Abstract
The zinc finger transcription factor, Krüppel-like factor 4 (KLF4), is expressed in the post-mitotic, differentiated epithelial cells lining the intestinal tract and exhibits a tumor suppressive effect on intestinal tumorigenesis. Here we report a role for KLF4 in maintaining homeostasis of intestinal epithelial cells. Mice with conditional ablation of the Klf4 gene from the intestinal epithelium were viable. However, both the rates of proliferation and migration of epithelial cells were increased in the small intestine of mutant mice. In addition, the brush-border alkaline phosphatase was reduced as was expression of ephrine-B1 in the small intestine, resulting in mispositioning of Paneth cells. In the colon of mutant mice, there was a reduction of the differentiation marker, carbonic anhydrase-1, and failure of differentiation of goblet cells. Mechanistically, deletion of Klf4 from the intestine resulted in a general activation of genes in the Wnt pathway and a global reduction in expression of genes encoding regulators of differentiation. Taken together, these data provide new insights into the function of KLF4 in regulating postnatal proliferation, migration, differentiation, and positioning of intestinal epithelial cells and demonstrate an essential role for KLF4 in maintaining normal intestinal epithelial homeostasis in vivo.
Keywords: KLF4, Wnt, Proliferation, Differentiation, Migration, Paneth Cells, Goblet Cells
INTRODUCTION
The mammalian intestinal epithelium is a dynamic system in which cell proliferation, differentiation, migration, and apoptosis are stringently coordinated to achieve homeostasis. The epithelium of the small and large intestine consists of a crypt/villus and crypt/surface epithelium unit, respectively. The bulk of the villus and surface epithelium is composed of differentiated columnar epithelial cells that are divided into absorptive cells (enterocytes) and secretory cells (including goblet, enteroendocrine, and Paneth cells; the last unique to the small intestine). The differentiated epithelial cells are descendants of the crypt progenitor cells, which are themselves derived from the multi-potent stem cells, also located in the crypt compartment (Barker et al., 2008; Scoville et al., 2008).
The zinc finger transcription factor, Krüppel-like factor 4 (KLF4) (Garrett-Sinha et al., 1996; Shields et al., 1996), is normally expressed in the differentiated epithelial cells of the intestine, suggesting that KLF4 may function in the switch from proliferation to differentiation. In vitro, KLF4 inhibits cell proliferation by functioning as a cell cycle checkpoint protein (Chen et al., 2001; Shields et al., 1996). In vivo, KLF4 exhibits a tumor suppressive effect on intestinal tumorigenesis (Ghaleb et al., 2007). Consistent with this finding, KLF4 is down-regulated in a variety of human cancers including esophageal, gastric, colorectal, and urinary bladder cancers (Kanai et al., 2006; Ohnishi et al., 2003; Wang et al., 2002; Wei et al., 2005; Zhao et al., 2004). However, KLF4 can promote tumorigenesis in a different context, for example, in the absence of p21CIP1 (Rowland et al., 2005; Rowland and Peeper, 2006).
Delineation of the physiologic function of KLF4 in the intestinal epithelium is hampered by the early lethality of mice lacking Klf4 (Katz et al., 2002; Segre et al., 1999). Klf4-null mice die within one day after birth and suffer from a loss of barrier function of the epidermis (Segre et al., 1999). Additionally, the colon of the Klf4-null mice has a 90% reduction in the number of goblet cells, suggesting that KLF4 plays a crucial role in colonic epithelial cell differentiation in vivo (Katz et al., 2002). Mice with conditional deletion of Klf4 from specific tissues have been described. Targeted deletion of Klf4 from the stomach and esophagus causes altered differentiation and precancerous changes (Katz et al., 2005; Tetreault et al., 2010). Here, we use the Cre recombinase system under control of the villin promoter to drive tissue-specific deletion of Klf4 in the intestinal epithelium. The resultant mutant mice had significantly altered homeostasis that involved proliferation, migration, differentiation, and positioning of intestinal epithelial cells. This study provides the first definitive evidence that KLF4 exerts a crucial function in maintaining intestinal epithelial cell homeostasis in vivo.
MATERIALS AND METHODS
Generation of mice with intestine-specific deletion of the Klf4 gene
C57BL/6 mice carrying floxed Klf4 gene (Klf4fl/fl) were previously described (Katz et al., 2002). C57BL/6 mice carrying Cre recombinase gene under the regulation of villin promoter (Vil/Cre) were purchased from The Jackson Laboratory in Bar Harbor, ME (Madison et al., 2002). Mice lacking Klf4 in their intestinal epithelium were generated by mating Klf4fl/fl mice with Vil/Cre mice followed by backcrossing to obtain Vil/Cre;Klf4fl/fl mice (designated Klf4ΔIS for intestine-specific deletion). All protocols involving mouse work have been approved by the Institutional Animal Care and Use Committee of Emory University (protocols #098-2007 and 099-2007).
Histology
The small and large intestines were removed from age-matched littermates of Klf4 mutant mice (Klf4ΔIS) and control (Klf4fl/fl) mice for histological and immunohistochemical characterization of the intestinal tract. Isolated small and large intestines were flushed with modified Bouin’s fixative (50% ethanol, 5% acetic acid, and 10% formaldehyde), and cut open longitudinally for gross examination. The intestines were then rolled into a Swiss-roll, fixed, and embedded in paraffin. Five-μm sections were cut and stained with hematoxylin and eosin (H & E). Age-matched littermate control and mutant mice were examined histologically at ages 3 weeks, 4, 7, and 10 months.
Alcian blue (AB) and Periodic acid-Schiff (PAS) staining
Goblet cell staining was carried out as described before (Ghaleb et al., 2008) with slight modifications. For AB staining, sections were deparaffinized in xylene, rehydrated in ethanol and brought to distilled water for 5 minutes. AB 8GX (Biocare Medical) was applied to the sections for 15 minutes at RT, followed by a 2 minutes wash in running tap water, counterstained with Nuclear Fast Red (Biocare Medical), followed by dehydration (twice in 95% EtOH and twice in 100% EtOH) and cover-slipped. For PAS staining, deparaffinized and rehydrated sections were treated with Periodic acid (Biocare Medical) for 5 minutes at RT. Slides were washed in distilled water then stained with Schiff’s reagent (Biocare Medical) for 15 minutes at RT, followed by a 5 minutes wash in running tap water. The sections were then counterstained with hematoxylin, washed in running tap water for 2 minutes, followed by dehydration (twice in 95% EtOH and twice in 100% EtOH) and cover-slipped.
Intestinal alkaline phosphatase staining
Deparaffinized and rehydrated sections were stained for endogenous intestinal alkaline phosphatase activity using Vulcan Fast Red Chromogen kit (Biocare Medical), following manufacturer’s recommendations.
