SUMMARY
We found that the receptor for erythropoietin (EpoR) is coexpressed with human epidermal growth factor receptor-2 (HER2) in a significant percentage of human breast tumor specimens and breast cancer cell lines. Exposure of HER2 and EpoR dual-positive breast cancer cells to recombinant human erythropoietin (rHuEPO) activated cell signaling. Concurrent treatment of the cells with rHuEPO and trastuzumab reduced the cells’ response to trastuzumab both in vitro and in vivo. We identified Jak2-mediated activation of Src and inactivation of PTEN as underlying mechanisms through which rHuEPO antagonizes trastuzumab-induced therapeutic effects. Furthermore, we found that compared with administration of trastuzumab alone, concurrent administration of rHuEPO and trastuzumab correlated with shorter progression-free and overall survival in patients with HER2-positive metastatic breast cancer.
Keywords: Breast cancer, rHuEPO, Trastuzumab, HER2, Jak2, Src, PTEN
INTRODUCTION
Erythropoietin (EPO) has long been known to be an important hematopoietic cytokine that regulates the survival, proliferation, and differentiation of the erythroid progenitor cells in the bone marrow (Krantz, 1991; Jelkmann, 1992). Recombinant human EPO (rHuEPO) has frequently been used in the treatment of cancer-related and chemotherapy-induced anemia and fatigue since the 1990s (Henry and Abels, 1994). Recent studies, however, have suggested that EPO, which was once thought to act solely on the erythroid compartment, is a pleiotropic cytokine (Lappin et al., 2002). A 2003 trial to investigate the effect of rHuEPO for the prevention of anemia on the survival of nonanemic patients with metastatic breast cancer was terminated earlier than planned because of a higher-than-expected mortality rate among patients in the group treated with epoetin alfa (Leyland-Jones, 2003). Another study in anemic patients with head and neck cancer showed that although epoetin beta was successful in correcting anemia, it failed to improve, and might even have impaired, cancer control and survival (Henke et al., 2003). Recently, two meta-analyses were reported of 53 and 52 published randomized trials, respectively, in which rHuEPO (epoetin alfa, epoetin beta, or darbepoetin alfa) was used for prophylaxis or treatment of anemia in patients with cancer (Bohlius et al., 2009; Tonelli et al., 2009). The authors concluded that overall survival was worse in patients treated with rHuEPO than in patients treated with placebo control. It should be noted that the increased mortality associated with EPO treatment was attributed mainly to an increase in adverse events (e.g., thromboembolic and cardiac complications) and not necessarily to a lower efficacy of chemotherapy, radiotherapy, or radiochemotherapy.
The functions of EPO are mediated by its specific cell-surface receptor, EpoR, which is now known to be found not only in erythroid progenitor cells but also in multiple types of normal and cancerous tissues [for a list, see (Hardee et al., 2006)]. In hematopoietic cells, EPO induces homodimerization of EpoR (Watowich et al., 1992), triggering activation of the receptor-associated kinase Jak2 and activation of STAT5 (Witthuhn et al., 1993). Adaptor proteins containing the Src homology 2 domain, such as Grb2 and Shc (Damen et al., 1993a; Liu et al., 1994), transduce EPO-induced cell signaling via interaction with specific tyrosine-phosphorylated regions within the activated EpoR, leading to activation of downstream signaling pathways, such as the MEK/Erk and PI3K/Akt pathways (Damen et al., 1993b; 1995; He et al., 1993; Miura et al., 1994).
These downstream signaling pathways activated by EPO via EpoR overlap substantially or interact with those activated by human epidermal growth factor receptor (HER)-2 (HER2), a member of the HER family, which is overexpressed in approximately 25% of breast cancers (Slamon et al., 1987). An anti-HER2 antibody, trastuzumab, is approved for use in combination with a taxane for HER2-overexpressing metastatic breast cancer (Slamon et al., 2001) and for use as adjuvant therapy in women with early-stage breast cancer to reduce the risk of cancer recurrence and/or metastasis after surgery or radiotherapy (Romond et al., 2005). However, clinical resistance to trastuzumab remains a challenging problem—only one third of patients with HER2-positive breast cancer, who would be expected to benefit from trastuzumab, actually respond to the treatment (Hortobagyi, 2005; Esteva et al., 2010). Recent studies have shown a relationship between poor response to trastuzumab and low PTEN levels (Nagata et al., 2004) or PIK3CA activating mutations (Berns et al., 2007). Mutationally activated PI3K can activate critical downstream targets, such as Akt, independently of HER2, thereby allowing cells to escape the effect of trastuzumab, which is believed to function in part through disruption of HER2/HER3/PI3K complexes (Junttila et al., 2009). However, low PTEN levels and PIK3CA activating mutations are not the only reason for trastuzumab resistance, as resistance to trastuzumab is also seen in patients whose tumors have normal PTEN and PIK3CA (Nagata et al., 2004; Berns et al., 2007).
It is currently unknown whether HER2 and EpoR are coexpressed in the same breast cancer cells. We hypothesized that, if HER2 and EpoR are coexpressed in the same breast cancer cells and patients are treated concurrently with rHuEPO and trastuzumab, rHuEPO may have antagonistic effects on trastuzumab-induced antitumor activity in HER2-positive breast cancer cells. In this article, we report our findings from testing this hypothesis.
RESULTS
HER2 and EpoR are Coexpressed in a Significant Proportion of Breast Cancer Cell Lines and Tumor Tissues
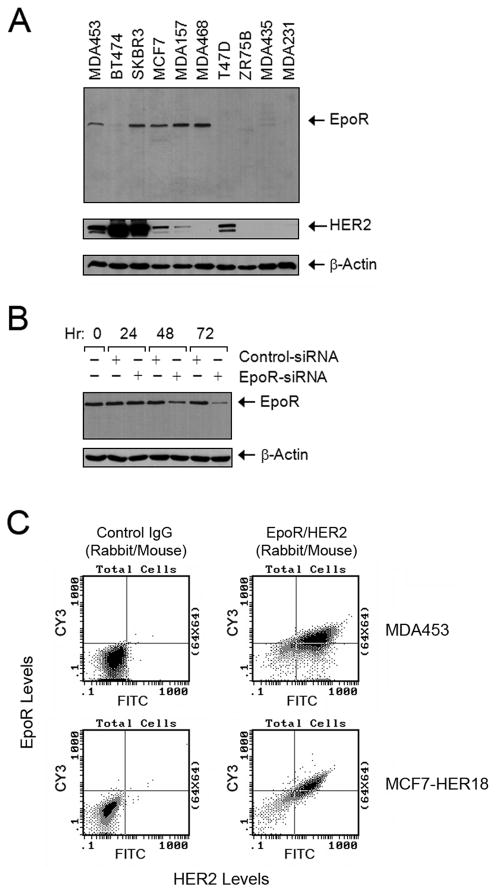
We analyzed the expression of HER2 and EpoR by Western blot analysis in a panel of 10 breast cancer cell lines. EpoR was readily detected in five of them: MDA453, SKBR3, MCF7, MDA157, and MDA468 (Figure 1A). Of these five cell lines, four also expressed HER2: SKBR3 expressed high levels of both HER2 and EpoR; MDA453 expressed intermediate levels of both receptors; and MCF7 and MDA157 expressed high levels of EpoR but relatively low levels of HER2. MDA468 expressed high levels of EpoR but no HER2.
