Abstract
Nitric oxide (NO) produced by neuronal nitric oxide synthase (nNOS) has a role in late-phase long-term potentiation (LTP) and long-term memory (LTM) formation. Our recent studies implicated NO signaling in contextual and auditory cued fear conditioning. The present study investigated the role of NO signaling in visually cued fear conditioning. First, visually cued fear conditioning was investigated in wild-type (WT) and nNOS knockout (KO) mice. Second, the effects of pharmacological modulators of NO signaling on the acquisition of visually cued fear conditioning were investigated. Third, plasma levels of corticosterone were measured to determine a relationship between physiological and behavioral responses to fear conditioning. Fourth, levels of ERK1/2 and CREB phosphorylation, downstream of NO signaling, were determined in the amygdala as potential correlates of fear learning. Mice underwent single or multiple (4) spaced trainings that consisted of a visual cue (blinking light) paired with footshock. WT mice acquired cued and contextual LTM following single and multiple trainings. nNOS KO mice acquired neither cued nor contextual LTM following a single training; however, multiple trainings improved contextual but not cued LTM. The selective nNOS inhibitor S-methylthiocitrulline (SMTC) impaired cued and contextual LTM in WT mice. The NO donor molsidomine recovered contextual LTM but had no effect on cued LTM in nNOS KO mice. Re-exposure to the visual cue 24h posttraining elicited freezing response and a marked increase in plasma corticosterone levels in WT but not nNOS KO mice. The expression of CREB phosphorylation (Ser-133) was significantly higher in naïve nNOS KO mice than in WT counterparts, and pharmacological modulators of NO had significant effects on levels of CREB phosphorylation and expression. These findings suggest that visual cue-dependent LTM is impaired in nNOS KO mice, and aberrant modulation of CREB in the absence of the nNOS gene may hinder cued and contextual LTM formation.
Keywords: Fear conditioning, Nitric oxide, neuronal nitric oxide synthase, Memory, corticosterone, Amygdala
Fear conditioning is an associative learning paradigm which is used to investigate cue and context-dependent long-term memory (LTM) formation. In the fear conditioning task, the presentation of an aversive unconditioned stimulus (US; foot shock) is temporally paired with the presentation of a neutral conditioned stimulus (CS; sensory cue) within a discrete context. The subject learns that the CS and the training context are predictive of the aversive US, and subsequent CS and context exposures elicit conditioned fear responses in the absence of the US. The fear response in rodents includes freezing behavior and the release of the stress hormone corticosterone (Rodrigues et al., 2009). The rodent responses to fear conditioning are considered analogous to the expression of the symptoms of posttraumatic stress disorder (PTSD) in humans (Mineka and Oehlberg, 2008). Understanding the mechanisms of LTM formation related to fear conditioning will facilitate the development of treatments for PTSD.
The neural pathways mediating cued and contextual fear conditioning have been extensively studied. Pharmacological and lesion studies suggest roles for the hippocampus and hippocampal long-term potentiation (LTP) in contextual fear conditioning (Ahi et al., 2004; Maren and Fanselow, 1995; Phillips and LeDoux, 1992). For cued fear conditioning, direct thalamo-amgydala projections rapidly transmit sensory information regarding the CS and US to the basolateral amygdala where Hebbian LTP and LTM permit development of conditioned response (Bauer et al., 2001; Rogan et al., 1997). Before reaching the basolateral amygdala, retinal projections relay visual CS information (via the superior colliculus) to the lateral geniculate nucleus and lateral posterior nucleus of the thalamus (Doron and Ledoux, 1999; Shi and Davis, 2001). The auditory CS pathway includes the medial geniculate nucleus and posterior intralaminar nucleus of the thalamus (Doron and Ledoux, 1999; LeDoux, 2000). It was recently shown that for auditory fear conditioning, nitric oxide (NO) signaling in the basolateral amygdala regulated retrograde extracellular signal-related kinase (ERK1/2)-mediated gene transcription in the aforementioned auditory thalamic nuclei in rats (Overeem et al., 2010). This was the first study to demonstrate a role for retrograde NO signaling in the direct thalamo-amygdala pathway for fear conditioning. A role for NO signaling in visually cued fear conditioning has not been investigated.
NO in the brain is primarily produced by neuronal nitric oxide synthase (nNOS) and has the role of retrograde neurotransmitter. The NO signal transduction pathway facilitates synaptic plasticity and late-phase LTP in the amygdala (Chien et al., 2003; Schafe et al., 2005) and hippocampus (Arancio et al., 1996; Lu et al., 1999). NO stimulates pre- and post-synaptic cyclic nucleotide production leading to the activation ERK1/2 and subsequently the transcription factor cyclic adenosine monophosphate response element binding protein (CREB) (Contestabile, 2008; Lu et al., 1999; Riccio et al., 2006). The nNOS gene and NO signaling have been implicated in contextual fear conditioning (Kelley et al., 2010; Kelley et al., 2009; Resstel et al., 2008) and in auditory cued fear conditioning (Ota et al., 2008; Schafe et al., 2005).
The present study was undertaken to investigate the role of NO signaling in visually cued fear conditioning. First, visually cued fear conditioning was investigated in wild-type (WT) and nNOS knockout (KO) mice. Second, the effect of pharmacological modulators of NO signaling on acquisition of visual fear conditioning was investigated. Third, plasma levels of the stress hormone corticosterone were measured to determine the relationship between physiological and behavioral responses to fear conditioning. Fourth, levels of ERK1/2 and CREB phosphorylation, which are downstream of NO signaling, were determined in the amygdala as potential correlates of fear learning.
1 Experimental Procedure
1.1 Subjects
Breeding of nNOS KO and WT mice was carried out in our facilities at the University of Miami, Miller School of Medicine, Miami, FL, as described previously (Balda et al., 2006). Adult male and female homozygote nNOS KO mice (B6;129S-Nos1; 6–8 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME). The targeted mutation of the nNOS gene in KO mice resulted in >95% inhibition of nNOS activity (Huang et al., 1993). The mice have not been further backcrossed onto any inbred strain, therefore the KO have approximately 1:1 ratio of the genetic backgrounds of the parental strains. Following arrival of the nNOS KO mice to the viral antibody free facilities at the University of Miami, Miller School of Medicine, animals were single-sex habituated to the new environment for 1 week before breeding. Each litter of newborn nNOS KO mice (n=6–8) routinely contained about equal numbers of males and females. Mice were weaned on postnatal day (PD) 21 and were housed in single-sex groups of 4–5 per cage.
WT mice were generated from the breeding of C57BL/6J females with SV129 males. The F2 progeny are more appropriate controls than the F1 hybrids because the parental alleles of F1 mice are not segregating like those on a mixed B6;129S background. The F2 generation has a 1:1 proportion of the genetic backgrounds of C57BL/6J and SV129 strains, approximating the genetic background of nNOS KO mice (Jackson Laboratories, Bar Harbor, ME). Adult SV129 males and C57BL/6J females (6–7 weeks old) were purchased from Jackson Laboratories and bred in our facilities at the University of Miami, Miller School of Medicine. Following a 1 week habituation period, mice were bred to generate B6;129F1 progeny. After weaning (PD21), the mating of F1 × F1 offspring (>PD60) generated B6;129F2 progeny (8–12 per litter). This latter progeny is considered the WT counterpart to nNOS KO mice, as it is congenic to the KO mouse strain (Itzhak et al., 1998). WT mice were weaned on PD21 and segregated according to sex into groups of 5 per cage.
Adult (8–10 week) males were used for all experiments. Routinely, each experimental group of mice (n=5–14) contained subjects from 3 to 4 litters. Animals were housed in a temperature- (22±0.5 °C) and humidity- (50%) controlled room and maintained on a 12-h light/dark schedule. Food and water were available ad libitum. Animal care was in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, 1996) and approved by the University of Miami Animal Care and Use Committee.
1.2 Drug Treatments
The preferential nNOS inhibitor S-methyl-L-thiocitrulline (SMTC; 100mg/kg) was dissolved in water (vehicle) and administered to WT mice. The NO donor molsidomine (10 and 20 mg/kg) was dissolved in saline (vehicle) and administered to nNOS KO and WT mice. Drugs were given intraperitoneally (IP; volume of 0.1mL/10mg) 30 min pretraining or 30 min prior to sacrifice for the western blotting studies. We have shown that SMTC (100 mg/kg) and molsidomine (10 mg/kg) modulated a) the development of contextual fear conditioning, and b) the levels of cyclic guanosine monophosphate (cGMP) in the amygdala and hippocampus of WT and nNOS KO mice (Kelley et al., 2010). In the current study, two doses of molsidomine were investigated, 10 and 20 mg/kg.
1.3 Fear conditioning apparatus and procedure
The fear conditioning apparatus used was previously described (Kelley et al., 2009). Briefly, fear conditioning training and testing occurred in Plexiglas chambers (30.5 × 30.5 × 43.5 cm; Noldus Information Technology Inc., Leesburg, VA). Each chamber was equipped with a stainless steel floor through which the electric footshock was delivered. An upper control unit housed a white light illuminating one corner of the chamber; a video camera and yellow light stimulus were located in the center. The chambers were housed in custom built sound-attenuating chambers which gave the appearance of black walls. The floors of the chamber were cleaned with a diluted soap solution (1% Alconox), rinsed with water, and dried after each animal.