Immunohistochemistry (IHC) and immunofluorescence (IF)
Mice were sacrificed by CO2 asphyxiation prior to IHC and IF examination. The entire small intestine and colon were dissected longitudinally and washed in modified Bouin’s fixative (50% EtOH, 5% acetic acid, and 10% formaldehyde). The small intestine was divided into 3 equal segments (proximal, middle, and distal). Both the small and large intestines were cut open along their longitudinal axis, rinsed briefly in phosphate-buffered saline (PBS), and examined under a dissecting microscope. Each segment of the intestine was then rolled in a Swiss-roll to allow for histopathological examination of the entire length of both the small and large intestines. Following the dissecting microscopic examination, intestinal tissues were fixed in 10% formalin in PBS and subsequently embedded in paraffin. Five μm-thick paraffin sections were cut and applied to Superfrost Plus slides (VWR). Some sections were used for standard H&E staining. For IHC, sections were deparaffinized in xylene, incubated in 3% hydrogen peroxide in methanol for 30 minutes, rehydrated in ethanol gradient, and then treated with 10 mM Na citrate buffer, pH 6.0, at 120°C for 10 minutes (except for Muc2 which was for 1 minute) in a pressure cooker. For lysozyme staining, antigen retrieval was done by Protienase K (Millipore) digestion (1:10 dilution in PBS, for 15 min at 37°C). All histological sections were incubated with a blocking buffer (2% non-fat dry milk and 0.01% Tween 20 in PBS) for 1 hour at RT. An avidin/biotin blocking kit (Vector Laboratories) was used in conjunction with the blocking buffer according to manufacturer’s directions to reduce background and nonspecific secondary antibody binding. Sections were then stained using goat anti-KLF4 (1:300 dilution; R&D), rabbit anti-lysozyme (1:200 dilution; Dako), goat anti-LBP (1:200 dilution; Santa Cruz), rabbit anti-Muc2 (1:500 dilution; Santa Cruz), mouse anti-BrdU (1:50 dilution; BD Pharmingen), rabbit anti-ephrin-B1 (1:500 dilution; Santa Cruz), goat anti-EphB2 and goat ant-EphB3 (1:500 dilution; R&D), rabbit anti-Ki67 (1:800 dilution; Leica Microsystems), rabbit anti-chromogranin A, rabbit anti-cleaved caspase-3 (1:500 dilution; Cell Signal), and rabbit anti-colonic carbonic anhydrase-1 (1:500 dilution; Santa Cruz). Detection of primary antibodies for IHC was carried out using appropriate biotinylated secondary antibodies at 1:500 dilutions for 30 minutes at 37°C, and color development was performed using the Vectastain ABC kit (Vector Laboratories). Sections were then counterstained with hematoxylin, dehydrated, and cover-slipped. Detection of primary antibodies for IF was carried out using appropriate AlexaFluor labeled secondary antibodies (Molecular Probes) at 1:500 dilutions in 3% bovine serum albumin (BSA) in PBS for 30 minutes at 37°C, counterstained with Hoechst 33258 (2 μg/ml), mounted with Prolong gold (Molecular Probes), and cover-slipped. Images were acquired using an Axioskop 2 plus microscope (Carl Zeiss MicroImaging, Thornwood, NY, USA) equipped with an AxioCam MRc5 CCD camera (Carl Zeiss MicroImaging, Thornwood, NY, USA).
5-Bromo-2-deoxyuridine (BrdU) labeling
Mice were injected intraperitoneally (IP) with BrdU (Sigma) at 50 μg/g body weight, then sacrificed at 4 and 24 hours post-injection. Following immunostaining for BrdU, the number and position of BrdU-positive cells were counted from at least 30 crypts per mouse per genotype per time point. Statistical significance for number of BrdU positive cells was performed by t-test and for cumulative frequency, Kolmogorov-Smirnov (K-S) test.
Ki67 staining and quantification
Following immunostaining for Ki67, the number of Ki67-positive cells was counted from at least 30 crypts per mouse per genotype. Statistical analysis for number of Ki67-positive cells was performed by t-test.
Goblet cells counting and size measuring
The number and diameter of Muc2-positive cells in the colon were counted and measured from at least 30 crypts per mouse per genotype. Diameter measurement was done using AxiovisionLE software (Carl Zeiss MicroImaging). Statistical analysis for number and diameter of Muc2-positive cells was performed by t-test.
Western blot analysis
Following euthanasia, intestines were removed, flushed once with cold PBS containing protease and phosphatase inhibitors. The intestines were then cut open longitudinally, and comparable regions from the intestines of both the control and Klf4ΔIS mice were used for protein extraction. Proteins were extracted by scraping the luminal side of the intestine onto a clean glass slide, using another clean glass slide. The scraped tissue was then homogenized in lysis buffer containing 50 mM Tris-HCl (pH 6.8) and 2% sodium dodecyl sulfate (SDS). Insoluble material was removed by centrifugation at 12,000 rpm, and the supernatant was collected for protein quantification and SDS gel electrophoresis. Following protein transfer, the membrane was immunoblotted with the following primary antibodies: rabbit anti-ephrin-B1 (1:500 dilution; Santa Cruz) and mouse monoclonal anti-β actin (1:2,000 dilution; Sigma), overnight at 4 C. The membrane was incubated for 1 hour at RT with appropriate secondary antibodies, and the signal detected by chemiluminscence.
Real-time PCR analysis
Total RNA was extracted from both the small and large intestines of three Klf4fl/fl control mice and three Klf4ΔIS mice. In brief, after the mice being euthanized, 2-cm segments from the mid section of the small intestine or the colon were dissected out and quickly flushed with cold PBS containing RNaseOut (1:500; Invitrogen). The segments were then cut open, placed with luminal side facing upward on a clean glass slide on ice, and the epithelium scraped off the segment using a clean glass slide. The scraped epithelium was immediately collected in a tube containing RNA extraction buffer, Trizol (Invitrogen) and extraction proceeded according to manufacturer’s recommendations. Equal amounts of extracted RNA from mice per group were pooled for cDNA synthesis. cDNA synthesis was done using RT2 First Strand kit (SABiosciences) according to manufacturer’s recommendation. Real-Time PCR array was done using RT2 Profiler PCR Array (SABiosciences) for mouse Wnt signaling pathway (cat. # PAMM-043A-2) or ready-made individual primers encoding regulatory genes of differentiation (QuantiTect Primer Assay, Qiagen). Samples were run in triplicates on Real-time thermal cycler Mastercycler® ep realplex machine (Eppendorf).
RESULTS
Mice with intestine-specific deletion of Klf4 are viable
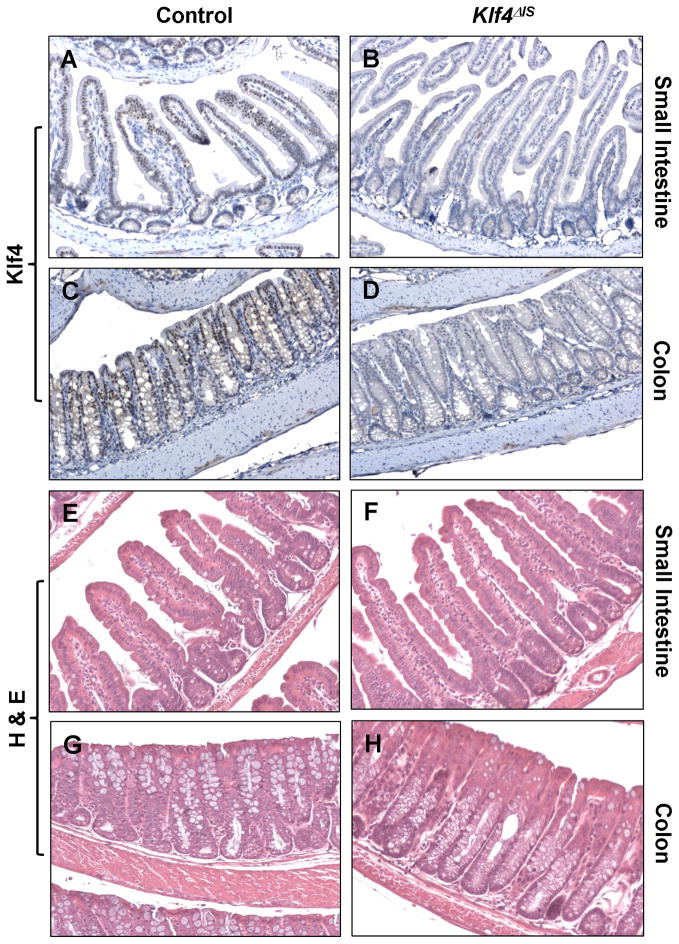
To delete Klf4 from the intestine, we employed Vil/Cre mice, which carry the Cre recombinase directed by 12.4 kb of the mouse villin promoter (Madison et al., 2002). Villin is normally expressed in the gut epithelium and the proximal tubule of the kidney beginning at embryonic day 11 (Maunoury et al., 1988; Maunoury et al., 1992). Vil/Cre mice were crossed with mice carrying floxed alleles of Klf4 (Klf4fl/fl) (Katz et al., 2005) and then backcrossed to obtain Vil/Cre;Klf4fl/fl (Klf4 ΔIS) mice. Klf4fl/fl mice without Cre served as controls. Klf4ΔIS mice were born in a Mendelian ratio, and at three weeks of age appeared grossly normal without any significant difference in weight from the control Klf4fl/fl or wild type mice. Deletion of the Klf4 gene in the intestine was confirmed by immunohistochemistry. Klf4 staining was seen in the differentiated intestinal epithelial cells of both the small and large intestines of the control mice (Fig. 1A and C), while it was absent from the epithelia of the small and large intestines of the Klf4ΔIS mice (Fig. 1B and D), confirming Cre-mediated deletion of Klf4 from the intestinal epithelium.
