Abstract
Arabidopsis thaliana SUPERMAN (SUP) plays an important role during flower development by maintaining the boundary between stamens and carpels in the inner two whorls. It was proposed that SUP maintains this boundary by regulating cell proliferation in both whorls, as loss-of-function superman mutants produce more stamens at the expense of carpels. However, the cellular mechanism that underlies SUP function remains unknown. Here Arabidopsis or tobacco (Nicotiana tabacum) SUP was overexpressed in tobacco plants to substantiate SUP's role as a regulator of cell proliferation and boundary definition and provide evidence that its biological role may be mediated via hormonal changes. It was found that moderate levels of SUP stimulated cell growth and proliferation, whereas high levels were inhibitory. SUP stimulated auxin- and cytokinin-regulated processes, and cells overexpressing SUP displayed reduced hormone dependency for proliferation and regeneration into plants. SUP also induced proliferation of female traits in the second and third flower whorls and promoted differentiation of petaloid properties in sepals, further supporting a role for SUP as a boundary regulator. Moreover, cytokinin suppressed stamen development and promoted differentiation of carpeloid tissues, suggesting that SUP may regulate male and female development via its effect on cytokinin signalling. Taken together, these observations suggest a model whereby the effect of SUP on cell growth and proliferation involves the modulation of auxin- and cytokinin-regulated processes. Furthermore, differential SUP expression or different sensitivities of different cell types to SUP may determine whether SUP stimulates or suppresses their proliferation.
Keywords: Auxin, cadastral genes, cell proliferation, cytokinin, flower development, SUPERMAN
Introduction
In many angiosperms, including Arabidopsis thaliana, flowers consist of four types of organs arranged into concentric whorls. From the outside to the inside of the flower, sepals, petals, stamens, and carpels can be distinguished and are referred to as whorls 1–4, respectively. Flower formation results from the sequential activity of floral meristem identity genes that specify floral meristem fate and of floral organ identity genes that determine the pattern of whorl formation in the flower. The function of floral meristem identity genes is to activate floral developmental programmes by activating floral organ identity genes. Where and which whorls form in the flower is controlled by the activity of three classes of homeotic floral organ identity genes, designated A, B, and C. The combinatorial interaction between these genes underlies the classic ABC model for flower development (Coen and Meyerowitz, 1991; Gustafson-Brown et al., 1994; Weigel and Meyerowitz, 1994). In Arabidopsis, the activity of the class A genes AP1 and APETALA2 (AP2) specifies sepal identity, while the simultaneous expression of class A and class B genes, APETALA3 (AP3) and PISTILLATA (PI), determines petal identity. B function combined with C function, conferred by the class C gene AGAMOUS (AG), specifies stamen identity, while C function alone specifies carpel identity. An additional class of floral organ identity genes, called E class genes, was subsequently identified; these are necessary for the specification of the inner three whorls (Ferrario et al., 2004).
The ABC model implies that expression patterns of floral organ identity genes must be tightly regulated both temporally and spatially. This is achieved by the activity of transcriptional regulators such as the floral meristem identity genes, and also by post-transcriptional regulation (Ng and Yanofsky, 2001; Aukerman and Sakai, 2003; Chen, 2004). Another level of regulation comes from the activity of cadastral genes that maintain whorl boundaries. One of these genes is SUPERMAN (SUP). Flowers of the superman mutant in Arabidopsis develop extra stamens at the expense of carpels (Schultz et al., 1991; Bowman et al., 1992; Sakai et al., 1995). In these mutant flowers, expression of the class B genes AP3 and PI is not only detected in the second and third whorls as expected, but also expands into the fourth whorl, leading to the formation of extra stamens; carpels either do not develop or are defective (Schultz et al., 1991; Bowman et al., 1992; Sakai et al., 1995). SUP functions are conserved in other plants, as illustrated by the petunia orthologue of SUP (PhSUP1). Plants lacking PhSUP1 also had increased stamen numbers and aberrant carpel and ovule development (Nakagawa et al., 2004).
SUP encodes a zinc-finger protein that has been proposed to act as a transcriptional repressor through a conserved motif at its C-terminus (Sakai et al., 1995; Hiratsu et al., 2002). In situ hybridization and promoter:GUS (β-glucuronidase) fusion analyses showed that SUP expression is restricted to the inner part of whorl 3 adjacent to whorl 4 (Sakai et al., 2000; Ito et al., 2003). It has been proposed that SUP controls the balance of cell proliferation at the boundary between stamens and carpels by regulating the transcription of genes that affect cell division (Hiratsu et al., 2002). In the absence of SUP, whorl 3 cells overproliferated at the expense of whorl 4 cells (Sakai et al., 1995, 2000). How this is achieved remains unclear. Genetic studies also established that SUP is present for a very short time, although the effects of SUP activity are required throughout flower development (Sakai et al., 2000). This suggests that SUP triggers a signalling cascade that is maintained after SUP expression ceases.
Ectopic expression of SUP in the flower affected not only organ identity but also organ size and number, reflecting an effect of SUP on cell division and/or cell expansion (Kater et al., 2000; Yun et al., 2002). When expressed ectopically throughout the entire plant, SUP causes dwarfism in tobacco and Arabidopsis plants, and this was attributed to an inhibitory effect on cell proliferation, expansion, and differentiation (Bereterbide et al., 2001; Hiratsu et al., 2002). Similar effects were observed when the PhSUP1 gene was ectopically expressed in petunia (Nakagawa et al., 2004). In rice, high levels of SUP expression caused dwarfism, while lower levels induced many floral defects, some suggesting increased feminization (Nandi et al., 2000).
Despite numerous studies suggesting a role for SUP as a regulator of cell division, growth, and differentiation (Gaiser et al., 1995; Kater et al., 2000; Bereterbide et al., 2001; Hiratsu et al., 2002; Yun et al., 2002), it is not yet known how this regulation is achieved or what the downstream effectors of SUP activity are. It is shown here that the function of SUP in cell proliferation and female tissue differentiation may be mediated by regulating auxin- and cytokinin-controlled processes. Moreover, the results also show that both the dosage of SUP and differential sensitivity towards SUP are important determinants for how SUP may act both as an inhibitor and an enhancer of cell proliferation to achieve border maintenance and regulation.
Materials and methods
cDNA isolation, chimeric gene construction, and RNA analysis
SUP cDNA was isolated by reverse transcription-PCR (RT-PCR) using A. thaliana floral RNA. NtSUP (GenBank accession GQ227844) was isolated by screening a Nicotiana tabacum var petite Havana SR1 floral λ-ZapII cDNA library using 32P-labelled SUP cDNA as a probe. The ARR5 and CYCB1;1 promoters were isolated by PCR from A. thaliana genomic DNA as previously described (Ferreira et al., 1994; D'Agostino et al., 2000). Chimeric CaMV35S (35S):SUP, 35S:NtSUP, 35S:SUP-GFP, ARR5:GUS, and CYCB1;1:GUS were constructed into pBluescript (pSK+) for transient transfection assays or into a T-DNA vector for Agrobacterium-mediated transformation using standard recombinant DNA methodology. The DR5:GUS and CDKA;1:GUS constructs were as described (Hemerly et al., 1993; Ulmasov et al., 1997; Tao et al., 2002). RNA isolation and blot analysis were performed as previously described (Cheung et al., 1993).
Plant growth and maintenance
Nicotiana tabacum var petite Havana SR1 (tobacco) plants were grown in the greenhouse under standard conditions with supplemental 16 h light. Arabidopsis thaliana ecotype Columbia plants were grown in a growth chamber at 22 °C under 16 h light/8 h dark. For in vitro growth, plants were germinated and maintained in basal medium containing Gamborg B5 salts and vitamins supplemented with 1% sucrose and 0.7% agar. For chemical treatments with 1-naphthalene acetic acid (NAA), 6-benzylaminopurine (BAP), N6-(2-isopentenyl) adenine (2iP), or p-chloro-phenoxy-isobutyric acid (PCIB), seeds were germinated in medium supplemented with these chemicals at the concentrations described in the text. Tissue culture regeneration assays from root and shoot explants were carried out on medium containing 1× MS salts and vitamins supplemented with 3% sucrose, 0.7% agar, and different hormones as described in the text. For de-etiolation experiments, seeds were germinated in basal medium and kept in the dark for 8 d. To test the effect of cytokinins during flower development, tobacco shoot apices were sprayed with 25, 50, or 75 μM BAP every day starting from floral bud emergence until the first flowers opened. In the case of Arabidopsis, the entire plant was sprayed with BAP (5, 10, 20, or 25 μM) every other day starting from inflorescence emergence.
