Abstract
Potted grapevines of 140 Ruggeri (Vitis berlandieri × Vitis rupestris), a good Cl− excluder, and K 51-40 (Vitis champinii × Vitis riparia ‘Gloire’), a poor Cl− excluder, and of a family obtained by crossing the two genotypes, were used to examine the inheritance of Cl− exclusion. Rooted leaves were then used to further investigate the mechanism for Cl− exclusion in 140 Ruggeri. In both a potting mix trial (plants watered with 50 mM Cl−) and a solution culture trial (plants grown in 25 mM Cl−), the variation in Cl− accumulation was continuous, indicating multiple rather than single gene control for Cl− exclusion between hybrids within the family. Upper limits of 42% and 35% of the phenotypic variation in Cl− concentration could be attributed to heritable sources in the potting mix and solution culture trials, respectively. Chloride transport in roots of rooted leaves of both genotypes appeared to be via the symplastic pathway, since addition of 8-hydroxy-1,3,6-pyrenetrisulphonic acid (PTS), an apoplastic tracer, revealed no obvious PTS fluorescence in the laminae of either genotype, despite significant accumulation of Cl− in laminae of K 51-40 during the PTS uptake period. There was no significant difference in either unidirectional 36Cl− flux (10 min) or 36Cl− uptake (3 h) into roots of rooted leaves exposed to 5, 10, or 25 mM Cl−. However, the percentage of 36Cl− transported to the lamina (3 h) was significantly lower in 140 Ruggeri than in K 51-40, supporting reduced Cl− loading into xylem and implicating the root stele in the Cl− exclusion mechanism.
Keywords: Chloride exclusion, genotype, K 51-40, rooted leaf, salinity, 140 Ruggeri
Introduction
Grapevines are considered moderately sensitive to root-zone salinity (Maas and Hoffman, 1977; Fisarakis et al., 2001) and in many situations accumulate chloride (Cl−) to a greater extent than sodium (Na+) in shoot tissues (Walker et al., 2004, 2010). Accumulation of Cl− and Na+ in grapevines may result in physiological disturbances leading to reductions in growth, vegetative biomass, and fruit yield (Downton, 1977a; Walker et al., 1997, 2002, 2004). Moreover, excessive Cl− accumulation in grape berries may result in wines that exceed the recommended limit of 607 mg l−1 Cl−, as specified by Standard P4 of the Australian Food Standards code (Commonwealth of Australia, 2009).
It has long been known that variation in salt exclusion exists between grapevine species and cultivars (Sauer, 1968; Bernstein et al., 1969; Downton, 1977b). When used as a grapevine rootstock, 140 Ruggeri (Vitis berlandieri×Vitis rupestris) is known for its ability as a Cl− excluder, whereas K 51-40 (Vitis champinii×Vitis riparia ‘Gloire’) is a poor excluder and scions grafted to it accumulate high concentrations of Cl− when grown under saline conditions (Tregeagle et al., 2006; Walker et al., 2010). This wide variation in Cl− exclusion indicates the potential for breeding new salt-excluding cultivars or rootstocks. However, the inheritance of Cl− exclusion in grapevine remains unclear. In one study involving hybrids of a cross between cultivars Ramsey (V. champinii) and Sultana (Vitis vinifera), there was a continuous variation in Cl− accumulation between the hybrids, suggesting quantitative, heritable Cl− exclusion (Sykes, 1985). In another study (Sykes, 1987) involving hybrids and backcrosses of V. berlandieri and V. vinifera under glasshouse conditions, the ratio of genotypic to phenotypic variance was greater for backcross families than for F1 hybrids (V. berlandieri×V. vinifera), which suggested that a dominant gene for Cl− exclusion may be derived from V. berlandieri.
Furthermore, while advances have been made in understanding the mechanism for Cl− exclusion in grapevines (Schachtman and Thomas, 2003; Tregeagle et al., 2010), the precise mechanism remains to be established. Rooted leaves, on which roots are induced from the proximal end of the leaf petiole, have proved to be a suitable model system for ion transport, especially for woody perennial plants like grapevine (Schachtman and Thomas, 2003). Initial comparative data between rooted leaves of 140 Ruggeri and K 51-40 indicate similar Cl− uptake rates into roots of the two genotypes over a 3-h period in 10 mM Cl−, but lower concentrations of Cl− were measured in xylem of 140 Ruggeri (Tregeagle et al., 2010).
In this study, first, we investigated the variation for Cl− exclusion between replicated individuals propagated from 60 hybrids within a family obtained by crossing K 51-40 (seed parent) with 140 Ruggeri (pollen parent). Secondly, rooted leaves propagated from 140 Ruggeri and K 51-40 grapevines were used to compare differences in Cl− uptake and transport in order to further elucidate the Cl− exclusion mechanism. The aims were to determine whether one or more genes were involved, to quantify heritable versus non-heritable variation in Cl− exclusion within the hybrid family, and to provide a physiological and genetic basis for future research aimed at cloning Cl− transporter genes.
Materials and methods
Experiments
Experiments 1 and 2 were conducted with grapevines growing in potting mix (PM) and solution culture (SC), respectively, to investigate the variation for Cl− exclusion in a family between 140 Ruggeri and K 51-40. These contrasting media were chosen because the Cl−-exclusion capacity of 140 Ruggeri is diminished when grown in SC relative to PM (Tregeagle et al., 2010). Furthermore, the lower salinity level of 25 mM Cl− (SC) relative to 50 mM Cl− (PM) was chosen because of concerns that the diminished Cl− exclusion capacity of at least some genotypes in SC (Tregeagle et al., 2010) may lead to premature Cl− toxicity at 50 mM Cl−.
Experiments 3–6 were with rooted leaves. Experiment 3 compared the time-course of accumulation of Cl− in lamina, petiole, and roots of rooted leaves over a 2-week period. Experiment 4 used a fluorescent apoplast tracer to determine whether Cl− transport through the root apoplast was similar between the two genotypes, or whether roots of the poor Cl− excluder, K 51-40, had a higher level that facilitated greater Cl− transport through the root apoplast than that of 140 Ruggeri. Experiments 5 and 6 were laboratory-based flux experiments and were conducted to determine unidirectional influx of 36Cl− into roots over a 10-min period, and 36Cl− uptake into rooted leaves over a 3-h uptake period when treated with 5, 10, or 25 mM Cl−, respectively.
