Abstract
Background
Echocardiography is the imaging modality of choice for evaluation of coronary artery (CA) abnormalities in Kawasaki disease (KD). Small series have established high specificity and sensitivity for detecting abnormalities, yet visualization rates of individual CA segments and factors associated with success are unknown.
Methods and Results
In the Pediatric Heart Network’s randomized trial on primary steroid treatment for KD, echocardiograms were interpreted locally and by a core laboratory (lab). We explored univariate and multivariate predictors of CA visualization by the local lab as determined by the core lab and assessed agreement of CA size measured locally and by the core lab. A total of 589 echoes from 199 patients were obtained over 27 months. Visualization rates for the left main, proximal/distal left anterior descending and proximal right CAs ranged from 91–98%, but were lower for the distal right (65%), circumflex (86%) and posterior descending (54%) CAs. For the distal right and circumflex CAs, visualization rates improved over the course of the study (p<0.05). In multivariate analysis, local center, CA segment, and time from study start to echocardiogram were independent predictors of visualization (all p<0.001). For segments for which visualization rates varied by center, higher percentage visualization was associated with larger center volume (p=0.001). Routine sedation use was also associated with higher visualization rates.
Conclusion
Successful CA visualization in KD is associated with the segment being evaluated, and is influenced by center volume and sedation use. Increased visualization rates over time suggest a learning curve and underscore the value of core lab oversight in pediatric multi-center trials.
INTRODUCTION
Kawasaki disease (KD) is a systemic vasculitis of unknown etiology that affects predominantly infants and young children 1. Inflammatory cells cause segmental destruction of the elastica interna of medium-sized, muscular extraparenchymal arteries, particularly the coronary arteries (CA) in locations similar to those found in atherosclerotic coronary artery disease 2. Almost all morbidity in KD after the first week of illness is related to CA aneurysms, which develop in up to 25% of untreated children 3, and fewer than 5% of those appropriately treated 4, 5.
Echocardiography is the mainstay of diagnosis and long-term surveillance of CA aneurysms in children with confirmed KD 6. Additionally, because there is no specific diagnostic test for KD and not all clinical features may be present particularly in young infants, echocardiography has a seminal role in the evaluation of children with suspected or incomplete KD in the 2004 American Heart Association recommendations 1. The sensitivity and specificity of echocardiography for detection of proximal CA dilation and aneurysms are high in single-center series 7, 8 compared to the gold standard of angiography. Despite the reliance upon echocardiography in the management of KD, however, few studies have examined rates of visualization of CA segments, with or without aneurysms or dilation, across clinical centers or the patient characteristics and institutional factors that correlate with successful CA imaging.
We sought to describe the frequency of successful visualization of individual coronary artery segments both by local and core laboratory (lab) assessments; to define potential factors associated with imaging success; to describe how that frequency changes over the course of a multi-center trial; and to compare visualization rates between local and core lab interpreters. Finally, we sought to compare measurements obtained by the individual sites with those obtained on the same examination by the core lab. These goals were pursued using the database of the Pediatric Heart Network (PHN) randomized trial of pulse steroids in primary treatment of KD 9.
METHODS
Subjects
PHN investigators evaluated the efficacy of corticosteroids for the primary treatment of KD in a multicenter, randomized, double-blind, and placebo-controlled trial9. Subjects were recruited for the trial between December 2002 and December 2004 from eight clinical centers in North America (seven U.S. and one Canadian). Informed consent was obtained from each subject’s parent or guardian prior to participation. Patients meeting the criteria for acute KD were enrolled between days 4 and 10 of illness and randomly assigned to receive a single-dose of intravenous methylprednisolone or placebo in addition to conventional therapy with intravenous immunoglobulin and aspirin. The primary trial outcome was maximum CA dimension z-score adjusted for body surface area (BSA) of either the proximal left anterior descending (LAD) segment or right coronary artery (RCA). No difference between treatment groups was found. The study was conducted in accordance with the guidelines of the PHN’s Data and Safety Monitoring Board and of each center’s Institutional Review Board.
Echocardiographic Methods
Echocardiograms were obtained at baseline and again at 7.8±1.8 days (median 8.0, allowable window 3–14 days) and 36.5±4.3 days (median 36.0, allowable window 21–49 days) after randomization. Sedation was given according to local practice. The cardiac ultrasound examination was performed using transducers with the highest frequency possible.