Tobacco plant transformation and crosses
Agrobacterium-mediated N. tabacum (SR1) leaf disc transformation was carried out as previously described (Delebrese et al., 1986). Phenotypes were observed during the T0 generation and detailed analyses carried out in subsequent generations. Transformed plants homozygous for a single insertion were used for reciprocal crosses with GUS marker lines. Representative progeny from crosses between the marker lines and multiple 35S:SUP lines were used for further studies.
Cell culture and protoplast isolation, transfection, and analysis
Suspension cell cultures were produced from root explants from wild-type and 35S:SUP Curly plants (lines 3, 2, and 22) maintained in 1× MS salts and vitamins supplemented with 3% sucrose, 0.7% agar, and different auxin and cytokinin concentrations until callus formed. NAA at 1 μM was found to be adequate to support the growth of 35S:SUP Curly calli, and 5 μM NAA was adequate for wild-type callus regeneration. Calli were used to inoculate liquid basal medium (1× MS salts and vitamins supplemented with 3% sucrose) supplemented with different hormones as described in the text. Cultures were maintained with shaking in the dark. Tobacco and Arabidopsis protoplast isolation, transfection, and analyses were carried out as described (Tao et al., 2002). DR5:GUS, ARR5:GUS, and CYCB1;1:GUS were used as reporter genes and 35S:LUC as an internal transfection efficiency control. Results shown are averages ±SD from triplicate samples, and each experiment was repeated at least three times with similar observations.
GUS analysis and histological observations
In vitro grown tobacco seedlings were used for histological observations and histochemical analysis of GUS expression. For histological observation, 2-week-old tobacco seedlings were used and processed as previously described (Langdale et al., 1988). Sections of 8 μm were prepared and stained with toluidine blue and observed under a light microscope (Nikon Eclipse E800). Vascular strands were imaged on toluidine blue-stained sections by epifluorescence (Ex546/10 nm, DM575 nm; BA590 nm); under these conditions the vascular strands fluoresced red. For GUS histochemical analyses, 2-week-old seedlings were incubated in liquid medium with the indicated hormone concentrations for 6 h before GUS staining for 6–12 h. Seedlings and floral explants were prepared for scanning electron microscopy (SEM) observation as described (Lolle et al., 1992).
Results
Ectopic expression of SUP causes multiple developmental defects
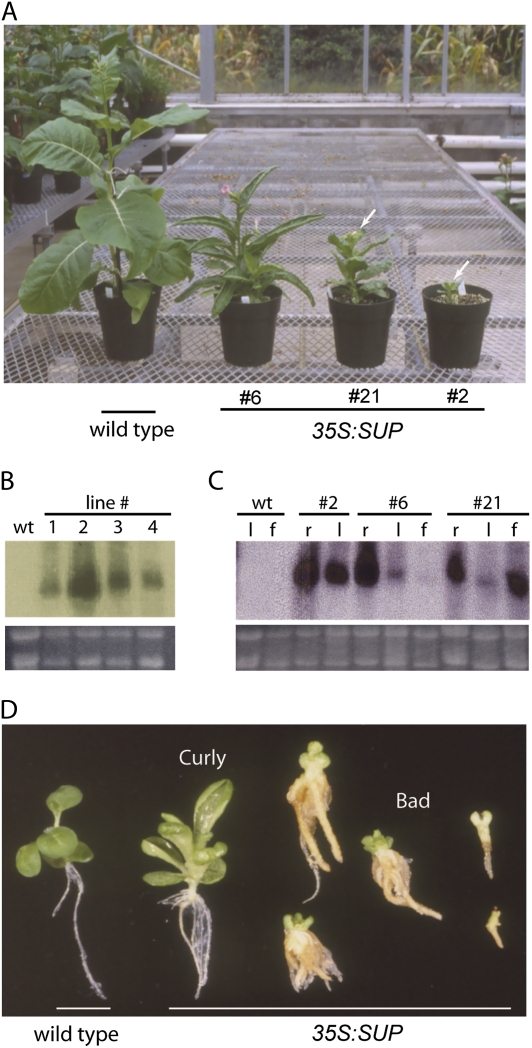
As studies in loss-of-function superman mutants in Arabidopsis did not reveal the cellular mechanism with which SUP mediates its function, SUP was overexpressed in N. tabacum to explore how it acts as a regulator of cell growth, proliferation, and differentiation. In transformed tobacco plants, distinct phenotypes were noticeable among some of the regenerating T0 plants, including prolific growth of roots and stunted development of shoots; however, the majority of these plants did not survive when transferred to soil (data not shown). The transgenic lines obtained showed a wide range of phenotypes, and representative lines that grew to maturity are shown in Fig. 1A. These lines were classified according to the severity of their phenotype into normal, Curly, and Bad (Supplementary Table S1 available at JXB online). The phenotypes of the progeny seedlings of all the lines reiterated those observed among the T0 plants and correlated with SUP expression (Supplementary Table S1; Fig. 1B–D). Normal plants with no or mild phenotypes had low levels of SUP transcript (e.g. Fig. 1B, lanes 1 and 4) and were not studied further. Only one severely defective T0 plant, SUP Bad (Line 2), survived to maturity; it was extremely dwarfed, its development seriously compromised, and its progeny seedlings accumulated high levels of SUP transcripts (Fig. 1A–D). The majority of transformed plants showed the intermediate Curly phenotype and had intermediate levels of SUP transcript (e.g. Fig. 1B, lane 3). SUP Curly plants (lines 3, 6, 21, 22, 25, and 26) developed readily recognizable curled-in leaves, bushy and thicker roots, and had reduced stature and apical dominance, in agreement with higher levels of expression in roots and leaves (Fig. 1C, D). Floral organs in a subset of this group (lines 21, 22, 25, and 26) showed developmental defects (see below), which correlated with increased expression of SUP transcript in their flowers (Fig. 1C, Curly 21).
Fig. 1.
Expression of Arabidopsis SUPERMAN (SUP) confers growth-related phenotypes in transgenic tobacco plants. (A) 35S:SUP primary transformants (T0 plants) grown in standard greenhouse conditions. Wild-type (left) and three independent 35S:SUP lines showing representative Curly and Bad phenotypes. Arrows point to flowers on dwarf plants. (B) RNA gel blot analysis of the expression levels of the SUP transgene in tobacco T1 seedlings from 35S:SUP-transformed plants. Line 1, SUP normal; line 2, SUP Bad; line 3, SUP Curly; line 4, SUP normal (see also Supplementary Table S1 at JXB online). rRNA is shown underneath the blot as a loading control. (C) RNA gel blot analysis of SUP expression levels in different tissues of the wild type and T2 progeny from 35S:SUP Curly and Bad plants. r, root; l, leaf; f, flower. rRNA is shown underneath the blot as a loading control. (D) Phenotypes of 25-day-old T1 35S:SUP seedlings. The increased severity of the phenotype (from left to right) correlates with the transgene expression level. (This figure is available in colour at JXB online.)
To complement the experiments using the Arabidopsis SUP gene, a tobacco homologue (NtSUP) was isolated. The NtSUP protein is 40% and 70% identical to SUP and PhSUP1, respectively and the zinc-finger domain is completely conserved (Supplementary Fig. S1A at JXB online). Similar to SUP, the 35S:NtSUP transgene induced strong phenotypic abnormalities that were evident in T0 transformants, and their progeny also segregated Curly and Bad phenotypes (Supplementary Fig. S1B, C). This observation suggests that the activity of SUP is largely conserved amongst these species.