Figure 1. Coexpression of HER2 and EpoR in Human Breast Cancer Cell Lines.
(A) Expression of HER2 and EpoR in human breast cancer cell lines. Exponentially proliferating breast cancer cells of the indicated lines were harvested by trypsinization. Equal amounts of cell lysates were subjected to Western blot analysis with specific antibodies directed against EpoR and HER2. The level of β-actin served as a protein-loading control.
(B) Expression knockdown of EpoR by RNAi. MCF7 cells were subjected to EpoR expression knockdown with EpoR-specific Dharmacon SMARTpool siRNA or control siRNA for the indicated durations. Cell lysates were prepared, and the levels of EpoR were measured by Western blot analysis with EpoR-specific antibody. The level of β-actin served as a protein-loading control.
(C) Coexpression of HER2 and EpoR. MDA453 and MCF7-HER18 cells were subjected to double-immunofluorescent staining with primary antibodies directed against EpoR (rabbit IgG) and HER2 (mouse IgG), followed by incubation with FITC-labeled goat anti-mouse IgG antibody and Cy3-labeled goat anti-rabbit IgG antibody. The cell suspensions were then analyzed by flow cytometry. See also Figure S1.
To ensure the specificity of the EpoR antibody used for the Western blotting, we knocked down EpoR expression in MCF7 cells by RNA interference (RNAi) using small interfering RNA (siRNA) and found that Western blot analysis with the antibody clearly demonstrated a decrease in EpoR expression level in the knockdown cells compared with the level in control siRNA– treated cells (Figure 1B). To further determine the identity of EpoR recognized by the antibody, we used a series of U6 promoter-driven pRS vectors constructed with short hairpin RNA (shRNA) complementary to various regions of the EpoR sequence (Figure S1A). We found that the antibody detected knockdown of EpoR expression in cells transfected with any of the EpoR shRNA vectors (E1–E6) but not in cells transfected with the backbone vector or a control vector constructed with a shRNA against green fluorescent protein (GFP). Because of the broad range of targeted sequences, it is virtually impossible that the expression-silenced protein was not EpoR. Therefore, we concluded that the antibody specifically detected EpoR expression in a fraction of the breast cancer cell lines in our study.
To further confirm coexpression of HER2 and EpoR, we conducted flow cytometric studies of cells double-immunofluorescently stained for HER2 and EpoR. Figure 1C shows the flow cytometric results for MDA453 cells, which have naturally occurring coexpression of HER2 and EpoR. We found that the majority of MDA453 cells were positive for both HER2 and EpoR, a result similar to that found in MCF7-HER18 cells, which have high levels of endogenous EpoR and were transfected for overexpression of exogenous HER2. A similar flow cytometric study of cells double-immunofluorescently stained for HER2 and EpoR showed that SKBR3 cells had a population of cells highly expressing both HER2 and EpoR, whereas BT474 cells only expressed a high level of HER2 (Figure S1B).
We next examined HER2 and EpoR expression in breast cancer tissue specimens from patients. First, we optimized immunohistochemical staining for EpoR in slides from paraffin-embedded cell blocks prepared identically to conventional tissue blocks. We confirmed that the EpoR antibody detected differences in EpoR levels between MCF7-HER18 cells with and without siRNA knockdown of EpoR expression (Figure S2). We then examined HER2 and EpoR expression in 55 paraffin-embedded breast cancer tissue sections, including 30 whole-tissue sections and 25 specimens assembled in a breast cancer tissue microarray. Overall, 13 of 15 HER2-positive samples and 33 of 40 HER2-negative samples had various degrees of positive staining for EpoR. The photomicrographs in Figure 2 were taken from two adjacent tissue sections from a paraffin-embedded tissue block. The pattern of HER2 positivity overlapped with the pattern of EpoR positivity, suggesting that this breast cancer sample coexpressed HER2 and EpoR. The table under Figure 2 summarizes the findings of our case series study. Overall EpoR positivity rates were similar in HER2-positive and HER2-negative cases. For all 55 breast cancer tissue sections, the rate of EpoR positivity, from weakly positive (+) to strongly positive (+++), was 83.6% (46 of 55 cases).
Figure 2. Expression of HER2 and EpoR in Human Breast Cancer Tissues from Patients.
Two adjacent tissue slides from a whole-tissue paraffin block of a breast cancer specimen were stained immunohistochemically for the expressions of HER2 (left panel, stained brown with diaminobenzidine tetrahydrochloride) and EpoR (right panel, stained red with 3-aminoethyl-carbazole). The nuclei were counterstained with hematoxylin. Selected fields under low-power magnification (scale bar = 400 μm) were then viewed under high-power magnification (scale bar = 50 μm) and had the same pattern of HER2 and EpoR expression in ductal carcinoma in situ and in invasive cancer. The table summarizes findings from all 55 cases examined. See also Figure S2.
rHuEPO Activates Cell Signaling in Breast Cancer Cells Expressing EpoR
To determine whether the EpoR expressed in breast cancer cells is functional, we exposed three breast cancer cell lines with moderate or high expression of EpoR—MDA453, SKBR3, and MCF7—to rHuEPO. We found that rHuEPO activated cell signaling in all three cell lines, as measured by Western blot analysis detecting activation-specific phosphorylation of Akt, Erk, and STAT5 (Figure 3A). An increase in S473-phosphorylated Akt was found in all three cell lines 30 minutes after rHuEPO stimulation, and this increase was most prominent in MCF7 cells. Stimulation with rHuEPO also increased Erk phosphorylation, particularly in SKBR3 cells. An increase in STAT5 phosphorylation seemed to be cell type–specific—it was found only in SKBR3 cells. Knockdown of EpoR in MCF7 cells remarkably abolished the effect of rHuEPO on the cells, indicating that the observed activation of cell signaling by rHuEPO was mediated specifically via the EpoR expressed in the cells (Figure 3B). Figure 3C shows the effect of rHuEPO in additional EpoR-positive and EpoR-undetectable breast cancer cells. rHuEPO activated cell signaling in MDA468 cells (EpoR-positive/HER2-negative), but not in BT474 cells (expressing high HER2) or T47D cells (expressing low HER2), in which EpoR was not detectable (Figure 1A). It is noteworthy that, although MDA468 cells are known to be PTEN-deficient (Lu et al., 2003), stimulation of the cells with rHuEPO still led to a mild increase in Akt phosphorylation, strong increase in Erk phosphorylation, but no increase in STAT5 phosphorylation. Together, these data indicate that rHuEPO activated cell signaling (mainly the Akt and Erk pathways) directly via the EpoR expressed in breast cancer cells.
Figure 3. Activation of Cell Signaling by rHuEPO in EpoR-positive but Not in EpoR-undetectable Breast Cancer Cells.
(A) Activation of cell signaling by rHuEPO in human breast cancer cell lines. The indicated cells were left untreated or were treated with 10 U/ml rHuEPO for 30 or 120 minutes in low-serum medium. Cell lysates were prepared, and equal amounts of cell lysates were subjected to Western blot analysis with antibodies directed against total and activation-specific phosphorylated Akt, Erk, and STAT5. The ratios represent quantitative analysis of densitometric values of specific band intensities normalized to the value of the corresponding untreated controls, which was arbitrarily set at 1. The level of β-actin served as a protein-loading control.
