2 µm-pore syringe filter (Corning, PES membrane), followed by a 0.1 µm-pore syringe filter (Whatman, inorganic membrane). The filtered nanocrystal solution was placed in an ultracentrifugation filter (Amicon Ultra, regenerated cellulose membrane, 50 kDa molecular weight cutoff) and centrifuged at 4000 xg for 4 minutes at room temperature. The colorless filtrate was discarded, and the concentrated nanocrystal retentate solution was diluted to 15.0 mL with aqueous, sterile-filtered phosphate buffered saline (PBS, 150 mM, pH 7.4). The ultracentrifugal filtration process was repeated two more times using PBS to dilute the nanocrystal retentate solution. The final aqueous nanocrystal solution was stored in a glass vial under ambient conditions until use.
Material characterization
XRD data were obtained with Si nanocrystals (5 mg) on quartz substrates using a Bruker-Nonius D8 Advance diffractometer. Scans of 2θ° were performed from 10 − 90° in 0.02 (2θ°) increments at a scan rate of 12.0° min−1 for 6 hours. XRD of freshly etched Si nanocrystals was collected immediately after removing the nanocrystals from the etching solution to minimize oxidation of the nanocrystals prior to the measurement. ATR-FTIR spectra (400 – 4000 cm−1) were acquired using a Thermo Mattson Infinity Gold FTIR with an attenuated total internal reflectance (ATR) stage. Samples were prepared by drop casting from chloroform dispersions after a 20 min ambient clean up in glass centrifuge tubes. XPS was performed on a Kratos photoelectron spectrometer equipped with a charge neutralizer and 180° hemispherical electron energy analyzer. XPS samples were prepared by dropcasting Si nanocrystals (~ 5 mg) onto indium tin oxide (ITO) coated glass substrates and degassed at 10−7 Torr for 1 day prior to analysis. XPS data were internally standardized with respect to the O1s peak position (530 eV). Small angle X-ray scattering (SAXS) was performed on a dilute dispersion of silicon nanocrystals in toluene enclosed in a stainless steel cell with kapton windows. Measurements were performed using a Molecular Metrology system with a rotating copper anode X-ray generator (Bruker Nonius; λ = 1.54 Å) operating at 3.0 kW. The scattered photons were collected on a 2D multiwire gas-filled detector (Molecular Metrology, Inc.) and the scattering angle was calibrated using a silver behenate (CH3(CH2)20COOAg) standard. Radial integrations of scattering intensity were performed using Datasqueeze. Experimental data was corrected for background scattering. Optical absorbance spectra were acquired with a Cary 500 UV/vis spectrophotometer using a quartz cuvette with a 10 mm optical path length. PL and PLE were acquired on a Varian Cary Eclipse Fluorescence Spectrophotometer using a quartz cuvette with a 10 mm optical path length. Si nanocrystal quantum yields were measured relative to a Rhodamine 101 standard, dissolved in anhydrous ethanol.
Zeta potential was measured by laser Doppler anemometry using a Zetasizer Nano ZS instrument (Malvern). Polymer-coated Si nanocrystals dispersed in aqueous PBS (~1016 nanocrystals mL−1) were loaded into a disposable folded capillary cell (Malvern, 1.5 mL volume) equipped with two electrodes. The cell was placed in the Zetasizer, which applied an alternating electric field across the cell. Particle mobility in the electric field was measured with the Zetasizer by recording the phase shift of an incident laser beam. Particle mobility was then converted to zeta potential by the Zetasizer software using Smoluchowski theory.[51] The average hydrodynamic diameter of the polymer coated Si nanocrystals in PBS was measured by dynamic light scattering using the Zetasizer instrument. The particles were placed in the disposable capillary cell at ~1016 nanocrystals mL−1 concentration for light scattering measurements. The Zetasizer detected the intensity of backscattered photons at a 173° angle from an incident 4 mW He-Ne (633 nm) laser over a time interval of 10 seconds, using a sample time (τ) of 0.5 microseconds. The hydrodynamic diameter was then extracted from the light scattering data using the method of cumulants.[52]
Biological imaging
A dispersion of polymer coated Si nanocrystals (150 uL, 1016 nanocrystals mL−1) were injected 3 mm below the surface of a store-bought piece of chicken breast. The tissue was imaged using a wide-field imaging system that consisted of: 1) a 337 nm nitrogen pulsed laser (NL-100, Stanford Research Systems), 2) a UV mirror to reflect the laser on the sample, 3) a 600 nm long pass filter to remove the excitation contribution in the image, 4) a focusing lens, and 5) a CCD to collect the grey scale fluorescence image (Grafteck Imaging Inc.). A background image of illuminated tissue prior to injection was collected and subtracted from the final image containing the Si nanocrystals to eliminate the autofluorescence contribution of the tissue.
Acknowledgments
We thank the National Science Foundation (Grant No. 0618242) and Texas Materials Institute for supporting the X-ray Photoelectron Spectrometer used in this work. We acknowledge the Robert A. Welch Foundation (Grant No.F-1464), the National Institutes of Health (Grant No. R01 CA132032), the Fundación Alfonso Martín Escudero, and the Natural Science and Engineering Research Council of Canada for financial support of this work.