Moderate overexpression of SUP correlates with increased cell number and cell size
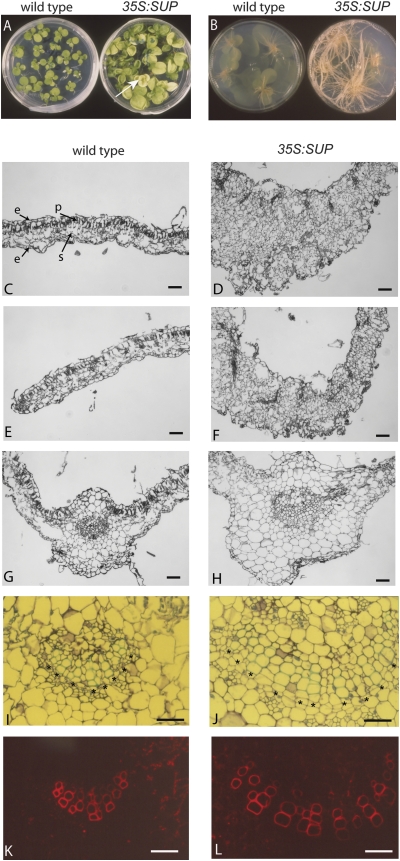
Plants with high levels of SUP expression, classified as SUP Bad, showed severe developmental defects similar to those previously reported (Bereterbide et al., 2001). The shoot and root system barely developed, and growth arrested at the seedling stage (Fig. 1D). More interesting was a class of SUP Curly plants with lower levels of SUP expression that showed robust growth of roots and shoots in tissue culture (Fig. 2A, B). Longitudinal tissue sections of the leaves of SUP Curly seedlings showed that the normal mesophyll organization of one layer of adaxial palisade and four layers of abaxial spongy mesophyll was disrupted (Fig. 2C–F). When compared with the wild type, SUP Curly had more spongy mesophyll cell layers that were more disorganized and comprised of more variably shaped cells (Fig. 2C–F). The mesophyll cells in the mid-vein regions of Curly leaves also showed irregular size and shape, but the basic vascular bundle organization was maintained (Fig. 2G–J). Moreover, the mid-vein of SUP Curly leaves showed an increased number of xylem files comprised of cells that were also larger than in the wild-type vein (Fig. 2I–L).
Fig. 2.
SUP Curly seedlings show enhanced growth and cell proliferation properties. (A and B) Enhanced shoot (A) and root (B) growth of 35S:SUP Curly when compared with the same age wild-type seedlings. The arrow points to a yellowish seedling, quite common amongst Curly progeny. (C–F) Leaf blade and leaf margin transverse sections showing the curly leaf phenotype of 35S:SUP. (G–L) Mid-rib transverse sections showing that the overall architecture is maintained but 35S:SUP leaves have an increased number of vascular strands. (K and L) fluorescence image of I and J, respectively, highlighting the xylem vascular strands (red). Scale bar=40 μm. Asterisks mark the xylem vascular strands. e, epidermis; p, palisade parenchyma; s, spongy parenchyma.
To ascertain that the severity of the SUP-induced phenotype was correlated with the level of transgene expression, transgenic plants carrying the 35S:SUP-Green Fluorescent Protein (GFP) construct were generated. A greater number of primary transformants grew to maturity and a range of phenotypes similar to those observed in 35S:SUP plants segregated from these plants, except that phenotypically normal seedlings predominated, suggesting that the SUP–GFP fusion preserved but attenuated SUP protein function (Supplementary Fig. S2A at JXB online). SUP–GFP localized to the nucleus (Supplementary Fig. S2B), consistent with its function as a transcription factor. Moreover, the levels of SUP–GFP correlated with the severity of the observed phenotype (Supplementary Fig. S2C).
Ectopic expression of SUP induces auxin-related phenotypes
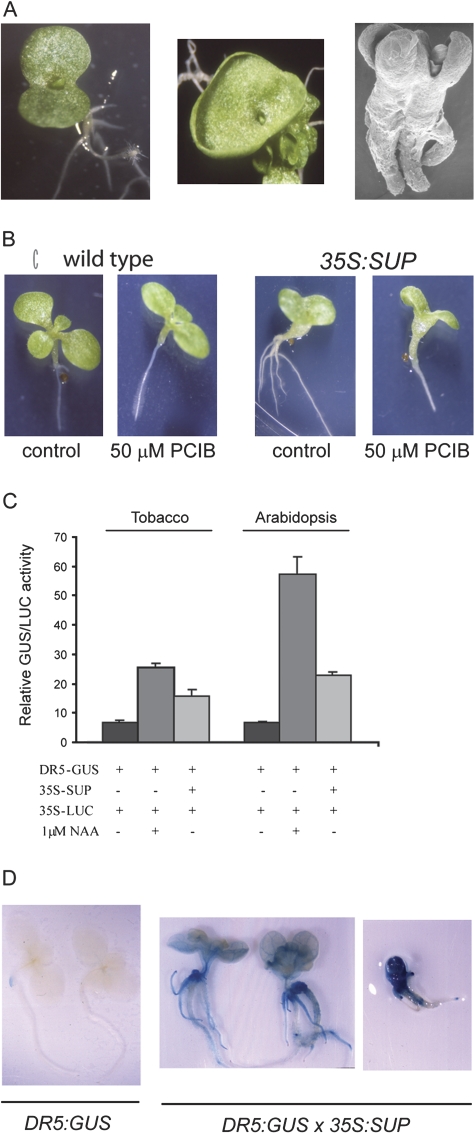
Some of the most obvious phenotypes in the 35S:SUP seedlings were the extensive root system and the upward curling of the leaves. These phenotypes are reminiscent of mutants with altered auxin signalling (Leyser et al., 1996; Hamann et al., 1999). Seedlings from 35S:SUP plants also frequently showed fused cotyledons and other patterning defects (Fig. 3A), suggesting that auxin distribution or auxin signalling was disrupted during embryo development (Liu et al., 1993). Besides being thick and long, roots from SUP Curly seedlings were also highly branched when compared with wild-type roots (Figs 1D, 2B, 3B), similar to what is observed in mutant plants with enhanced auxin levels (Boerjan et al., 1995; Barlier et al., 2000). Indeed, when SUP Curly seedlings were grown in medium containing an antagonist of auxin action, PCIB, the root phenotype was mitigated. On the other hand, the fused cotyledon phenotype was not affected, consistent with it being an embryonic phenotype (Fig. 3B).
Fig. 3.
Expression of SUP induces auxin-related phenotypes. (A) 35S:SUP seedlings with cup- and disc-shaped cotyledons and a fused twin seedling. (B) PCIB treatment rescues the root branching phenotype but has no effect in the fused cotyledon phenotype of 35S:SUP seedlings. (C) SUP stimulates transcription from DR5, an auxin-responsive promoter in protoplasts. Arabidopsis and tobacco protoplasts were transfected with the reporter gene DR5:GUS alone or together with 35S:SUP and incubated in the absence or presence of 1 μM NAA, as labelled. 35S:LUC was used as an internal control for transformation efficiency. (D) SUP expression increases transcription from the DR5 promoter in planta. Promoter activity was detected by GUS staining of control transgenic seedlings containing the DR5:GUS construct and two independent lines containing both the DR5:GUS and 35S:SUP constructs. (This figure is available in colour at JXB online.)
To test if auxin signalling pathways were affected by SUP, the GUS gene under the control of the auxin-responsive promoter DR5 (Ulmasov et al., 1997) was used as a reporter for auxin signalling. In both transiently transformed protoplasts and stably transformed plants, SUP expression, even in the absence of exogenous auxin, stimulated transcription from the DR5 promoter, suggesting that SUP alone is able to activate auxin-induced gene expression (Fig. 3C, D). Moreover, GUS activity was higher in plants with more severe phenotypes and, presumably, higher SUP expression levels. Similarly, 35S:NtSUP also stimulated DR5:GUS expression (Supplementary Fig. S1E at JXB online). Together, these observations suggest that SUP action involves auxin signalling pathways.
Ectopic expression of SUP induces cytokinin-related phenotypes
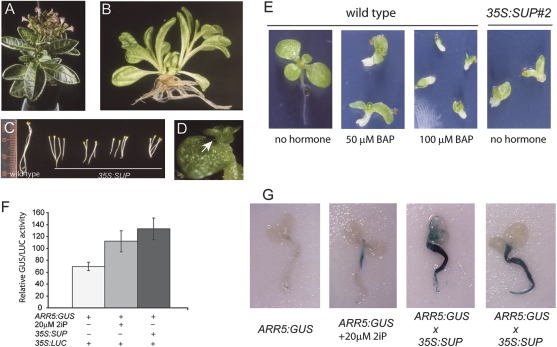
Some of the phenotypes suggested that cytokinin signalling might also be affected in 35S:SUP plants. The loss of apical dominance, shorter inflorescences that did not elongate and appeared as a big cluster, the occurrence of seedlings with multiple shoots, de-etiolation in the dark, the formation of meristems and plantlets on leaves (Fig. 4A–D), and the yellowing of young leaves (Fig. 2A) are all phenotypes reminiscent of mutants with altered cytokinin content and signalling (Estruch et al., 1991; Li et al., 1992; Rupp et al., 1999). These phenotypes were also observed in 35S:NtSUP plants (Supplementary Fig. S1C, D at JXB online, and data not shown). Furthermore, severe inhibition of root and shoot growth was observed when wild-type tobacco seedlings were grown in 50 μM of the cytokinin BAP (Fig. 4E). At higher concentrations (100 μM BAP), seedling growth was arrested early and these seedlings strongly resembled SUP Bad seedlings (Fig. 4E). In addition, expression from the cytokinin-inducible ARR5 promoter (D'Agostino et al., 2000) was stimulated by SUP in the absence of exogenously applied cytokinin in protoplasts and transgenic plants (Fig. 4F, G). These observations support a role for SUP in augmenting cytokinin-related processes.