(B) Dependence of rHuEPO-induced activation of cell signaling on EpoR expression. MCF7 cells were transfected for 72 hours with a control vector or one of two shRNA constructs targeting different regions of EpoR. The cells were stimulated with 10 U/ml rHuEPO for 30 minutes and immediately lysed for Western blot analysis with the indicated antibodies.
(C) Effect of rHuEPO on cell signaling in additional breast cancer cell lines. The indicated cell lines were treated and analyzed as described in (A).
rHuEPO Protects against Trastuzumab in HER2/EpoR Dual-positive Breast Cancer Cells
We next tested our hypothesis that cell signaling in response to concurrent administration of rHuEPO constitutes a mechanism of resistance to trastuzumab in HER2 and EpoR dual-positive breast cancer cells. Trastuzumab inhibited Akt phosphorylation and moderately inhibited Erk phosphorylation in breast cancer cell lines with naturally occurring intermediate to high levels of HER2 (MDA453 and SKBR3) or experimentally elevated levels of HER2 (MCF7-HER18) after overnight treatment (Figure 4A). In contrast, rHuEPO increased Akt and Erk phosphorylation in all three cell lines. Concurrent exposure of the three cell lines to rHuEPO and trastuzumab reduced trastuzumab’s inhibitory effect on Akt and Erk phosphorylation.
Figure 4. Antagonism by rHuEPO of Trastuzumab-Induced Inhibition of Cell Signaling and Antitumor Activities In Vitro.
(A) Antagonism by rHuEPO of trastuzumab-induced inhibition of cell signaling. The indicated cell lines were left untreated or were treated with 20 nM trastuzumab, 10 U/ml rHuEPO, or both in low-serum medium overnight (16 hours). Cell lysates were prepared for Western blot analysis with the indicated antibodies. The ratios represent quantitative analysis of densitometric values of specific band intensities normalized to the value of the corresponding untreated controls, which was arbitrarily set at 1.
(B) Antagonism by rHuEPO of trastuzumab-induced inhibition of cell growth. The indicated cell lines were treated as described in (A) for 5 days, and then an MTT colorimetric assay was performed to quantify relative survival and proliferation. The optical density value in each group of cells was directly plotted against the types of treatment.
(C) Antagonism by rHuEPO of trastuzumab-induced inhibition of cell invasion and motility. The indicated cell lines were used in a Boyden transwell chamber assay (Becton Dickinson, Franklin Lakes, NJ) with the membrane coated with Matrigel. The cells were left untreated or treated with 20 nM trastuzumab, 10 U/ml rHuEPO, or both for 24 hours, and then the cells that had penetrated through the membrane were stained and counted using an inverted microscope equipped with a ×10 objective. The data are shown as the average number of cells per field. All scale bars represent 80 μm.
(D) Antagonism by rHuEPO of trastuzumab-induced inhibition of cell clonogenic formation. MCF7-HER18 cells (500 cells per 60-mm dish) were exposed to trastuzumab in the presence or absence of 10 U/ml rHuEPO for 3 weeks. Numbers of cell colonies in trastuzumab-treated groups with or without current exposure to rHuEPO were normalized to the number of colonies in the control group (without any treatment).
Furthermore, after 5 days of culture, trastuzumab inhibited cell survival and proliferation in all three cell lines (Figure 4B), and rHuEPO stimulated cell survival and proliferation, with the effect ranging from prominent in MDA453 cells to marginal in MCF7-HER18 cells. We found that rHuEPO significantly antagonized trastuzumab-induced inhibition of cell growth: when rHuEPO was added to trastuzumab, the percentage of surviving cells increased from 58.2% to 80.4% in MDA453 cells, from 57.2% to 77.7% in SKBR3 cells, and from 34.0% to 57.5% in MCF7-HER18 cells.
In addition, rHuEPO significantly counteracted trastuzumab-induced inhibition of cell migration and invasion, measured in SKBR3 and MCF7-HER18 cells using Matrigel-coated transwell chamber assays (Figure 4C). [MDA453 cells had very low baseline levels of cell migration and invasion (data not shown).] rHuEPO also markedly decreased trastuzumab-induced inhibition of clonogenic survival, as shown by statistically significant differences in the number and size of surviving colonies between the trastuzumab-treated MCF7-HER18 cells with and without cotreatment with rHuEPO (Figure 4D). [MDA453 and SKBR3 cells did not form clearly individual colonies (data not shown).]
rHuEPO Counteracts Trastuzumab-Induced Inhibition of Breast Cancer Xenograft Growth
To determine whether concurrent administration of rHuEPO with trastuzumab would affect the therapeutic effect of trastuzumab in vivo, we used two cell lines: an MDA453 derivative (MDA453β) that we previously showed can produce tumor xenografts in immunodeficient mice compared with parental MDA453 cells that cannot (Hu et al., 2004), and a bioluminescent subline of MCF7-HER18 cells (MCF7-HER18/Fluc-GFP) that not only can grow in nude mice but also can be tracked using an in vivo imaging system to monitor tumor growth and metastasis potential. We used SCID mice for growing MDA453β xenografts (they grow more robustly in SCID mice than in nude mice) and used nude mice for growing MCF7-HER18/Fluc-GFP xenografts. On day 26 after inoculation of MDA453β cells and day 20 after inoculation of MCF7-HER18/Fluc-GFP cells into the mammary fat pads of the mice, the SCID mice that had developed tumors were divided into 4 groups (5 mice each) with similar average tumor volume, and the nude mice that had developed tumors were also divided into 4 groups (10 mice each) with similar average tumor volume.
To ensure that the data from our animal study would be closely related to clinical scenarios, we used epoetin alfa (Procrit), a prescription form of rHuEPO that was proven to activate cell signaling in our cell culture studies (data not shown). The mice were treated with epoetin alfa and trastuzumab alone and in combination. Phosphate-buffered saline was used as a control. The sizes of tumors in each group were measured with calipers and plotted as the average tumor volumes ± standard deviation for each group versus time. Whereas the xenografts in the control and epoetin alfa–treated groups grew robustly, the xenografts in the mice treated with trastuzumab alone shrank or stopped growing, and concurrent exposure of the mice to epoetin alfa and trastuzumab resulted in a reduced therapeutic response to treatment (Figure 5). These data were consistent with the findings from our cell culture study (Figure 4), but the difference in tumor volume between trastuzumab-alone-treated and trastuzumab-plus-rHuEPO-treated mice after 4 weeks of treatment was more striking than the difference in the proportion of surviving cells between trastuzumab-alone-treated and trastuzumab-plus-rHuEPO-treated cells after 5-day culture.
Figure 5. Antagonism by rHuEPO of Trastuzumab-Induced Inhibition of Breast Cancer Xenograft Growth in Mouse Mammary Fat Pads.