During training, mice were transferred in a covered carrier cage from the housing colony to the experimental room. Mice were placed in the training context (context A) and allowed to habituate to the novel environment for 134 s. Next, the corner white light was turned off and the center yellow light began blinking for 16 s (2 s on/off intervals). Pretraining mobility (%) in the context and during the visual cue was determined at this time as a measure of baseline activity which is depicted in the figures. The final 2 s blink of the yellow light (CS) co-terminated with a 2 s footshock (0.75 mA) and then mice were returned to the home cage 30 s later. In the multiple (4) training experiments, mice underwent a series of four of the previously described trainings with an intertrial interval (ITI) of 10–12 min, during which the mice were returned to home cage. Results of each experimental group were compared to a control group that underwent the same treatments in the absence of the footshock (referred as “no shock” in the figures).
For testing, contextual fear conditioning was measured in context A (training context) and consisted of digitally recording the subject’s percentage of time spent “freezing” while in the chamber for 2 min. Freezing was defined as a complete lack of movement besides respiration and was measured automatically using EthoVision v3.1 software (Noldus Information Technology, Inc.) with the following parameters: 6 samples/s and immobility threshold of 5% (Pham et al., 2009). Freezing is expressed as a percentage of the total time for each test. Visually cued fear conditioning was measured in a different context (context B), which utilized a smooth white foam pad floor that covered the shock grid, four opaque white walls, and an olfactory enrichment of pure orange extract that was affixed to the chamber ceiling. To further distinguish the contextual and cued LTM tests, the subjects were transferred to the experimental room on a wheeled cart and the lighting in the experimental room was dimmed. Thus, multiple sensory cues were changed to differentiate the contextual and cued LTM test conditions. After a 2 min habituation period in context B, the yellow light cue blinked for 2 min. Contextual and visually cued fear conditioning tests were separated by a 4 h interval and took place 24 h and 7 d posttraining. Results of the 24 h and 7 d tests following a single training are depicted in Fig. 1. Since the results of the 24 h and 7 d tests following multiple trainings were similar, only the results of the 24 h tests are depicted in Fig. 2.
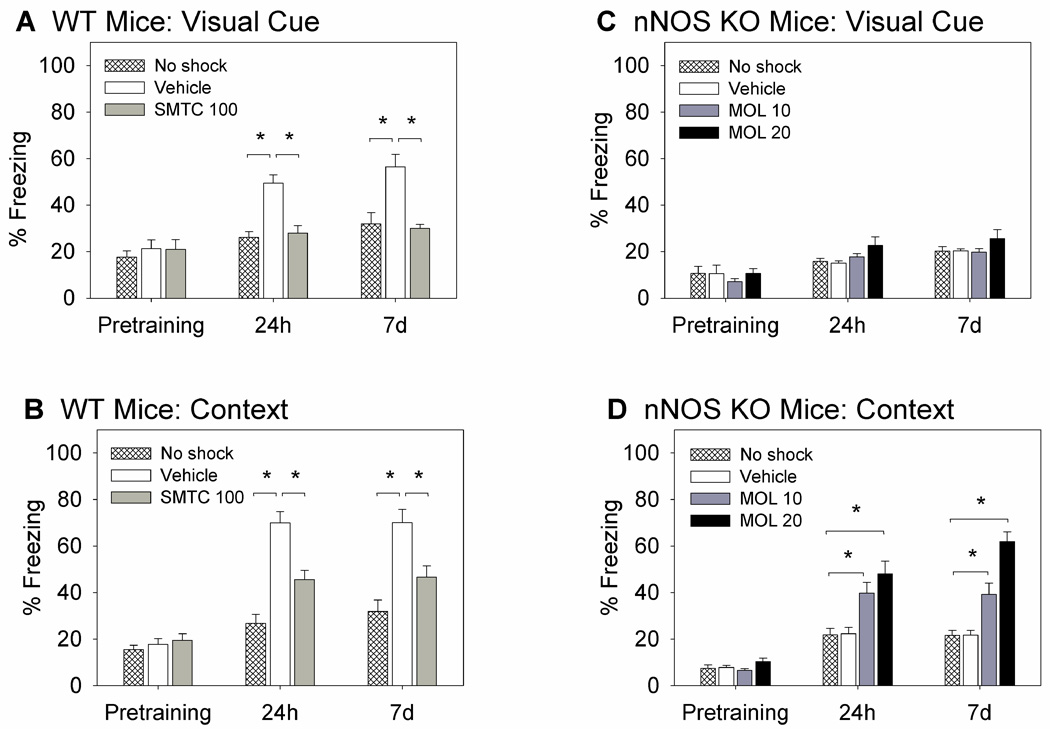
Figure 1.
Long-term memory (LTM) of visually cued and contextual fear conditioning in WT and nNOS KO mice. Mice (n=6–10/group) underwent a single training and LTM was determined 24 h and 7 d posttraining. Results are expressed as percent of total time spent freezing. “No shock” controls underwent the same training but did not receive the footshock. WT mice received the nNOS inhibitor SMTC (100 mg/kg) or vehicle and nNOS KO mice received the NO donor molsidomine (10 and 20 mg/kg) or vehicle 30 min before fear conditioning. Pretraining measurements reflect immobility before the footshock; no differences in % freezing between the groups were observed in baseline activity during the pretraining period. (A) WT mice acquired visual cued LTM, and the nNOS inhibitor impaired visual cued LTM (*P<0.05). (B) WT mice acquired contextual LTM, and the nNOS inhibitor impaired contextual LTM (*P<0.05). (C) nNOS KO mice failed to acquire visual cued LTM, and the NO donor did not facilitate visual cued LTM. (D) nNOS KO mice failed to acquire contextual LTM, but the NO donor dose-dependently improved contextual LTM (*P<0.05).
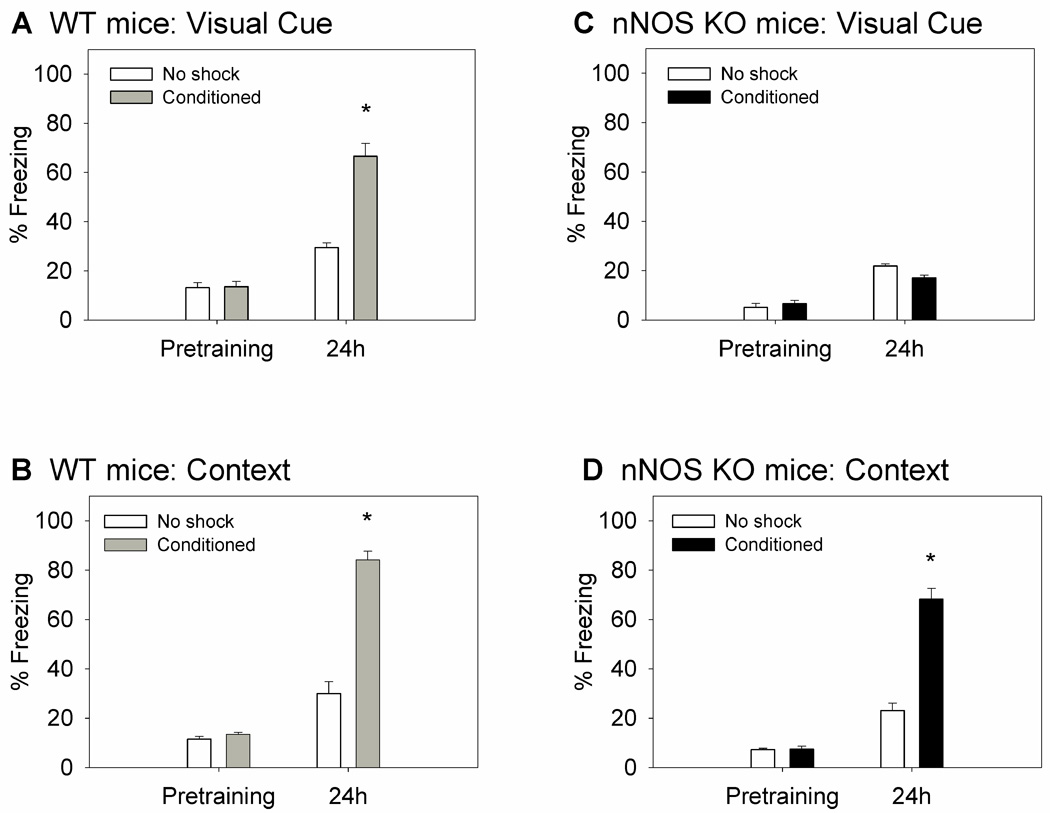
Figure 2.
LTM for visually cued and contextual fear conditioning after multiple (4) trainings in WT and nNOS KO mice. Mice (n=5–8/group) underwent four trainings (10–12 min intertrial interval); “no shock” controls underwent the same trainings but did not receive the footshocks. Cued and contextual freezing were measured pretraining and LTM was measured 24 h posttraining. (A) The multiple trainings improved visual cued LTM in WT mice (67±5% freezing vs. 49±4% freezing following a single training; Fig. 1A) (*P<0.001 conditioned vs. no shock). (B) WT mice acquired contextual LTM after multiple trainings (*P<0.001). (C) nNOS KO mice failed to acquire visual cued LTM after the multiple trainings. (D) The multiple trainings restored the contextual learning deficit observed after a single training (Fig. 1D) in nNOS KO mice (*P<0.001).
1.4 Plasma corticosterone measurements
Corticosterone assays were performed as previously described (Kelley et al., 2009). On Day 1, 15 min before, and 15 min after fear conditioning training, blood samples (80µl) were drawn from the retroorbital venous plexus using heparinized microcapillary tubes. On Day 2, blood samples were drawn 15 min after the visual CS test. Samples were then treated according to instructions provided in a corticosterone EIA kit (Immunodiagnostic Systems, Inc.), and analyzed on a spectrophotometer at 450nm.