Fig. 4.
Expression of SUP induces cytokinin-related phenotypes. (A) A representative adult 35S:SUP plant whose inflorescences fail to elongate. (B) A representative 35S:SUP seedling with shoots developed from multiple shoot apical meristems. (C) De-etiolation phenotype of 35S:SUP seedlings from four independent parents (lines 3, 6, 21, and 22 from left to right) when grown in the dark. (D) Ectopic plantlet developing on a leaf of a 35S:SUP seedling (arrow). (E) Exogenous application of cytokinin (BAP) to wild-type seedlings phenocopies the 35S:SUP Bad phenotype. (F) SUP stimulates the transcription from ARR5, a cytokinin-responsive promoter in protoplasts. Tobacco protoplasts were transfected with the reporter gene ARR5:GUS alone or together with 35S:SUP and incubated in the absence or presence of 20 μM of the cytokinin 2iP, as labelled. 35S:LUC was used as an internal control. (G) SUP expression increases transcription from ARR5 in planta. Promoter activity was detected by GUS staining of seedlings containing the ARR5:GUS construct and two independent lines containing both the ARR5:GUS and the 35S:SUP construct, in the presence or absence of the cytokinin 2iP. (This figure is available in colour at JXB online.)
Dependence on exogenous hormones under tissue culture conditions is reduced by SUP
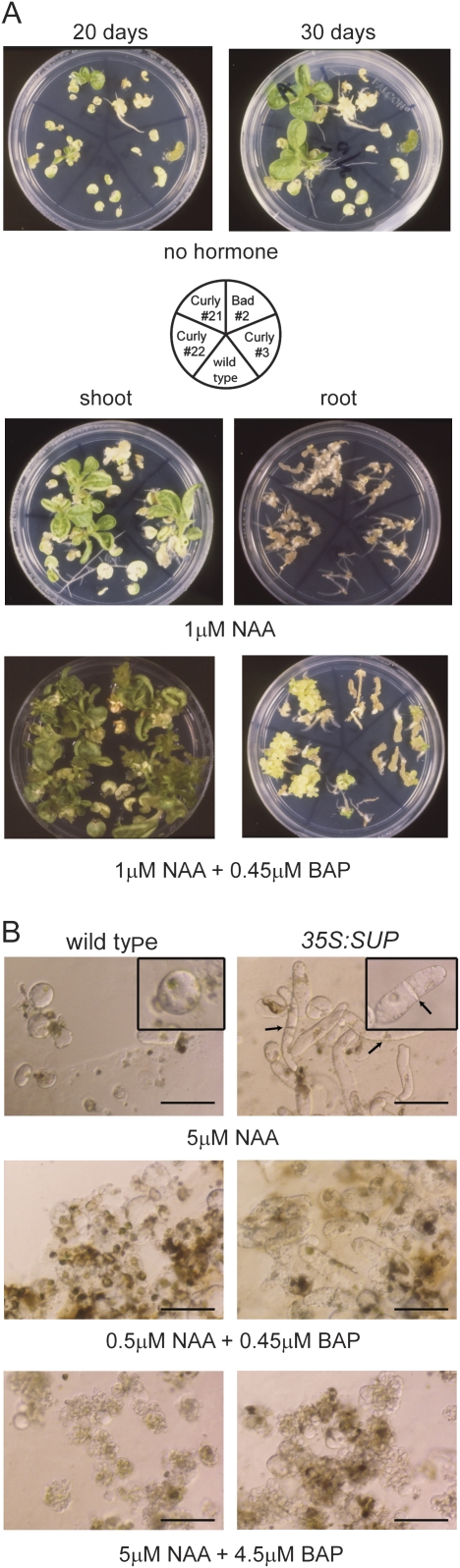
The effect of SUP on auxin and cytokinin signalling pathways prompted the examination of whether SUP affects hormone-dependent organogenesis in tissue culture. With the right balance of exogenously applied hormones, organ explants are able to regenerate into new plants through a process of de-differentiation, cell division, and re-differentiation. Explants from the leaves of wild-type, Curly, and Bad plants were excised and put on basal medium with no exogenous hormones. Under these conditions, wild-type leaf explants failed to regenerate whereas leaf explants from a subset of Curly plants (e.g. lines 21 and 22, and, to a much lesser extent, 3) showed extensive regeneration of shoots and roots (Fig. 5A). This observed hormone autonomy is consistent with the idea that moderate levels of SUP activity promote cell division.
Fig. 5.
35:SUP tissue has reduced dependence on exogenous hormones under tissue culture conditions. (A) Hormone independence of 35S:SUP explants under tissue culture regeneration conditions. The diagram in the middle shows locations of the different lines used in the assay. Regeneration assays of wild-type and 35S:SUP explants in hormone-free medium after 20 d and 30 d in culture (upper panel), or in medium with 1 μM NAA (middle panel) and 1 μM NAA+0.45 μM BAP (bottom panel). (B) Behaviour of 14-day-old wild-type (left panels) and SUP Curly-derived (right panels) suspension cells in tissue culture conditions with the indicated hormone concentrations. Insets, cell detail. Arrows indicate cell division planes in SUP-Ox cells. Scale bar=100 μm.
On the other hand, explants from SUP Bad plants rarely regenerated; when they did, they differentiated root tissues but growth was not sustained and the tissues became necrotic, consistent with the idea that high levels of SUP activity are inhibitory to growth (Fig. 5A).
When wild-type shoot and root explants were incubated in a medium supplemented with 1 μM NAA, no regeneration was observed in shoot explants after 30 d in culture, while root explants regenerated few roots (Fig. 5A). Under the same conditions, SUP Curly shoot explants regenerate into shoots and roots, and root explants formed extensive calli from which many hairy roots differentiated (Fig. 5A). When cytokinin (0.45 μM BAP) was also added to the medium, a more rapid tissue proliferation of SUP Curly tissue was observed relative to proliferation of wild-type tissue (Fig. 5A). SUP Bad explants were much slower at regenerating under the same conditions, and prolonged culturing could not be sustained (Fig. 5A).
Similarly, SUP Curly-derived suspension cell cultures grew faster than wild-type cell cultures under a range of hormonal conditions tested (Fig. 5B). Moreover, cultured SUP Curly cells were morphologically different from wild-type cells. Wild-type cells remained round while SUP-overexpressing (SUP-Ox) cells elongated and started to divide (Fig. 5B, insets). In order to obtain wild-type cells with morphologies similar to the elongated morphology of SUP Curly cells and cell division patterns that produced linear arrays of cells, they needed to be cultured in the presence of 0.9–9 nM BAP in addition to 5 μM NAA (Supplementary Fig. S3A at JXB online). This further implies higher endogenous cytokinin-related activities in the SUP Curly-derived cells. Tissue derived from SUP Bad plants could not be propagated, presumably due to the negative effects of high SUP levels.
These observations, together with the effect of SUP on auxin and cytokinin signalling pathways, suggest that the reduced hormone dependence of SUP-Ox tissue had resulted from increased SUP-induced endogenous hormone-related activities in these tissues.