Starting on day 26 after inoculation of MDA453β cells into the mammary fat pads of female ICR SCID mice (4–6 weeks old, 5 mice/group) (A) and starting on day 20 after inoculation of MCF7-HER18/Fluc-GFP cells into the mammary fat pads of female Swiss nude mice (4–6 weeks old, 10 mice/group) (B), the mice were treated with PBS (control), trastuzumab (0.5 mg/mouse twice a week), epoetin alfa (100 U/mouse daily on weekdays), or trastuzumab plus epoetin alfa (same doses and schedules as each treatment alone) for 4 weeks or until mice were sacrificed, whichever came first. Tumor sizes were measured with a digital caliper every other day and plotted as a function of days after tumor cell inoculation. See also Figure S3.
MCF7-HER18 cells do not metastasize when they grow in the mammary fat pads of nude mice. Administration of rHuEPO did not seem to make these cells metastatic in our study, either at 2 weeks after the treatment (by bioluminescent imaging of MCF7-HER18/Fluc-GFP) or at the point when the mice had to be sacrificed. Figure S3A illustrated the protective effect of rHuEPO against trastuzumab-mediated antitumor activity by bioluminescent imaging of the tumors 2 weeks after the initiation of treatment. Because most tumors in the trastuzumab-alone-treated group disappeared after extended treatment, we examined markers of cell proliferation (PCNA, Figure S3B), angiogenesis (CD31, Figure S3C), apoptosis (TUNEL, Figure S3D), and cell signaling (Figure S3E) by immunohistochemical staining of tumor specimens of MCF7-HER18/Fluc-GFP xenografts obtained only at the 2-week mark. The differences in the levels of these markers between trastuzumab-alone-treated and trastuzumab-plus-rHuEPO-treated mice at the 2-week mark were similar to the differences in cell growth in vitro (Figure 4) and tumor volumes in vivo at the 2-week mark (Figure 5). It seems that rHuEPO had an effect on angiogenesis (Figure S3C), which is consistent with reports in the literature (Hardee et al., 2007; Okazaki et al., 2008).
rHuEPO Activates Cell Signaling via Promoting Associations between Src and EpoR/Jak2 and between Src and HER2 in HER2/EpoR Dual-Positive Breast Cancer Cells
To elucidate the mechanisms by which rHuEPO rescues HER2 and EpoR dual-positive breast cancer cells from trastuzumab treatment, we first examined the dependence of rHuEPO-induced effects in the breast cancer cells on Jak2 expression and activity, which are known to be required for EPO- and rHuEPO-mediated effects in hematopoietic cells (Witthuhn et al., 1993). We found that AG490, a Jak2 inhibitor, markedly inhibited rHuEPO-induced activation of Jak2, Akt, and Erk in MCF7-HER18 cells (Figure 6A). Similar results were found in cells in which Jak2 expression was silenced by RNAi (Figure 6B), indicating an essential role of Jak2 in mediating rHuEPO-induced signaling in the breast cancer cells.
Figure 6. Roles of Jak2 and Src in Mediating rHuEPO-Induced Cell Signaling in HER2 and EpoR Dual-positive Breast Cancer Cells.
(A and B) Dependence of rHuEPO-induced activation of cell signaling on Jak2 activity and expression. MCF7-HER18 cells were pre-exposed to 50 μM AG490 or DMSO in low-serum medium overnight (A) or subjected to expression knockdown of Jak2 by transient transfection with one of two different Jak2 shRNA constructs or control vector for 72 hours (B). The cells were then stimulated with 10 U/ml rHuEPO for 30 minutes, followed by cell lysis and Western blot analysis with the indicated antibodies.
(C) Increased associations between Src and Jak2 and between Src and HER2 after rHuEPO stimulation. MCF7-HER18 and MDA453β cells were treated or untreated with 10 U/ml rHuEPO for 30 minutes or not. Cell lysates were subjected to immunoprecipitation with a Src antibody or mock antibody, followed by Western blot analysis with antibodies direct against Jak2 and HER2. (D and E) Role of Jak2 in rHuEPO-induced associations between Src and EpoR and between Src and HER2, and in rHuEPO-induced Src activation. MCF7-HER18 cells were transiently transfected with Jak2 shRNA constructs or control vector as described in (B) and then stimulated with 10 U/ml rHuEPO for 30 minutes. Src-immunoprecipitates (D) and whole cell lysates (E) were subjected to Western blot analysis with the indicated antibodies. See also Figure S4.
Because Src is known to be activated by association with HER2 in HER2-overexpressing cells (Belsches-Jablonski et al., 2001), we hypothesized that Src mediates rHuEPO-induced resistance against trastuzumab by acting as a bridge between the HER2 and EpoR/Jak2 pathways. To address this hypothesis, we examined the levels of HER2 and Jak2 coimmunoprecipitated with Src by a Src antibody after rHuEPO stimulation. We found that there was an increase in protein associations between Src and HER2 and between Src and Jak2 after rHuEPO stimulation in both MCF7-HER18 and MDA453β cells (Figure 6C). Furthermore, we detected an enhanced association between Src and EpoR after rHuEPO stimulation; both the association between Src and HER2 and the association between Src and EpoR were reduced when the expression of Jak2 was knocked down (Figure 6D). Figure 6E shows that Src was robustly activated upon rHuEPO stimulation in a Jak2-dependent manner. Together, these data indicate that Src is activated by rHuEPO stimulation via association with EpoR/Jak2 and plays an important role in the interaction between EpoR and HER2. Because Src is associated with both HER2 and Jak2, and because Jak2 is associated with EpoR, HER2 and EpoR may co-exist in the complex; however, we were unable to detect direct association between EpoR and HER2 in our experiments.
A recent study showed that trastuzumab reduces Akt phosphorylation by disrupting HER2/HER3/PI3Kp85 complex, resulting in dissociation of PI3Kp85 from HER3 (Junttila et al., 2009). We therefore examined whether rHuEPO has any effects in stimulating interactions between HER2, HER3, and PI3Kp85. We found basal associations between HER2 and HER3, HER2 and PI3Kp85, and HER3 and PI3Kp85 in both MCF7-HER18 and MDA453β cells by immunoblotting HER2 immunoprecipitates with anti-HER3 and PI3Kp85 antibodies and immunoblotting HER3 immunoprecipitates with anti-HER2 and PI3Kp85 antibodies; however, we did not find notable changes in associations between HER2 and HER3, HER2 and PI3Kp85, and HER3 and PI3Kp85 in MDA453β or MCF7-HER18 cells after rHuEPO treatment (Figure S4). These data indicate that rHuEPO does not stimulate HER2, HER3, and PI3Kp85 interaction in the cell models used in our studies.