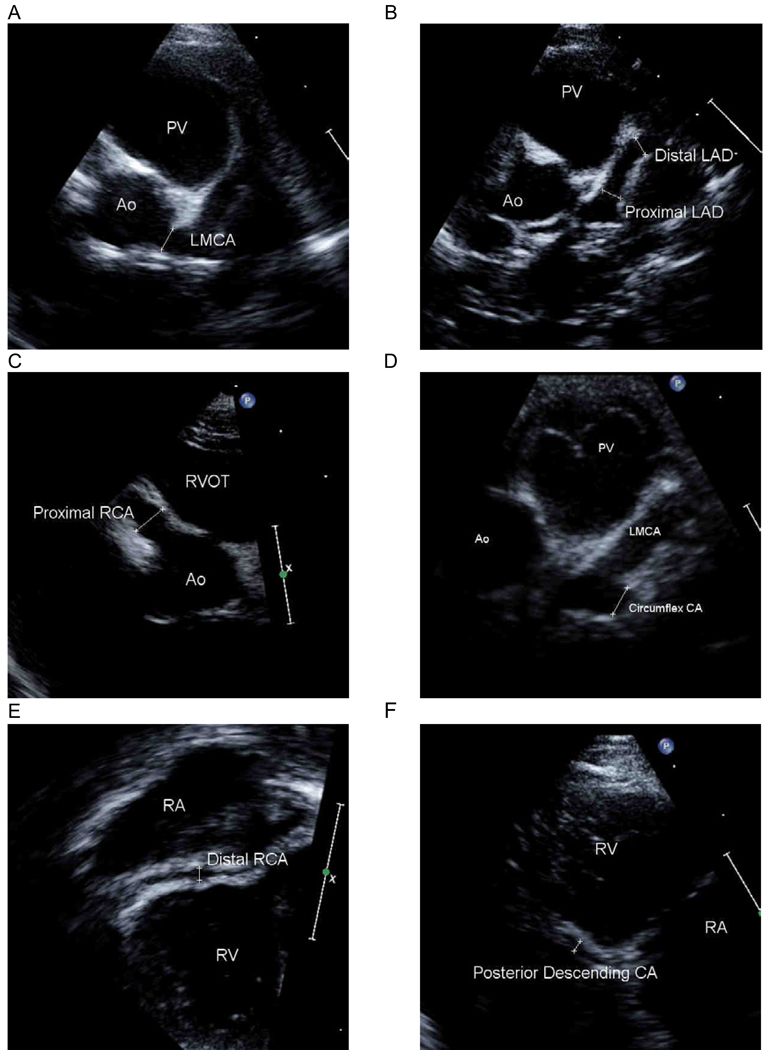
Echocardiographers at the participating centers acquired the studies according to a uniform, predetermined protocol 1 which included display of seven CA segments. The left main (LMCA), left circumflex and posterior descending (PD) segments were treated as single segments. The left anterior descending (LAD) was divided into proximal and distal segments. The proximal LAD was defined as the portion from the bifurcation of the LMCA into the circumflex CA and LAD to the point where the artery crossed the plane of the pulmonary valve. The distal LAD was defined as the segment beyond the plane of the pulmonary valve. The right coronary artery (RCA) was also divided into proximal and distal segments. The proximal RCA was defined as the portion of the artery between the ostium and the lateral border of the tricuspid valve. The distal RCA was measured at the mid-segment of the atrioventricular groove in apical imaging planes. On-line measurements were made of the internal lumen diameters of arterial segments using electronic calipers during image acquisition and the largest measurement obtained from all views was recorded for the trial. Measurements were made from internal surface to internal surface and excluded points of branching, as these normally exceed adjacent vessel diameter. Figure 1 demonstrates the measurements made of each coronary artery segment in a patient with significant dilation of all of the CA segments.
Figure 1.
Representative coronary artery segment imaging views and measurements. Ao: aorta; LAD: left anterior descending coronary artery; LMCA: left main coronary artery; PV: pulmonary valve; RA: right atrium; RCA: right coronary artery; RV: right ventricle; RVOT: right ventricular outflow tract.
Studies were recorded in a dynamic video format, either digitally or in standard 0.5 inch super-VHS tape, and submitted to the core lab for interpretation by a single observer (SDC), blinded to subject identification, treatment, and study visit. Video examinations were converted to digital imaging sets to allow calibrated measurements. Dimensions of the LMCA, proximal LAD, and proximal RCA were adjusted for BSA and expressed in standard deviation units (z-scores) to assess their size compared to the normal population10 The core lab provided training materials for the local centers for image standardization at study launch and periodic additional site-specific feedback on image quality.
Statistical Analyses
The primary outcomes for this analysis were the visualization rates of the seven CA segments. Candidate factors associated with successful visualization included time from study onset to echo, artery segment, center, study visit, age at echo, BSA, body mass index (BMI), use of sedation, and type of imaging media (digital versus videotape). Time was modeled as days from date of study launch to date of echocardiogram acquisition as a continuous variable, as well as in three nine-month time blocks, to determine if rates of visualization improved with time over the study period. Univariate and multivariate associations between visualization rate and potential predictors (including patient factors as well as source of reading – local vs. central) were estimated using a generalized linear mixed model (binomial link), which incorporates a random effect for subject to account for multiple echocardiograms obtained from the same subject. If the estimate of the common variance of the random effect was zero, so that within-subject correlation could be ignored, we used simple logistic regression with a fixed effect only. A p-value of <0.05 was considered statistically significant. An intraclass correlation coefficient was used to estimate core lab vs. local center agreement on CA dimensions. Confidence intervals for the intra-class correlation coefficients derived from longitudinal data were obtained by bootstrapping with 1000 samples. The Kappa statistic was used to estimate core lab vs. local center agreement with respect to whether a CA z-score was above normal (z>2.0). All analyses were performed using SAS statistical software version 9 (SAS Institute, Inc, Cary NC).
RESULTS
We enrolled 199 patients (62% male, with a mean age of 3.3 ± 2.2 years and a mean BSA of 0.6 ± 0.2 m2), in whom 589 echocardiograms were obtained. Two echocardiograms were assessed by the core lab to be unacceptable, i.e. none of the primary measurements (LMCA, proximal LAD, proximal RCA) were able to be made. Ten additional studies were obtained outside of protocol windows and were excluded from analysis of association between visualization and study visit portions of these results. One patient found to have a single right CA was excluded from analysis of visualization rate of the LMCA.
The overall rates of visualization of coronary segments as determined by core lab analysis are presented in Table 1. The LMCA, proximal LAD and proximal RCA each were visualized on almost all studies (98% or more). Visualization rates for the distal RCA, circumflex and PD arteries were lower (65%, 86% and 54% respectively).
Table 1.