1.5 Western blotting
WT mice received injections of SMTC (100 mg/kg), molsidomine (20 mg/kg), or vehicle and nNOS KO mice received molsidomine (20 mg/kg) or vehicle 30 min before sacrifice by cervical dislocation. The amygdala was immediately microdissected according to The Mouse Brain Atlas (Paxinos and Franklin, 2001). Tissue was homogenized in ice-cold buffer (20mM Tris-HCl, pH 7.4, 0.32 sucrose, 1mM EDTA, 1mM EGTA, 1mM PMSF, 50mM NaF, phosphatase inhibitors (Cocktail II, Sigma) and protease inhibitors (Complete Mini tablets; Roche Diagnostics). Lanes were loaded with 50µg protein/well, separated using 10% SDS-PAGE, and transferred to PVDF membranes. Membranes were incubated first for 1h at room temperature (RT) in blocking buffer: TBS + 50mM NaF + 0.1% Tween-20 (TBS-T) + 0.4% I-BLOCK. Next, membranes were incubated with anti-phosphorylated CREB (Ser133) (1:1000) at 4°C for 72 h or anti-phosphorylated ERK1/2 (1:2000) at RT for 1 h (both Cell Signaling, Beverly, MA) diluted in TBS-T + 5% BSA. After incubation, membranes were washed 3× for 5 min with TBS-T and then incubated (1h at RT) with the secondary antibody, horseradish peroxidase-conjugated goat anti-rabbit (dilutions of 1:1500 for pCREB; 1:2000 for pERK1/2) (Cell Signaling) and washed 3× for 5 min. The signal was visualized using an enhanced chemiluminescent (ECL) substrate for HRP enzyme (Pierce, Rockford, IL). Following development of the immunoblots for the phosphorylated protein, the immunoblots were stripped with Restore western blot stripping buffer (Pierce) for 15 min. After 3 quick rinses in TBS-T, the membranes were blocked again as above and then incubated with anti-total CREB (1:1000) or anti-total ERK1/2 (1:3000) antibodies (both Cell Signaling) for 1h at RT in TBS-T + 5% BSA. Next, membranes were washed and treated as described for pCREB and pERK1/2 except the secondary antibody was diluted at 1:3000. To control for protein loading membranes were stripped once more and probed for β-tubulin. Quantification of the bands corresponding to changes in protein levels were calculated by scanned image densitometric analysis UN-SCAN-IT gel analysis software (v6.1, Silk Scientific Inc., Orem, UT). Relative density units are expressed as the phosphorylated/total isoform and total isoform/ β-tubulin.
1.6 Statistical Analysis
Results of cued and contextual freezing following a single training were analyzed by two-way ANOVA (group × time; pretraining, 24 h or 7d) for each genotype. Results of cued and contextual freezing following multiple trainings were analyzed by two-way ANOVA (group × time; pretraining or 24 h) for each genotype. The corticosterone results were analyzed by three-way ANOVAs (genotype × group × time) for the single and multiple training experiments. A relationship between the magnitude of cued freezing and corticosterone levels following re-exposure to the visual cue 24 h posttraining was determined by Pearson product moment correlation. ANOVAs were followed by post hoc analysis using Bonferroni correction to determine differences between multiple groups. Amygdalar protein levels in the Western blotting studies were compared using unpaired, two-tailed Student’s t tests. All results are shown as mean ± standard error of the mean. A P value of <0.05 was considered statistically significant.
2 Results
2.1 Fear conditioning in WT and nNOS KO mice following a single training
2.1.1. WT mice
WT mice acquired visually cued and contextual fear conditioning following a single training (Fig. 1A, 1B). Pharmacological inhibition of nNOS impaired both types of learning (Fig. 1A, 1B). For visually cued freezing, there were significant effects for group (F (2,55) = 20.002, P<0.001), time (F(2,55) = 25.819, P<0.001), and the interaction between the two variables (F(4,55) = 4.464, P<0.003). Post hoc analysis of 24 h results revealed significantly higher freezing in the vehicle group (49±3%) than the SMTC group (28±4%, P=0.006) and the no shock group (26±4%, P<0.001). Analysis of the 7 d results also showed significantly higher freezing for the vehicle group (56±4%) than the SMTC group (30±4%, P<0.001) and the no shock group (35±4%, P=0.01).
For contextual freezing, there were significant effects for group (F(2,55) = 37.720, P<0.001), time (F(2,55) = 72.107, P < 0.001), and the interaction between the two variables (F(4,55) = 9.314, P<0.001). Post hoc analysis of the 24 h results revealed significantly higher freezing in the vehicle group (70±4%) than the SMTC group (46±4%, P<0.001) and the no shock group (28±4%, P<0.001). Analysis of the 7 d results showed significantly higher freezing for the vehicle group (70±4%) than the SMTC group (47±4%, P=0.004) and the no shock group (35±4%, P<0.001). The results of the effects of SMTC on contextual fear conditioning are in agreement with our previous studies (Kelley et al., 2010).
2.1.2 nNOS KO mice
nNOS KO mice showed impairments in both visually cued and contextual fear conditioning (Fig. 1C, 1D). The NO donor molsidomine improved contextual but not cued fear conditioning (Fig. 1C, 1D). For visually cued freezing, the ANOVA revealed a significant time effect (F(2,69) = 23.148, P<0.001) and non-significant group effect. A trend of increased immobility on day 7 (20±3%) compared to pretraining (11±3%) and 24 h (16±3%) in the no shock group is likely due to habituation to the light cue. In rodents, habituation occurs following repeated exposure to a novel context in the absence of a biologically relevant consequence (i.e. no shock); as a result there is a decrease in exploratory behavior (Leussis and Bolivar, 2006). Decreased exploratory behavior resulted in a higher magnitude of immobility, or “freezing” as it is represented in the figures. Also, it was observed that pretraining, or baseline, immobility in the novel context was reduced in nNOS KO compared to WT counterparts (WT: 17±1% vs. KO: 9±1%; Figs. 1A, 1B). This suggested that nNOS KO mice are slightly more active and exhibited increased exploratory behavior in a novel context compared to WT, which we (Kelley et al., 2010) and others (Tanda et al., 2009) have previously reported. Importantly, in nNOS KO mice there was no significant group effect, suggesting that molsidomine at all doses tested had no effect on cued fear conditioning (Fig. 1C).
Conversely, molsidomine dose-dependently improved contextual fear conditioning (Fig. 1D). ANOVA revealed significant effects for group (F(3,69) = 36.397, P<0.001), time (F(3,69) = 92.642, P<0.001), and the interaction between the two variables (F(6,69) = 8.605, P<0.001). For the molsidomine (10 mg/kg) group, contextual freezing after 24 h reached 40±3%, which was increased from the vehicle group (23±3%; P=0.005) and no shock group (22±3%; P=0.004). Freezing after 7 d in the molsidomine (10 mg/kg) group reached 39±4% which was increased from the vehicle group (22±3%, P<0.05) and the no shock group (22±3, P<0.05). For the molsidomine (20 mg/kg) group, contextual freezing reached 48±3% after 24 h and 62±3% after 7 d. The freezing in the molsidomine (20 mg/kg) group was significantly increased from all other groups (P<0.001 compared to vehicle and no shock groups; P<0.05 compared to molsidomine (10 mg/kg) group). The results of molsidomine effects on contextual freezing are similar to our previous studies where only a single dose of the NO donor was tested (10 mg/kg; Kelley et al., 2010).
2.2 Fear conditioning by multiple trainings improves LTM
Results of multiple (4) trainings of WT and nNOS KO mice are depicted in Fig. 2. Only the results of the 24 h tests are presented for LTM because no significant differences between the results of 24 h and 7 d tests were observed.
2.2.1 WT mice
Mice underwent four spaced trainings with intertrial intervals of 10–12 min. WT mice acquired cued and contextual fear conditioning after multiple trainings (Fig. 2A, 2B). For visually cued freezing there was a significant group effect (F(1,26) = 36.499, P<0.001), time effect (F(1,26) = 124.632, P<0.001), and interaction (F(1,26) = 34.714, P<0.001). Post hoc analysis revealed significantly higher freezing in the conditioned group (67±3%) compared to the no shock group (30±3%) during re-exposure to the visual CS (P<0.001). The small increase in immobility (15.7% increase) in the no shock group from pretraining (14±2%; P<0.05) may be due to habituation to the light cue.
For contextual freezing there was a significant group effect (F(1,26) = 100.867, P<0.001), time effect (F(1,26) = 254.042, P=0.007), and interaction (F(1,26) = 87.495, P<0.001). Freezing in the conditioned group (84±2%) was significantly higher than in the no shock group (30±3%; P<0.001). The significant differences between control (no shock) and conditioned (shocked) subjects in the magnitude of freezing to the light cue (37% increase) and the training context (54% increase) suggest that the augmented freezing in the conditioned group was due to the acquisition of conditioned fear response.
2.2.2 nNOS KO mice
nNOS KO mice failed to acquire visually cued fear conditioning (Fig. 2C) but did acquire contextual fear conditioning (Fig. 2D) after multiple trainings. For visually cued freezing there was a significant time effect (F(1,20) = 110.814, P<0.001), but no significant group effect (P>0.05), and a significant interaction between group and time (F(1,20) = 5.759, P<0.05). Post hoc analysis revealed slightly increased freezing in the conditioned group (17±1%) and the no shock group (22±1%) compared to pretraining levels (6±1% and 5±2%, respectively) which may be due to habituation to the light cue. The finding that no differences were observed between the conditioned and no shock groups after 24 h suggests that the impairments for visually cued fear conditioning persisted after the multiple trainings.