Src Activation and PTEN Inactivation Contribute to rHuEPO-Mediated Resistance to Trastuzumab Treatment
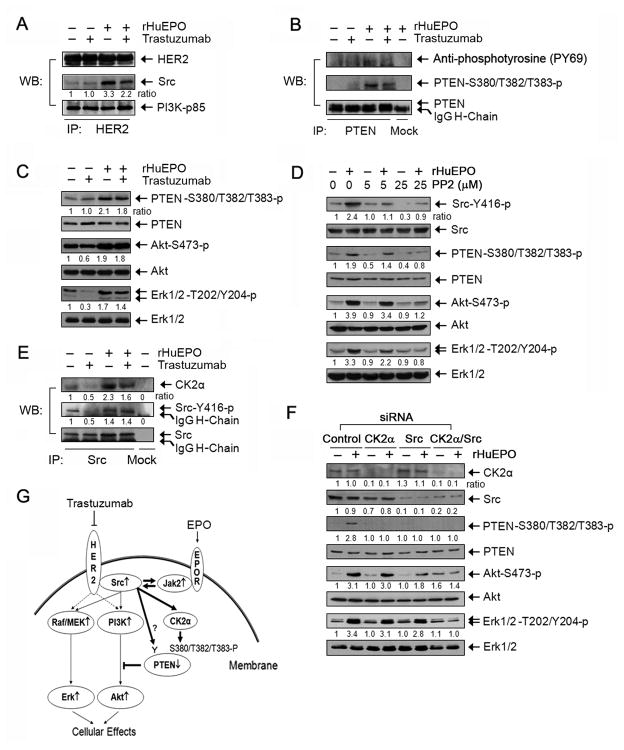
It was previously shown that trastuzumab reduced association between Src and HER2 in BT474 cells and that this effect of trastuzumab is important for trastuzumab-induced therapeutic effects (Nagata et al., 2004). In the present study, we confirmed this phenomenon in BT474 cells but found that trastuzumab did not reduce the association between Src and HER2 in MCF7-HER18 or MDA453β cells, suggesting that the reduction in association between Src and HER2 after trastuzumab treatment is cell type-specific (Figure S5). However, rHuEPO stimulated association between Src and HER2 in these cells, and this increased association remained, albeit modestly less, when cells were treated concurrently with rHuEPO and trastuzumab, suggesting that rHuEPO can confer resistance to trastuzumab in MCF7-HER18 and MDA453β cells through enhancing the association between Src and HER2 (Figure 7A).
Figure 7. Roles of Src Activation and PTEN Inactivation in Conferring Resistance to Trastuzumab by rHuEPO.
(A) Effects of rHuEPO and trastuzumab on association between Src and HER2. MCF7-HER18 cells were cultured in the presence or absence of 20 nM trastuzumab overnight in low serum medium and then stimulated with 10 U/ml rHuEPO or not for 30 minutes. The cell lysates were subjected to immunoprecipitation of HER2 with an anti-HER2 antibody, followed by Western blot analysis with the indicated antibodies.
(B) Phosphorylation of PTEN upon rHuEPO stimulation. MCF7-HER18 cells were treated as described in (A). Cell lysates were prepared and subjected to immunoprecipitation for PTEN, followed by Western blot analysis with the indicated antibodies.
(C) Correlation of rHuEPO-induced phosphorylation of PTEN with its antagonizing effects on trastuzumab-mediated inhibition of cell signaling. MCF7-HER18 cells were treated as described in (A). Cell lysates were subjected to Western blot analysis with the indicated antibodies.
(D) Dependence of rHuEPO-induced cell signaling on Src activity. MCF7-HER18 cells were left untreated or treated with 5 μM or 25 μM PP2 (Src inhibitor) in 0.5% FBS medium overnight and then stimulated with 10 U/ml rHuEPO or not for 30 minutes, followed by cell lysis and Western blot analysis with the indicated antibodies.
(E) Effects of rHuEPO and trastuzumab on Src and CK2α association. MCF7-HER18 cells were treated as described in (A). Cell lysates were prepared and subjected to immunoprecipitation of Src, followed by Western blot analysis of the immunoprecipitates with the indicated antibodies.
(F) Roles of Src and CK2α in rHuEPO-mediated phosphorylation of Akt and Erk. MCF7-HER18 cells were transfected with siRNA for expression knockdown of CK2α, Src, or CK2α and Src, as indicated. After 72 hours, the cells were stimulated with 10 U/ml rHuEPO or not for 30 minutes, followed by cell lysis and Western blot analysis with the indicated antibodies.
(G) Schematic of our working model. Trastuzumab binds to HER2 expressed by breast cancer cells, preventing activation of HER2-mediated cell signaling (dashed lines). rHuEPO binds to EpoR expressed by the same breast cancer cells, leading to activation of EpoR-associated protein tyrosine kinase Jak2 and subsequent activation of Src and inactivation of PTEN. The thick arrows indicate the pathways identified in the current study. The thick arrow with a question mark indicates a need for further confirmation. All of the experiments (A–F) were repeated at least once with similar findings. See also Figure S5.
An increased association between Src and HER2 can potentiate HER2 downstream pathways through activation of Ras/Raf/MEK and PI3K, thereby conferring resistance to trastuzumab. To explore additional mechanisms by which activation of Src following rHuEPO stimulation protects breast cancer cells from trastuzumab treatment, we examined whether PTEN, which counteracts the effect of PI3K and the function of which can be inactivated by Src through phosphorylation on tyrosine (Lu et al., 2003) and indirectly on serine/threonine at the carboxyl terminal (S380/T382/T383) of PTEN [the latter phosphorylation leads to increased stability of PTEN but loss of its functions (Vazquez et al., 2000)], plays a role in rHuEPO-mediated resistance to trastuzumab. Figure 7B shows that rHuEPO induced a moderate increase in tyrosine phosphorylation of PTEN, but the increase in phosphorylation was more convincing on the triple residues (S380/T382/T383). Figure 7C further shows that the level of phosphorylation of the triple residues correlated well with the effects of rHuEPO on protecting MCF7-HER18 cells from trastuzumab-induced inhibition of Akt and Erk phosphorylation.
To confirm the role of Src in rHuEPO-mediated PTEN phosphorylation on the triple residues, we treated the cells with PP2, a small-molecule inhibitor of Src, and found that PP2 clearly decreased rHuEPO-mediated PTEN phosphorylation as well as Akt and Erk phosphorylation in a PP2-dose-dependent manner (Figure 7D). Phosphorylation of PTEN on the triple residues is catalyzed directly by a threonine/serine kinase called casein kinase 2 alpha (CK2α), which is a known substrate of Src (Vazquez et al., 2000). We thus further examined association between Src and CK2α in the cells treated with trastuzumab and rHuEPO, alone and together. Although trastuzumab did not reduce the association between Src and HER2 (Figure 7A), there were a reduced association between Src and CK2α and decrease in the level of activation-specific phosphorylation of Src in trastuzumab-treated cells compared with untreated cells. The levels of Src/CK2α association and Src phosphorylation were markedly increased by rHuEPO treatment, which prevented trastuzumab-mediated decrease in the levels of Src/CK2α association and Src phosphorylation (Figure 7E).
To further confirm the roles of Src and CK2α in mediating rHuEPO-induced activation of Akt and Erk, we knocked down the expression of Src and CK2α alone and together. Figure 7F shows that knockdown of either Src or CK2α markedly decreased rHuEPO-mediated PTEN phosphorylation on S380/T382/T383, but decrease of the accompanying Akt phosphorylation was more prominent with Src knockdown alone than with CK2α knockdown alone. The level of rHuEPO-induced Erk phosphorylation was less affected than the level of Akt phosphorylation. Dual knockdown of Src and CK2α resulted in a slight rebound in basal Akt phosphorylation level, but it completely abolished rHuEPO-induced increase in Akt and Erk phosphorylation (Figure 7F). Together, these results indicate that both Src activation and Src activation-induced PTEN inactivation are involved in rHuEPO-mediated antagonism of trastuzumab treatment. On the basis of these data, we propose the model in Figure 7G for the pathway through which rHuEPO antagonizes trastuzumab in HER2-overexpressing breast cancer cells.