Core laboratory coronary artery segment visualization rates by study month. Time from study start to echocardiogram acquisition was analyzed as a continuous variable as well as in 9-month time blocks.
| CA segment | Overall (%)* |
Visualized (%) | Categorical | Continuous | ||
|---|---|---|---|---|---|---|
| 0–9 months |
10–18 months |
19–27 months |
P-value | P-value | ||
| N | 589 | 254 | 225 | 110 | ||
| LMCA | 583 (99) | 98 | 99 | 100 | 0.80 | 0.18 |
| Proximal LAD | 579 (98) | 98 | 99 | 99 | 0.55 | 0.15 |
| Distal LAD | 538 (91) | 91 | 91 | 93 | 0.86 | 0.44 |
| Proximal RCA | 576 (98) | 98 | 98 | 97 | 0.84 | 0.81 |
| Distal RCA | 385 (65) | 59 | 72 | 67 | 0.08 | 0.03 |
| Circumflex | 509 (86) | 82 | 89 | 91 | 0.12 | 0.03 |
| PD | 316 (54) | 48 | 61 | 52 | 0.05 | 0.12 |
CA: coronary artery; LAD: left anterior descending; LMCA: left main coronary artery; PD: posterior descending; RCA: right coronary artery
Counts indicate number of echocardiograms, not number of subjects
Univariate analyses
We evaluated the rate of CA visualization by the core lab as a function of the time since study launch, both in terms of tertile of study period (categorical) and the number of days since study onset (continuous) variables (Table 1). The distal RCA and circumflex CA were visualized more often in echocardiograms performed later in the study period. Visualization of the PD improved from the first nine-month block of the study to the second, but did not reach significance in the continuous model.
Visualization rates as determined by the core lab stratified by center are shown in Table 2. Rates for three of the most reliably identified segments - LMCA, proximal LAD and proximal RCA – were similar among the clinical sites. The four least commonly visualized distal coronary segments demonstrated significant variation amongst the eight sites, even when the least successful center was excluded.
Table 2.
Coronary artery visualization rates as determined by core laboratory stratified by local center.
| CA segment | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 | Site 8 | P-value |
|---|---|---|---|---|---|---|---|---|---|
| N* | 93 | 154 | 80 | 71 | 30 | 51 | 81 | 29 | |
| LMCA (%) | 100 | 98 | 100 | 97 | 100 | 100 | 100 | 97 | 0.99 |
| Proximal LAD (%) | 100 | 100 | 100 | 93 | 100 | 96 | 99 | 93 | 0.89 |
| Distal LAD (%) | 93 | 98 | 98 | 82 | 93 | 84 | 93 | 66 | <.001 |
| Proximal RCA (%) | 98 | 100 | 99 | 92 | 100 | 96 | 100 | 93 | 0.59 |
| Distal RCA (%) | 67 | 92 | 36 | 28 | 77 | 45 | 94 | 38 | <.001 |
| Circumflex (%) | 90 | 92 | 85 | 68 | 90 | 80 | 95 | 79 | 0.005 |
| PD (%) | 51 | 67 | 48 | 39 | 47 | 41 | 78 | 7 | <.001 |
CA: coronary artery; LAD: left anterior descending; LMCA: left main coronary artery; PD: posterior descending; RCA: right coronary artery
Counts indicate number of echocardiograms, not number of subjects
In an effort to define center characteristics that would account for this difference in visualization rates, we examined whether patient sedation was associated with improved visualization. In all, 299 echocardiograms were obtained in patients younger than 3 years of age, and 72% of these were obtained with sedation. The sedation strategy used most frequently was chloral hydrate (81% of sedated studies). The next most frequent medication, midazolam, was used in 16% of sedated exams, usually in combination with other medications (ketamine, nubain, nembutal and chloral hydrate). Overall sedation use in this subset of young patients varied significantly among the eight local centers, ranging from 6% to 100%. Visualization of the distal LAD, distal RCA and PD was more often successful in echocardiograms obtained with sedation than in those without sedation (Table 3). We also investigated whether time to randomization of first patient (with a longer time indicating a greater delay in implementing the imaging protocol after centralized training) or center volume affected visualization rates. The number of days from study onset to first patient randomization ranged from 10 to 42, with a median of 16 days. Centers that randomized their first patient at or earlier than the median of 16 days were more likely to visualize the distal RCA and posterior descending than centers randomizing later (Table 4). Local center volume was categorized by total number of echocardiograms submitted for analysis: high was defined as > 90 studies (two centers), medium as 70 – 90 studies (three centers), and low as < 70 studies (three centers). For the distal LAD, distal RCA and PD the odds of visualization at high volume centers were three to five times higher than the odds at low volume centers (Table 4).
Table 3.
Core laboratory coronary artery visualization rate by sedation use in patients less than 3 years of age.
| CA segment | Visualization Rate | Odds Ratio for Visualization | ||
|---|---|---|---|---|
| Sedation (%) | No Sedation (%) | Sedation vs. No sedation | ||
| (95%) CI | P-value | |||
| LMCA | 99 | 99 | 2.57 (0.16, 42.40) | 0.51 |
| Proximal LAD | 99 | 95 | 5.39 (0.96, 30.31) | 0.06 |
| Distal LAD | 94 | 82 | 3.76 (1.50, 9.40) | 0.005 |
| Proximal RCA | 99 | 96 | 2.35 (0.41, 13.56) | 0.34 |
| Distal RCA | 80 | 42 | 5.59 (2.74, 11.41) | <.001 |
| Circumflex | 90 | 81 | 2.03 (0.83, 4.96) | 0.12 |
| PD | 63 | 34 | 3.16 (1.58, 6.33) | 0.001 |
CA: coronary artery; CI: confidence interval; LAD: left anterior descending; LMCA: left main coronary artery; PD: posterior descending; RCA: right coronary artery
Table 4.