Variation for Cl− exclusion within the K 51-40×140 Ruggeri family (Experiments 1 and 2)
Hybrid family development and establishment of ‘source vines’ in the field: Controlled crosses were made between K 51-40 and 140 Ruggeri in 1985 (progeny code ‘M’) and 2005 (progeny code ‘H’). Following seed germination, 80 hybrids were planted and maintained subsequently as mature ‘source’ grapevines in CSIRO's experimental vineyard at Merbein, Victoria, Australia (34°13′41′′S, 142º2′38′′E). Soil characteristics, vineyard management, and irrigation practices were the same for the ‘M’ and ‘H’ grapevines. Irrigation water was taken from the River Murray and was generally of low salinity (<0.35 dSm−1).
Grapevine propagation: Uniform (30-cm), dormant cuttings of each hybrid, their parents, and two standard rootstocks, Ramsey (V. champinii) and 1103 Paulsen (V. berlandieri×V. rupestris), were placed in PM held in plastic boxes (30×37 cm) resting on heat-beds in a mist house held at or below 25 °C. The heat beds were set at 25 °C to encourage callus formation and root growth. Only 60 hybrids (11 ‘M’ and 49 ‘H’) were used because the vigour of the remaining grapevines was weak such that cuttings, if available, were thin and did not form callus or roots. Six uniform grapevines (∼20–30 cm in height) of each hybrid, their parents, and the two standard rootstocks were selected for each experiment.
PM experiment
Experimental grapevines were propagated in August 2007, planted in late October 2007 in a mixture of sand and red loam (40:60 v/v), held in 4.5-l pots and set up on benches in a glasshouse as randomized blocks with each genotype replicated once per block. The grapevines were watered daily with excess nutrient solution to ensure adequate leaching using an automated drip-irrigation system. The solution contained the following nutrients (mM): Ca2+, 1.2; K+, 1.7; Mg2+, 0.4; NH4+, 0.5; NO3−, 4.2; SO42–, 0.4; H2PO4−, 0.3; H3BO3, 8.3×10−4; Zn, 1.9×10−3; Cu, 1.4×10−3; Mn, 4.4×10−3; Mo, 3.2×10−5; EDTA–Fe, 3.0×10−2. The grapevines were maintained as single shoots by removing laterals as they arose. After 28 d, salt was added to the nutrient solution at a rate of 10 mM Cl− (with cations Na+:Ca2+:Mg2+ in the ratio 6:1:1) per day until 50 mM Cl− was reached. The grapevines were maintained under these conditions for a further 27 d when they were destructively harvested and 20% of each shoot proximal to the apex and 20% distal to the shoot base was discarded. All laminae and petioles in the middle 60% of each shoot were retained for analysis. A large composite root sample (>25% of total root mass) was also retained from each grapevine in four of the blocks. Aerial and root-zone temperatures were monitored in January 2008, the averages being 27.3 °C (17.7–39.8°C) and 27.3 °C (18.8–35.3 °C), respectively.
SC experiment
Experimental grapevines were propagated in September 2008 and transferred in mid-November 2008 to aerated solution cultures held in 450-l fibreglass tanks (surface area 1 m2) under glasshouse conditions. One replicate grapevine per genotype was randomly positioned in each of six tanks. The nutrient solution was as used in the PM experiment except that NH4+ was absent and total NO3− was 3.8 mM. Three weeks after grapevine transfer, the concentration of EDTA–Fe in the culture solution was increased from 30 μM to 71 μM, and supplementary lighting was used to extend the photoperiod by 2 h. One week later, the youngest leaf on each grapevine was labelled using a loosely tied cotton thread, shoot length was recorded, and Cl− was added to the cultures at a rate of 12.5 mM d−1 (with cations Na+:Ca2+:Mg2+ in the ratio 6:1:1) to achieve a final concentration of 25 mM. The grapevines were maintained under these conditions as single shoots by removing laterals as they arose. After a further 27 d, total shoot length and the length between the labelled leaf and the shoot apex were recorded, and the grapevines were destructively harvested. As in the PM experiment, all laminae and petioles in the middle 60% of each shoot were retained for analysis. As far as was possible, the complete root system of each grapevine was also retained. Aerial and root-zone temperatures were monitored in January 2009, the averages being 25.9 °C (15.3–33.3 °C) and 25.6 °C (range 24.5–27.4 °C), respectively.
Preparation of rooted leaves of K 51-40 and 140 Ruggeri (Experiments 3–6)
Container-grown grapevines of K 51-40 and 140 Ruggeri, established from cuttings, were maintained in a glasshouse and watered daily with non-saline nutrient solution (see PM experiment section). Mature leaves were excised from these plants and the proximal ends of petioles were dipped in a hormone gel containing 1.5 g l−1 indole-3-butryic acid (Clonex Growth Technology, Taunton, Somerset, UK) before planting them in propagation boxes containing (v:v) 50% vermiculite:50% perlite (Australian Vermiculite and Perlite Pty Ltd, Campbellfield, Victoria, Australia). The propagation boxes (33×28 cm) were placed upon heat beds (30 °C) in a mini-shadehouse within the glasshouse. The mini-shadehouse was covered with 90% shadecloth and the leaves were misted with fine sprays for 1 min every hour during the day between 7 a.m. and 7 p.m. About 3 weeks later, rooted leaves were transferred to aerated modified Hoagland #1 solution culture (Hoagland and Arnon, 1938) containing the following nutrients (mM) for a 2-week pretreatment period (except for 36Cl− flux experiment, in which there was a 3-d pretreatment): KNO3, 1.0; Ca(NO3)2·4H2O, 1.0; MgSO4·7H2O, 0.4; KH2PO4, 0.2; H3BO3, 4.6×10−2; MnCl2·4H2O, 9.1×10−3; ZnSO4·7H2O, 7.6×10−4; CuSO4·5H2O, 3.2×10−4; Na2MoO4·2H2O, 2.4×10−4; EDTA–Fe–Na, 7.1×10−2.
Cl− accumulation in intact rooted leaves of K 51-40 and 140 Ruggeri (Experiment 3)
Following pretreatment, the rooted leaves were subjected to 25 mM Cl− (Na+:Ca2+:Mg2+=6:1:1) in nutrient solution for up to 14 d. At each harvest, six rooted leaves of K 51-40 and 140 Ruggeri were separated into lamina, petiole, and roots, which were weighed before being dried in an oven at 60 °C and retained for Cl− analysis.