Contributor Information
Colin M. Hessel, Department of Chemical Engineering, Texas Materials Institute, and Center for Nano- and Molecular Science and Technology, The University of Texas at Austin, Austin, Texas 78712 (USA)
Michael R. Rasch, Department of Chemical Engineering, Texas Materials Institute, and Center for Nano- and Molecular Science and Technology, The University of Texas at Austin, Austin, Texas 78712 (USA)
Jose L. Hueso, Department of Chemical Engineering, Texas Materials Institute, and Center for Nano- and Molecular Science and Technology, The University of Texas at Austin, Austin, Texas 78712 (USA)
Brian W. Goodfellow, Department of Chemical Engineering, Texas Materials Institute, and Center for Nano- and Molecular Science and Technology, The University of Texas at Austin, Austin, Texas 78712 (USA)
Vahid A. Akhavan, Department of Chemical Engineering, Texas Materials Institute, and Center for Nano- and Molecular Science and Technology, The University of Texas at Austin, Austin, Texas 78712 (USA)
Priyaveena Puvanakrishnan, Department of Biomedical Engineering, The University of Texas at Austin, Austin, Texas 78712 (USA).
James W. Tunnell, Department of Biomedical Engineering, The University of Texas at Austin, Austin, Texas 78712 (USA)
Brian A. Korgel, Email: korgel@che.utexas.edu, Department of Chemical Engineering, Texas Materials Institute, and Center for Nano- and Molecular Science and Technology, The University of Texas at Austin, Austin, Texas 78712 (USA).
References
- 1.Li ZF, Ruckenstein E. Nano Lett. 2004;4:1463–1467. [Google Scholar]
- 2.Erogbogbo F, Yong KT, Roy I, Xu GX, Prasad PN, Swihart MT. ACS Nano. 2008;2:873–878. doi: 10.1021/nn700319z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alivisatos AP, Gu W, Larabell C. Annu. Rev. Biomed. Eng. 2005;7:55–76. doi: 10.1146/annurev.bioeng.7.060804.100432. [DOI] [PubMed] [Google Scholar]
- 4.Hua F, Swihart MT, Ruckenstein E. Langmuir. 2005;21:6054–6062. doi: 10.1021/la0509394. [DOI] [PubMed] [Google Scholar]
- 5.Li X, He Y, Talukdar SS, Swihart MT. Langmuir. 2003;19:8490–8496. [Google Scholar]
- 6.Weissleder R. Nat. Biotechnol. 2001;19:316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 7.Bayliss SC, Heald R, Fletcher DI, Buckberry LD. Adv. Mater. 1999;11:318–321. [Google Scholar]
- 8.Canham LT. Adv. Mater. 1995;7:1033–1037. [Google Scholar]
- 9.Popplewell JF, King SJ, Day JP, Ackrill P, Fifield LK, Cresswell RG, Di Tada ML, Liu K. J. Inorg. Biochem. 1998;69:177–180. doi: 10.1016/s0162-0134(97)10016-2. [DOI] [PubMed] [Google Scholar]
- 10.Park JH, Gu L, Von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Nature Mater. 2009;8:331–336. doi: 10.1038/nmat2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derfus AM, Chan WCW, Bhatia SN. Nano Lett. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 13.Chan WCW, Nie S. Science. 1998;281:2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 14.Larson DR, Zipfel WR, Williams RM, Clark SW, Bruchez MP, Wise FW, Webb WW. Science. 2003;300:1434–1436. doi: 10.1126/science.1083780. [DOI] [PubMed] [Google Scholar]
- 15.Clapp AR, Medintz IL, Tetsuo Uyeda H, Fisher BR, Goldman ER, Bawendi MG, Mattoussi H. J. Am. Chem. Soc. 2005;127:18212–18221. doi: 10.1021/ja054630i. [DOI] [PubMed] [Google Scholar]
- 16.Osaki F, Kanamori T, Sando S, Sera T, Aoyama Y. J. Am. Chem. Soc. 2004;126:6520–6521. doi: 10.1021/ja048792a. [DOI] [PubMed] [Google Scholar]
- 17.Soo Choi H, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Nat. Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadauskas E, Wallin H, Stoltenberg M, Vogel U, Doering P, Larsen A, Danscher G. Part. Fibre Toxicol. 2007;4:10–17. doi: 10.1186/1743-8977-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang W, Kim BYS, Rutka JT, Chan WCW. Nat. Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 20.Gao H, Shi W, Freund LB. Proc. Natl. Acad. Sci. USA. 2005;102:9469–9474. doi: 10.1073/pnas.0503879102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Neiner D, Wang S, Louie AY, Kauzlarich SM. Nanotechnology. 2007;18:095601–095617. doi: 10.1088/0957-4484/18/9/095601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperling RA, Pellegrino T, Li JK, Chang WH, Parak WJ. Adv. Funct. Mater. 2006;16:943–948. [Google Scholar]