Odds ratios and 95% CI for coronary artery visualization by local center characteristics.
| Time to first randomization following training: Early vs. Late* | Local Center Volume: High vs. Low† | |||
|---|---|---|---|---|
| CA segment | Odds ratio (95%CI) | P-value | Odds ratio (95%CI) | P-value |
| Distal LAD | 1.61 (9.83, 3.14) | 0.16 | 5.07 (2.10,12.30) | 0.002 |
| Distal RCA | 2.30 (1.33, 4.00) | 0.003 | 4.93 (2.41,10.10) | <0.001 |
| Circumflex | 1.76 (0.96, 3.22) | 0.07 | 2.18 (0.95, 5.00) | 0.59 |
| PD | 1.67 (1.03, 2.71) | 0.039 | 3.41 (1.79, 6.48) | 0.001 |
CA: coronary artery; CI: confidence interval; LAD: left anterior descending; PD: posterior descending; RCA: right coronary artery
Early: time to first randomization following training ≤ 16 days; late: time to first randomization following training > 16 days
High center volume: > 90 echocardiograms submitted for analysis; low center volume: < 70 echocardiograms submitted for analysis
The percentage of visualized CA segments did not vary according to whether the patient’s echocardiogram was performed at baseline, one week, or five weeks post-randomization. Similarly, patient BSA, BMI and age at echocardiogram did not affect rates of visualization, except for lower visualization of the distal RCA in patients with higher BSA (p = 0.009) and with higher BMI (p = 0.002). Two of the eight sites submitted images in both videotape and DICOM formats; the other sites utilized only videotape. A total of 64 (11%) of examinations were DICOM. Visualization of the distal RCA only was better in DICOM examinations than videotape: 81% vs. 63% respectively (odds ratio 2.63, 95% confidence interval 1.06, 6.53), p=0.037.
Multivariate Model
Using a mixed logistic model, we explored independent factors associated with visualization. Starting with all stated candidate predictors (time from the onset of study enrollment to echocardiogram, artery segment, local center, study visit, age at echocardiogram, BMI and BSA at echocardiogram), variables were eliminated sequentially based on highest p-values. The first model included 4,123 observations: seven segments in all 589 echocardiograms. Variability in visualization rates was most strongly and independently associated with local center and the particular CA segment being imaged, with sedation having a moderate association after accounting for local center and CA segment (odds ratio 1.41, p=0.047; Table 5). The second model included only those patients less than three years of age. A total of 2,093 observations were performed in seven CA segments in 299 echocardiograms. In this model, local center, CA segment, and sedation (odds ratio 1.89, p=0.024) all remained independently associated with visualization; increasing time from trial start to the echocardiogram acquisition was also significant in this model (Table 5).
Table 5.
Multivariate Models for coronary artery visualization by the core laboratory.
| Variable | All echocardiograms (4,123 observations) | Echocardiograms on patients < 3 years of age (2,093 observations) | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value | |
| Local Center | <0.001 | <0.001 | ||
| CA Segment | <0.001 | <0.001 | ||
| Days from trial start to echocardiogram | N/A | 1.12* (1.02, 1.24) | 0.02 | |
| Sedation | 1.41 (1.004, 1.97) | 0.047 | 1.89 (1.09, 3.27) | 0.02 |
CA: coronary artery; CI = confidence interval
Odd ratio per 90 day increase in interval from trial start to echo acquisition
Comparison of Local Center and Core Lab Assessments
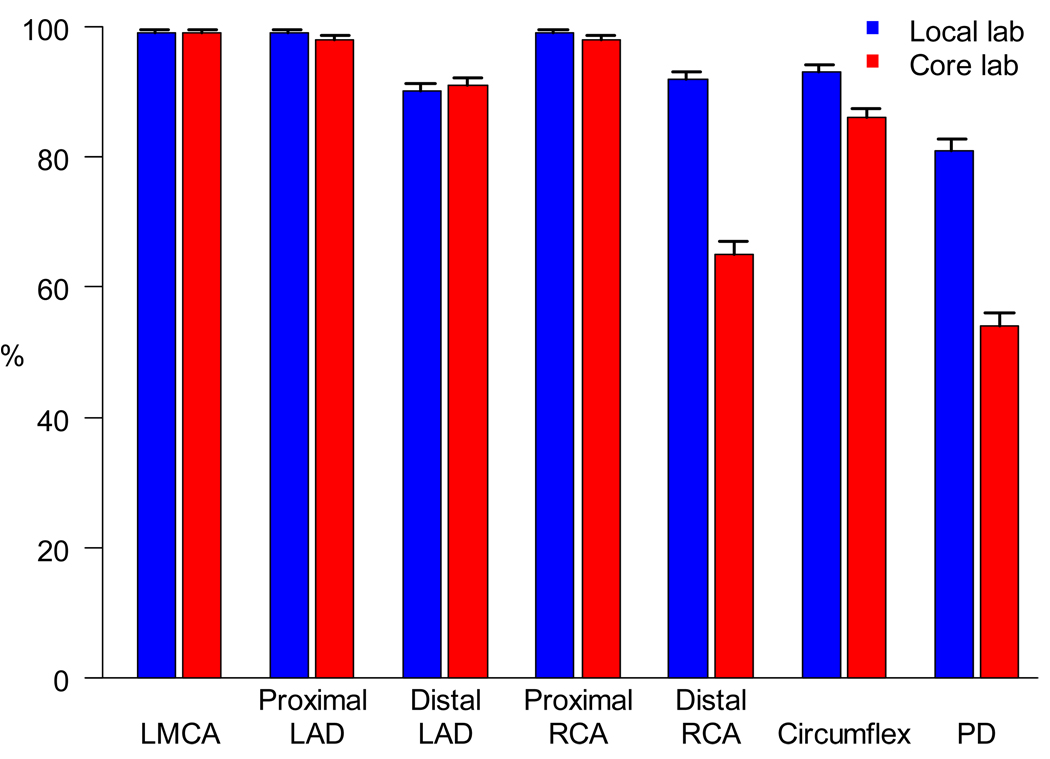
We explored the extent to which local centers and the core lab concurred on whether an individual arterial segment had been visualized on a given echocardiogram (Figure 2). Formal agreement analysis was not conducted for the proximal and left main arteries due to the very high visualization rates. However, the more challenging segments (e.g., the distal RCA, circumflex and the PD arteries) were significantly more likely to have been classified by the local center as having been visualized than they were by the core lab (p <.001).