8-hydroxy-1,3,6-pyrenetrisulphonic acid uptake (Experiment 4)
Following pretreatment, rooted leaves were subjected to 25 mM Cl− (Na+:Ca2+:Mg2+=6:1:1) in nutrient solution±100 mg l−1 of 8-hydroxy-1,3,6-pyrenetrisulphonic acid (PTS) for 24 h. Six rooted leaves from the control and 12 from the PTS treatment for each genotype were harvested and retained for Cl− and fluorescence analysis. Leaves and roots were extracted in 40 ml deionized water at 90 °C for 2 h. PTS fluorescence was determined at λexcitation of 400 nm and λexcitation of 520 nm with a FLUOstar Omega microplate reader (BMG LABTECH GmbH, Offenburg, Germany).
36Cl− flux (Experiments 5 and 6)
Three days after being transferred to the modified Hoagland #1 solution, rooted leaves were subjected to 10 mM Cl− in the form of NaCl and CaCl2 in addition to nutrients for 6 d. The rooted leaves were then transferred to 5, 10, or 25 mM Cl− solution (n=6 for each genotype at each Cl− treatment) without background nutrients for 24 h, after which they were used for a 36Cl− flux experiment. Ca2+ activity was always kept at 0.69 mM during this experiment.
36Cl− flux experiments were conducted at 25 °C under a fluorescent light bank at the Australian Centre for Plant Functional Genomics. Two experiments were conducted, which measured unidirectional Cl− flux into roots over a 10-min period and net Cl− flux into roots over a 3-h period. Rooted leaves were transferred to 100 ml of radioactive 36Cl solution (0.1 μCi/ml) with roots lowered into the solution. The radioactive flux solution was agitated continuously on a rotating shaker (40 r.p.m).
Three 20-μl standards (radioactive solution) were collected at the start and end of each flux experiment. These standards were mixed with scintillation fluid, measured with the scintillation counter, and were used along with its specific activity (radioactivity divided by total nmoles of nominal Cl− in this 20-μl radioactive solution) in the conversion of 36Cl− content in plant tissues to nominal Cl− content.
In the 3-h uptake experiment, loss of volume due to evapotranspiration (∼1–1.5 ml h−1) by the radioactive flux solutions was corrected for by weighing the container plus rooted leaves at the start of the experiment, and then by reweighing and replacing the lost weight with deionized water every 30–60 min. Similarly, the loss of weight caused by the removal of a rooted leaf from the radioactive flux solution was corrected for by adding deionized water. Following removal of the rooted leaf, the new weight for the radioactive flux solution was used as the basis for subsequent checks for evapotranspiration losses.
At the end of the flux time, rooted leaves were carefully removed from the radioactive flux solution, and roots and petiole rinsed twice in an ice-cold 5, 10, or 25 mM Cl− rinse solution for 2 and 3 min, respectively. The rinsed roots were blotted dry, and each rooted leaf was separated into roots, callus, petiole, and lamina, which were put into scintillation vials and weighed. Scintillation fluid was then added and the samples were shaken, placed in the dark, and then analysed with a liquid scintillation counter at least 1 h later.
To prevent the presence of chlorophyll within the lamina and petiole samples from increasing the scintillation counts, every sample was first bleached in sunlight for a week and then with 200 μl of domestic bleach (containing sodium hypochlorite 49.9 g l−1, sodium hydroxide 12 g l−1, alkaline salts 0.5 g l−1) added to each vial. Samples were then kept in the dark for a week before being run on the scintillation counter.
Ion analyses
Laminae, petiole, and root samples were dried at 60 °C for at least 72 h and finely powdered in a hammer mill to pass through a 0.5-mm mesh. Chloride concentration was measured by silver ion titration with a chloridometer (Model 442-5150; Buchler Instruments, Lenexa, KS, USA) from extracts prepared by digesting 20- to 100-mg dry samples in 4 ml of an acid solution containing 10% (v/v) CH3COOH and 0.1 M HNO3 overnight before analysis.
Statistical analysis
Data were subjected to analysis of variance using GenStat Release 11.1 software. Where F tests were significant (P<0.05), means were separated by least significant differences (Snedecor and Cochran, 1967). Significant differences between data for ‘H’ and ‘M’ hybrids were explored and none were demonstrated, so the data were combined.
Results
Variation for Cl− exclusion in the hybrid family (PM: Experiment 1)
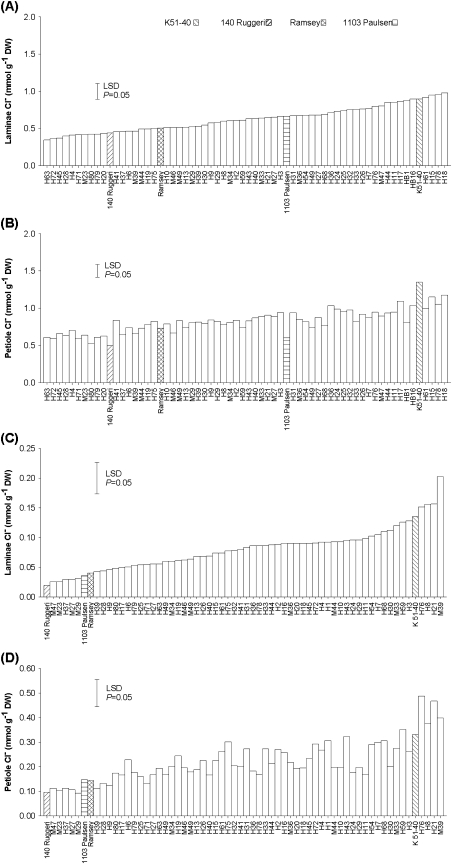
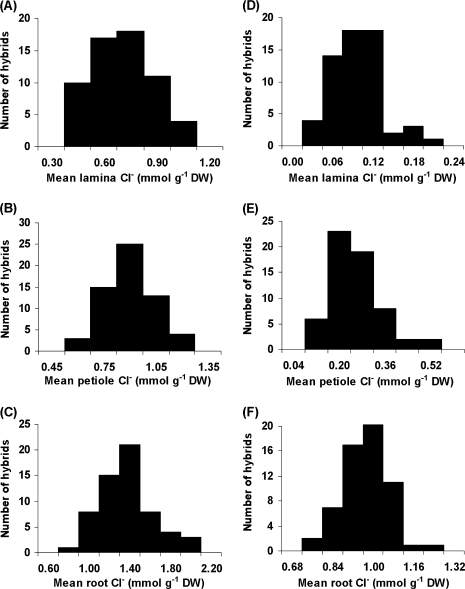
There were significant differences (P<0.001) between hybrids for mean Cl− concentration in laminae (Table 1), this variation being continuous (Fig. 1A) and normally distributed (Fig. 2A). Hybrid ‘H63’ was found to have the lowest laminae Cl− concentration and ‘H18’ the highest (Fig. 1A). There was a significant difference (P<0.001) in laminae Cl− concentrations between the two parents, with the male parent 140 Ruggeri containing half that of the female parent K 51-40 (Table 1). The mean laminae Cl− concentration for the hybrid family was calculated to be 0.62 mmol g−1 dry weight (DW), which is higher than 140 Ruggeri but lower than K 51-40. No evidence was found of transgressive segregation, there being no significant differences between the parent cultivars and the hybrids that fell at the end of the distributions (Table 1, Fig. 1A).