For contextual freezing there was a significant group effect (F(1,20) = 56.361, P<0.001), time effect (F(1,20) = 160.902, P<0.001), and interaction (F(1,20) = 55.730, P<0.001). Freezing was significantly higher in the conditioned group (68±3%) compared to the no shock group (23±3%; P<0.001), suggesting that the multiple spaced trainings facilitated LTM for contextual fear conditioning. These results are in agreement with our previous studies in nNOS KO mice, showing improvement in contextual fear conditioning following multiple trainings (Kelley et al., 2009).
2.3 Plasma corticosterone response correlates with fear learning
2.3.1 Single training
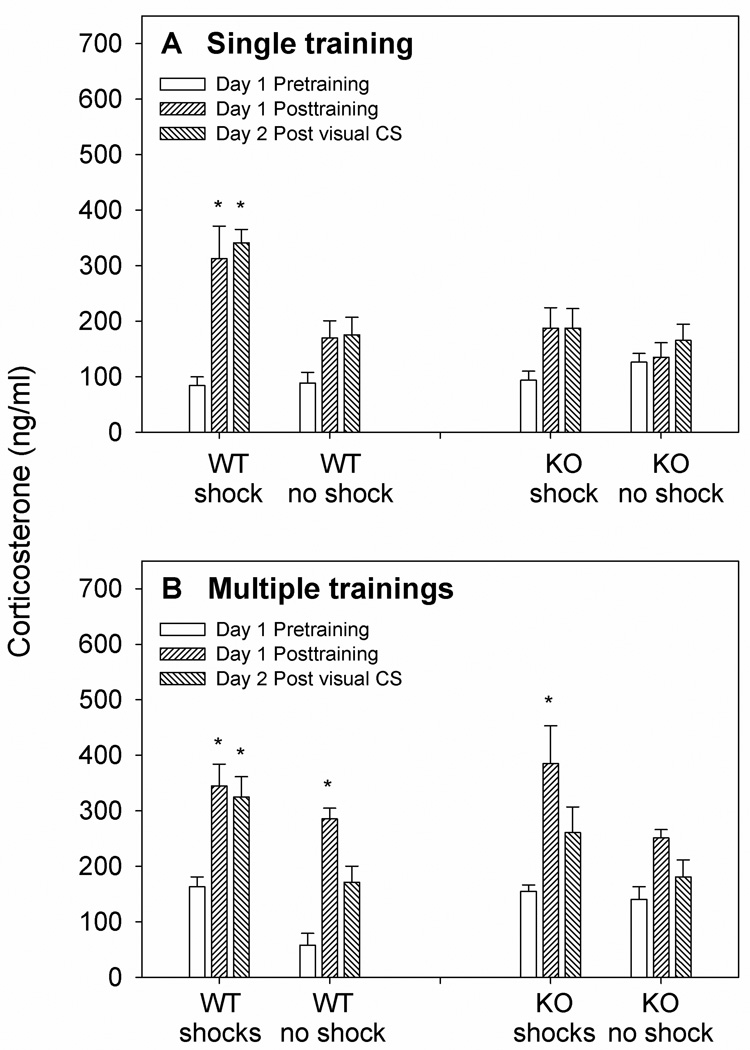
Plasma corticosterone levels were measured on day 1, both 15 min pretraining and 15 min posttraining. Subsequently, after 24 h, we investigated whether re-exposure to the visual CS elicits an increase in the stress hormone. Fig. 3A depicts results from WT and nNOS KO mice using a single training. There was a significant group effect (F(1,63) = 4.422, P<0.05), time effect (F(2,63) = 15.774; P<0.001), and interaction between genotype and group (F(1,63) = 4.811; P<0.05). Post hoc tests showed no significant differences between pretraining corticosterone levels across all groups. In the WT shock group, there were significant increases from pretraining to posttraining and Day 2 post visual CS exposure (P<0.001). In nNOS KO mice, neither the exposure to the shock nor the visual CS increased plasma corticosterone levels (Fig. 3A). Results show that the expression of LTM of visually fearful cue (freezing) is associated with increase in corticosterone levels.
Figure 3.
Corticosterone levels before and after single and multiple trainings (n=5–8/group). (A) For single training experiments, WT shock mice exhibited a significant increase in corticosterone levels from pretraining to 15 min posttraining, on Day 1, and 15 min post-exposure to the visual CS on Day 2 (*P<0.001). WT no shock control mice did not show significant increases in corticosterone levels. nNOS KO mice did not show significant increases in corticosterone levels after the administration of a single footshock training. (B) For multiple training experiments, WT mice showed a significant increase in corticosterone levels from pretraining to 15 min posttraining, on Day 1, and 15 min post-exposure to the visual CS on Day 2 (*P<0.001). Control no shock WT mice showed an increase in corticosterone 15 min post pseudo-training (*P<0.01), but not after visual CS exposure on Day 2. nNOS KO shock group exhibited a significant increase in corticosterone levels from pretraining to 15 min posttraining on Day 1 (*P<0.001). However, exposure to the visual CS on Day 2 did not cause a significant increase in corticosterone levels.
2.3.2 Multiple trainings
Fig. 3B depicts results of corticosterone levels following multiple (4) fear conditioning trainings. There were significant effects of group (F(1,72) = 9.681; P=0.003) and time (F(2,72) = 16.512; P<0.001). The WT mice exhibited posttraining increases in corticosterone following multiple (4) US (P<0.001) and pseudotrainings (P<0.05) on Day 1. Increased corticosterone in the psuedotrained control mice suggests that the repeated handling and transfers were stressful. Importantly, re-exposure to the visual CS on Day 2 only elicited corticosterone response in the WT shock group (P<0.001). The nNOS KO shock group exhibited an increase in corticosterone levels from pretraining to posttraining (P<0.001) but not on Day 2 after visual CS re-exposure. The hormonal response of nNOS KO mice 15 min following four trainings appears to correlate with acquisition of contextual fear conditioning as we have previously reported (Kelley et al., 2009). The lack of hormonal response to the visual cue in nNOS KO mice on Day 2 correlated with the impaired acquisition of cued fear conditioning.
2.3.3 Correlation analysis
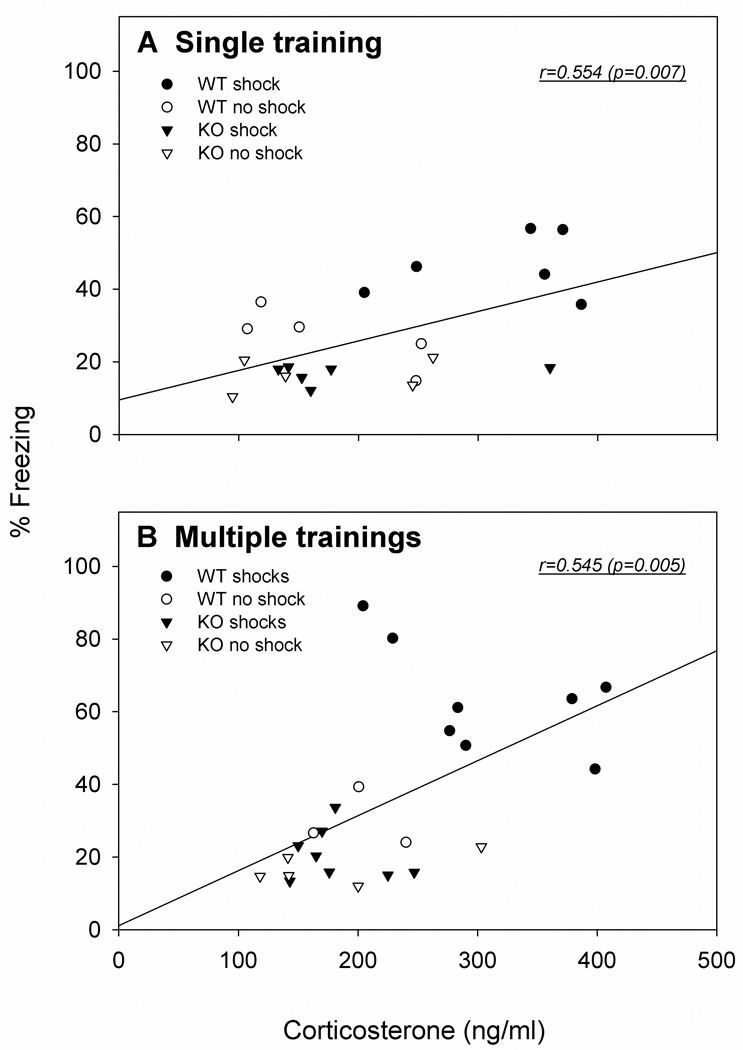
Correlations between corticosterone levels 15 min following re-exposure to the visual CS (Day 2) and behavioral freezing in the LTM test are shown in Fig. 4. Significant positive correlations were obtained for corticosterone and freezing responses after a single training (Fig. 4A; r=+0.554,p=0.007) and after multiple trainings (Fig. 4B; r=+0.545, p=0.005). These results suggested that the magnitudes of physiological and behavioral responses to conditioned fearful stimuli were correlated for both intensities of training.
Figure 4.
Correlations between the expression of visually cued LTM (% Freezing) and plasma corticosterone (ng/ml) 15 min after the LTM tests. (A) For a single training, the magnitude of freezing during the visual cue test correlated with the magnitude of corticosterone responses in WT and KO mice: WT shock (●, n=6); WT no shock (○, n=5); nNOS KO shock (▼, n=6); KO no shock (▽, n=5). (B) For multiple trainings, the magnitude of freezing during the visual cue test correlated with corticosterone responses in WT and nNOS KO mice: WT shock (●, n=8); WT no shock (○, n=3); nNOS KO shock (▼, n=8); nNOS KO no shock (▽, n=5).