SUP affects cell cycle regulation
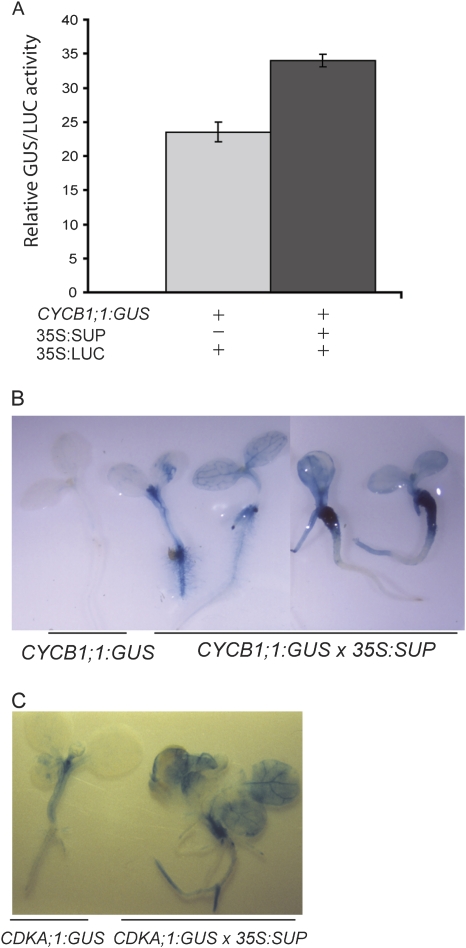
The cell culture studies suggested an increased rate of cell divisions in SUP Curly-derived tissue. The effect of SUP on the activity of cell cycle genes involved in regulating cell division in plants was therefore examined. The CYCB1;1:GUS promoter controls the transcription of a cyclin gene that is only active when the cells initiate division, and can be activated by auxin and cytokinin (Ferreira et al., 1994). In both transiently transfected protoplasts and stably transformed plants, SUP expression stimulates expression of CYCB1;1:GUS (Fig. 6A, B). The observed intensity of GUS staining in stably transformed plants correlated with the severity of the phenotype conferred by SUP expression (Fig. 6B). Similar results were obtained when promoter activity from another cell cycle-regulated gene, the cyclin-dependent kinase CDKA;1 (Hemerly et al., 1993), was examined in SUP-Ox plants (Fig. 6C). Moreover, experiments using [3H]thymidine incorporation as a measure for cell division in a subset of SUP Curly-derived culture lines were also consistent with these cell lines having increased cell division rates in the presence of cytokinin alone or in conjunction with auxin (Supplementary Fig. S3B at JXB online).
Fig. 6.
SUP expression affects the expression of cell cycle genes. (A) SUP stimulates the expression from the CYCB1;1 promoter. Tobacco protoplasts were transfected with CYCB1;1:GUS alone, or together with 35S:SUP. 35S:LUC was used as an internal control for transfection efficiency. (B) SUP stimulates the expression from the CYCB1;1 promoter in planta. Plants carrying the CYCB1;1:GUS construct were crossed with plants carrying the 35S:SUP construct and the level of activity of the CYCB1;1 promoter was assayed by GUS staining. GUS activity correlated with the severity of the SUP-induced phenotype. (C) SUP stimulates the expression from the CDKA;1 promoter in planta. Plants carrying the CDKA;1:GUS construct were crossed with plants carrying the 35S:SUP construct and the level of promoter activity was assayed by GUS staining. (This figure is available in colour at JXB online.)
Together, the present observations are consistent with 35S:SUP Curly plants having enhanced cell cycle activities and that more cells throughout the plant acquire competence to divide due to increased SUP activity.
Ectopic expression of SUP induces altered organ boundaries and increased feminization in flowers
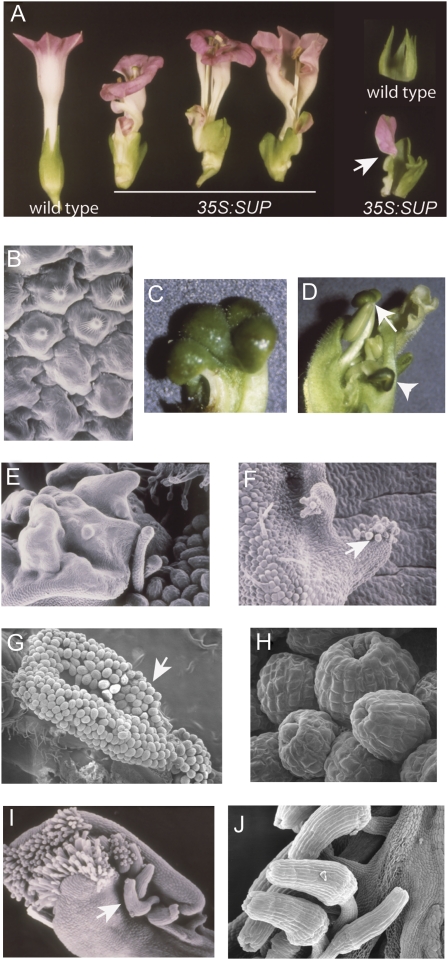
A subset of 35S:SUP plants with high levels of SUP expression in flowers showed floral phenotypes that were considerably more pronounced than previously reported (e.g. Curly 21, Fig. 1C, Supplementary Table S1 at JXB online) (Kater et al., 2000; Nandi et al., 2000; Bereterbide et al., 2001; Yun et al., 2002; Nakagawa et al., 2004) and provide more information about the function of SUP during floral organ differentiation. In wild-type tobacco flowers, the first whorl, or calyx, is composed of five green sepals that are fused at the base. In the second whorl, five petals are fused for most of their length, creating a corolla tube. The third whorl is composed of five stamens and the fourth whorl is composed of two fused carpels (Fig. 7A). In 35S:SUP flowers the calyx and corollas were often not fused and petaloid sepals were frequently observed (Fig. 7A). SEM images of these petaloid sepals revealed that the typical irregular-shaped sepal epidermal cells shifted abruptly into conical cells characteristic of petals, consistent with their chimeric nature and a relaxed whorl boundary definition (Fig. 7B).
Fig. 7.
SUP expression alters flower morphology and increases the appearance of female-related phenotypes. (A) 35S:SUP flowers show split calyxes and corollas and chimeric petaloid sepals (arrow). (B) SEM image of the transition region of a petaloid sepal. Note the conical cells typical of petals on the upper half and irregular flat cells typical of sepals on the lower half. (C) Detail of a four-lobed stigma. (D) Detail of a 35S:SUP flower with a tri-carpeloid pistil (arrow) and a stigmatoid anther (arrowhead). (E–J) SEM images of 35S:SUP flowers. (E) Carpeloid tissue developing on a petal. (F and G) Ovule-like structures (arrows) developing on the surface of petals. (H) Detail of the ovule-like structures. (I) Pistil showing an ectopic ovule and prolific papillar development at the carpel fusion line. Some ovules fail to bend (arrow). (J) Detail of the tubular-shaped ovules.
SUP ectopic expression also induced increased feminization in some SUP Curly lines (Supplementary Table S1 at JXB online). Pistils with more than two carpels were often observed (Fig. 7C), whereas stamens were often dwarfed and stigmatoid tissue developed at their apices (Fig. 7D). Large patches of carpeloid tissue containing ovule-like structures differentiated on the surface of petals (Fig. 7E–H). In addition, prolific papillae-like structures and ectopic ovules often developed along carpel fusion lines (Fig. 7I). Some of these ovules were straight rather than bent (Fig. 7I,J), similar to those in superman flowers (Gaiser et al., 1995). The acquisition of carpeloid properties in tissues of the outer whorls also reflects relaxed boundary definitions when SUP is overexpressed. The formation of female tissues occurred most prevalently where petal tissues became adherent to form the corolla tube (Fig. 7E–G) and along the carpel fusion line (Fig. 7I), possibly indicating that cells along fusion lines are more plastic for differentiation and thus more sensitive to altered growth regulation conditions (Siegel and Verbeke, 1989; Lolle et al., 1992).
Cytokinin enhances female differentiation and suppresses male development
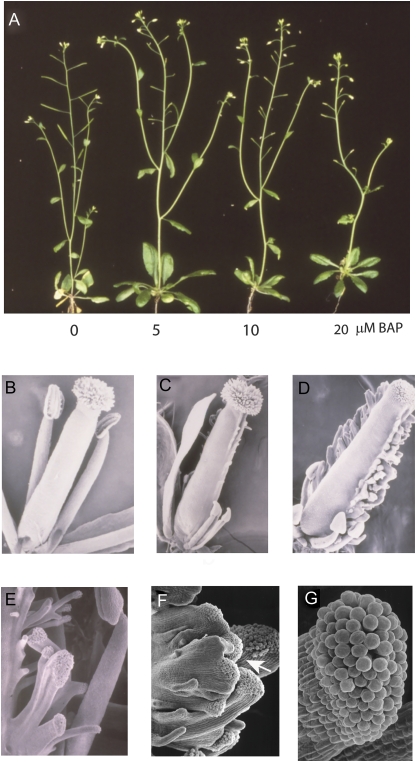
Cytokinin is known to stimulate flavonoid biosynthesis and to be associated with female sex determination in some plants (Durand and Durand, 1991; Menendez et al., 2009; Deikman and Hammer, 1995). Indeed, the petaloid sepals observed in SUP-Ox plants were phenocopied by exogenous application of cytokinin to wild-type tobacco plants (Supplementary Fig. S4 at JXB online), consistent with cytokinin-related processes being affected by overexpression of SUP. Moreover, Arabidopsis plants sprayed with the cytokinin BAP showed a dose-dependent decrease in fertility as observed by the short siliques produced (Fig. 8A). This decrease in fertility was probably a consequence of the reduced stamen size, precluding self-pollination of the stigma (Fig. 8C, D). In addition, dramatic tissue outgrowths occurred along the carpel fusion line of BAP-treated plants, and many stigmatoid protrusions containing papillar cell-like structures developed at the apex of these protrusions (Fig. 8D–G). Taken together, these observations establish a feminizing role for cytokinin during tobacco flower development and suggest that SUP may act through the modulation of cytokinin pathways to regulate male and female organogenesis.