Concurrent Treatment with rHuEPO Correlates with Poor Progression-Free Survival and Overall Survival in Patients Treated with Trastuzumab
We conducted a retrospective case-control study to determine the impact of concurrent rHuEPO and trastuzumab on progression-free survival (PFS) and overall survival (OS) in patients with HER2-overexpressing metastatic breast cancer. The Department of Pharmacy Informatics identified 1,941 women with breast cancer treated with rHuEPO (epoetin alfa or darbepoetin) at MD Anderson from December 1998 to February 2006. Complete treatment data and outcomes were available for 1,482 patients in our Breast Cancer Management System database. Two hundred seventy-three of these patients had received trastuzumab for breast cancer. Because of the retrospective nature of the analysis and to reduce potential selection bias, we used specific criteria to select patients treated with trastuzumab (± chemotherapy) and concurrent rHuEPO (rHuEPO group) and similar patients treated with trastuzumab (± chemotherapy) without rHuEPO (control group). The rHuEPO group included patients who had received trastuzumab as first-, second-, or third-line treatment for metastatic breast cancer and who had not received prior trastuzumab in the adjuvant or metastatic settings. Because the longer the PFS, the higher the probability that a patient would receive rHuEPO, only patients for whom rHuEPO was started within 40 days from the beginning of trastuzumab were included in the rHuEPO group. Inclusion criteria for the control group were the same as inclusion criteria for the rHuEPO group except that patients in the control group received trastuzumab (± chemotherapy) without rHuEPO.
Thirty-seven patients met the criteria for the rHuEPO group. To serve as a control group, 74 patients (1:2 ratio of case-control matching to enhance the power of comparison) were identified from the Breast Cancer Management System database. Because the number of patients eligible as controls exceeded the number of patients required for the matching process, a random selection of the patients was applied. Age, hormone receptor status, type of chemotherapy used in combination with trastuzumab, nuclear grade, prior adjuvant or neoadjuvant chemotherapy, and sites of metastatic disease were well balanced between the rHuEPO and control groups (Supplemental Table 1). Twenty-four of 37 patients (64.9%) received rHuEPO concurrently with trastuzumab for more than 50% of the duration of trastuzumab administration. The PFS curves for the rHuEPO and control groups are shown in Figure 8A. The PFS curves separate after 6 months, and the difference becomes statistically significant at 1 year, when the PFS rates were 40% (95% confidence interval [CI], 31–53%) in the control group and 19% (95% CI, 10–37%) in the rHuEPO group (chi-squared test p=0.039), although the overall difference in PFS was not statistically significant on either univariate analysis (hazard ratio [HR], 1.40 (0.92–2.13); p=0.11) or multivariate analysis (HR, 1.41 (0.90–2.22); p=0.14) (Supplemental Table 2). OS curves are shown in Figure 8B. The control group had better OS than the rHuEPO group. The OS difference was statistically significant on multivariate analysis (HR, 1.69 (1.04–2.73); p=0.03) but not on univariate analysis (HR, 1.51 (0.97–2.37); p=0.07).
Figure 8. Progression-free Survival and Overall Survival of Patients with HER2-positive Metastatic Breast Cancer Treated with Trastuzumab-based Chemotherapy with or without Concurrent rHuEPO.
(A) Progression-free survival.
(B) Overall survival. See also Figure S6 and Tables S1 and S2.
We were able to retrieve 8 tumor specimens of patients in the rHuEPO-treated group from our institutional tumor bank. Immunohistochemical staining showed that 5 of the 8 tumor specimens were positive for EpoR, and 5 of the 8 specimens had PTEN protein expressed. Figure S6 shows one case of PTEN-negative and two representative cases of PTEN-positive staining, one being focal positive and the other being 100% positive. Using a mass spectrometry-based method recently described (Berns et al., 2007), we identified a homozygous PI3K mutation (PIK3CA_E542K_G1624A) in 1 of the 8 specimens, which happened to be PTEN positive. Together, 4 of the 8 tumor specimens (3 PTEN-negative specimens and 1 specimen with the PI3K mutation) had aberrations in the PI3K pathway. Although the number of tumor specimens is small, this result clearly indicates that the PI3K pathway is not aberrantly activated in all trastuzumab-resistant breast cancers. Up to 50 % of the patients in our series may have had cancers that were PTEN-positive and had wild-type PI3K. Because the patients in our rHuEPO group were treated concurrently with rHuEPO and trastuzumab, our analysis of clinical data, together with our preclinical studies on cell signaling and animal models, support our conclusion that concurrent use of rHuEPO constitutes a resistance mechanism to trastuzumab in patients with HER2-positive breast cancer.
DISCUSSION
In this study, we generated preclinical and clinical data demonstrating the antagonizing effect of rHuEPO on trastuzumab-induced antitumor activity in HER2 and EpoR dual-positive breast cancer cells and elucidated underlying mechanisms by which rHuEPO protects HER2-positive breast cancer from trastuzumab. We show that HER2 and EpoR are coexpressed in a significant percentage of HER2-positive breast cancer cell lines and patient specimens. Our study differs from previous studies reporting detrimental outcomes associated with rHuEPO use in cancer patients receiving chemotherapy for two main reasons. First, our study focused on targeted therapy (i.e., trastuzumab) in a specific breast cancer subtype (i.e., HER2-positive disease) rather than nontargeted chemotherapy in unselected populations. Second, our study was based on a clear rationale that enabled us to demonstrate the underlying mechanisms and causally explain how rHuEPO protects breast cancer cells against trastuzumab treatment using preclinical models. The previous studies, in contrast, were correlative in nature and did not examine underlying mechanisms.
Our data clearly showed that rHuEPO activated cell signaling; however, rHuEPO alone did not seem to have a noticeable impact on the growth of the breast cancer cell lines used in the current study in vivo. This result is consistent with findings from a few studies reporting minimal or no effect of rHuEPO on the behavior of cancer cells in vivo (Hardee et al., 2006). A possible explanation is the self-sufficiency of these cells growing in vivo; according to this explanation, tumors would not respond to additional growth and survival signals produce by rHuEPO unless cell signaling became inadequate because of interruption of a major pathway for cell proliferation and survival, such as the inhibition of HER2 by trastuzumab in our study.
Although additional mechanisms potentially underlying rHuEPO-mediated antagonism of trastuzumab’s effects in breast cancer need to be explored—such as the potential involvement of STATs, which are major downstream mediators of EpoR/Jak2 in hematopoietic cells in response to rHuEPO stimulation—our current study identified the underlying mechanism in which Src is activated as a result of rHuEPO-induced associations between Src and EpoR/Jak2 and between Src and HER2 in breast cancer. Increased association between Src and HER2 has been reported in the literature to contribute to resistance to trastuzumab (Nagata et al., 2004). The activation of common downstream pathways resulting from rHuEPO-stimulated associations between Src and EpoR/Jak2 and between Src and HER2 collectively contributes to rHuEPO-mediated cellular resistance to trastuzumab.