Table 1.
A summary of Cl− concentration (mmol g−1 DW) in laminae, petioles, and roots of plants grown in PM (Experiment 1)
The grapevines were irrigated daily with a complete nutrient solution containing 50 mM Cl– (Na+:Ca2+:Mg2+=6:1:1) for 27 d under glasshouse conditions.
| Parents |
Hybrids (60) |
||||||||
| K 51-40 | 140 Ruggeri | SEM | F value | Range | Mean | SEM | F value | ta | |
| Lamina Cl− | 0.90 | 0.44 | 0.06 | 33.41** | 0.35–0.98 | 0.62 | 0.08 | 4.86*** | 0.39 |
| Petiole Cl− | 1.35 | 0.50 | 0.06 | 99.36*** | 0.50–1.35 | 0.83 | 0.06 | 5.31*** | 0.42 |
| Root Cl− | 1.16 | 1.36 | 0.13 | NS | 0.79–1.99 | 1.27 | 0.13 | 3.72*** | 0.40 |
NS, not significant at P≤0.05; **P<0.01; ***P<0.001.
t, intraclass correlation coefficient, which was obtained from the analysis of variance as follows:
Fig. 1.
Mean Cl− concentrations (mmol g−1 DW) in laminae and petioles of grapevines propagated clonally from K 51-40×140 Ruggeri hybrids grown in PM (A and B) (Experiment 1) or aerated nutrient culture solution (C and D) (Experiment 2) in glasshouse conditions. In the PM experiment, the grapevines were irrigated daily with a complete nutrient solution containing 50 mM Cl−. In the SC experiment, the grapevines were grown in a complete nutrient solution containing 25 mM Cl−. Salt treatment lasted 27 d in both experiments. LSD, least significant difference.
Fig. 2.
Distribution of mean (n=6) Cl− concentrations (mmol g−1 DW) in laminae, petioles, and roots of grapevines propagated clonally from K 51-40×140 Ruggeri hybrids grown in PM (A–C) (Experiment 1) or aerated nutrient culture solution (D–F) (Experiment 2) in glasshouse conditions. In the PM experiment, the grapevines were irrigated daily with a complete nutrient solution containing 50 mM Cl−. In the SC experiment, the grapevines were grown in a complete nutrient solution containing 25 mM Cl−. Salt treatment lasted 27 d in both experiments.
| Source | df | Mean square |
| Between genotypes | n–1 | σw2+p σg2 |
| Within genotypes | n (p–1) | σw2 |
Where n=number of genotypes; p=number of replicates; genotypic variance=σg2; phenotypic variance=σg2+σw2; t=σg2/(σg2+σw2).
Similar results were observed for petiole Cl− concentrations (Table 1, Figs 1B, 2B). There was a strong correlation between laminae and petiole Cl− concentrations (r2=0.76; P<0.001).
In the root, there were also significant differences (P<0.001) between hybrids for mean Cl− concentration, with ‘H49’ and ‘H20’ having the highest and lowest Cl− concentrations, respectively (data not shown). There was no significant difference for mean root Cl− concentration between K 51-40 and 140 Ruggeri grapevines (Table 1). Three hybrids (H10, H25, and H49) had higher root Cl− concentrations compared with their parents and this might be due to transgressive segregation. There was no relationship between root Cl− concentrations and those for either laminae or petioles.
The intraclass correlation coefficient for Cl− concentration was in the range 0.39–0.42 for laminae, petiole, and root (Table 1).
Variation for Cl− exclusion in the hybrid family (SC: Experiment 2)
There were significant differences (P<0.001) between hybrids for laminae Cl− concentration and the variation was continuous (Table 2, Fig. 1C) and normally distributed (Fig. 2D). There was a 7-fold difference between the parent cultivars for mean laminae Cl− concentration (Table 2). Hybrid M39 had the highest mean lamina Cl− concentration in the family, this being significantly greater than that for K 51-40 (Fig. 1C). K 51-40 grapevines had a 3.3-fold higher mean petiole Cl− concentration than those of 140 Ruggeri (Table 2). Two hybrids, H21 and H76, had significantly higher mean petiole Cl− concentrations than K 51-40 (Fig. 1D). There was a strong correlation between laminae and petiole Cl− concentrations (r2=of 0.71; P<0.001). Roots of K 51-40 had 26.5% higher mean Cl− concentrations than those of 140 Ruggeri grapevines (Table 2). The distribution of mean root Cl− concentrations was continuous in the hybrid family (data not shown) and normally distributed (Fig. 2F), but the variation obtained in the SC experiment (Table 2) was less than that observed in the PM experiment (Table 1). There was no correlation between mean root Cl− concentrations and those for either laminae or petioles.
Table 2.
A summary of Cl− concentration (mmol g−1 DW) in laminae, petioles, and roots of plants grown in a complete nutrient solution with addition of 25 mM Cl− (Na+:Ca2+:Mg2+=6:1:1) for 27 d (Experiment 2)
| Parents |
Hybrids (60) |
||||||||
| K 51-40 | 140 Ruggeri | SEM | F value | Range | Mean | SEM | F value | ta | |
| Lamina Cl− | 0.14 | 0.02 | 0.02 | 25.49** | 0.03–0.20 | 0.08 | 0.02 | 3.09*** | 0.26 |
| Petiole Cl− | 0.33 | 0.10 | 0.03 | 34.98** | 0.09–0.49 | 0.22 | 0.04 | 4.18*** | 0.35 |
| Root Cl− | 1.05 | 0.83 | 0.05 | 11.89* | 0.74–1.16 | 0.94 | 0.06 | 2.28*** | 0.18 |
t, intraclass correlation coefficient. The calculation is as shown in Table 1.