Figure 1. Deletion of Klf4 from the intestine Klf4ΔIS mice.
The small intestine and colon of control Klf4fl/fl mice (A and C) and Vil/Cre;Klf4fl/fl (Klf4ΔIS) mice (B and D) were immunostained with anti-Klf4 followed by secondary antibodies as described in Materials and Methods. Sections were also stained with H & E (E–H).
Macroscopically, both the small and large intestines of the Klf4ΔIS mice appeared normal. At 3 weeks of age, histology of the small intestinal epithelium of Klf4ΔIS mice appeared normal (Fig. 1F) as compared to controls (Fig. 1E). However, the colonic epithelium of the Klf4ΔIS mice had a distorted architecture with increased glandular formation and reduced numbers of goblet cells (compare Fig. 1G and H). No evidence of hypertrophy or hyperplasia was observed in either the small or large intestine of the Klf4ΔIS mice up to 10 months of age.
Intestinal deletion of Klf4 increases rates of proliferation and migration along the crypt-villus axis
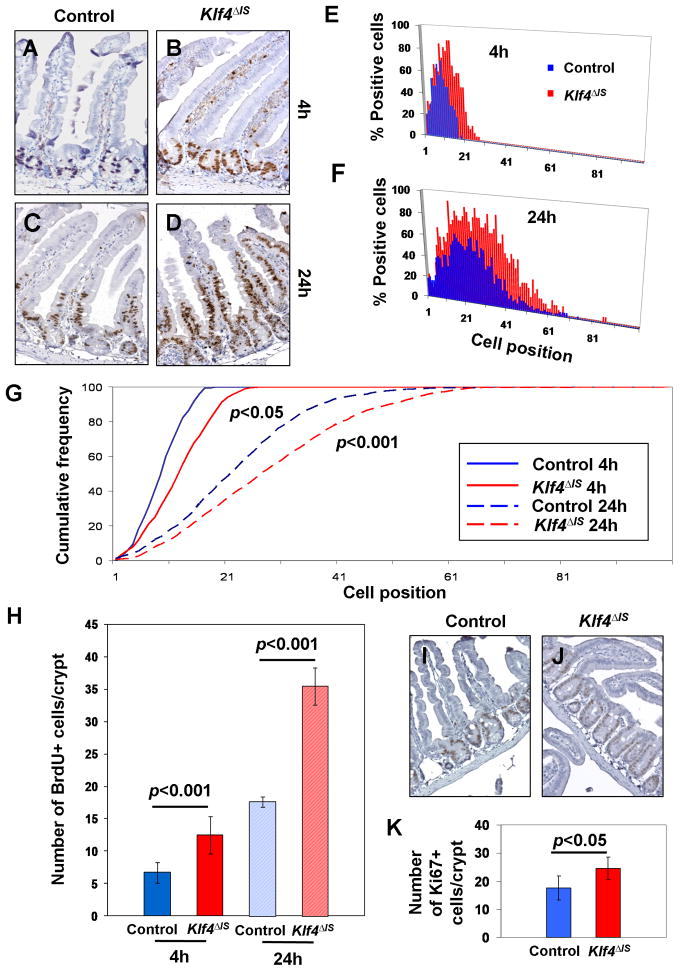
Since KLF4 negatively regulates cellular proliferation, we examined the effect of Klf4 deletion on the rate of proliferation of intestinal epithelial cells in vivo. At 3 weeks of age, mice were pulse-labeled in the S-phase with bromodeoxyuridine (BrdU) and sacrificed at 4 and 24 hours later for immunohistochemical examination. The number of BrdU-positive cells was higher in the small intestine of Klf4ΔIS mice compared to the control littermates at both 4 hours (p<0.001) and 24 hours (p<0.001) (Fig. 2A–F & H). The rate of migration of epithelial cells as defined by the cumulative frequency of BrdU-positive cells along the crypt-villus axis was also higher in the small intestine of Klf4ΔIS mice compared to the control littermates at 4 hours (p<0.05) and 24 hours (p<0.001) (Fig. 2G). There was also a significant difference (p<0.05) in the number of cells positive for the proliferation marker, Ki67, in the crypts between control and Klf4ΔIS mice (Fig. 2I–K). These results suggest that the number of progenitor cells or stem cells is increased by Klf4 deletion leading to an increase in the number of cells incorporating BrdU.
Figure 2. Intestinal deletion of Klf4 increases the rate of proliferation and migration of small intestinal epithelial cells.
Control (Klf4fl/fl) or Klf4ΔIS mice were injected with BrdU intraperitoneally and euthanized 4 or 24 hours later. Intestinal sections were immunostained for BrdU as described in Materials and Methods. Shown are representative sections of intestines from control (A and C) and Klf4ΔIS (B and D) mice at 4 and 24 hours, respectively. (E and F) are the percentages of BrdU-positive cells according to their cell positions in the crypts (position 1 is at the very bottom of the crypt) of control and Klf4ΔIS mice at 4 and 24 hours. At least 30 crypts were examined per mouse per genotype per time point. (G) Cumulative frequency of BrdU-positive cells of control and Klf4ΔIS mice along the crypt-villus axis. The rates of migration between control and Klf4ΔIS mice were significantly different at both 4 hours (p<0.05) and 24 hours (p<0.001). (H) The total number of BrdU-positive cells per crypt were compared between control and Klf4ΔIS mice at 4 and 24 hours. At least 30 crypts were examined per mouse per genotype per time point. (I and J) Representative patterns of immunostaining for Ki67 in the small intestine of control and Klf4ΔIS mice. (K) The number of Ki67-positive cells per crypt in the small intestine of control and Klf4ΔIS mice. At least 30 crypts were examined per mouse per genotype.
In contrast to the small intestine, there was no difference in the number of BrdU-positive cells in the colon of Klf4ΔIS mice compared to control mice (Suppl. Fig. S1A–C). We also stained the small and large intestines of the mutant and control mice for cleaved caspase-3 but found no difference in the rate of apoptosis between the two groups (data not shown). These results indicate that both the rates of proliferation and migration along the crypt-villus axis are increased when Klf4 is deleted from the small intestine.