Fig. 8.
Cytokinin application induces feminization in Arabidopsis plants. (A) Arabidopsis plants show a dose-dependent decrease in fertility with exogenous cytokinin (BAP) application, as assessed by silique size. (B) SEM image of a wild-type Arabidopsis flower with no treatment (petals and sepals removed). (C and D) SEM image of a wild-type Arabidopsis flower sprayed with 10 μM (C) and 25 μM (D) BAP. Note the short stamens and the development of stigmatoid protrusions from the carpel fusion line. (E–G) Detail of the stigmatoid protrusions containing stigma-like papillae (arrow) forming on the carpel fusion line. (This figure is available in colour at JXB online.)
Discussion
A threshold for SUP as an enhancer or suppressor of cell growth and differentiation
Mis- or overexpression of SUP in different plant species was previously reported to produce severely dwarfed plants and organs (Kater et al., 2000; Nandi et al., 2000; Bereterbide et al., 2001; Hiratsu et al., 2002; Yun et al., 2002). However, the mechanisms proposed for growth inhibition varied, probably reflecting the complexity of SUP activity as the results show here. In the present studies a correlation was found between SUP expression levels and the continuum of growth phenotypes (ranging from growth enhancement in SUP Curly to severe inhibition in SUP Bad), indicating a dosage effect of SUP activity.
Even amongst SUP Curly plants, higher SUP expression in flowers correlated with the development of floral phenotypes, while plants with lower SUP floral expression had normal flowers (Fig. 1, and Supplementary Table S1 at JXB online). Even within the same tissue, such as in the leaves of Curly plants, a dosage effect clearly underlies the difference between the curled-up leaf edge and the relatively normal leaf blade area, as evident by the differential expression of the SUP–GFP protein in normal and curled tissue (Supplementary Fig. S2).
The enhancement of cell proliferation caused by SUP overexpression (Figs 2, 5, 6) is consistent with the up-regulation of cell cycle gene expression in SUP Curly seedlings. On the other hand, the stunted growth and inhibited cell proliferation properties seen in SUP Bad plants and in vitro derived tissues indicate a tolerance limit to SUP activity: once this threshold is crossed, SUP becomes inhibitory to growth.
While dosage is clearly an important factor, the ultimate effect of SUP, in particular in cellular differentiation, is probably influenced by the differential sensitivity with which various cell and tissue types respond to SUP. This is most evident in the striking transition from sepal to petal tissues in the petaloid sepals of SUP Curly flowers and in petal tissues where individual cells or patches of cells assume carpel tissue identities (Fig. 7). The ability to fine-tune local responses would provide a versatile mechanism whereby small differences in SUP expression along the whorl 3 and 4 border could result in opposing proliferative effects on the cells in these neighbouring whorls.
SUP function involves auxin and cytokinin actions
It is well established that plant growth and differentiation relies on a tight balance between cytokinin and auxin activities. Some of the phenotypes observed in 35S:SUP plants mimic those found in mutants defective in some aspects of auxin and cytokinin pathways, and a subset of these can be phenocopied by cytokinin application or relieved by an antagonist of auxin action (Figs 3, 4). Hormone autonomous regeneration into plantlets from some SUP Curly explants also reflects elevated auxin and cytokinin functional activity in these tissues (Fig. 5), as does the observation that SUP stimulates the expression of auxin- and cytokinin-inducible promoters (Figs 3, 4). Preliminary efforts in IAA quantification (Tam and Normanly, 1998) and immunodetection of cytokinin (PhytodetektZR ELISA kit, Idetek Inc., San Bruno, CA, USA) did not yield reproducible differences among 13-day-old wild-type, SUP Bad and SUP Curly seedlings (data not shown), plausibly due to SUP's effect being more on hormone signalling rather than on hormone levels.
Auxin and cytokinin regulate the rate of cell division by affecting expression of cell cycle genes (Coenen and Lomax, 1997). The observation that SUP stimulated expression from the CYCB1;1 promoter, which has previously been shown to be regulated by the synergistic action of auxin and cytokinin (Fig. 6; Ferreira et al., 1994), further supports the idea that SUP's ability to regulate cell proliferation is probably mediated through the combined actions of these two hormones. Different levels of SUP should affect auxin- and cytokinin-regulated pathways in a dose-dependent manner, and the combined activity from these pathways would differentially regulate cell proliferation. In addition, different cell types and tissues have different sensitivities and responses to auxin and cytokinin. These together could provide the basis for the variable phenotypic effects caused by SUP in different tissues and organs observed among transgenic plants described here and previously (Kater et al., 2000; Nandi et al., 2000; Bereterbide et al., 2001; Hiratsu et al., 2002; Yun et al., 2002; Nakagawa et al., 2004).
The role of SUP-activated cytokinin signalling pathways in sex determination
SUP expression enhances feminization in tobacco flowers by suppressing stamen development and increasing the appearance of female traits in other whorls (Fig. 8). SUP-enhanced feminization was also observed in Arabidopsis (Yun et al., 2002) and rice (Nandi et al., 2000). In the dioecious species Silene latifolia, the SUP orthologue, SlSUP, is expressed only in female flowers, apparently playing a role in the female flower developmental pathway (Kazama et al., 2009).
Exogenous cytokinin application to Arabidopsis flowers increases the appearance of female characters but suppresses stamen development, suggesting that cytokinins are important factors in the establishment of female organs (Fig. 8). Moreover, the present results indicating that SUP expression affects cytokinin-related processes (Figs 5, 6) strongly suggest that SUP activity along the border between whorls 3 and 4 involves cytokinin signalling that ultimately results in the promotion of female organ differentiation in the fourth whorl.
Conserved functions of the SUP-related protein family
Studies thus far on SUP and homologues from various plant species, including petunia PhSUP1 and tobacco NtSUP, suggest that SUP functions are broadly conserved in plants (Kater et al., 2000; Nandi et al., 2000; Bereterbide et al., 2001; Nakagawa et al., 2004). The present results show that this conservation extends to regulation of hormonal signalling pathways (Supplementary Fig. S1 at JXB online). Regulation through hormone signalling by SUP proteins may be an effective strategy to control complex responses in the plant, such as morphogenesis and organogenesis. This strategy might also be used to fine-tune the regulatory activity from developmentally important transcription factors as observed for other SUP-like genes (Rupp et al., 1999; Nakagawa et al., 2005; Jiang et al., 2008).
The partial homeotic conversion of sepals into petals in tobacco plants overexpressing SUP denotes an alteration of the boundary between whorls 1 and 2, suggesting that the ectopic expression of SUP in those whorls allowed for B function genes to ‘spill over’ to the first whorl. Similarly, homeotic differentiation of carpeloid features in stamens denotes a disruption of the boundary between whorls 3 and 4, normally kept in check by a tightly delimited SUP expression domain. In addition, overexpression of SUP has been found to recover male sterility caused by stamen–carpel fusion in tobacco, by restoring the boundaries between the third and fourth whorls (Bereterbide et al, 2002). Taken together, these results point to an overall disruption in the maintenance of floral organ identities in SUP overexpressors, in accordance with its cadastral role. Cadastral genes are necessary to establish and maintain boundaries; mutations in these genes result in the production of fused organs. Interestingly, KNUCKLES, a zinc-finger protein similar to SUP, is also proposed to function in boundary maintenance, in this case carpel–ovule (Payne et al., 2004). Another zinc-finger protein, JAGGED (JAG), results in fused organs, cup-shaped cotyledons, and leaf-like outgrowths when overexpressed, suggesting it acts by increasing cell proliferation (Ohno et al., 2004). Taken together, these studies and the results reported here suggest that the basic function of the SUP family of transcription factors is broadly maintained. They control organ growth, development, and patterning by regulating cell proliferative activities, often via hormone signalling pathways.