*P<0.05; **P<0.01**; ***P<0.001.
The intraclass correlation coefficient for Cl− concentration was in the range 0.18–0.35 (Table 2). Regression coefficients (b) between lamina or petiole Cl− concentrations and shoot growth parameters (except shoot DW) were significant (P<0.05) (Table 3).
Table 3.
Correlation between Cl− concentrations and shoot growth parameters for hybrids grown in SC (Experiment 2)
| Shoot growth parameter | Cl− concentration (mmol g−1 DW) | r | y=a+bx | b±SE | P |
| Shoot length at stress start (cm) | Lamina | 0.136 | y=0.103–0.3×10−3x | –0.3×10−3±0.1×10−3 | 0.01 |
| Petiole | 0.182 | y=0.290–0.8×10−3x | –0.8×10−3±0.2×10−3 | <0.001 | |
| Shoot growth (length from tag to tip, cm) | Lamina | 0.249 | y=0.141–0.4×10−3x | –0.4×10−3±0.1×10−3 | <0.001 |
| Petiole | 0.321 | y=0.399–1.1×10−3x | –1.1×10−3±0.2×10−3 | <0.001 | |
| Shoot length at harvest (cm) | Lamina | 0.222 | y=0.131–0.2×10−3x | –0.2×10−3±0.5×10−4 | <0.001 |
| Petiole | 0.292 | y=0.371–0.6×10−3x | –0.6×10−3±0.1×10−3 | <0.001 | |
| Shoot dry weight (g) | Lamina | 0.003 | y=0.081–1.1×10−5x | –1.1×10−5±2.0×10−4 | 0.96 |
| Petiole | 0.041 | y=0.229–0.4×10−3x | –0.4×10−3±0.5×10−3 | 0.44 |
r=correlation coefficient; a=the intercept; b=the slope.
Comparison of data obtained in PM and SC trials
No overall relationship was found between mean Cl− concentrations of grapevines grown in the SC experiment and those from the PM experiment (data not shown). However, mean Cl− concentrations for some hybrids were similar in the two experiments. For example, H28, H37, H63, H71, H79, H80, and M23 had low, and H76 had relatively high lamina and petiole Cl− concentrations, irrespective of root medium. In contrast, the mean lamina and petiole Cl− concentrations of H17, M39, and M47 were dissimilar between experiments. H17 and M47 had relatively high lamina and petiole Cl− concentrations in the PM experiment, but low in the SC experiment. M39 had relatively low lamina and petiole Cl− concentrations in the PM experiment, but was found to be one of the poorest Cl− excluders in the SC experiment. This may be due to the relatively poor vigour of M39 at the commencement of salt treatment in the SC experiment.
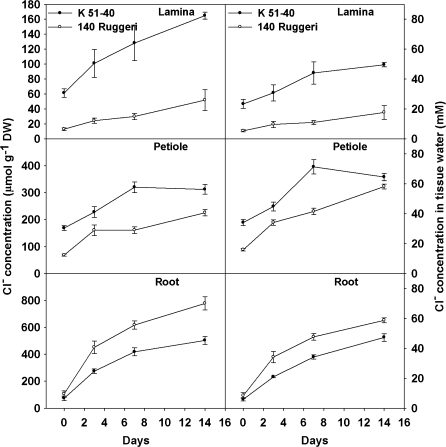
Cl− accumulation in rooted leaves of K 51-40 and 140 Ruggeri (Experiment 3)
In the presence of additional Cl− in culture solution, rooted leaves of K 51-40 and 140 Ruggeri showed differences in Cl− accumulation in lamina, petiole, and roots (Fig. 3). K 51-40 rooted leaves always had higher mean lamina and petiole Cl− concentrations than 140 Ruggeri, but those in roots were consistently lower.
Fig. 3.
Mean Cl− concentrations in laminae, petioles, and roots of rooted leaves of K 51-40 and 140 Ruggeri at time points during a 2-week period when grown in modified Hoagland solution with addition of 25 mM Cl− (Experiment 3). At each harvest, the rooted leaf was separated into lamina, petiole, and roots, which were weighed, then dried in an oven and retained for Cl− analysis. Bars represent ±SEM (n=6).
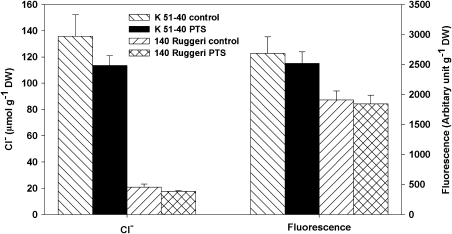
Apoplastic transport (Experiment 4)
The addition of PTS, an apoplastic tracer (Yeo et al., 1999), significantly increased (P<0.001) the fluorescence in the roots of both K 51-40 and 140 Ruggeri rooted leaves under Cl− treatment. No significant PTS fluorescence was observed above background readings in leaves of either K 51-40 or 140 Ruggeri (Fig. 4). However, during the 24-h PTS uptake period, Cl− concentration in the shoot (lamina+petiole) of K 51-40 increased from a background value of ∼80 μmol g−1DW to 135.8±16.5 μmol g−1 DW for the (–) PTS and 113.5±7.5 μmol g−1 DW for the (+) PTS treatment, whereas there was no significant increase in Cl− concentration in shoots of 140 Ruggeri in (+) and (–) PTS treatments. The difference in Cl− concentration in shoots of the two genotypes after the 24-h period was 6.5-fold (Fig. 4).
Fig. 4.
Fluorescence and Cl− concentrations of rooted leaves (lamina+petiole) of K 51-40 and 140 Ruggeri as affected by salt and PTS treatment (Experiment 4). Two weeks after being transferred to modified Hoagland nutrient solution, the rooted leaves were subjected to 25 mM Cl− (Na+:Ca2+:Mg2+=6:1:1) without or with 100 mg/l PTS for 24 h, after which the plants were harvested for Cl− and fluorescence analysis. Bars represent standard errors of means (n=6 for control and 12 for PTS treatment).