Intestinal deletion of Klf4 results in altered goblet cell maturation and differentiation
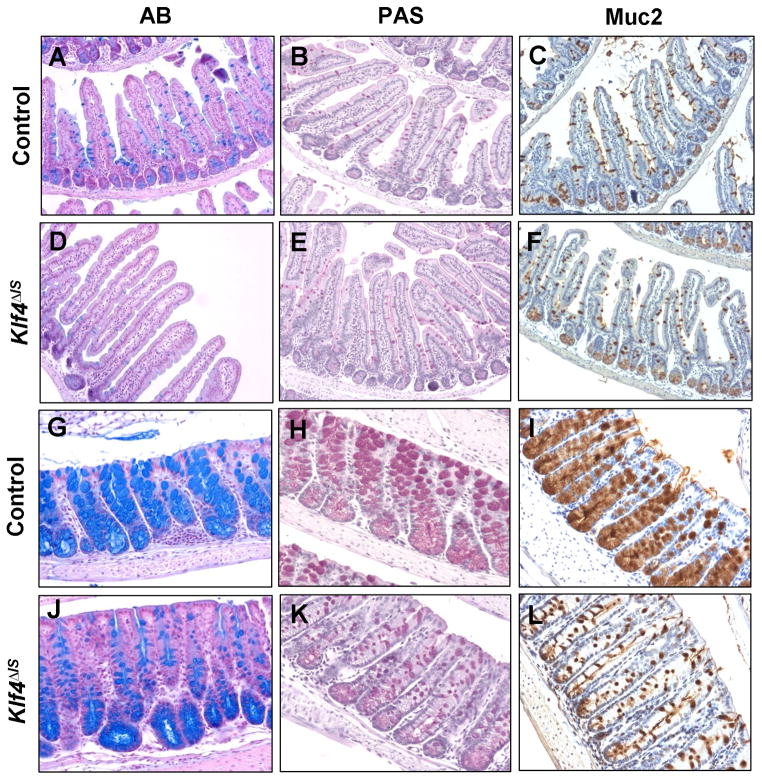
The effect of intestinal Klf4 deletion on cellular differentiation was then investigated. Differentiation of intestinal epithelial cells into goblet cells was examined by staining for acidic, neutral, and Muc2 mucins using Alcian blue (AB), Periodic-acid Schiff (PAS), and anti-Muc2 stains, respectively. Compared to the controls, Klf4ΔIS mice showed a reduction in acidic mucin expression, as demonstrated by AB staining, in the small intestine but no change in neutral mucin or Muc2 mucin expression (Fig. 3A–F). In contrast, the colon of Klf4ΔIS mice showed an overall altered goblet cell maturation and differentiation as indicated by the reduction in staining for all three mucins (Fig. 3G–L), as well as a reduction in the number and size of goblet cells, when compared to the controls (Suppl. Fig. S2A & B). These results support the previous finding that Klf4 is required for terminal differentiation of goblet cells in the colon of one day-old Klf4-null mice (Katz et al., 2002).
Figure 3. Intestinal deletion of Klf4 perturbs terminal differentiation and maturation of goblet cells.
Histochemical staining for acidic mucin by Alcian blue (AB) (A, D, G, and J), neutral mucin by Periodic acid-Schiff’s (PAS) (B, E, H, and K), and immunohistochemical staining for Muc2 mucin (C, F, I, and L) were conducted in the small (A–F) and large (G–L) intestines of control (A–C; G–I) and Klf4ΔIS (D–F; J–L) mice.
Deletion of Klf4 alters terminal differentiation of intestinal enterocytes and colonocytes
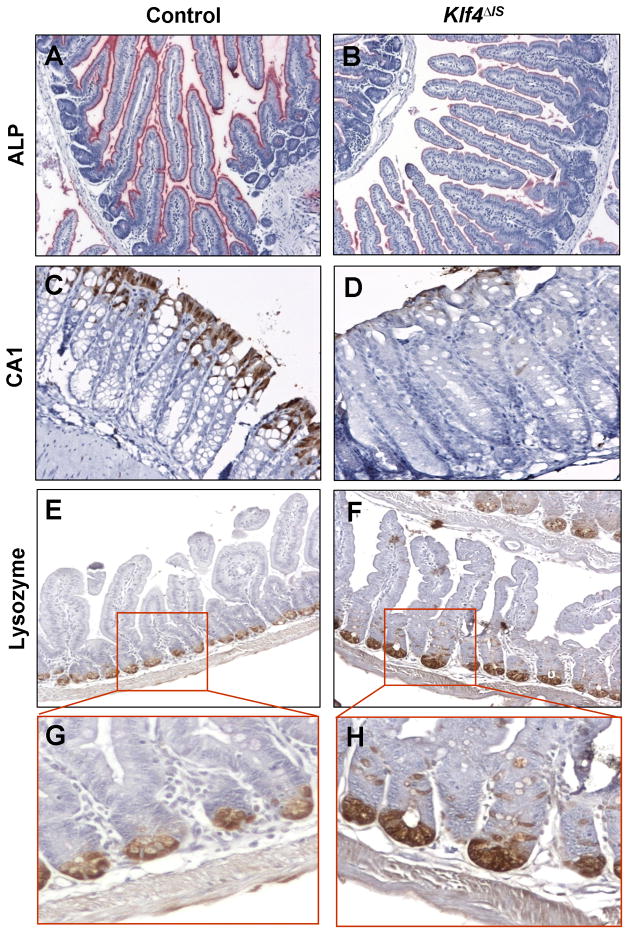
The intestinal alkaline phosphatase (ALP) gene is only expressed in differentiated enterocytes of the small intestine and has been shown to be an in vitro target gene of KLF4 (Hinnebusch et al., 2004). To investigate the effect of intestinal Klf4 deletion on the terminal differentiation of enterocytes, we stained the small intestine for ALP. Compared to the controls, the small intestine of Klf4ΔIS mice showed a reduction in ALP staining, indicating altered differentiation of intestinal enterocytes (Fig. 4A and B). In the colon, the effect of Klf4 deletion on the terminal differentiation of colonocytes was determined by staining for carbonic anhydrase-1 (CA1). CA1 is expressed in the colonic epithelium but not the small intestine (Parkkila et al., 1994; Sowden et al., 1993). The staining of CA1 in the colon of Klf4ΔIS mice varied from weak to absent (Fig. 4D), while it was present throughout the colon in the upper one-third of the colonic epithelium of control mice (Fig. 4C). These results indicate that KLF4 is required for terminal differentiation of both small intestinal enterocytes and colonocytes.
Figure 4. Intestinal Klf4 deletion results in altered terminal differentiation of enterocytes and colonocytes and mispositioning of Paneth cells.
(A and B) Alkaline phosphatase (ALP) staining of small intestinal enterocytes in control and Klf4ΔIS mice. (C and D) Carbonic anhydrase-1 (CA1) staining of colonocytes in control and Klf4ΔIS mice. (E and F) Lysozme staining for Paneth cells in the small intestines of control and Klf4ΔIS mice. (G and H) are enlargement of the inserts in panels E and F.