Evolutionary implications of the cadastral role of SUP
The marked consequences on floral morphogenesis of changes in the domain of SUP expression observed here are likely to have evolutionary implications. It has been hypothesized that the floral diversity observed in angiosperms may be explained in part by a ‘sliding boundary’ model (Kramer et al., 2003; Theissen and Melzer, 2007). In this model, shifts in the expression domains of the ABC genes may have been selected during evolution, resulting, for example, in flowers with two whorls of petaloid organs due to expansion of B function to the first whorl (Kanno et al., 2003), flowers with two whorls of sepaloid organs due to contraction of B expression (Ainsworth et al., 1995), or unisexual flowers due to homeotic conversions between reproductive organs (Di Stilio et al., 2005; Pfent et al., 2005; Sather et al., 2005). Moreover, non-Eudicot angiosperms exhibiting spiral rather than whorled phyllotaxy have gradual transitions between organs, consistent with leaky organ identity boundaries also known as the ‘fading borders’ model (Buzgo et al., 2004; Soltis et al., 2007). In this context, changes to SUP function, probably mediated by hormones as indicated by this and previous studies, provide an intriguing novel target for the genetic dissection of angiosperm diversification.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Isolation and characterization of the Nicotiana tabacum SUPERMAN gene (NtSUP).
Figure S2. Plants constitutively expressing SUP-GFP have similar phenotypes to 35S:SUP plants.
Figure S3. Effect of hormones on the growth of wild-type and SUP-Ox tobacco cells.
Figure S4. Cytokinin application induces petaloid sepals in tobacco plants.
Table S1. Transgenic 35S:SUP lines used in this study.
Acknowledgments
We thank Professors Tom Guilfoyle for the DR5:GUS and Dirk Inzé for the CDKA;1:GUS constructs. We thank Dr. S. McCormick for comments on the manuscript. CN was supported by a PhD grant from the Fundação para a Ciência e Tecnologia, Portugal, and a Gilgut Fellowship from the Plant Biology Graduate Program at the University of Massachusetts, Amherst. This work was partially supported by Cooperative State Research, Education and Extension Service, 2004-03419, and by the National Science Foundation IOB-0544222.
References
- Ainsworth C, Crossley S, Buchanan-Wollaston V, Thangavelu M, Parker J. Male and female flowers of the dioecious plant sorrel show different patterns of MADS box gene expression. The Plant Cell. 1995;7:1583–1598. doi: 10.1105/tpc.7.10.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukerman MJ, Sakai H. Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-Like target genes. The Plant Cell. 2003;15:2730–2741. doi: 10.1105/tpc.016238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlier I, Kowalczyk M, Marchant A, Ljung K, Bhalerao R, Bennett M, Sandberg G, Bellini C. The SUR2 gene of Arabidopsis thaliana encodes the cytochrome P450CYP83B1, a modulator of auxin homeostasis. Proceedings of the National Academy of Sciences, USA. 2000;97:14819–14824. doi: 10.1073/pnas.260502697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereterbide A, Hernould M, Castera S, Mouras A. Inhibition of cell proliferation, cell expansion and differentiation by the Arabidopsis SUPERMAN gene in transgenic tobacco plants. Planta. 2001;214:22–29. doi: 10.1007/s004250100584. [DOI] [PubMed] [Google Scholar]
- Bereterbide A, Hernould M, Farbos I, Glimelius K, Mouras A. Restoration of stamen development and production of functional pollen in an alloplasmic CMS tobacco line by ectopic expression of the Arabidopsis thaliana SUPERMAN gene. The Plant Journal. 2002;29:607–615. doi: 10.1046/j.0960-7412.2001.01243.x. [DOI] [PubMed] [Google Scholar]
- Boerjan W, Cervera MT, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Vanonckelen H, Vanmontagu M, Inze D. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. The Plant Cell. 1995;7:1405–1419. doi: 10.1105/tpc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JL, Sakai H, Jack T, Weigel D, Mayer U, Meyerowitz EM. SUPERMAN a regulator of floral homeotic genes in Arabidopsis. Development. 1992;114:599–615. doi: 10.1242/dev.114.3.599. [DOI] [PubMed] [Google Scholar]
- Buzgo M, Soltis PS, Soltis DE. Floral developmental morphology of Amborella trichopoda (Amborellaceae) International Journal of Plant Sciences. 2004;165:925–947. [Google Scholar]
- Chen X. A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science. 2004;303:2022–2025. doi: 10.1126/science.1088060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, May B, Kawata EE, Gu Q, Wu H-M. Characterization of cDNAs for stylar transmitting tissue-specific proline-rich proteins in tobacco. The Plant Journal. 1993;3:151–160. [PubMed] [Google Scholar]
- Coen E, Meyerowitz EM. The war of the whorls—genetic interactions controlling flower development. Nature. 1991;353:31–37. doi: 10.1038/353031a0. [DOI] [PubMed] [Google Scholar]
- Coenen C, Lomax TL. Auxin–cytokinin interactions in higher plants: old problems and new tools. Current Opinion in Plant Biology. 1997;2:351–356. doi: 10.1016/S1360-1385(97)84623-7. [DOI] [PubMed] [Google Scholar]
- D'Agostino IB, Deruere J, Kieber JJ. Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiology. 2000;124:1706–1717. doi: 10.1104/pp.124.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deikman J, Hammer PE. Induction of anthocyanin accumulation by cytokinins in Arabidopsis thaliana. Plant Physiology. 1995;108:47–57. doi: 10.1104/pp.108.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delebrese R, Reynaert A, Hofte H, Hernalsteen HP, Leemans J, Van Montagu M. Vectors for cloning in plant cells. Methods in Enzymology. 1986;153:277–290. [Google Scholar]
- Di Stilio VS, Kramer EM, Baum DA. Floral MADS box genes and homeotic gender dimorphism in Thalictrum dioicum (Ranunculaceae)—a new model for the study of dioecy. The Plant Journal. 2005;41:755–766. doi: 10.1111/j.1365-313X.2005.02336.x. [DOI] [PubMed] [Google Scholar]
- Durand B, Durand R. Sex determination and reproductive organ differentiation in Mercurialis. Plant Science. 1991;80:49–65. [Google Scholar]
- Estruch JJ, Prinsen E, Van Onckelen H, Schell J, Spena A. Viviparous leaves produced by somatic activation of an inactive cytokinin-synthesizing gene. Science. 1991;254:1364–1367. doi: 10.1126/science.254.5036.1364. [DOI] [PubMed] [Google Scholar]
- Ferrario S, Immink RGH, Angenent GC. Conservation and diversity in flower land. Current Opinion in Plant Biology. 2004;7:84–91. doi: 10.1016/j.pbi.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Ferreira P, Hemerly AS, Engler J, Montagu MV, Engler G, Inze D. Developmental expression of the Arabidopsis cyclin gene cyc1At. The Plant Cell. 1994;6:1763–1774. doi: 10.1105/tpc.6.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiser J, Robinsonbeer K, Gasser CS. The Arabidopsis SUPERMAN gene mediates asymmetric growth of the outer integument of ovules. The Plant Cell. 1995;7:333–345. doi: 10.1105/tpc.7.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson-Brown C, Savidge B, Yanofsky MF. Regulation of the Arabidopsis floral homeotic gene APETALA1. Cell. 1994;76:131–143. doi: 10.1016/0092-8674(94)90178-3. [DOI] [PubMed] [Google Scholar]
- Hamann T, Mayer U, Jurgens G. The auxin-insensitive bodenlos mutation affects primary root formation and apical–basal patterning in the Arabidopsis embryo. Development. 1999;126:1387–1395. doi: 10.1242/dev.126.7.1387. [DOI] [PubMed] [Google Scholar]
- Hemerly AS, Ferreira P, de Almeida Engler J, Van Montagu M, Engler G, Inze D. cdc2a expression in Arabidopsis is linked with competence for cell division. The Plant Cell. 1993;5:1711–1723. doi: 10.1105/tpc.5.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsu K, Ohta M, Matsui K, Ohme-Takagi M. The SUPERMAN protein is an active repressor whose carboxy-terminal repression domain is required for the development of normal flowers. FEBS Letters. 2002;514:351–354. doi: 10.1016/s0014-5793(02)02435-3. [DOI] [PubMed] [Google Scholar]