Short-term Cl− uptake and transport in rooted leaves: 36Cl− flux (Experiments 5 and 6)
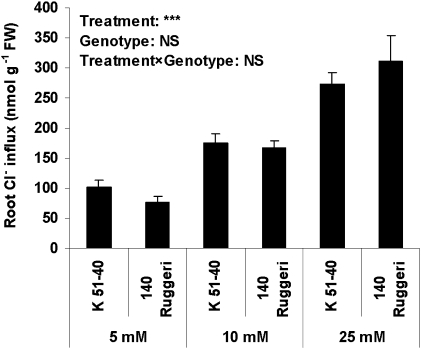
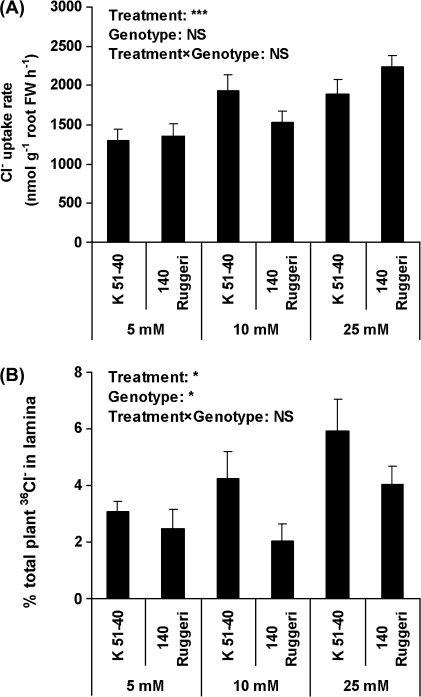
There was no significant difference in the unidirectional Cl− flux into the root of rooted leaves between the two genotypes (Fig. 5). 36Cl− uptake over 3 h also did not show a significant difference between rooted leaves of the two genotypes (Fig. 6A). With the increase in Cl− concentration in the external solution from 5 to 25 mM, root Cl− uptake significantly increased (Fig. 6A). The percentage of 36Cl− transported to the lamina was significantly (P<0.05) lower in 140 Ruggeri than in K 51-40 (Fig. 6B).
Fig. 5.
Unidirectional influx of Cl− measured by 36Cl− over 10 min in the rooted leaves of K 51-40 and 140 Ruggeri (Experiment 5). Three days after being transferred to modified Hoagland solution, the rooted leaves were subjected to 10 mM Cl− in the form of NaCl and CaCl2 in addition to nutrients for 6 d. The rooted leaves were then subjected to 5, 10, or 25 mM Cl− treatment without nutrients for 24 h, after which the whole intact plants were used for the 36Cl− flux experiment. The Ca2+ activity was always kept at 0.69 mM during the experiment. Bars represent SEM (n=6). ***P<0.001; NS, not significant.
Fig. 6.
Chloride uptake measured by 36Cl− influx over 3 h (A) and percentage of 36Cl− transport into the lamina as compared with total 36Cl− uptake (B) in rooted leaves of K 51-40 and 140 Ruggeri (Experiment 6). Plant treatment was the same as described in Fig. 5 legend. Bars represent SEM (n=6). *P<0.05; ***P<0.001; NS, not significant.
Discussion
Chloride exclusion has been defined by Teakle and Tyerman (2010) as the ability of plants to restrict uptake of Cl− from the soil and subsequent transport in the xylem to the shoot. Genotypes with good Cl−-excluding capacity, e.g. 140 Ruggeri, can be distinguished from poor Cl−-excluding genotypes, e.g. K 51-40, by their lower concentrations of Cl− in above-ground vegetative tissues, e.g. laminae and petioles.
Comparison of hybrids grown in PM and SC
The investigation of Cl− exclusion in a family of 60 140 Ruggeri×K 51-40 hybrids revealed a continuous variation in Cl− accumulation in both laminae and petioles. This suggests involvement of more than one gene in control of Cl− exclusion within the family, which is consistent with the conclusion by Sykes (1985), who observed a continuous variation in Cl− accumulation in shoots in the hybrid family of Ramsey (V. champinii)×Sultana (V. vinifera). These results, however, are in contrast to those of Newman and Antcliff (1984) and Sykes (1987) who observed that hybrids between V. berlandieri and V. vinifera had low petiole Cl− concentrations and discontinuous distributions within backcrosses to V. vinifera under vineyard conditions, suggesting a single dominant gene for Cl− exclusion in V. berlandieri. As one of the parents in our study (140 Ruggeri) was a hybrid of V. berlandieri, a single dominant gene (Newman and Antcliff, 1984; Sykes, 1987) may have been transmitted, only to be masked by possible modifier genes, which may have been derived from the different grapevine species background of the parents, and by other factors.
Chloride concentrations in laminae, petioles, and roots were found to be lower in the SC experiment than in the PM experiment. This most likely was related to the difference in salinity level and to the growth conditions. The mean Cl− concentration in the roots for the hybrids grown in the SC experiment was 74% of that in PM experiment, indicating that Cl− accumulation in roots is less affected than Cl− accumulation in laminae and petioles by the type of growing medium. For example, the average Cl− concentrations in laminae and petioles for the hybrids in the SC experiment were 13% and 27% of those in the PM experiment, respectively. Besides the difference in Cl− concentration in the nutrient solution (50 mM in the PM experiment and 25 mM in the SC experiment), reduced Cl− transport from the root to the shoot in the SC experiment may have resulted from differences in aerial and root-zone temperatures. Higher root-zone temperature can increase ion accumulation in plants (Zelleke and Kliewer, 1980) and accordingly the higher maximum root-zone temperatures in the PM experiment may have enhanced Cl− uptake in the roots. Higher air temperature is known to stimulate leaf transpiration (Reddy et al., 1995), which may have increased total Cl− flux in the xylem. A link between transpiration and total Cl− accumulated has been reported for citrus genotypes by Moya et al. (2003).
The variability for Cl− concentration in leaves and roots reported here may indicate a genetic variation for the ability by the hybrids to exclude this ion from their leaves. The values obtained for the intra-class correlation coefficient, a statistic that estimates the degree of genotypic determination (Falconer and Mackay, 1996) and, thus, an upper estimate of the broad sense heritability, supported this argument. As the grapevines grown in the experiments were clonal, estimates of broad sense heritability from intra-class correlation coefficients indicated that upper limits of 42% and 35% of the total phenotypic variation in Cl− exclusion of this hybrid family could be attributed to heritable sources in PM and SC experiments, respectively.