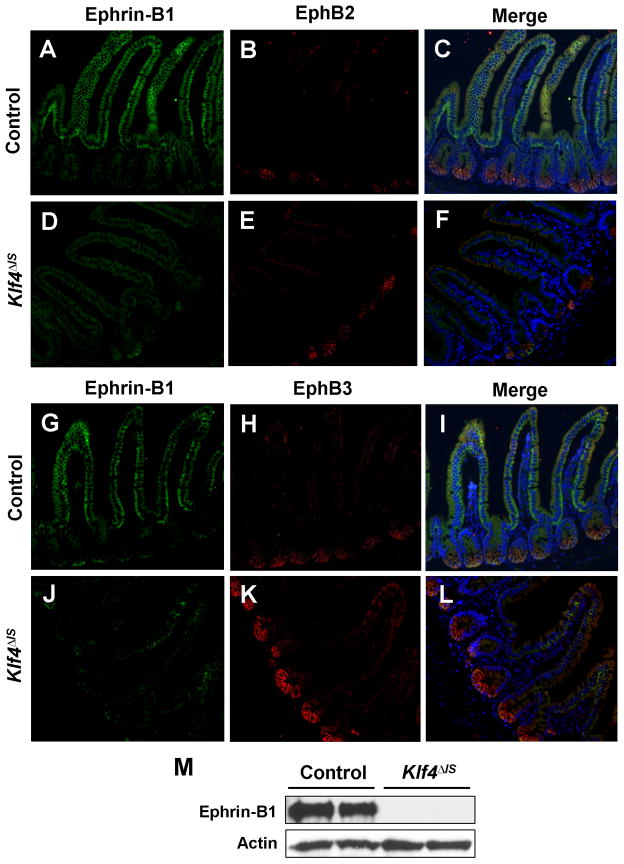
Intestinal Klf4 deletion causes mislocalization of Paneth cells and decreased ephrin-B1 expression
The effect of Klf4 deletion of enteroendocrine and Paneth cell differentiation was also investigated. As reported before (Katz et al., 2002), we found no difference in enteroendocrine cells differentiation between the intestines of control and Klf4ΔIS mice (data not shown). However, compared to the controls, Klf4ΔIS mice had mislocalized Paneth cells in the small intestine as demonstrated by the displacement of the cells normally residing at the bottom of the crypts when stained for Paneth cell markers lysozyme (Fig. 4E–H) and lipopolysaccharide-binding protein (LBP) (Suppl. Fig. S3A–D).
Ephrin-B/EphB signaling has been shown to regulate Paneth cell positioning in the intestine. In both EphB3-null mice (Batlle et al., 2002) and mice with conditional ephrin-B1 deletion (Cortina et al., 2007), Paneth cells are no longer restricted to the crypt base. To determine whether mispositioning of Paneth cells in the Klf4ΔIS mice was due to alterations in ephrin-B/EphB signaling, we stained the intestine for ephrin-B1, EphB2, and EphB3. Compared to the controls, ephrin-B1 staining in the small intestine was significantly reduced in Klf4ΔIS mice (Fig. 5A, D, G, and J); and while there was no change in EphB2 staining between the two groups (Fig. 5B and E), there was a relative increase in EphB3 staining in Klf4ΔIS mice (Fig. 5H and K). The effect Klf4 deletion on ephrin-B1 was confirmed by Western blot analysis, which showed an absence of ephrin-B1 expression from the small intestine of Klf4ΔIS mice (Fig 5M).
Figure 5. Intestinal deletion of Klf4 causes a decrease in ephrin-B1 expression in the small intestine.
Immunofluorescence staining for ephrin-B1 (A, D, G, and J), EphB2 (B and E), and EphB3 (H and K) were performed on sections of small intestines from control (A, B, G, and H) and Klf4ΔIS (D, E, J, and K) mice. Panels C, F, I, and L are merged images. (M) Western blot analysis of ephrin-B1 and actin in small intestines of control and Klf4ΔIS mice. Shown are representative results of two individual mice from each group.
Intestinal Klf4 deletion leads to perturbation of proliferation and differentiation pathways
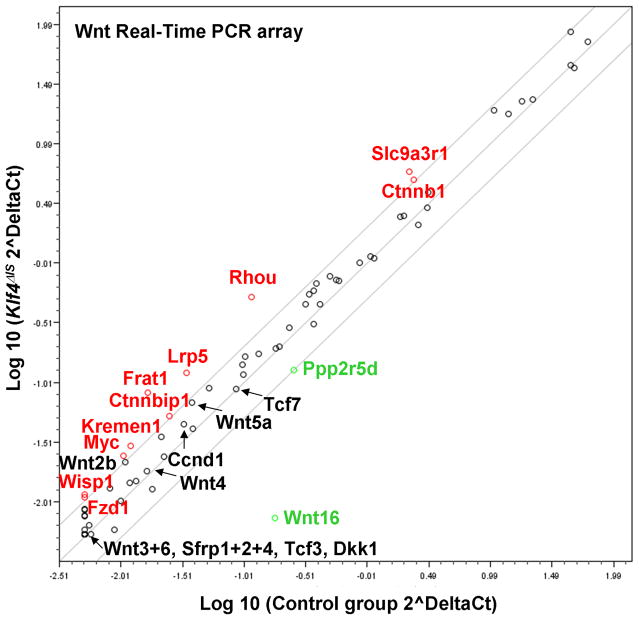
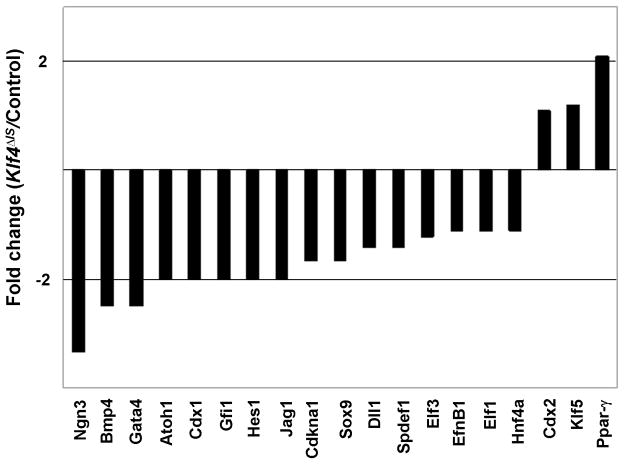
Since deletion of Klf4 from the small intestine results in increased epithelial proliferation, we examined the consequence of Klf4 deletion on expression of components of the Wnt pathway using a real-time PCR array. As shown in Fig. 6 and Suppl. Table S1, there was an overall increase in the transcript levels of many of the genes in the Wnt pathway, including β-catenin (Ctnnb1) and c-Myc, in the small intestine of Klf4 ΔIS mice compared to controls. This is consistent with the observed increase in cellular proliferation in the small intestinal epithelium in the absence of Klf4. The result also suggests that Klf4 is a negative regulator of Wnt signaling in vivo. Because deletion of Klf4 appears to perturb differentiation more in the colon than the small intestine, we compared the transcript levels of a panel of genes involved in regulation of differentiation between Klf4 ΔIS and control mice. As seen in Fig. 7, there was a general decrease in the expression levels of these genes in the colon of Klf4 ΔIS mice compared to controls. This result suggests that Klf4 is involved in global regulation of colonocyte differentiation.
Figure 6. Comparison of expression of Wnt pathway genes in the small intestines of Klf4ΔIS and control mice.
Shown is a scatter plot of expression levels of Wnt pathway genes in the intestines of Klf4 mutant and control mice as determined by real-time PCR analysis. The analysis was conducted with pooled RNA specimens from three mice for each genotype. Transcripts with two or more fold-increase in the Klf4 mutant mice compared to the controls are labeled in red and those with two or more fold-decrease in the Klf4 mutant mice are labeled in green. Detailed results are shown in Suppl. Table S1.
Figure 7. Comparison of expression of genes involved in regulating differentiation in the colon of Klf4ΔIS and control mice.
Real-time PCR analysis of transcript levels in pooled RNA specimens of colonic epithelial cells from three mutant or control mice was conducted on a panel of genes that are involved in regulating epithelial differentiation. Shown are the fold-changes in transcript levels between Klf4ΔIS and control mice.
DISCUSSION
Much has been learned in the recent past about the mechanisms by which differentiation and development of the intestinal tract are regulated (Barker et al., 2008; de Santa Barbara et al., 2003; Hauck et al., 2005; Jedlicka and Gutierrez-Hartmann, 2008; Scoville et al., 2008). It is clear from these studies that several signaling pathways including Wnt, Notch, hedgehog, and bone morphogenetic protein (BMP), play critical roles in the regulatory process. The roles of individual regulatory proteins in the epithelium in controlling proliferation, differentiation, and migration of intestinal epithelial cells, however, are still not well defined. Using targeted deletion of Klf4 from the intestinal epithelium, we demonstrate for the first time that the zinc finger transcription factor, KLF4, has a crucial role in regulating intestinal epithelial cell homeostasis in vivo.