Figure 2.
Bar graph of visualization rates ± one standard error of coronary artery segment by the core laboratory and by local center assessment. LAD: left anterior descending; LMCA: left main coronary artery; PD: posterior descending; RCA: right coronary artery.
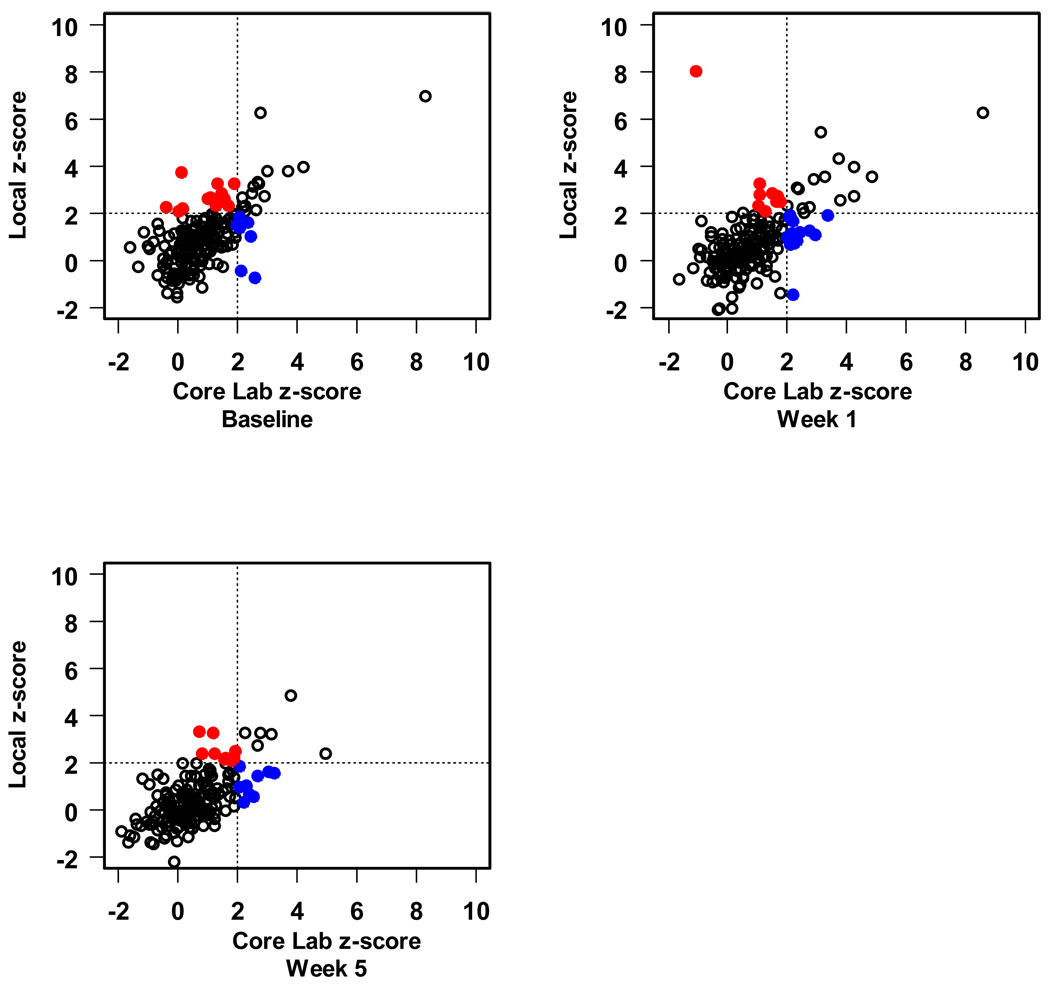
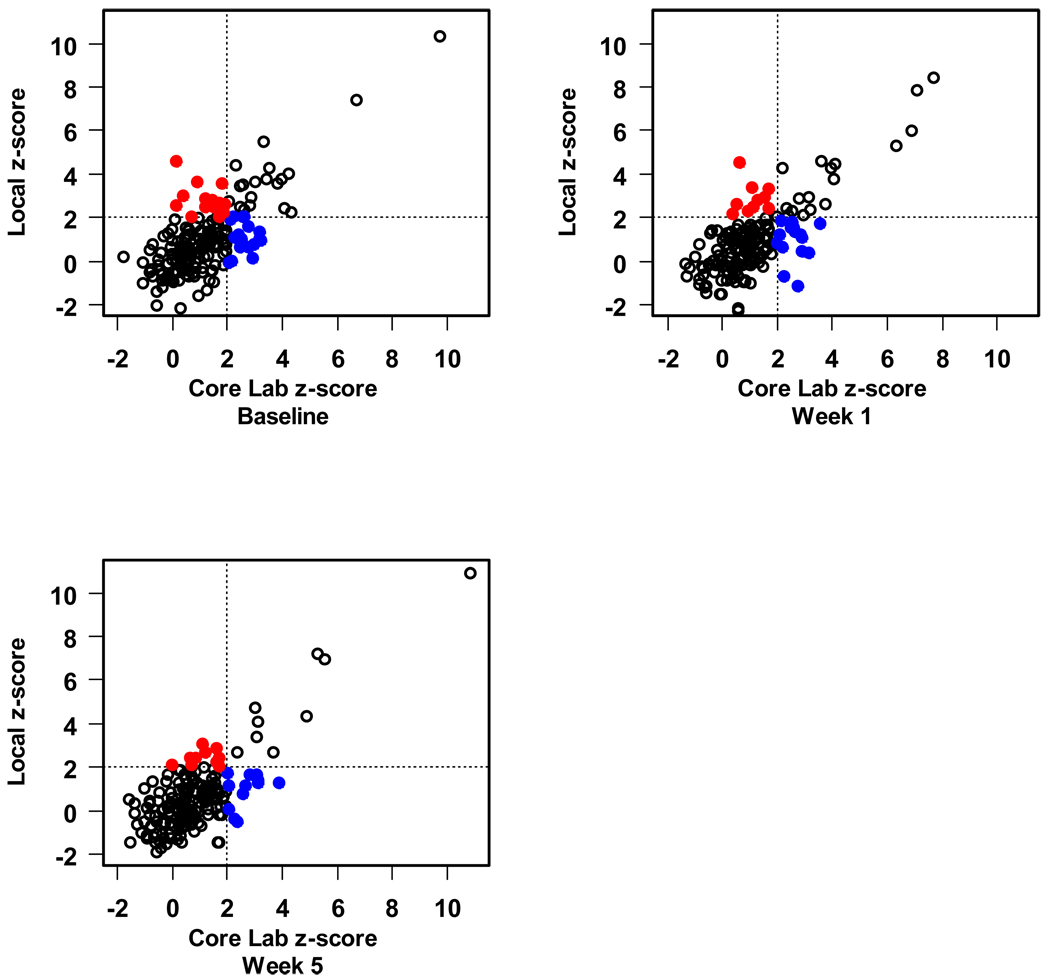
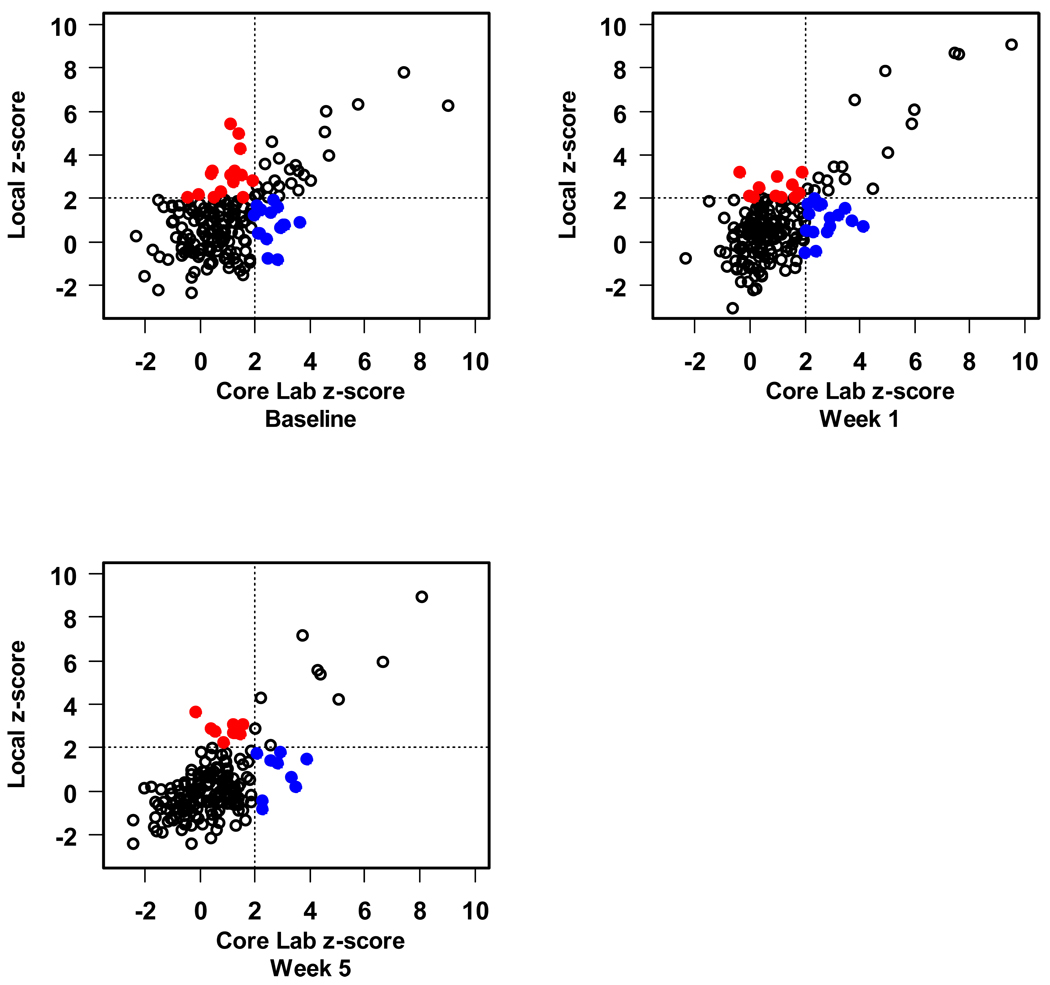
Finally, we compared local measurement of the CA diameter with those obtained by the core lab as to whether or not the core lab and the local center agreed that the CA dimension z-score was above normal (z>2.0) or not. For the CAs for which z-scores were available (LMCA, and the proximal RCA and LAD), we found moderate agreement with regard to classification of the artery size as above normal (kappa statistics ranged from 0.37 to 0.54 depending on the segment and study visit). Sensitivity was lower than specificity. For example, the core lab classified 40 baseline proximal LAD CA measurements as having a z-score > 2, of which 25 (63%) were similarly classified by the local center. Amongst 151 baseline proximal LAD measurements with z ≤ 2 according to the core lab, 136 (90%) were also classified as normal size by the local center. In this example, the kappa statistic was 0.53 with 95% confidence interval 0.38 to 0.67. Figures 3–5 present the scatter plots of the z-scores by local center vs. core lab reading for the LMCA, proximal RCA and proximal LAD.
Figure 3.
Scatter plot of left main coronary artery z-score. The blue solid dots indicate those which the local lab has declared as a normal size CA that was abnormal according to the core lab. The red solid dots indicate the opposite; both represent discordant assessments.
Figure 5.