- Ito T, Sakai H, Meyerowitz EM. Whorl-specific expression of the SUPERMAN gene of Arabidopsis is mediated by cis elements in the transcribed region. Current Biology. 2003;13:1524–1530. doi: 10.1016/s0960-9822(03)00612-2. [DOI] [PubMed] [Google Scholar]
- Jiang C-J, Aono M, Tamaoki M, Maeda S, Sugano S, Mori M, Takatsuji H. SAZ a new SUPERMAN-like protein, negatively regulates a subset of ABA-responsive genes in Arabidopsis. Molecular Genetics and Genomics. 2008;279:183–192. doi: 10.1007/s00438-007-0306-1. [DOI] [PubMed] [Google Scholar]
- Kanno A, Saeki H, Kameya T, Saedler H, Theissen G. Heterotopic expression of class B floral homeotic genes supports a modified ABC model for tulip (Tulipa gesneriana) Plant Molecular Biology. 2003;52:831–841. doi: 10.1023/a:1025070827979. [DOI] [PubMed] [Google Scholar]
- Kater MM, Franken J, van Aelst A, Angenent GC. Suppression of cell expansion by ectopic expression of the Arabidopsis SUPERMAN gene in transgenic petunia and tobacco. The Plant Journal. 2000;23:407–413. doi: 10.1046/j.1365-313x.2000.00785.x. [DOI] [PubMed] [Google Scholar]
- Kazama Y, Fujiwara MT, Koizumi A, Nishihara K, Nishiyama R, Kifune E, Abe T, Kawano S. A SUPERMAN-like gene is exclusively expressed in female flowers of the dioecious plant Silene latifolia. Plant and Cell Physiology. 2009;50:1127–1141. doi: 10.1093/pcp/pcp064. [DOI] [PubMed] [Google Scholar]
- Kramer EM, Di Stilio VS, Schluter P. Complex patterns of gene duplication in the APETALA3 and PISTILLATA lineages of the Ranunculaceae. International Journal of Plant Science. 2003;164:1–11. [Google Scholar]
- Langdale JA, Zelitch I, Miller E, Nelson T. Cell position and light influence C4 versus C3 patterns of photosynthetic gene expression in maize. EMBO Journal. 1988;7:3643–3651. doi: 10.1002/j.1460-2075.1988.tb03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser HMO, Pickett FB, Dharmasiri S, Estelle M. Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. The Plant Journal. 1996;10:403–413. doi: 10.1046/j.1365-313x.1996.10030403.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Hagen G, Guilfoyle TJ. Altered morphology in transgenic tobacco plants that overproduce cytokinins in specific tissues and organs. Developmental Biology. 1992;153:386–395. doi: 10.1016/0012-1606(92)90123-x. [DOI] [PubMed] [Google Scholar]
- Liu C, Xu Z, Chua NH. Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. The Plant Cell. 1993;5:621–630. doi: 10.1105/tpc.5.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolle SJ, Cheung AY, Sussex IM. Fiddlehead: an Arabidopsis mutant constitutively expressing an organ fusion program that involves interactions between epidermal cells. Developmental Biology. 1992;152:383–392. doi: 10.1016/0012-1606(92)90145-7. [DOI] [PubMed] [Google Scholar]
- Menendez V, Revilla MA, Fal MA, Fernandez H. The effect of cytokinins on growth and sexual organ development in the gametophyte of Blechnum spicant L. Plant Cell Tissue and Organ Culture. 2009;96:245–250. [Google Scholar]
- Nakagawa H, Ferrario S, Angenent GC, Kobayashi A, Takatsuji H. The Petunia ortholog of Arabidopsis SUPERMAN plays a distinct role in floral organ morphogenesis. The Plant Cell. 2004;16:920–932. doi: 10.1105/tpc.018838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Jiang C-J, Sakakibara H, et al. Overexpression of a petunia zinc-finger gene alters cytokinin metabolism and plant forms. The Plant Journal. 2005;41:512–523. doi: 10.1111/j.1365-313X.2004.02316.x. [DOI] [PubMed] [Google Scholar]
- Nandi AK, Kushalappa K, Prasad K, Vijayraghavan U. A conserved function for Arabidopsis SUPERMAN in regulating floral-whorl cell proliferation in rice, a monocotyledonous plant. Current Biology. 2000;10:215–218. doi: 10.1016/s0960-9822(00)00341-9. [DOI] [PubMed] [Google Scholar]
- Ng M, Yanofsky MF. Activation of the Arabidopsis B class homeotic genes by APETALA1. The Plant Cell. 2001;13:739–754. doi: 10.1105/tpc.13.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno CK, Reddy GV, Heisler MGB, Meyerowitz EM. The Arabidopsis JAGGED gene encodes a zinc finger protein that promotes leaf tissue development. Development. 2004;131:1111–1122. doi: 10.1242/dev.00991. [DOI] [PubMed] [Google Scholar]
- Payne T, Johnson SD, Koltunow AM. KNUCKLES (KNU) encodes a C2H2 zinc-finger protein that regulates development of basal pattern elements of the Arabidopsis gynoecium. Development. 2004;131:3737–3749. doi: 10.1242/dev.01216. [DOI] [PubMed] [Google Scholar]
- Pfent C, Pobursky K, Sather D, Golenberg E. Characterization of SpAPETALA3 and SpPISTILLATA, B class floral identity genes in Spinacia oleracea, and their relationship to sexual dimorphism. Development, Genes and Evolution. 2005;215:132–142. doi: 10.1007/s00427-004-0459-4. [DOI] [PubMed] [Google Scholar]
- Rupp H-M, Frank M, Werner T, Strnad M, Schmülling T. Increased steady state mRNA levels of the STM and KNAT1 homeobox genes in cytokinin overproducing Arabidopsis thaliana indicate a role for cytokinins in the shoot apical meristem. The Plant Journal. 1999;18:557–563. doi: 10.1046/j.1365-313x.1999.00472.x. [DOI] [PubMed] [Google Scholar]
- Sakai H, Krizek BA, Jacobsen SE, Meyerowitz EN. Regulation of SUP expression identifies multiple regulators involved in Arabidopsis floral meristem development. The Plant Cell. 2000;12:1607–1618. doi: 10.1105/tpc.12.9.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Medrano LJ, Meyerowitz EM. Role of SUPERMAN in maintaining Arabidopsis floral whorl boundaries. Nature. 1995;378:199–203. doi: 10.1038/378199a0. [DOI] [PubMed] [Google Scholar]
- Sather DN, York A, Pobursky KJ, Golenberg EM. Sequence evolution and sex-specific expression patterns of the C class floral identity gene, SpAGAMOUS, in dioecious Spinacia oleracea L. Planta. 2005;222:284–292. doi: 10.1007/s00425-005-1544-2. [DOI] [PubMed] [Google Scholar]
- Schultz EA, Pickett FB, Haughn GW. The FLO10 gene product regulates the expression domain of homeotic genes AP3 and PI in Arabidopsis flowers. The Plant Cell. 1991;3:1221–1237. doi: 10.1105/tpc.3.11.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel BA, Verbeke JA. Diffusible factors essential for epidermal cell redifferentiaion in Catharanthus roseus. Science. 1989;244:580–582. doi: 10.1126/science.244.4904.580. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Chanderbali AS, Kim S, Buzgo M, Soltis PS. The ABC model and its applicability to basal angiosperms. Annals of Botany. 2007;100:155–163. doi: 10.1093/aob/mcm117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam YY, Normanly J. Determination of indole-3-pyruvic acid levels in Arabidopsis thaliana by gas chromatography-selected ion monitoring-mass spectrometry. Journal of Chromatography. 1998;800:101–108. doi: 10.1016/s0021-9673(97)01051-0. [DOI] [PubMed] [Google Scholar]
- Takatsuji H. Zinc-finger transcription factors in plants. Cellular and Molecular Life Sciences. 1998;54:582–596. doi: 10.1007/s000180050186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao LZ, Cheung AY, Wu HM. Plant Rac-like GTPases are activated by auxin and mediate auxin-responsive gene expression. The Plant Cell. 2002;14:2745–2760. doi: 10.1105/tpc.006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theissen G, Melzer R. Molecular mechanisms underlying origin and diversification of the angiosperm flower. Annals of Botany. 2007;100:603–619. doi: 10.1093/aob/mcm143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. The Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Meyerowitz EM. The ABCs of floral homeotic genes. Cell. 1994;78:203–209. doi: 10.1016/0092-8674(94)90291-7. [DOI] [PubMed] [Google Scholar]
- Yun JY, Weigel D, Lee I. Ectopic expression of SUPERMAN suppresses development of petals and stamens. Plant and Cell Physiology. 2002;43:52–57. doi: 10.1093/pcp/pcf018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.