Previous studies indicate that KLF4 is mainly expressed in the post-mitotic, differentiated epithelial cells in the small intestine and colon (McConnell et al., 2007; Shields et al., 1996). Consistent with this observation, expression of KLF4 is associated with growth arrest in vitro (Shields et al., 1996) and forced expression of KLF4 leads to cell cycle arrest (Chen et al., 2001; Shields et al., 1996). Results of the current study showing that conditional deletion of Klf4 from the small intestinal epithelium causes an increased rate of proliferation (Fig. 2) further substantiate the inhibitory function of KLF4 on cell proliferation. These results suggest that one of KLF4’s physiologic functions in the intestinal tract is to regulate proliferation of stem cells or progenitor cells and that its deletion results in an increase in the rate of the division of such cells. This increase could also explain the observed increase in migration of epithelial cells along the crypt-villus axis. In contrast, these changes were not observed in the colonic epithelial cells, suggesting that additional mechanisms may guard against increased proliferation of colonic epithelial cells. The increased proliferation of small intestinal epithelial cells following loss of Klf4 is similar to that observed in mice with conditional deletion of Klf4 from the esophagus and stomach (Katz et al., 2005; Tetreault et al., 2010).
Homeostasis in epithelial tissues is balanced between proliferation and apoptosis (Guasch et al., 2007; Squier and Kremer, 2001; Walker and Stappenbeck, 2008). This is demonstrated by the finding that conditional deletion of the tumor suppressor gene, Apc, from the small intestine led to an increase in both proliferation and apoptosis (Sansom et al., 2004). In contrast, loss of Klf4 did not result in an increased rate of apoptosis in the intestine. We also did not observe any intestinal epithelial hyperplasia as a consequence of Klf4 deletion, as might be expected from the combination of increased proliferation and unchanged apoptotic rates. This could potentially be explained by the increased migratory rate, which may be accompanied by increased shedding of epithelial cells into the lumen. It is worth noting that mice with intestine-specific deletion of Apc was accompanied by abrogated epithelial cell migration along the crypt-villus axis, which led to the retention of Apc mutant cells, allowing them to acquire additional genetic changes and produce daughter cells that exhibit properties of premalignant cells (Sansom et al., 2004). In this regard, the increased migration of epithelial cells in the intestine of Klf4 mutant mice may offset the acquisition of any genetically altered premalignant cells by increasing their migration and eventual shedding in the lumen. This may also explain the lack of any intestinal neoplasia from the intestine of Klf4 mutant mice up to 10 months of life. A similar finding of increased proliferation with unchanged apoptosis has been observed in the intestine of mice with conditional deletion of the transcription factor, Math1 (Shroyer et al., 2007).
Despite the differences in apoptosis and migration of intestinal epithelial cells, Klf4 mutant mice shared several features with those with intestine-specific deletion of Apc, aside from the increased epithelial cell proliferation. For example, Apc mutant mice had reduced staining for alkaline phosphatase in the brush border, reduced goblet cells, and mispositioned Paneth cells in their small intestine (Sansom et al., 2004). The last observation is particularly intriguing as the mispositioning of Paneth cells in the Apc mutant mice is a consequence of perturbed ephrin-B/EphB signaling system (Sansom et al., 2004). In the intestine of Apc mutant mice, both EphB2 and EphB3 are up-regulated while ephrin-B2 is down-regulated. This is similar to our findings of up-regulation of EphB3 and down-regulation of ephrin-B1 in the intestine lacking Klf4 (Fig. 5). A similar phenotype of mispositioned Paneth cells has also been described in the intestine of mice null for EphB3 (Batlle et al., 2002) or with conditional deletion of ephrin-B1 (Cortina et al., 2007). These studies underscore the importance of the ephrin-B/EphB repulsion system in determining cell positioning. Significantly, consistent with previous reports that KLF4 is a downstream mediator of APC (Dang et al., 2001; Stone et al., 2002; Zhang et al., 2006), the phenotypic overlaps between Klf4 and Apc mutant mouse intestines lend further support to this notion. To this end, KLF4, ephrin-B1, intestinal alkaline phosphatase, Muc2, and p21CIP1 are among the highest up-regulated markers of intestinal differentiation in colorectal cancer cells with abrogated Wnt signaling (which functions in opposition to APC) (van de Wetering et al., 2002). Previous studies have already established that KLF4 directly regulates intestinal alkaline phosphatase and p21CIP1 expression (Chen et al., 2001; Chen et al., 2003; Hinnebusch et al., 2004; Zhang et al., 2000). The results of the current study suggest that Klf4 is also responsible for the expression of ephrin-B1 in the small intestinal epithelium (Fig. 5) and possibly Muc2 and CA-1 in the colonic epithelium (Figs. 3 and 4). In addition, results of our recent microarray analysis showed that transcripts for ephrin-B1, B2, and B4 were among those down-regulated in mouse embryonic fibroblasts null for Klf4 (Hagos et al., 2010), suggesting that Klf4 regulates expression of the ephrin-B family of ligands, perhaps at the transcriptional level. Finally, the observation that Klf4 deletion leads to a general up-regulation of genes in the Wnt pathway (Fig. 6 and Suppl. Table S1) is highly suggestive that Klf4 is involved in the network of Wnt signaling.
Results of our study also suggest that intestine-specific Klf4 deletion alters differentiation and maturation of goblet cells, albeit to a different extent between the small intestine and colon (Fig. 3). In the small bowel of Klf4 mutant mice, there is a reduction in staining for acidic mucin (as demonstrated by the Alcian blue stain) but no change in staining for neutral mucin (as demonstrated by the PAS stain) and the goblet cell-specific marker, Muc2. As acidic mucin is considered the more mature form of mucin produced by goblet cells (Fontaine et al., 1998), these findings suggest that Klf4 is required for maturation of goblet cells in the small intestine. In the colon, Klf4 is required not only for the maturation but differentiation of goblet cells as demonstrated by the significant reduction in the number and size of goblet cells, as well as in staining for Muc2, and both acidic and neural mucins in the colon of Klf4ΔIS mice compared to controls (Fig. 3 and Suppl. Fig. S2). These results are similar to those observed in the colon of one day-old newborn mice lacking Klf4 (Klf4−/−) (Katz et al., 2002). Combining the results of these studies, it is apparent that Klf4 functions as a colonic goblet cell-differentiation factor in vivo.
Perturbations in goblet cell homeostasis in the intestine have been described in several other mouse models in which specific genes were deleted from the intestine. Mice with intestine-specific ablation of Math1 (Atoh1) lost cells of the secretory lineage from their intestine including goblet, Paneth, and enteroendocrine cells (Shroyer et al., 2007). The transcription repressor, Gfi1, functions downstream of Math1 in intestinal secretory lineage differentiation–Gfi1-null mice lacked Paneth cells, had fewer goblet cells, and supernumerary enteroendocrine cells (Shroyer et al., 2005). Another transcription factor, Spdef, when over-expressed in the intestine, caused the expansion of goblet cells and a corresponding reduction in Paneth and enteroendocrine cells (Noah et al., 2010). It is of interest to note that the transcript levels of several factors involved in goblet cell differentiation including Atoh1, Gfi1, Spdef1 and Elf3, are lower in the colonic epithelial cells from Klf4 mutant mice than controls (Fig. 7). In addition, Klf4 deletion results in the down-regulation of several other genes known to regulate epithelial differentiation such as Ngn3, Bmp4, Gata4, Sox9 and Hnf4a (Fig. 7). These results suggest that Klf4 has a global function in regulating epithelial differentiation, which may be linked to its ability to regulate epithelial proliferation.