2.4 Western blotting studies
2.4.1 Expression of ERK1/2 and CREB in WT and nNOS KO mice
We performed western blot analysis of amygdala tissue from naïve WT and nNOS KO mice to determine if there were genotypic differences in the expression of ERK1/2, CREB, and the respective phosphorylated isoforms, pERK1/2 and pCREB (Fig. 5). The levels of pERK1/2 and pCREB were normalized to total ERK1/2 and CREB levels, respectively, and expressed as arbitrary relative density units (Fig. 5B, 5E). The levels of total protein were normalized to β-tubulin loading control (Fig. 5C, 5F). Student’s t-tests (unpaired, two-tail) revealed elevated pCREB in nNOS KO mice compared to WT mice (2-fold; P=0.005; Fig. 5E). The levels of pERK1/2, ERK1/2, and total CREB were not different. The results suggest that elevated CREB phosphorylation in the amygdala of nNOS KO mice is independent of total CREB expression and ERK1/2 activity.
Figure 5.
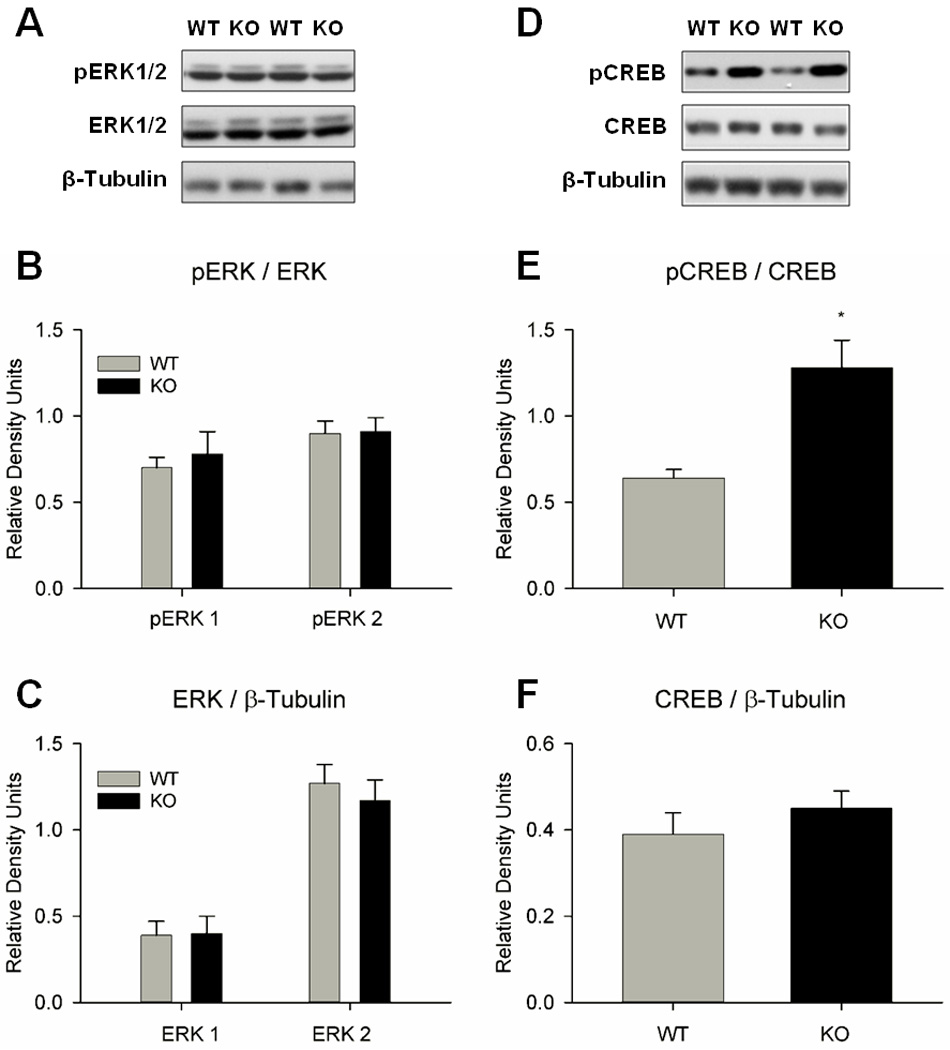
Genotypic comparisons of ERK1/2 and CREB expressions in the amygdala of naïve WT and nNOS KO mice (n=9–14/group). No differences in ERK1/2 phosphorylation or expression were observed, however pCREB was significantly elevated in nNOS KO mice compared to WT mice. (A) Representative immunoblots for ERK1/2 experiments. (B) No genotypic differences were observed for pERK1/2. (C) No genotypic differences were observed for ERK1/2 expression. (D) Representative immunoblots for CREB experiments. (E) pCREB levels were 2-fold higher in nNOS KO mice than in WT mice (*P =0.005). (F) No genotypic differences were observed for total CREB expression.
2.4.2 Effects of SMTC and molsidomine on CREB in the amygdala of WT mice
We investigated the effects of the behaviorally effective doses of NO signaling modulators on CREB in the amygdala 30 min following drug administration in WT mice. Results are summarized in Fig. 6. The nNOS inhibitor SMTC reduced pCREB expression by 75% of control vehicle (Fig. 6B; P<0.001) and total CREB expression by 44% of control vehicle (Fig. 6C; P<0.05). Conversely, the NO donor molsidomine increased pCREB expression by 51% of control vehicle (Fig. 6E; P=0.007) but had no effect on total CREB expression (Fig. 6F). The findings suggest that inhibition and activation of NO signaling pathway influenced CREB activation and expression in WT mice, which is consistent with the NO-cGMP transduction pathway.
Figure 6.
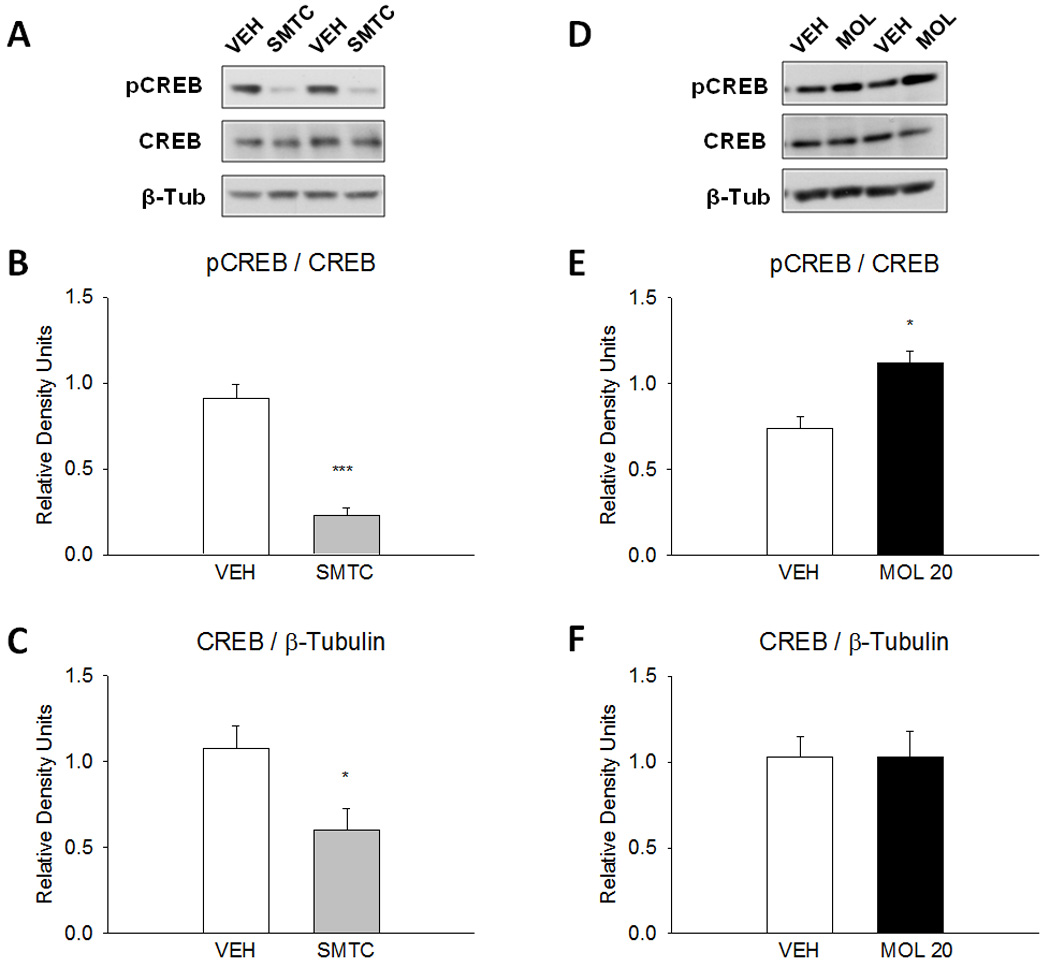
Effects of pharmacological modulators of NO on downstream CREB in the amygdala of WT mice (n=5/group). Mice received vehicle, SMTC (100 mg/kg) or molsidomine (20 mg/kg) in the home cage 30 min before sacrifice. The nNOS inhibitor and NO donor affected CREB phosphorylation in a manner that is consistent with the NO signal transduction pathway. (A) Representative immunoblots following vehicle and SMTC administrations. (B) SMTC reduced CREB phosphorylation by 75% of control vehicle (***P<0.001). (C) SMTC reduced total CREB expression by 45% of control vehicle (*P<0.05). (D) Representative immunoblots following vehicle and molsidomine administrations. (E) Molsidomine increased CREB phosphorylation by 34% of control vehicle (*P<0.05). (F) Total CREB expression was not affected by molsidomine administration.