Our clinical data strongly support our preclinical findings; however, the finding of potential detrimental effects of rHuEPO in patients undergoing trastuzumab-based therapy should be interpreted with caution because our data are based on a small retrospective study. Patients treated with rHuEPO might have had more extensive disease (i.e., subclinical bone marrow infiltration) or been in worse general condition at the time of treatment (performance status at that time was not available) than patients not treated with rHuEPO. The difference observed in OS between the rHuEPO and control groups could also be explained by an unfavorable effect related to rHuEPO administration alone, as described in other malignancies, such as head and neck cancer (Bohlius et al., 2009; Tonelli et al., 2009). Another theoretically possible explanation for the differences between patients treated and not treated with rHuEPO is differences in PTEN levels and PIK3CA status (Nagata et al., 2004; Berns et al., 2007). However, our study with a relatively small number of patient specimens suggested that as many as 50 % of the tumor specimens in our current series had normal PI3K pathway functions. A future, larger study should better address the potential impact of PTEN and PIK3CA status and concurrent use of rHuEPO on trastuzumab resistance by multivariate analyses.
Interestingly, the observation of a significant difference in PFS between the two groups observed 6 months after treatment with rHuEPO and trastuzumab raises the possibility that antagonism of trastuzumab by rHuEPO could be related to the duration of treatment; this possibility can also be addressed later in a larger study. Because of these potential biases and to confirm our intriguing findings, prospective clinical trials are warranted. If confirmed, our findings would have a strong impact on clinical care for patients with HER2-overexpressing breast cancer.
EXPERIMENTAL PROCEDURES
Materials
We purchased epoetin alfa (Procrit; 4,000 U/ml, Amgen, Inc., Thousand Oaks, CA) and trastuzumab (Herceptin, Genentech, Inc., San Francisco, CA) from the pharmacy of MD Anderson and the cell culture–grade rHuEPO from R&D Systems (Minneapolis, MN). The antibodies we used for Western blot analysis, immunoprecipitation, and immunohistochemical staining are summarized in Supplemental Experimental Procedures. We purchased all other materials from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Cell Lines and Culture
We maintained all breast cancer cell lines in Dulbecco’s minimal essential medium containing high glucose levels and 10% fetal bovine serum (FBS).
Western Blotting, Immunoprecipitation, and Immunohistochemical Staining
We performed Western blot analysis and immunoprecipitation analysis as previously described (Li et al., 2008). We obtained human tumor specimens in accordance with Institutional Review Board protocols and the tumor specimens from nude mice in accordance with Institutional Animal Care and Use Committee (IACUC) protocols. Details of experimental procedures are provided in Supplemental Information.
Terminal Deoxynucleotidyl Transferase-mediated dUTP-X Nick End Labeling (TUNEL) Assay
We used a kit from R&D Systems to detect apoptosis in 5-μm paraffin tissue sections from whole tissue. Details are provided in Supplemental Information.
RNA Interference
We ordered the SMARTpool siRNA against EpoR, Jak2, Src, and CK2α and nontargeting siRNA from Dharmacon, Inc. (Lafayette, CO) and the shRNA constructs (pRS) against EpoR and Jak2 from OriGene Technologies, Inc. (Rockville, MD). We used a ratio of 50 pmol siRNA/μl FuGENE-6 (Roche, Indianapolis, IN) to mix them in 300 μl of serum-free medium for 20 minutes and then used the siRNA/FuGENE6 mixture to transiently transfect the cells overnight. After siRNA transfection, we cultured the cells for an additional 48 hours to allow knockdown of expression of the targeted genes.
Cell Proliferation Assay
We allowed the cells to attach to 24-well culture plates by overnight culture in the regular medium, after which we replaced the medium with fresh culture medium containing various treatment additions as described in the figure legends. At the end of treatment, we subjected the cells to an MTT assay described in detail in Supplemental Information.
Cell Invasion and Migration Assay
We performed in vitro invasion and migration assays using a modified Boyden chamber in which the filter surfaces had been coated with Matrigel. The procedures are described in detail in Supplemental Information.
Monolayer Clonogenic Assay
We seeded exponentially growing cells onto 60-mm dishes at a low density overnight prior to treating the cells with various additions in 10% FBS culture medium for extended periods. We then fixed, stained, and counted the surviving colonies using procedures detailed in Supplemental Information.
Animal Studies and Imaging of Tumor Bioluminescence
We used 4- to 6-week-old female ICR SCID mice (Taconic, Hudson, NY) and Swiss nude mice (nu/nu, colony maintained by the Department of Experimental Radiation Oncology, MD Anderson Cancer Center) for inoculation of MDA453β cells and MCF7-HER18/Fluc-GFP cells, respectively, into the mammary fat pads (1×107 cells/mouse in 100 μl) of the mice. We started treatment 26 days after implantation of MDA453β cells and 20 days after implantation of MCF7-HER18/Fluc-GFP cells. We administered trastuzumab (0.5 mg/mouse) intraperitoneally twice a week for 4 weeks (Wang et al., 2005). We administered epoetin alfa (100 U/mouse) through subcutis injection into the nape of the neck daily on weekdays for 4 weeks, compared to 40,000-60,000 U epoetin alfa once weekly up to 16 weeks in human (Gabrilove et al., 2001). Additional information can be found in Supplemental Information.
All mouse experiments were approved by MD Anderson Cancer Center Institutional Animal Care and Use Committee. Mice were cared for in accordance with guidelines set forth by the American Association for Accreditation of Laboratory Animal Care and the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Patient Data and Tumor Specimens
MD Anderson Cancer Center Institutional Review Board (IRB) approved the retrospective case-control study and waived the requirement for informed consent. The IRB also approved the study of patient tumor specimens, which was exempt from the requirement for informed consent because it used previously collected residual tissue samples.
Statistical Analysis
We assessed the results using Student’s t-test to compare two groups or one-way analysis of variance for multiple comparisons and expressed the results as means ± standard deviation. P values < 0.05 were considered statistically significant. For patient survival analysis, we used the R survival and Design package (http://www.r-project.org). PFS was defined as the time from the beginning of trastuzumab treatment to the development of progressive disease. OS was defined as the time from the beginning of trastuzumab treatment to death. Kaplan-Meier plots were compared using log-rank tests. The univariate and multivariate HRs and 95% CIs were estimated using Cox regression analysis. In multivariate models, patients with missing variables were excluded.
SIGNIFICANCE.
Recombinant human erythropoietin (rHuEPO) has been used widely to treat cancer-related and chemotherapy-induced anemia and fatigue, including in patients with breast cancer. EPO has strong activity in protecting cells from apoptosis by activating cell signaling pathways that overlap substantially with several growth factor receptor–mediated cell signaling pathways, including that of the human epidermal growth factor receptor-2 (HER2). Our results provide important preclinical and clinical evidence and mechanistic insights indicating that concurrent administration of rHuEPO and trastuzumab, an anti-HER2 antibody, may be contraindicated in patients with breast cancer that is positive for both HER2 and EpoR. Our study warrants further evaluation of clinical outcomes of patients with HER2-positive breast cancer treated with concurrent rHuEPO and trastuzumab.