The down-regulation of expression of some components of the Notch pathway such as Hes1, Jag1 and Dll1 (Fig. 7), is somewhat unexpected since Notch signaling has been shown to inhibit differentiation of the secretory lineage including goblet cells (Jensen et al., 2000; van Es et al., 2005). Notch regulates terminal differentiation by Hes1-dependent repression of Math1, which is required for commitment to the secretory cell lineage in the mouse intestine (Yang et al., 2001). Notch also inhibits expression of KLF4 in colonic epithelial cells and in the mouse intestine (Ghaleb et al., 2008; Zheng et al., 2009). It is possible that KLF4 is involved in a feedback loop by positively regulating Notch signaling, thus explaining the reduction in transcript levels of the Notch pathway components in the colon of Klf4 mutant mice (Fig. 7). However, the concomitant reduction in the transcript levels of Atoh1, which is required for the differentiation effects of Notch inhibitors (Kazanjian et al., 2010), in Klf4 mutant mice may account for the lack of goblet cell differentiation in the colon.
In contrast to the previous finding that haploinsufficiency of Klf4 results in increased intestinal tumor formation in the ApcMin mouse background (Ghaleb et al., 2007), Klf4ΔIS mice remain tumor-free up to 10 months of age despite evidence for increased intestinal epithelial proliferation. These results suggest that Klf4 deletion alone is not sufficient to initiate tumor formation in the mouse, and that it may require a “second hit” in order to do so. We can also conclude that perturbed maturation and differentiation of goblet cells in the colon of Klf4ΔIS mice, which also occurs in Klf4−/− newborn mice, does not play a role in the premature death of Klf4-null mice (Katz et al., 2002; Segre et al., 1999). The additional phenotypic changes such as increased proliferation and migration of epithelial cells observed in the Klf4ΔIS mice but not in Klf4−/− mice likely reflect a true physiologic function of Klf4 in the intestine since the former survived to adulthood. Additionally, ablation of goblet cells from the colon results in a reduction in susceptibility to chemical-induced colonic injury (Itoh et al., 1999). On the other hand, chronic inflammation such as ulcerative colitis is characterized by goblet cell depletion (Podolsky, 1997; Tytgat et al., 1996a; Tytgat et al., 1996b; Van Klinken et al., 1999) and loss of Muc2 in mice leads to the development of spontaneous colitis and rectal tumors on a permissive genetic background (Chutkan, 2001; Van der Sluis et al., 2006; Velcich et al., 2002). The physiologic role of KLF4 in mediating the epithelial response to injury therefore remains to be determined.
CONCLUSION
The results of our study provide new insights into the in vivo function of KLF4 in the postnatal proliferation, migration, differentiation, and positioning of intestinal epithelial cells, and highlight an essential role for KLF4 in maintaining normal intestinal epithelial cell homeostasis.
Supplementary Material
Acknowledgments
We thank Dr. Jonathan Katz for providing reagents and helpful input into the manuscript. This work was in part contributed by grants from the Nationals Institutes of Health (DK52230, DK64399, DK76742, CA84197, and CA130308).
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- Chen X, Johns DC, Geiman DE, Marban E, Dang DT, Hamlin G, Sun R, Yang VW. Kruppel-like factor 4 (gut-enriched Kruppel-like factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle. J Biol Chem. 2001;276:30423–30428. doi: 10.1074/jbc.M101194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Whitney EM, Gao SY, Yang VW. Transcriptional profiling of Kruppel-like factor 4 reveals a function in cell cycle regulation and epithelial differentiation. J Mol Biol. 2003;326:665–677. doi: 10.1016/S0022-2836(02)01449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutkan RK. Inflammatory bowel disease. Prim Care. 2001;28:539–556. vi. doi: 10.1016/s0095-4543(05)70052-x. [DOI] [PubMed] [Google Scholar]
- Cortina C, Palomo-Ponce S, Iglesias M, Fernandez-Masip JL, Vivancos A, Whissell G, Huma M, Peiro N, Gallego L, Jonkheer S, Davy A, Lloreta J, Sancho E, Batlle E. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet. 2007;39:1376–1383. doi: 10.1038/ng.2007.11. [DOI] [PubMed] [Google Scholar]
- Dang DT, Mahatan CS, Dang LH, Agboola IA, Yang VW. Expression of the gut-enriched Kruppel-like factor (Kruppel-like factor 4) gene in the human colon cancer cell line RKO is dependent on CDX2. Oncogene. 2001;20:4884–4890. doi: 10.1038/sj.onc.1204645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Santa Barbara P, van den Brink GR, Roberts DJ. Development and differentiation of the intestinal epithelium. Cell Mol Life Sci. 2003;60:1322–1332. doi: 10.1007/s00018-003-2289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine N, Meslin JC, Dore J. Selective in vitro degradation of the sialylated fraction of germ-free rat mucins by the caecal flora of the rat. Reprod Nutr Dev. 1998;38:289–296. doi: 10.1051/rnd:19980309. [DOI] [PubMed] [Google Scholar]
- Garrett-Sinha LA, Eberspaecher H, Seldin MF, de Crombrugghe B. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J Biol Chem. 1996;271:31384–31390. doi: 10.1074/jbc.271.49.31384. [DOI] [PubMed] [Google Scholar]
- Ghaleb AM, Aggarwal G, Bialkowska AB, Nandan MO, Yang VW. Notch inhibits expression of the Kruppel-like factor 4 tumor suppressor in the intestinal epithelium. Mol Cancer Res. 2008;6:1920–1927. doi: 10.1158/1541-7786.MCR-08-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaleb AM, McConnell BB, Nandan MO, Katz JP, Kaestner KH, Yang VW. Haploinsufficiency of Kruppel-like factor 4 promotes adenomatous polyposis coli dependent intestinal tumorigenesis. Cancer Res. 2007;67:7147–7154. doi: 10.1158/0008-5472.CAN-07-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch G, Schober M, Pasolli HA, Conn EB, Polak L, Fuchs E. Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell. 2007;12:313–327. doi: 10.1016/j.ccr.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagos GH, Ghaleb AM, Kumar A, Neish AS, Yang VW. Expression profiling and pathway analysis of Krüppel-like factor 4 in mouse embrynoic fibroblasts. Amer J Cancer Res. 2010 in press. [PMC free article] [PubMed] [Google Scholar]
- Hauck AL, Swanson KS, Kenis PJ, Leckband DE, Gaskins HR, Schook LB. Twists and turns in the development and maintenance of the mammalian small intestine epithelium. Birth Defects Res C Embryo Today. 2005;75:58–71. doi: 10.1002/bdrc.20032. [DOI] [PubMed] [Google Scholar]
- Hinnebusch BF, Siddique A, Henderson JW, Malo MS, Zhang W, Athaide CP, Abedrapo MA, Chen X, Yang VW, Hodin RA. Enterocyte differentiation marker intestinal alkaline phosphatase is a target gene of the gut-enriched Kruppel-like factor. Am J Physiol Gastrointest Liver Physiol. 2004;286:G23–30. doi: 10.1152/ajpgi.00203.2003. [DOI] [PubMed] [Google Scholar]
- Itoh H, Beck PL, Inoue N, Xavier R, Podolsky DK. A paradoxical reduction in susceptibility to colonic injury upon targeted transgenic ablation of goblet cells. J Clin Invest. 1999;104:1539–1547. doi: 10.1172/JCI6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedlicka P, Gutierrez-Hartmann A. Ets transcription factors in intestinal morphogenesis, homeostasis and disease. Histol Histopathol. 2008;23:1417–1424. doi: 10.14670/hh-23.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Kanai M, Wei D, Li Q, Jia Z, Ajani J, Le X, Yao J, Xie K. Loss of Kruppel-like factor 4 expression contributes to Sp1 overexpression and human gastric cancer development and progression. Clin Cancer Res. 2006;12:6395–6402. doi: 10.1158/1078-0432.CCR-06-1034. [DOI] [PubMed] [Google Scholar]