2.4.3 Effects of NO donor molsidomine on pCREB expression in the amygdala of nNOS KO mice
Unexpectedly, molsidomine (20 mg/kg) reduced pCREB expression in the amygdala of nNOS KO mice by 48% of control vehicle (Fig. 7B; P <0.05). The drug had no effect on total CREB expression (Fig. 7C).
Figure 7.
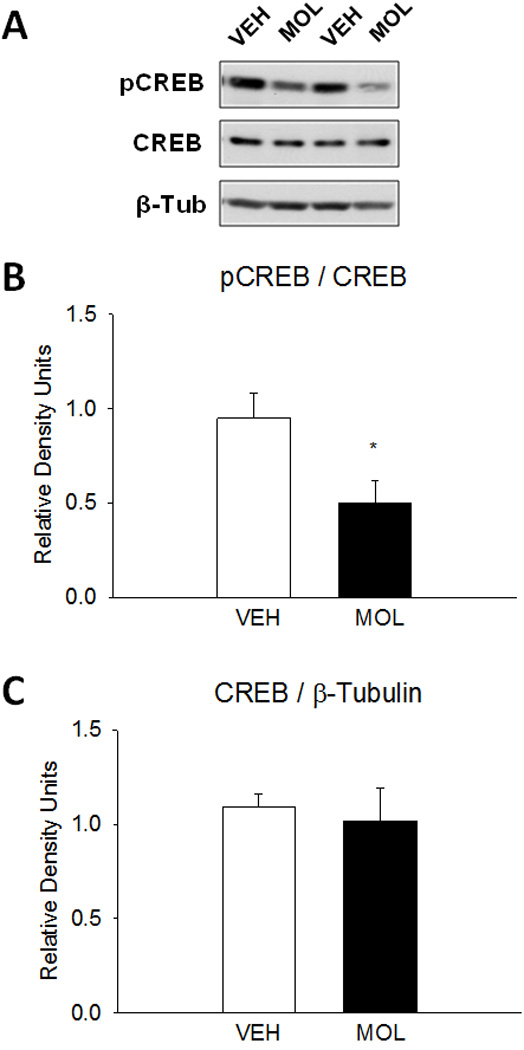
Effects of the NO donor molsidomine on downstream CREB in the amygdala of nNOS KO mice (n=5/group). nNOS KO mice received vehicle or molsidomine (20 mg/kg) in home cage 30 min before sacrifice. (A) Representative immunoblots following vehicle and molsidomine administrations. (B) Molsidomine reduced CREB phosphorylation by 47%.of control vehicle (*P<0.05) (C) Total CREB expression was not affected by molsidomine administration.
3 Discussion
3.1 Genetic deletion and pharmacological inhibition of nNOS impair cued and contextual fear conditioning
Previous studies have implicated NO signaling in contextual (Kelley et al., 2010; Kelley et al., 2009; Resstel et al., 2008) and auditory cued (Ota et al., 2008; Overeem et al., 2010; Schafe et al., 2005) fear conditioning. Our previous studies showed that nNOS KO mice had short- and long-term memory deficits for auditory cued (minor) and contextual (major) fear conditioning (Kelley et al., 2009) that were partially rescued by pretraining, but not posttraining, administration of NO donor (Kelley et al., 2010). Thus, our previous studies implicated NO in the acquisition of fear learning. The present study investigated the effects of genetic deletion and acute pharmacological inhibition of nNOS on LTM for visually cued and contextual fear conditioning. The results of acute pharmacological inhibition of nNOS by SMTC corroborated the results of the genetic deletion of nNOS, suggesting a requirement for NO signaling in the acquisition and subsequent consolidation of LTM for visually cued and contextual fear conditioning. A deficit in visually cued LTM was also observed in nNOS KO mice following appetitive conditioning by cocaine; nNOS KO mice acquired preference for cocaine-associated context but not cocaine-associated light cue (Itzhak et al., 2010). Yet, the finding that the NO donor molsidomine did not improve visually cued fear conditioning in nNOS KO mice suggests that exogenously administered NO donor may be insufficient to reverse this particular impairment. Previous studies from our laboratory showed that lower doses of molsidomine (4 – 10 mg/kg) improved a) cocaine-induced conditioned place preference (Itzhak and Anderson, 2007), and b) contextual fear conditioning (Kelley et al., 2010) in nNOS KO mice. The present study showed a dose-dependent improvement in contextual fear conditioning in nNOS KO mice following administration of molsidomine (Fig. 1D). Molsidomine (10 mg/kg) caused a 5.7-fold increase in cGMP in the hippocampus of nNOS KO mice (Kelley et al., 2010) which may be related to the improvements in contextual LTM.
It has been suggested that pairing a discrete visual CS with footshock may require multiple CS-US pairings to elicit fear conditioned response (Heldt et al., 2000; Newton et al., 2004). Our results show, however, that WT mice acquired visually cued fear conditioning after a single training and that cued LTM was only moderately improved (19% increase in freezing) by multiple (4) spaced trainings. Further, the multiple spaced trainings failed to improve visually cued LTM in nNOS KO mice. These outcomes are similar to our previous studies with auditory cued fear conditioning in which multiple (4) trainings moderately improved auditory cued LTM in WT mice but not nNOS KO mice (Kelley et al., 2009). However, the magnitude of auditory cued LTM deficits in nNOS KO mice was much smaller (10–15% less freezing than WT; Kelley et al., 2009) than the magnitude of visual cue LTM deficits (Fig. 1A, 1C). Differential dependency of NO signaling in the auditory and visual CS processing pathways, such as within the basolateral amygdala, may underlie the differences between auditory and visually cued fear conditioning.
The current findings demonstrate that the acquisition of contextual LTM is facilitated by multiple (4) spaced trainings in WT and nNOS KO. Several studies suggest that single and multiple spaced trainings may recruit different mechanisms and substrates for contextual LTM. For instance, spaced trainings improved LTM for contextual but not auditory cued fear conditioning through enhancement of hippocampal LTP (Scharf et al., 2002). Also, multiple trainings and the presence of an intertrial interval (spacing) can supersede the requirements of CREB (Kogan et al., 1997) and the hippocampus (Wiltgen et al., 2006) for contextual fear conditioning. Mutant CREB-deficient mice acquired LTM for contextual fear conditioning after spaced but not massed trainings (Kogan et al., 1997). Additionally, rats with hippocampal lesions acquired contextual LTM when multiple shocks, but not a single shock, were used (Wiltgen et al., 2006).
Our results suggest that LTM formation following a single fear conditioning training is NO-dependent, while LTM formation following multiple (4) spaced trainings may be NO-independent. First, nNOS KO mice had deficits in both cued and contextual fear memory following a single training (Fig. 1). Second, administration of the nNOS inhibitor SMTC prior to a single training suppressed both cued and contextual fear memory in WT mice (Figs. 1A, 1B). Third, administration of the NO donor molsidomine prior to a single training dose-dependently improved contextual fear conditioning in nNOS KO (Fig. 1D). Fourth, nNOS KO mice acquired optimal contextual fear response following multiple (4) spaced trainings (Fig. 2D), suggesting that formation of LTM following multiple trainings no longer depends on NO signaling pathway.
3.2 nNOS and the visual system
The findings that NO donor and multiple trainings did not improve LTM for visually cued fear conditioning in nNOS KO mice raises the possibility that neural adaptations in the KO mice may impede visual processing. Several studies have examined roles for nNOS and NO signaling in visual processing pathways (Cudeiro and Rivadulla, 1999). For example, in the mouse retina NO production is stimulated by light and can modulate light-evoked responses (Pang et al., 2010; Wang et al., 2007). Also, in the avian retina NO has been shown to stimulate ERK and CREB phosphorylation (Socodato et al., 2009). In the lateral geniculate nucleus, NO may play a role in neuronal light/dark responses (Nucci et al., 2003). Finally, in the mouse visual cortex NO can contribute to LTP through modulation of cGMP production (Haghikia et al., 2007). The visual cortex may be relevant because indirect thalamo-cortico-amgydala pathways play a role in the development and maintenance of visually cued fear response (Shi and Davis, 2001). However, it should be noted that acute (Knepper and Kurylo, 1998) and chronic (Tobin et al., 1995) pharmacological inhibitions of NO signaling by the NOS inhibitor L-nitroarginine methyl-ester (L-NAME; IP) in rats did not impede visual discrimination task performance when a light cue was used. This suggests that nNOS inhibition does not prevent discrimination of the visual CS. We hypothesize that deficient NO signaling in the amygdala, rather than disturbances of visual processing, impairs visual cue-dependent learning. Inhibition of NO signaling in the amygdala should be sufficient to impair fear learning across modalities because the amygdala is the convergence point of CS, US, and hippocampal processing pathways in fear conditioning (LeDoux, 2000).
3.3 Plasma corticosterone and fear conditioning
The stress hormone corticosterone has been implicated in LTM formation for cued and contextual fear conditioning (Rodrigues et al., 2009). The current study investigated a relationship between elevations of plasma corticosterone and the magnitude of conditioned freezing elicited by reexposure to the visual CS. Results showed that there is a positive correlation between the magnitudes of visually cued freezing and corticosterone responses 15 min following re-exposure to the visual CS 24 h posttraining (Fig. 4). Overall, the results show a relationship between the physiological response to stress (increase in corticosterone levels) and the behavioral fear response (freezing) to a visual cue that had been associated with aversive US. These findings suggest that measurements of neuroendocrine responses to cues associated with traumatic events may facilitate the monitoring of clinical treatments of PTSD.