Supplementary Material
Acknowledgments
This work was supported by an NIH R01 award (CA129036 to Z.F.), by research awards from the Breast Cancer Research Foundation (to F.J.E., G.B.M., G.N.H., J.M., M-C.H, and Z.F.), and by the Cancer Center Support Grant CA016672 from the National Cancer Institute. We thank Drs. Wenya Xia and Xiaoming Xie for technical support with immunohistochemical staining and the animal experiments, Stephanie Deming for editorial assistance, and the staff of the Breast Cancer Management System, Department of Breast Medical Oncology, MD Anderson Cancer Center, for management of the patient data used in the current study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belsches-Jablonski AP, Biscardi JS, Peavy DR, Tice DA, Romney DA, Parsons SJ. Src family kinases and HER2 interactions in human breast cancer cell growth and survival. Oncogene. 2001;20:1465–1475. doi: 10.1038/sj.onc.1204205. [DOI] [PubMed] [Google Scholar]
- Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke M, Weingart O, Kluge S, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373:1532–1542. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- Damen JE, Cutler RL, Jiao H, Yi T, Krystal G. Phosphorylation of tyrosine 503 in the erythropoietin receptor (EpR) is essential for binding the P85 subunit of phosphatidylinositol (PI) 3-kinase and for EpR-associated PI 3-kinase activity. J Biol Chem. 1995;270:23402–23408. doi: 10.1074/jbc.270.40.23402. [DOI] [PubMed] [Google Scholar]
- Damen JE, Liu L, Cutler RL, Krystal G. Erythropoietin stimulates the tyrosine phosphorylation of Shc and its association with Grb2 and a 145-Kd tyrosine phosphorylated protein. Blood. 1993a;82:2296–2303. [PubMed] [Google Scholar]
- Damen JE, Mui AL, Puil L, Pawson T, Krystal G. Phosphatidylinositol 3-kinase associates, via its Src homology 2 domains, with the activated erythropoietin receptor. Blood. 1993b;81:3204–3210. [PubMed] [Google Scholar]
- Esteva FJ, Yu D, Hung MC, Hortobagyi GN. Molecular predictors of response to trastuzumab and lapatinib in breast cancer. Nat Rev Clin Oncol. 2010;7:98–107. doi: 10.1038/nrclinonc.2009.216. [DOI] [PubMed] [Google Scholar]
- Gabrilove JL, Cleeland CS, Livingston RB, Sarokhan B, Winer E, Einhorn LH. Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. J Clin Oncol. 2001;19:2875–2882. doi: 10.1200/JCO.2001.19.11.2875. [DOI] [PubMed] [Google Scholar]
- Hardee ME, Arcasoy MO, Blackwell KL, Kirkpatrick JP, Dewhirst MW. Erythropoietin biology in cancer. Clin Cancer Res. 2006;12:332–339. doi: 10.1158/1078-0432.CCR-05-1771. [DOI] [PubMed] [Google Scholar]
- Hardee ME, Cao Y, Fu P, Jiang X, Zhao Y, Rabbani ZN, Vujaskovic Z, Dewhirst MW, Arcasoy MO. Erythropoietin blockade inhibits the induction of tumor angiogenesis and progression. PLoS One. 2007;2:e549. doi: 10.1371/journal.pone.0000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC, Zhuang H, Jiang N, Waterfield MD, Wojchowski DM. Association of the p85 regulatory subunit of phosphatidylinositol 3-kinase with an essential erythropoietin receptor subdomain. Blood. 1993;82:3530–3538. [PubMed] [Google Scholar]
- Henke M, Laszig R, Rube C, Schafer U, Haase KD, Schilcher B, Mose S, Beer KT, Burger U, Dougherty C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- Henry DH, Abels RI. Recombinant human erythropoietin in the treatment of cancer and chemotherapy-induced anemia: results of double-blind and open-label follow-up studies. Semin Oncol. 1994;21:21–28. [PubMed] [Google Scholar]
- Hortobagyi GN. Trastuzumab in the treatment of breast cancer. N Engl J Med. 2005;353:1734–1736. doi: 10.1056/NEJMe058196. [DOI] [PubMed] [Google Scholar]
- Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- Jelkmann W. Erythropoietin: structure, control of production, and function. Physiol Rev. 1992;72:449–489. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Krantz SB. Erythropoietin. Blood. 1991;77:419–434. [PubMed] [Google Scholar]
- Lappin TR, Maxwell AP, Johnston PG. EPO’s alter ego: erythropoietin has multiple actions. Stem Cells. 2002;20:485–492. doi: 10.1634/stemcells.20-6-485. [DOI] [PubMed] [Google Scholar]
- Leyland-Jones B. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol. 2003;4:459–460. doi: 10.1016/s1470-2045(03)01163-x. [DOI] [PubMed] [Google Scholar]
- Li X, Lu Y, Liang K, Pan T, Mendelsohn J, Fan Z. Requirement of hypoxia-inducible factor-1alpha down-regulation in mediating the antitumor activity of the anti-epidermal growth factor receptor monoclonal antibody cetuximab. Mol Cancer Ther. 2008;7:1207–1217. doi: 10.1158/1535-7163.MCT-07-2187. [DOI] [PubMed] [Google Scholar]
- Liu L, Damen JE, Cutler RL, Krystal G. Multiple cytokines stimulate the binding of a common 145-kilodalton protein to Shc at the Grb2 recognition site of Shc. Mol Cell Biol. 1994;14:6926–6935. doi: 10.1128/mcb.14.10.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Yu Q, Liu JH, Zhang J, Wang H, Koul D, McMurray JS, Fang X, Yung WK, Siminovitch KA, et al. Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-kinase/AKT cascades. J Biol Chem. 2003;278:40057–40066. doi: 10.1074/jbc.M303621200. [DOI] [PubMed] [Google Scholar]
- Miura O, Nakamura N, Ihle JN, Aoki N. Erythropoietin-dependent association of phosphatidylinositol 3-kinase with tyrosine-phosphorylated erythropoietin receptor. J Biol Chem. 1994;269:614–620. [PubMed] [Google Scholar]
- Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Ebihara S, Asada M, Yamanda S, Niu K, Arai H. Erythropoietin promotes the growth of tumors lacking its receptor and decreases survival of tumor-bearing mice by enhancing angiogenesis. Neoplasia. 2008;10:932–939. doi: 10.1593/neo.08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- Tonelli M, Hemmelgarn B, Reiman T, Manns B, Reaume MN, Lloyd A, Wiebe N, Klarenbach S. Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysis. CMAJ. 2009;180:E62–E71. doi: 10.1503/cmaj.090470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20:5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CX, Koay DC, Edwards A, Lu Z, Mor G, Ocal IT, Digiovanna MP. In vitro and in vivo effects of combination of trastuzumab (Herceptin) and tamoxifen in breast cancer. Breast Cancer Res Treat. 2005;92:251–263. doi: 10.1007/s10549-005-3375-z. [DOI] [PubMed] [Google Scholar]
- Watowich SS, Yoshimura A, Longmore GD, Hilton DJ, Yoshimura Y, Lodish HF. Homodimerization and constitutive activation of the erythropoietin receptor. Proc Natl Acad Sci U S A. 1992;89:2140–2144. doi: 10.1073/pnas.89.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthuhn BA, Quelle FW, Silvennoinen O, Yi T, Tang B, Miura O, Ihle JN. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.