- 23.Hermanson GT. Bioconjugate Techniques. San Diego, CA: Academic Press; 1996. [Google Scholar]
- 24.Lin CAJ, Sperling RA, Li JK, Yang TY, Li PY, Zanella M, Chang WH, Parak WJ. Small. 2008;4:334–341. doi: 10.1002/smll.200700654. [DOI] [PubMed] [Google Scholar]
- 25.Franchina JG, Lackowski WM, Dermody DL, Crooks RM, Bergbreiter DE, Sirkar K, Russell RJ, Pishko MV. Anal. Chem. 1999;71:3133–3139. doi: 10.1021/ac981300q. [DOI] [PubMed] [Google Scholar]
- 26.Li ZF, Kang ET, Neoh KG, Tan KL. Biomaterials. 1998;19:45–53. doi: 10.1016/s0142-9612(97)00154-3. [DOI] [PubMed] [Google Scholar]
- 27.Veinot JGC. Chem. Commun. 2006:4160–4168. doi: 10.1039/b607476f. [DOI] [PubMed] [Google Scholar]
- 28.Hessel CM, Henderson EJ, Veinot JGC. J. Phys. Chem. C. 2007;111:6956–6961. [Google Scholar]
- 29.Hessel CM, Henderson EJ, Veinot JGC. Chem. Mater. 2006;18:6139–6146. [Google Scholar]
- 30.Williams KR, Gupta K, Wasilik M. J. Micromech. Syst. 2003;12:761–778. [Google Scholar]
- 31.Hessel CM, Henderson EJ, Kelly JA, Cavell RG, Sham TK, Veinot JGC. J. Phys. Chem. C. 2008;112:14247–14254. [Google Scholar]
- 32.Luh DA, Miller T, Chiang TC. Phys. Rev. Lett. 1997;79:3014–3017. [Google Scholar]
- 33.Marra DC, Edelberg EA, Naone RL, Aydil ES. J. Vac. Sci. Technol., A. 1998;16:3199–3210. [Google Scholar]
- 34.Henderson EJ, Kelly JA, Veinot JGC. Chem. Mater. 2009;21:5426–5434. [Google Scholar]
- 35.Henderson EJ, Veinot JGC. J. Am. Chem. Soc. 2009;131:809–815. doi: 10.1021/ja807701y. [DOI] [PubMed] [Google Scholar]
- 36.Avila A, Montero I, Galán L, Ripalda JM, Levy R. J. Appl. Phys. 2001;89:212–216. [Google Scholar]
- 37.Matsumoto T, Suzuki JI, Ohnuma M, Kanemitsu Y, Masumoto Y. Phys. Rev. B.: Condens. 2001;63:1953221–1953225. [Google Scholar]
- 38.Bernstorff S, Dubƒçek P, Kovaƒçeviƒá I, Radiƒá N, Pivac B. Thin Solid Films. 2007;515:5637–5640. [Google Scholar]
- 39.Gracin D, Bernstorff S, Dubcek P, Gajovic A, Juraic K. J. Appl. Crystallogr. 2007;40:s373–s376. [Google Scholar]
- 40.Korgel BA, Fitzmaurice D. Phys. Rev. B.: Condens. 1999;59:14191–14201. [Google Scholar]
- 41.Korgel BA, Fullam S, Connolly S, Fitzmaurice D. J. Phys. Chem. B. 1998;102:8379–8388. [Google Scholar]
- 42.Glatter OD, O, editors. Small-Angle X-ray Scattering. New York: Academic Press; 1982. [Google Scholar]
- 43.Guinier AF, G . Small-angle Scattering of X-rays. New York: Wiley; 1955. [Google Scholar]
- 44.Li X, He Y, Swihart MT. Langmuir. 2004;20:4720–4727. doi: 10.1021/la036219j. [DOI] [PubMed] [Google Scholar]
- 45.Pi XD, Liptak RW, Deneen Nowak J, Wells NP, Carter CB, Campbell SA, Kortshagen U. Nanotechnology. 2008;19:245603–245608. doi: 10.1088/0957-4484/19/24/245603. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Z, Brus L, Friesner R. Nano Lett. 2003;3:163–167. [Google Scholar]
- 47.Wolkin MV, Jorne J, Fauchet PM, Allan G, Delerue C. Phys. Rev. Lett. 1999;82:197–200. [Google Scholar]
- 48.Yong KT, Roy I, Swihart MT, Prasad PN. J. Mater. Chem. 2009;19:4655–4672. doi: 10.1039/b817667c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puvanakrishnan P, Park J, Diagaradjane P, Schwartz JA, Coleman CL, Gill-Sharp KL, Sang KL, Payne JD, Krishnan S, Tunnell JW. J. Biomed. Opt. 2009;14:024044. doi: 10.1117/1.3120494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu R, Kay BK, Jiang S, Chen S. MRS Bull. 2009;34:432–440. [Google Scholar]
- 51.Smoluchowski MV. Bull. Int. Acad. Sci. Cracovie. 1903;184 [Google Scholar]
- 52.Frisken BJ. Appl. Opt. 2001;40:4087–4091. doi: 10.1364/ao.40.004087. [DOI] [PubMed] [Google Scholar]