3.4 ERK1/2 and CREB expression in WT and nNOS KO mice
Regulation of CREB activity in the basolateral and central nuclei of the amygdala is essential for the acquisition and consolidation of LTM of fear conditioning. Specifically, amygdalar activations of ERK1/2 (Schafe et al., 2000) and CREB (Han et al., 2007; Kida et al., 2002; Viosca et al., 2009a) are required for the development of cued fear conditioned response. We investigated the expression ERK1/2 (upstream of CREB) and CREB and their phosphorylated isoforms in the amygdala of naïve WT and nNOS KO mice because they are a) required for fear conditioning, and b) downstream of NO-cGMP signal transduction.
In the amygdala of naive nNOS KO mice, pCREB levels were 2-fold higher than in WT mice while total CREB expression was the same (Fig. 5E, 5F). This finding suggests a dysregulation of the mechanisms of CREB phosphorylation or dephosphorylation, rather than of overall CREB expression, in the absence of the nNOS gene. The reason for enhanced pCREB expression in the amygdala is not clear, however several studies have showed that genetic and chronic pharmacological inhibition of NO signaling led to increased CREB phosphorylation (Ser-133) in the dentate gyrus of the hippocampus and the subventricular zone of the olfactory bulb (Moreno-Lopez et al., 2004; Packer et al., 2003; Zhu et al., 2006). These studies demonstrated that NO can act as a negative regulator of neurogenesis and CREB phosphorylation; the mechanism of this interaction is unknown (Contestabile, 2008). The findings that pERK1/2 and total ERK1/2 were similar in both genotypes suggest that aberrant pCREB expression is independent of ERK1/2. It is possible that enhanced pCREB expression is the consequence of a compensatory mechanism in the nNOS KO mice, whereby the absence of NO signaling stimulates other transduction pathways that modulate the phosphorylation state of CREB.
Several studies have demonstrated that viral vector-mediated overexpression of CREB in rodent amygdala facilitated auditory cued LTM of fear conditioning (Han et al., 2007; Josselyn et al., 2001; Viosca et al., 2009a; Wallace et al., 2004). It has been suggested that overexpression of active CREB enhances learning by 1) reducing the threshold for late-phase LTP induction and 2) overexpression of plasticity-related proteins enabling rapid consolidation of LTM (Viosca et al. 2009a). Interestingly however, nNOS KO mice have increased amygdalar pCREB levels concurrent with significant deficits in cued LTM (Figs. 1, 2 and Itzhak et al., 2010). Although these findings are difficult to reconcile, the consequences of enhanced pCREB expression in nNOS KO mice may be different than that in WT mice. First, the absence of nNOS leads to a deficit in late-phase LTP in nNOS KO mice (Hopper and Garthwaite, 2006). Second, a consequence of increased CREB transcription would normally be overexpression of nNOS because these form a positive feedback loop (Sasaki et al., 2000). Enhancement of nNOS and NO signaling in the CREB overexpression studies would be expected to facilitate LTM (Chien et al., 2005; Ota et al., 2008). Conversely, other studies have shown that overexpression of CREB impairs learning. For example, overexpression of CREB in the olfactory bulb reduced odor preference learning in rats (Yuan et al., 2003), and overexpression of an active isoform of CREB in the hippocampus impaired acquisition and retrieval of spatial LTM in Morris Water Maze task (Viosca et al., 2009b). Also, it has been shown that overexpression of CREB in the nucleus accumbens was shown to decrease natural and drug reward behavior, while reduction in CREB expression enhanced natural and drug reward behavior (Nestler, 2004). These studies demonstrated that the effects of increased CREB are not always associated with facilitation of learning and memory. The results of genetic and pharmacological studies support the conclusion that nNOS inhibition precludes potential gains in learning and memory associated with elevated pCREB.
Unlike chronic inhibition of nNOS, either pharmacologic or genetic, we found that acute inhibition of nNOS by SMTC reduced expression of pCREB and total CREB. Our previous studies demonstrated that the same treatment decreased cGMP by 56% in the amygdala of WT mice (Kelley et al., 2010). This implies that the reduced production of cyclic nucleotides also inhibits downstream CREB phosphorylation (Lu et al., 1999; Puzzo et al.; 2006). In agreement with this is the finding that the NO donor molsidomine (20mg/kg) increased CREB phosphorylation in WT mice (Fig. 6E). The finding that SMTC reduced total CREB expression supports evidence that nNOS and CREB form a positive feedback loop (Sasaki et al., 2000). Under typical conditions, CREB phosphorylation in the basolateral and central nuclei of the amygdala is required for fear-related LTM formation. We posit that inhibition of CREB phosphorylation and transcription by acute administration SMTC during fear conditioning underlies the behavioral impairments observed in fear conditioning (Fig. 1).
Interestingly, the NO donor had opposing effects on amygdalar pCREB expression in WT and nNOS KO mice. Molsidomine enhanced and decreased pCREB expression in WT and nNOS KO mice, respectively. The possibility of NO acting as a negative regulator of pCREB in nNOS KO mice is at odds with the previously described NO-cGMP transduction pathway; however, it may be consistent with the hypothesis that NO negatively regulates pCREB expression (Packer et al., 2003; Zhu et al., 2006). The differences of the two functions of NO may be reconciled by considering the roles and characteristics of the pathways. For learning and memory, NO signaling positively regulates CREB in a phasic manner that is dependent on neuronal activity and the N-methyl-D-aspartate (NMDA) receptor. For neurogenesis, the tone of neural proliferation and CREB activity is negatively regulated by NO through an alternate and currently unknown mechanism. The 47% reduction of pCREB in the amygdala of molsidomine-treated nNOS KO mice suggests that the net effect of the NO donor was inhibition of pCREB (Fig. 7B). The reason for the opposite effects of molsidomine in WT and nNOS KO mice is not known, however the results may be related to the baseline genotypic differences in pCREB expression (Fig. 5E). Regardless of the mechanism, exogenous administration of NO to nNOS KO mice may function to restore (i.e. reduce) aberrant CREB phosphorylation and consequently to aid recovery of optimal contextual fear conditioning.
3.5 Conclusions
The major findings of the present study are: 1) Results of both genetic and pharmacological modulations of NO signaling suggest that the development of visually cued fear conditioning is nNOS-dependent. 2) The physiological (plasma corticosterone) and behavioral (freezing) responses to visual aversive CS correlated with fear learning ability. 3) Modulation of pCREB expression in the amygdala by the NO signaling pathway may be implicated in the consolidation of visual cue-dependent LTM.
Acknowledgements
This work was supported by partially by the Louis Pope Life Fellowship (JBK) and RO1 DA026878 from the National Institute on Drug Abuse, National Institutes of Health, USA (YI)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahi J, Radulovic J, Spiess J. The role of hippocampal signaling cascades in consolidation of fear memory. Behav Brain Res. 2004;149:17–31. doi: 10.1016/s0166-4328(03)00207-9. [DOI] [PubMed] [Google Scholar]
- Arancio O, Kiebler M, Lee CJ, Lev-Ram V, Tsien RY, Kandel ER, Hawkins RD. Nitric oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal neurons. Cell. 1996;87:1025–1035. doi: 10.1016/s0092-8674(00)81797-3. [DOI] [PubMed] [Google Scholar]
- Balda MA, Anderson KL, Itzhak Y. Adolescent and adult responsiveness to the incentive value of cocaine reward in mice: role of neuronal nitric oxide synthase (nNOS) gene. Neuropharmacology. 2006;51:341–349. doi: 10.1016/j.neuropharm.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Bauer EP, LeDoux JE, Nader K. Fear conditioning and LTP in the lateral amygdala are sensitive to the same stimulus contingencies. Nat Neurosci. 2001;4:687–688. doi: 10.1038/89465. [DOI] [PubMed] [Google Scholar]
- Chien WL, Liang KC, Teng CM, Kuo SC, Lee FY, Fu WM. Enhancement of long-term potentiation by a potent nitric oxide-guanylyl cyclase activator, 3-(5-hydroxymethyl-2-furyl)-1-benzyl-indazole. Mol Pharmacol. 2003;63:1322–1328. doi: 10.1124/mol.63.6.1322. [DOI] [PubMed] [Google Scholar]
- Chien WL, Liang KC, Teng CM, Kuo SC, Lee FY, Fu WM. Enhancement of learning behaviour by a potent nitric oxide-guanylate cyclase activator YC-1. Eur J Neurosci. 2005;21:1679–1688. doi: 10.1111/j.1460-9568.2005.03993.x. [DOI] [PubMed] [Google Scholar]
- Contestabile A. Regulation of transcription factors by nitric oxide in neurons and in neural-derived tumor cells. Prog Neurobiol. 2008;84:317–328. doi: 10.1016/j.pneurobio.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Cudeiro J, Rivadulla C. Sight and insight--on the physiological role of nitric oxide in the visual system. Trends Neurosci. 1999;22:109–116. doi: 10.1016/s0166-2236(98)01299-5. [DOI] [PubMed] [Google Scholar]
- Doron NN, Ledoux JE. Organization of projections to the lateral amygdala from auditory and visual areas of the thalamus in the rat. J Comp Neurol. 1999;412:383–409. [PubMed] [Google Scholar]