Scatter plot of proximal right coronary artery z-score. The blue solid dots indicate those which the local lab has declared as a normal size CA that was abnormal according to the core lab. The red solid dots indicate the opposite; both represent discordant assessments.
Comparisons of specific coronary artery segment measurements between the core lab and the local center are included in Table 6. The overall ICC ranged from 0.38 for the PD to 0.75 for the proximal RCA, indicating moderate correlation for the larger vessels and low correlation for the segments that were more difficult to image.
Table 6.
Agreement of coronary artery segment dimensions by local center vs. core laboratory assessment.
| CA segment | Local center (cm) | Core Laboratory(cm) | ICC | 95% CI |
|---|---|---|---|---|
| LMCA | 0.27±0.06 (577) | 0.27±0.06 (577) | 0.70 | 0.68, 0.72 |
| Proximal LAD | 0.21±0.06 (573) | 0.21±0.05 (573) | 0.63 | 0.60, 0.67 |
| Distal LAD | 0.19±0.05 (495) | 0.18±0.05 (495) | 0.62 | 0.59, 0.66 |
| Proximal RCA | 0.22±0.07 (572) | 0.23±0.06 (572) | 0.75 | 0.72, 0.78 |
| Distal RCA | 0.15±0.04 (373) | 0.14±0.04 (373) | 0.64 | 0.54, 0.71 |
| Circumflex | 0.17±0.06 (480) | 0.17±0.05 (480) | 0.55 | 0.45, 0.65 |
| PD | 0.14±0.06 (286) | 0.13±0.05 (286) | 0.38 | 0.14, 0.68 |
CA: coronary artery; CI: confidence interval; ICC: intraclass correlation coefficients; LAD: left anterior descending; LMCA: left main coronary artery; PD: posterior descending; RCA: right coronary artery
DISCUSSION
In this report, we have demonstrated that the ability to visualize CA segments in KD varies with the particular coronary segment, the local center performing the examination, and the use of sedation. Visualization rates for the more difficult segments improved over the time course of this research protocol. For CA segments measured by both the clinical center and core lab, the agreement of diameter measurements was moderate, and demonstrates that a dichotomous assignment of normal versus abnormal CA dimension becomes somewhat arbitrary when the data points are clustered around the line that defines normal. Patient characteristics had little influence upon visualization rates. The largest coronary segments were reliably imaged across the range of age and BSA, with only the distal RCA visualized less frequently in larger patients. Visualization rates did not vary among the three study visits, which reflected acute, subacute, and convalescent phases of illness.
Imaging of distal coronary segments is technically challenging, and visualization of these segments varied importantly by clinical center. Our data indicate that reliance on assessment of distal CA segments as an endpoint for future research efforts should be avoided. Although it is uncommon to have aneurysms in distal vessels without proximal abnormalities 7, treatment duration relies upon documentation of aneurysm regression, both proximal and distal, thus emphasizing the importance of imaging distal vessels in clinical practice. At the start of the trial, local centers were provided with a detailed predetermined protocol. Centers that randomized more quickly after training had highest visualization rates for the distal RCA and PD artery. In addition, imaging of distal segments by the local centers improved over time, likely reflecting both cumulative experience with an established protocol and response to core lab feedback. Other studies have also confirmed the value of core lab oversight in large multi-center clinical trials that utilize echocardiographic measurements for clinical endpoints, particularly in circumstances where the need to detect small changes necessitates reduction in variability 11–13.
While several studies have reported reduced variability of echocardiographic parameters that result from employing a core lab in adult medicine, relatively fewer trials make use of echocardiographic endpoints in pediatric cardiology. One notable investigation of the utility of core laboratory involvement in pediatrics was the P2C2 HIV study, a natural history description of cardiac and pulmonary complications of vertically transmitted HIV. Significant variability in left ventricular dimension, wall thickness and shortening fraction was demonstrated between local centers and central measurements in this trial as well14. Other pediatric trials have employed echocardiographic core labs; however, reports of resultant added utility or critical analysis of variability in measurements are lacking.
The organization of the core lab also impacts results. For example, in the PROSPECT study of echocardiographic assessment of ventricular mechanical synchrony, the utilization of multiple observers in three separate core labs was felt to have contributed to the negative result of the study 15, 16. In the present study, the core lab consisted of a single experienced observer, reducing such potential random error.
An important finding in our study was that use of sedation in young children was more likely to result in visualization of coronary segments, particularly the smaller and more distal vessels. Although sedation is included as a recommendation in the 2004 American Heart Association guidelines for diagnosis and treatment of KD (1), it is not a universal practice. Because KD patients are generally young and often febrile and irritable, they are likely to be agitated during echocardiography, thus limiting the ability to visualize the CAs. However, sedation requires increased resources for adequate evaluation and monitoring and may not be available in all settings.
Oral chloral hydrate was the most commonly utilized method for sedation across the local centers involved in this study. It has been demonstrated to have a good safety profile in pediatric patients with heart disease 17. Although many reports demonstrate the efficacy of chloral hydrate in achieving sedation for echocardiography 18–20, none have demonstrated that such a strategy improves the data obtained from these examinations. Our report is the first that provides evidence that the use of sedation improves the ability to evaluate CAs by echocardiography.
Limitations
Some limitations of this study merit attention. The echocardiograms represented in this report were performed at academic institutions in a research setting, and the results may not be generalizable to community practice. Additionally, information regarding the experience level of specific sonographers performing the examinations and differences in imaging equipment was not gathered, and could contribute to center variability. Because no gold standard for dimensions was used in this study, the comparisons of the core lab and local center measurements are limited to agreement, rather than validation.
Finally, the study was performed during a transition period of videotape to digital format. Important evolution in echocardiographic imaging has occurred since the time of image acquisition for this study. Factors such as enhanced harmonic imaging, digital storage and higher frequency transducers would likely have contributed to improved image quality. Reliance on still-frame or single-beat clips common in some laboratories in the digital era may negatively impact imaging of very small vessels in this setting. Although only one segment demonstrated a statistically significant difference in visualization between the two modalities, the higher quality of digital images overall in echocardiography is not disputed by our findings.