- Katz JP, Perreault N, Goldstein BG, Actman L, McNally SR, Silberg DG, Furth EE, Kaestner KH. Loss of Klf4 in mice causes altered proliferation and differentiation and precancerous changes in the adult stomach. Gastroenterology. 2005;128:935–945. doi: 10.1053/j.gastro.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, Kaestner KH. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanjian A, Noah T, Brown D, Burkart J, Shroyer NF. Atonal homolog 1 is required for growth and differentiation effects of notch/gamma-secretase inhibitors on normal and cancerous intestinal epithelial cells. Gastroenterology. 2010;139:918–928. 928, e911–916. doi: 10.1053/j.gastro.2010.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- Maunoury R, Robine S, Pringault E, Huet C, Guenet JL, Gaillard JA, Louvard D. Villin expression in the visceral endoderm and in the gut anlage during early mouse embryogenesis. EMBO J. 1988;7:3321–3329. doi: 10.1002/j.1460-2075.1988.tb03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunoury R, Robine S, Pringault E, Leonard N, Gaillard JA, Louvard D. Developmental regulation of villin gene expression in the epithelial cell lineages of mouse digestive and urogenital tracts. Development. 1992;115:717–728. doi: 10.1242/dev.115.3.717. [DOI] [PubMed] [Google Scholar]
- McConnell BB, Ghaleb AM, Nandan MO, Yang VW. The diverse functions of Kruppel-like factors 4 and 5 in epithelial biology and pathobiology. Bioessays. 2007;29:549–557. doi: 10.1002/bies.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noah TK, Kazanjian A, Whitsett J, Shroyer NF. SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp Cell Res. 2010;316:452–465. doi: 10.1016/j.yexcr.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi S, Ohnami S, Laub F, Aoki K, Suzuki K, Kanai Y, Haga K, Asaka M, Ramirez F, Yoshida T. Downregulation and growth inhibitory effect of epithelial-type Kruppel-like transcription factor KLF4, but not KLF5, in bladder cancer. Biochem Biophys Res Commun. 2003;308:251–256. doi: 10.1016/s0006-291x(03)01356-1. [DOI] [PubMed] [Google Scholar]
- Parkkila S, Parkkila AK, Juvonen T, Rajaniemi H. Distribution of the carbonic anhydrase isoenzymes I, II, and VI in the human alimentary tract. Gut. 1994;35:646–650. doi: 10.1136/gut.35.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky DK. Lessons from genetic models of inflammatory bowel disease. Acta Gastroenterol Belg. 1997;60:163–165. [PubMed] [Google Scholar]
- Rowland BD, Bernards R, Peeper DS. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat Cell Biol. 2005;7:1074–1082. doi: 10.1038/ncb1314. [DOI] [PubMed] [Google Scholar]
- Rowland BD, Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer. 2006;6:11–23. doi: 10.1038/nrc1780. [DOI] [PubMed] [Google Scholar]
- Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS, Clarke AR, Winton DJ. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Kruppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroyer NF, Helmrath MA, Wang VY, Antalffy B, Henning SJ, Zoghbi HY. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology. 2007;132:2478–2488. doi: 10.1053/j.gastro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- Shroyer NF, Wallis D, Venken KJ, Bellen HJ, Zoghbi HY. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev. 2005;19:2412–2417. doi: 10.1101/gad.1353905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowden J, Leigh S, Talbot I, Delhanty J, Edwards Y. Expression from the proximal promoter of the carbonic anhydrase 1 gene as a marker for differentiation in colon epithelia. Differentiation. 1993;53:67–74. doi: 10.1111/j.1432-0436.1993.tb00647.x. [DOI] [PubMed] [Google Scholar]
- Squier CA, Kremer MJ. Biology of oral mucosa and esophagus. J Natl Cancer Inst Monogr. 2001:7–15. doi: 10.1093/oxfordjournals.jncimonographs.a003443. [DOI] [PubMed] [Google Scholar]
- Stone CD, Chen ZY, Tseng CC. Gut-enriched Kruppel-like factor regulates colonic cell growth through APC/beta-catenin pathway. FEBS Lett. 2002;530:147–152. doi: 10.1016/s0014-5793(02)03449-x. [DOI] [PubMed] [Google Scholar]
- Tetreault MP, Yang Y, Travis J, Yu QC, Klein-Szanto A, Tobias JW, Katz JP. Esophageal squamous cell dysplasia and delayed differentiation with deletion of Klf4 in murine esophagus. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat KM, Opdam FJ, Einerhand AW, Buller HA, Dekker J. MUC2 is the prominent colonic mucin expressed in ulcerative colitis. Gut. 1996a;38:554–563. doi: 10.1136/gut.38.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat KM, van der Wal JW, Einerhand AW, Buller HA, Dekker J. Quantitative analysis of MUC2 synthesis in ulcerative colitis. Biochem Biophys Res Commun. 1996b;224:397–405. doi: 10.1006/bbrc.1996.1039. [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- Van Klinken BJ, Van der Wal JW, Einerhand AW, Buller HA, Dekker J. Sulphation and secretion of the predominant secretory human colonic mucin MUC2 in ulcerative colitis. Gut. 1999;44:387–393. doi: 10.1136/gut.44.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- Walker MR, Stappenbeck TS. Deciphering the ‘black box’ of the intestinal stem cell niche: taking direction from other systems. Curr Opin Gastroenterol. 2008;24:115–120. doi: 10.1097/MOG.0b013e3282f4954f. [DOI] [PubMed] [Google Scholar]
- Wang N, Liu ZH, Ding F, Wang XQ, Zhou CN, Wu M. Down-regulation of gut-enriched Kruppel-like factor expression in esophageal cancer. World J Gastroenterol. 2002;8:966–970. doi: 10.3748/wjg.v8.i6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D, Gong W, Kanai M, Schlunk C, Wang L, Yao JC, Wu TT, Huang S, Xie K. Drastic down-regulation of Kruppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res. 2005;65:2746–2754. doi: 10.1158/0008-5472.CAN-04-3619. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- Zhang W, Chen X, Kato Y, Evans PM, Yuan S, Yang J, Rychahou PG, Yang VW, He X, Evers BM, Liu C. Novel cross talk of Kruppel-like factor 4 and beta-catenin regulates normal intestinal homeostasis and tumor repression. Mol Cell Biol. 2006;26:2055–2064. doi: 10.1128/MCB.26.6.2055-2064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Geiman DE, Shields JM, Dang DT, Mahatan CS, Kaestner KH, Biggs JR, Kraft AS, Yang VW. The gut-enriched Kruppel-like factor (Kruppel-like factor 4) mediates the transactivating effect of p53 on the p21WAF1/Cip1 promoter. J Biol Chem. 2000;275:18391–18398. doi: 10.1074/jbc.C000062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Hisamuddin IM, Nandan MO, Babbin BA, Lamb NE, Yang VW. Identification of Kruppel-like factor 4 as a potential tumor suppressor gene in colorectal cancer. Oncogene. 2004;23:395–402. doi: 10.1038/sj.onc.1207067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Pritchard DM, Yang X, Bennett E, Liu G, Liu C, Ai W. KLF4 gene expression is inhibited by the notch signaling pathway that controls goblet cell differentiation in mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G490–498. doi: 10.1152/ajpgi.90393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.