- Haghikia A, Mergia E, Friebe A, Eysel UT, Koesling D, Mittmann T. Long-term potentiation in the visual cortex requires both nitric oxide receptor guanylyl cyclases. J Neurosci. 2007;27:818–823. doi: 10.1523/JNEUROSCI.4706-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Heldt S, Sundin V, Willott JF, Falls WA. Posttraining lesions of the amygdala interfere with fear-potentiated startle to both visual and auditory conditioned stimuli in C57BL/6J mice. Behav Neurosci. 2000;114:749–759. [PubMed] [Google Scholar]
- Hopper RA, Garthwaite J. Tonic and phasic nitric oxide signals in hippocampal long-term potentiation. J Neurosci. 2006;26:11513–11521. doi: 10.1523/JNEUROSCI.2259-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF, Martin JL, Black MD, Huang PL. Resistance of neuronal nitric oxide synthase-deficient mice to cocaine-induced locomotor sensitization. Psychopharmacology. 1998;140:378–386. doi: 10.1007/s002130050779. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Anderson KL. Memory reconsolidation of cocaine-associated context requires nitric oxide signaling. Synapse. 2007;61:1002–1005. doi: 10.1002/syn.20446. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Roger-Sanchez C, Kelley JB, Anderson KL. Discrimination between cocaine-associated context and cue in a modified conditioned place preference paradigm: role of the nNOS gene in cue conditioning. Int J Neuropsychopharmacol. 2010;13:171–180. doi: 10.1017/S1461145709990666. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Shi C, Carlezon WA, Jr, Neve RL, Nestler EJ, Davis M. Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J Neurosci. 2001;21:2404–2412. doi: 10.1523/JNEUROSCI.21-07-02404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley JB, Anderson KL, Itzhak Y. Pharmacological modulators of nitric oxide signaling and contextual fear conditioning in mice. Psychopharmacology (Berl) 2010;210:65–74. doi: 10.1007/s00213-010-1817-8. [DOI] [PubMed] [Google Scholar]
- Kelley JB, Balda MA, Anderson KL, Itzhak Y. Impairments in fear conditioning in mice lacking the nNOS gene. Learn Mem. 2009;16:371–378. doi: 10.1101/lm.1329209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, Pena de Ortiz S, Kogan JH, Chevere I, Masushige S, Silva AJ. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- Knepper BR, Kurylo DD. Effects of nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester on spatial and cued leaning. Neuroscience. 1998;83:837–841. doi: 10.1016/s0306-4522(97)00457-0. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Blendy JA, Coblentz J, Marowitz Z, Schutz G, Silva AJ. Spaced training induces normal long-term memory in CREB mutant mice. Curr Biol. 1997;7:1–11. doi: 10.1016/s0960-9822(06)00022-4. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Bolivar VJ. Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci Biobehav Rev. 2006;30:1045–1064. doi: 10.1016/j.neubiorev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Lu YF, Kandel ER, Hawkins RD. Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. J Neurosci. 1999;19:10250–10261. doi: 10.1523/JNEUROSCI.19-23-10250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychol (Amst) 2008;127:567–580. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez B, Romero-Grimaldi C, Noval JA, Murillo-Carretero M, Matarredona ER, Estrada C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J Neurosci. 2004;24:85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington D.C: National Academies Press; 1996. [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47 Suppl 1:24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Newton JR, Ellsworth C, Miyakawa T, Tonegawa S, Sur M. Acceleration of visually cued conditioned fear through the auditory pathway. Nat Neurosci. 2004;7:968–973. doi: 10.1038/nn1306. [DOI] [PubMed] [Google Scholar]
- Nucci C, Morrone L, Rombola L, Nistico R, Piccirilli S, Cerulli L. Multifaceted roles of nitric oxide in the lateral geniculate nucleus: from visual signal transduction to neuronal apoptosis. Toxicol Lett. 2003;139:163–173. doi: 10.1016/s0378-4274(02)00430-7. [DOI] [PubMed] [Google Scholar]
- Ota KT, Pierre VJ, Ploski JE, Queen K, Schafe GE. The NO-cGMP-PKG signaling pathway regulates synaptic plasticity and fear memory consolidation in the lateral amygdala via activation of ERK/MAP kinase. Learn Mem. 2008;15:792–805. doi: 10.1101/lm.1114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overeem KA, Ota KT, Monsey MS, Ploski JE, Schafe GE. A role for nitric oxide-driven retrograde signaling in the consolidation of a fear memory. Front Behav Neurosci. 2010;4:2. doi: 10.3389/neuro.08.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer MA, Stasiv Y, Benraiss A, Chmielnicki E, Grinberg A, Westphal H, Goldman SA, Enikolopov G. Nitric oxide negatively regulates mammalian adult neurogenesis. Proc Natl Acad Sci U S A. 2003;100:9566–9571. doi: 10.1073/pnas.1633579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light responses and morphology of bNOS-immunoreactive neurons in the mouse retina. J Comp Neurol. 2010;518:2456–2474. doi: 10.1002/cne.22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin BJ. The mouse brain in stereotaxic coordinates. 2nd ed. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Pham J, Cabrera SM, Sanchis-Segura C, Wood MA. Automated scoring of fear-related behavior using EthoVision software. J Neurosci Methods. 2009;178:323–326. doi: 10.1016/j.jneumeth.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Correa FM, Guimaraes FS. The expression of contextual fear conditioning involves activation of an NMDA receptor-nitric oxide pathway in the medial prefrontal cortex. Cereb Cortex. 2008;18:2027–2035. doi: 10.1093/cercor/bhm232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Alvania RS, Lonze BE, Ramanan N, Kim T, Huang Y, Dawson TM, Snyder SH, Ginty DD. A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Mol Cell. 2006;21:283–294. doi: 10.1016/j.molcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Gonzalez-Zulueta M, Huang H, Herring WJ, Ahn S, Ginty DD, Dawson VL, Dawson TM. Dynamic regulation of neuronal NO synthase transcription by calcium influx through a CREB family transcription factor-dependent mechanism. Proc Natl Acad Sci U S A. 2000;97:8617–8622. doi: 10.1073/pnas.97.15.8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Bauer EP, Rosis S, Farb CR, Rodrigues SM, LeDoux JE. Memory consolidation of Pavlovian fear conditioning requires nitric oxide signaling in the lateral amygdala. Eur J Neurosci. 2005;22:201–211. doi: 10.1111/j.1460-9568.2005.04209.x. [DOI] [PubMed] [Google Scholar]
- Scharf MT, Woo NH, Lattal KM, Young JZ, Nguyen PV, Abel T. Protein synthesis is required for the enhancement of long-term potentiation and long-term memory by spaced training. J Neurophysiol. 2002;87:2770–2777. doi: 10.1152/jn.2002.87.6.2770. [DOI] [PubMed] [Google Scholar]
- Shi C, Davis M. Visual pathways involved in fear conditioning measured with fear-potentiated startle: behavioral and anatomic studies. J Neurosci. 2001;21:9844–9855. doi: 10.1523/JNEUROSCI.21-24-09844.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socodato RE, Magalhaes CR, Paes-de-Carvalho R. Glutamate and nitric oxide modulate ERK and CREB phosphorylation in the avian retina: evidence for direct signaling from neurons to Muller glial cells. J Neurochem. 2009;108:417–429. doi: 10.1111/j.1471-4159.2008.05778.x. [DOI] [PubMed] [Google Scholar]
- Tanda K, Nishi A, Matsuo N, Nakanishi K, Yamasaki N, Sugimoto T, Toyama K, Takao K, Miyakawa T. Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol Brain. 2009;2:19. doi: 10.1186/1756-6606-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin JR, Gorman LK, Baxter MG, Traystman RJ. Nitric oxide synthase inhibition does not impair visual or spatial discrimination learning. Brain Res. 1995;694:177–182. doi: 10.1016/0006-8993(95)00693-k. [DOI] [PubMed] [Google Scholar]
- Viosca J, Lopez de Armentia M, Jancic D, Barco A. Enhanced CREB-dependent gene expression increases the excitability of neurons in the basal amygdala and primes the consolidation of contextual and cued fear memory. Learn Mem. 2009a;16:193–197. doi: 10.1101/lm.1254209. [DOI] [PubMed] [Google Scholar]
- Viosca J, Malleret G, Bourtchouladze R, Benito E, Vronskava S, Kandel ER, Barco A. Chronic enhancement of CREB activity in the hippocampus interferes with the retrieval of spatial information. Learn Mem. 2009b;16:198–209. doi: 10.1101/lm.1220309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TL, Stellitano KE, Neve RL, Duman RS. Effects of cyclic adenosine monophosphate response element binding protein overexpression in the basolateral amygdala on behavioral models of depression and anxiety. Biol Psychiatry. 2004;56:151–160. doi: 10.1016/j.biopsych.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Wang GY, van der List DA, Nemargut JP, Coombs JL, Chalupa LM. The sensitivity of light-evoked responses of retinal ganglion cells is decreased in nitric oxide synthase gene knockout mice. J Vis. 2007;7:1–13. doi: 10.1167/7.14.7. 7. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26:5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, Darby-King A, Neve RL, McLean JH. Early odor preference learning in the rat: bidirectional effects of cAMP response element-binding protein (CREB) and mutant CREB support a causal role for phosphorylated CREB. J Neurosci. 2003;23:4760–4765. doi: 10.1523/JNEUROSCI.23-11-04760.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XJ, Hua Y, Jiang J, Zhou QG, Luo CX, Han X, Lu YM, Zhu DY. Neuronal nitric oxide synthase-derived nitric oxide inhibits neurogenesis in the adult dentate gyrus by down-regulating cyclic AMP response element binding protein phosphorylation. Neuroscience. 2006;141:827–836. doi: 10.1016/j.neuroscience.2006.04.032. [DOI] [PubMed] [Google Scholar]