In summary, successful visualization of CAs in KD is independently associated with the local center at which the study was performed, the segment being evaluated and sedation use. This report provides evidence supporting the use of echocardiographic core laboratories in pediatric trials as well as the use of sedation to improve the quality of echocardiograms in young children.
Figure 4.
Scatter plot of proximal left anterior descending coronary artery z-score. The blue solid dots indicate those which the local lab has declared as a normal size CA that was abnormal according to the core lab. The red solid dots indicate the opposite; both represent discordant assessments.
Acknowledgments
Supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors state no conflicts of interest exist.
References
- 1.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 2.Naoe S, Takahashi K, Masuda H, Tanaka N. Kawasaki disease. With particular emphasis on arterial lesions. Acta Pathologica Japonica. 1991;41(11):785–797. doi: 10.1111/j.1440-1827.1991.tb01620.x. [DOI] [PubMed] [Google Scholar]
- 3.Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, Kazue T, Eto G, Yamakawa R. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94(6):1379–1385. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 4.Durongpisitkul K, Gururaj VJ, Park JM, Martin CF. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics. 1995;96(6):1057–1061. [PubMed] [Google Scholar]
- 5.Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315(6):341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 6.Mavrogeni S, Papadopoulos G, Karanasios E, Cokkinos DV. How to image Kawasaki disease: a validation of different imaging techniques. Int J Cardiol. 2008;124(1):27–31. doi: 10.1016/j.ijcard.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 7.Capannari TE, Daniels SR, Meyer RA, Schwartz DC, Kaplan S. Sensitivity, specificity and predictive value of two-dimensional echocardiography in detecting coronary artery aneurysms in patients with Kawasaki disease. J Am Coll Cardiol. 1986;7(2):355–360. doi: 10.1016/s0735-1097(86)80505-8. [DOI] [PubMed] [Google Scholar]
- 8.Hiraishi S, Misawa H, Takeda N, Horiguchi Y, Fujino N, Ogawa N, et al. Transthoracic ultrasonic visualisation of coronary aneurysm, stenosis, and occlusion in Kawasaki disease. Heart. 2000;83(4):400–405. doi: 10.1136/heart.83.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newburger JW, Sleeper LA, McCrindle BW, Minich LL, Gersony W, Vetter VL, et al. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med. 2007;356(7):663–675. doi: 10.1056/NEJMoa061235. [DOI] [PubMed] [Google Scholar]
- 10.McCrindle BW, Li JS, Minich LL, Colan SD, Atz AM, Takahashi M, et al. Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007;116(2):174–179. doi: 10.1161/CIRCULATIONAHA.107.690875. [DOI] [PubMed] [Google Scholar]
- 11.Baur LH, Schipperheyn JJ, van der Velde EA, van der Wall EE, Reiber JH, van der Geest RJ, et al. Reproducibility of left ventricular size, shape and mass with echocardiography, magnetic resonance imaging and radionuclide angiography in patients with anterior wall infarction. A plea for core laboratories. Int J Card Imaging. 1996;12(4):233–240. doi: 10.1007/BF01797736. [DOI] [PubMed] [Google Scholar]
- 12.Hole T, Otterstad JE, St John Sutton M, Froland G, Holme I, Skjaerpe T. Differences between echocardiographic measurements of left ventricular dimensions and function by local investigators and a core laboratory in a 2-year follow-up study of patients with an acute myocardial infarction. Eur J Echocardiogr. 2002;3(4):263–270. [PubMed] [Google Scholar]
- 13.Wong M, Staszewsky L, Volpi A, Latini R, Barlera S, Hoglund C. Quality assessment and quality control of echocardiographic performance in a large multicenter international study: Valsartan in heart failure trial (Val-HeFT) J Am Soc Echocardiogr. 2002;15(4):293–301. doi: 10.1067/mje.2001.115103. [DOI] [PubMed] [Google Scholar]
- 14.Lipshultz SE, Easley KA, Orav EJ, Kaplan S, Starc TJ, Bricker JT, Lai WW, Moodie DS, Sopko G, Schluchter MD, Colan SD. Reliability of multicenter pediatric echocardiographic measurements of left ventricular structure and function: the prospective P(2)C(2) HIV study. Circulation. 2001;104(3):310–316. doi: 10.1161/01.cir.104.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117(20):2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 16.Marwick TH. Hype and hope in the use of echocardiography for selection for cardiac resynchronization therapy: the Tower of Babel revisited. Circulation. 2008;117(20):2573–2576. doi: 10.1161/CIRCULATIONAHA.108.772475. [DOI] [PubMed] [Google Scholar]
- 17.Heistein LC, Ramaciotti C, Scott WA, Coursey M, Sheeran PW, Lemler MS. Chloral hydrate sedation for pediatric echocardiography: physiologic responses, adverse events, and risk factors. Pediatrics. 2006;117(3):e434–e441. doi: 10.1542/peds.2005-1445. [DOI] [PubMed] [Google Scholar]
- 18.Coskun S, Yuksel H, Onag A. Chloralhydrate in children undergoing echocardiography. Indian J Pediatr. 2001;68(4):319–322. doi: 10.1007/BF02721836. [DOI] [PubMed] [Google Scholar]
- 19.Lipshitz M, Marino BL, Sanders ST. Chloral hydrate side effects in young children: causes and management. Heart Lung. 1993;22(5):408–414. [PubMed] [Google Scholar]
- 20.Napoli KL, Ingall CG, Martin GR. Safety and efficacy of chloral hydrate sedation in children undergoing echocardiography. J Pediatr. 1996;129(2):287–291. doi: 10.1016/s0022-3476(96)70256-1. [DOI] [PubMed] [Google Scholar]