The full spectrum of vigour in the hybrid family may not have been represented in the hybrids used in the PM and SC experiments due to the propagation loss of weak individuals. However, differences in Cl− accumulation among the hybrids used in the PM and SC experiments were not strongly associated with plant vigour, shown by low r values (Table 3). A similar observation was made by Walker et al. (2004), where Cl− concentrations in petioles at flowering, laminae at harvest, and grape juice at harvest showed no correlation with either yield or weight of 1-year-old pruning wood in a 5-year study involving mature Sultana grapevines on a range of rootstocks irrigated with water having electrical conductivities of 0.40, 1.75, and 3.50 dS/m. However, grapevine rootstocks that lead to high concentrations of Cl− in laminae, petioles, and grape juice of scions grafted to them, e.g. rootstock K 51-40 (Tregeagle et al., 2006; Walker et al., 2010), can lead to significant leaf damage and impairment to fruit development and yield of scions under saline conditions (Walker et al., 2010). Determination of grapevine vigour and salt tolerance index was not an objective of our study, mainly because the large size of the experiments (384 grapevines in each case) precluded inclusion of a nutrient-solution-only control.
Studies with mature grapevines on a range of rootstocks in a range of environments and salinities have shown that the difference between a strong (e.g. 140 Ruggeri) and a poor (e.g. K 51-40) Cl− excluder, is maintained across the range of environments and salinities (Walker et al., 2010). This is supported by the results of our study, which has shown that in PM and SC experiments, 140 Ruggeri showed a better capacity than K 51-40 for Cl− exclusion from laminae and petioles (Tables 1, 2). However, the mean Cl− concentrations of grapevines of all genotypes grown in the SC experiment showed no overall relationship with those from the PM experiment (r=0.15 and 0.07 for lamina and petiole Cl−, respectively). The lack of a relationship between the results of the PM and SC experiments suggests that genotype–environment interactions were important. Possible differences in root morphology and function between PM- and SC-grown plants (Storey, 1995), which may have been greater for some hybrids than others, could be responsible for the poor correlation between the results of the PM and SC experiments.
Cl− fluxes in rooted leaves of the two parent genotypes
A previous study involving citrus rootstocks showed that genotype capacity for Cl− exclusion was linked to lower water use (Moya et al., 2003). However, comparative studies between 140 Ruggeri and K 51-40 have demonstrated that the better Cl−-excluding capacity of 140 Ruggeri (Tregeagle et al., 2006; Walker et al., 2010) cannot be explained by differences in water use by the genotypes (Tregeagle et al., 2010). An obvious question to follow from the earlier work (Tregeagle et al., 2010) was whether there is a difference in transport through the apoplast of the two genotypes, or more specifically whether roots of the poor Cl− excluder, K 51-40, had a higher level of apoplast Cl− transport. Previous studies found no difference in hydraulic conductivity of the roots of 140 Ruggeri and K 51-40 (J. Tregeagle, J.Tisdall, R.Walker, and S.Tyerman, unpublished data). Furthermore, no published data relating to apoplast flow and Cl− accumulation appear to be available for grapevine. In this study, using the apoplast tracer PTS (Yeo et al., 1999), no obvious PTS fluorescence was observed in the leaves of either genotype (Fig. 4), despite a significant increase in concentration of Cl− accumulated in the ‘shoot’ (lamina+petiole) of K 51-40 during the PTS treatment period, indicating no significant difference in Cl− transport via the apoplast in the roots of these two genotypes. This suggests that Cl− transport through the symplastic pathway contributes to the difference in Cl− accumulation in the leaves of both genotypes.
A previous study (Tregeagle et al., 2010) also demonstrated no significant difference in the unidirectional Cl− flux into excised roots of 140 Ruggeri and K 51-40. This study has extended that observation and further demonstrated no significant difference in the unidirectional Cl− flux into roots of intact rooted leaves. Furthermore, in Cl− influx studies involving three concentrations of Cl− in solution culture (5, 10, and 25 mM Cl−), no significant difference between the genotypes in net Cl− influx into whole plants could be demonstrated. However, similar to the earlier observation of Tregeagle et al. (2010), the percentage of 36Cl− transported to the lamina was significantly lower in 140 Ruggeri than in K 51-40 (Fig. 6B), which is consistent with lower xylem Cl− concentrations and lower root to shoot transport of Cl− in 140 Ruggeri relative to K 51-40 (Tregeagle et al., 2010). In addition, this study has shown higher concentrations of Cl− in roots of intact rooted leaves of 140 Ruggeri treated with 10 mM Cl−, evident after 3 d and maintained for the experiment duration (2 weeks), similar to that demonstrated for intact rooted leaves after 14 d treatment with 10 mM Cl− (Tregeagle et al., 2010). This may be a consequence of similar unidirectional Cl− influx (10 min) and Cl− uptake (3 h) of the two genotypes (Figs 5, 6A), but lower release of Cl− into xylem of 140 Ruggeri, therefore leading to higher concentrations of Cl− in roots of 140 Ruggeri (Fig. 3). However, it is notable that Cl− concentrations in root samples from whole grapevines of 140 Ruggeri and K 51-40 grown in PM and treated with 50 mM Cl− for 27 d were similar. Furthermore, Cl− concentrations in root systems of whole grapevines of 140 Ruggeri grown in SC with 25 mM Cl− for 27 d were lower than in K 51-40, similar to observations made with excised roots (Tregeagle et al., 2010). This indicates that a range of factors, e.g. plant–root system age, size and morphology, and applied salt concentration, potentially impact on the comparative response of the two genotypes in terms of Cl− concentration accumulated in roots. Similar factors could also act to modify the expression of genes controlling Cl− exclusion, which, in a highly heterozygous plant such as the grapevine, could mask any major gene effects.
In summary, the variation in Cl− exclusion within the hybrid family from a cross between 140 Ruggeri and K 51-40 appears to be controlled by more than one gene. Studies with rooted leaves over three root-zone salinity concentrations (5, 10, and 25 mM Cl−) confirmed similar unidirectional Cl− influx and similar Cl− uptake rate into roots of the two genotypes, but the percentage of total plant Cl− accumulated in laminae was significantly lower for 140 Ruggeri, consistent with lower Cl− transport from the root to the shoot. The results further our understanding of the underlying genetic and physiological mechanisms contributing to Cl− exclusion in grapevine genotypes. It also indicates the symplast as the key pathway for Cl− transport in grapevine roots and suggests that stelar cells may be important sites of the Cl− exclusion mechanism. Forward and reverse genetic approaches are currently being undertaken to further understand the regulation of Cl− uptake and transport.
Acknowledgments
We acknowledge Australia's grape growers and winemakers for financial support through the Grape and Wine Research and Development Corporation with matching funds from the Federal Government. We thank Dr Mandy Walker of CSIRO Plant Industry for her valuable suggestions.
Glossary
Abbreviations
- DW
dry weight
- PM
potting mixture
- PTS
8-hydroxy-1,3,6-pyrenetrisulphonic acid
- SC
solution culture
References
- Bernstein L, Ehlig CF, Clark RA. Effect of grape rootstocks on chloride accumulation in leaves. Journal of American Society for Horticultural Science. 1969;94:584–590. [Google Scholar]
- Commonwealth of Australia. 2009. Wine Production Requirements (Australia only) http://www.foodstandards.gov.au/thecode/foodstandardscode/standard451wineprodu4297.cfm (accessed 2 October 2009) [Google Scholar]
- Downton WJS. Photosynthesis in salt-stressed grapevines. Australian Journal of Plant Physiology. 1977a;4:183–192. [Google Scholar]
- Downton WJS. Chloride accumulation in different species of grapevines. Scientia Horticulturae. 1977b;7:249–253. [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. 4th edn. New York: Longman; 1996. [Google Scholar]
- Fisarakis I, Chartzoulakis K, Stavrakas D. Response of Sultana vines (V. vinifera L.) on six rootstocks to NaCl salinity exposure and recovery. Agricultural Water Management. 2001;51:13–27. [Google Scholar]
- Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. 1938 Circular 347 (University of California College of Agriculture, Berkeley, CA, USA) [Google Scholar]
- Maas EV, Hoffman GJ. Crop salt-tolerance – current assessment. Journal of the Irrigation and Drainage Division of the American Society of Civil Engineers. 1977;103:115–134. [Google Scholar]
- Moya JL, Gomez-Cadenas A, Primo-Millo E, Talon M. Chloride absorption in salt-sensitive Carrizo Citrange and salt-tolerant Cleopatra Mandarin citrus rootstocks is linked to water use. Journal of Experimental Botany. 2003;54:825–833. doi: 10.1093/jxb/erg064. [DOI] [PubMed] [Google Scholar]
- Newman HP, Antcliff AJ. Chloride accumulation in some hybrids and backcrosses of Vitis berlandieri and Vitis vinifera. Vitis. 1984;23:106–112. [Google Scholar]
- Reddy VR, Reddy KR, Hodges HF. Carbon dioxide enrichment and temperature effects on cotton canopy photosynthesis, transpiration, and water use efficiency. Field Crops Research. 1995;41:13–23. [Google Scholar]
- Sauer MR. Effects of vine rootstocks on chloride concentration in Sultana scions. Vitis. 1968;7:223–226. [Google Scholar]
- Schachtman DP, Thomas MR. A rapid method for generating sufficient amounts of uniform genotype-specific material from the woody perennial grapevine for ion transport studies. Plant Soil. 2003;253:195–199. [Google Scholar]
- Snedecor GW, Cochran WG. Statistical methods. 6th edn. Ames, IA: Iowa State University Press, Iowa, USA; 1967. [Google Scholar]
- Storey R. Salt tolerance, ion relations and the effect of root medium on the response of citrus to salinity. Australian Journal of Plant Physiology. 1995;22:101–114. [Google Scholar]
- Sykes SR. Variation in chloride accumulation by hybrid vines from crosses involving the cultivars Ramsey, Villard Blanc, and Sultana. American Journal of Enology and Viticulture. 1985;36:30–37. [Google Scholar]
- Sykes SR. Variation in chloride accumulation in hybrids and backcrosses of Vitis berlandieri and Vitis vinifera under glasshouse conditions. American Journal of Enology and Viticulture. 1987;38:313–320. [Google Scholar]
- Teakle NL, Tyerman SD. Mechanisms of Cl− transport contributing to salt tolerance. Plant, Cell and Environment. 2010;33:566–589. doi: 10.1111/j.1365-3040.2009.02060.x. [DOI] [PubMed] [Google Scholar]
- Tregeagle JM, Tisdall JM, Blackmore DH, Walker RR. A diminished capacity for chloride exclusion by grapevine rootstocks following long-term saline irrigation in an inland versus coastal region of Australia. Australian Journal of Grape and Wine Research. 2006;12:178–191. [Google Scholar]
- Tregeagle JM, Tisdall JM, Tester M, Walker RR. Cl− uptake, transport and accumulation in grapevine rootstocks of differing capacity for Cl− exclusion. Functional Plant Biology. 2010;37:665–673. [Google Scholar]
- Walker RR, Blackmore DH, Clingeleffer PR. Impact of rootstock on yield and ion concentrations in petioles, juice and wine of Shiraz and Chardonnay in different viticultural environments with different irrigation water salinity. Australian Journal of Grape and Wine Research. 2010;16:243–258. [Google Scholar]
- Walker RR, Blackmore DH, Clingeleffer PR, Correll RL. Rootstock effects on salt tolerance of irrigated field-grown grapevines (Vitis vinifera L. cv. Sultana) 1. Yield and vigour inter-relationships. Australian Journal of Grape and Wine Research. 2002;8:3–14. [Google Scholar]
- Walker RR, Blackmore DH, Clingeleffer PR, Correll RL. Rootstock effects on salt tolerance of irrigated field-grown grapevines (Vitis vinifera L. cv. Sultana) 2. Ion concentrations in leaves and juice. Australian Journal of Grape and Wine Research. 2004;10:90–99. [Google Scholar]
- Walker RR, Blackmore DH, Clingeleffer PR, Iacono F. Effect of salinity and Ramsey rootstock on ion concentrations and carbon dioxide assimilation in leaves of drip-irrigated, field-grown grapevines (Vitis vinifera L. cv. Sultana) Australia Journal of Grape and Wine Research. 1997;3:66–74. [Google Scholar]
- Yeo AR, Flowers SA, Rao G, Welfare K, Sennanayake N, Flowers TJ. Silicon reduces sodium uptake in rice (Oryza sativa L.) in saline conditions and this is accounted for by a reduction in the transpirational bypass flow. Plant, Cell and Environment. 1999;22:559–565. [Google Scholar]
- Zelleke A, Kliewer WM. Effect of root temperature, rootstock and fertilization on bud-break, shoot growth and composition of ‘Cabernet Sauvignon’ grapevines. Scientia Horticulturae. 1980;13:339–347. [Google Scholar]