Abstract
Seed dormancy in wild Lolium rigidum Gaud (annual ryegrass) populations is highly variable and not well characterized at the biochemical level. To identify some of the determinants of dormancy level in these seeds, the proteomes of subpopulations selected for low and high levels of primary dormancy were compared by two-dimensional polyacrylamide gel electrophoresis of extracts from mature, dry seeds. High-dormancy seeds showed higher expression of small heat shock proteins, enolase, and glyoxalase I than the low-dormancy seeds. The functional relevance of these differences in protein expression was confirmed by the fact that high-dormancy seeds were more tolerant to high temperatures imposed at imbibition and had consistently higher glyoxalase I activity over 0–42 d dark stratification. Higher expression of a putative glutathione peroxidase in low-dormancy seeds was not accompanied by higher activity, but these seeds had a slightly more oxidized glutathione pool and higher total peroxidase activity. Overall, these biochemical and physiological differences suggest that L. rigidum seeds selected for low dormancy are more prepared for rapid germination via peroxidase-mediated cell wall weakening, whilst seeds selected for high dormancy are constitutively prepared to survive environmental stresses, even in the absence of stress during seed development.
Keywords: Dormancy, glutathione, glyoxalase I, heat shock protein, Lolium rigidum, peroxidase, proteomics, seed
Introduction
Lolium rigidum Gaud (annual ryegrass) is a highly successful weed in cereal production systems in many regions of the world. Contributing to its success is the fact that its seeds are dormant (classified as non-deep physiological dormancy; Finch-Savage and Leubner-Metzger, 2006) at maturity, thus avoiding germination in response to transient rainfall during the hot, dry summer months (Chauhan et al., 2006). Different levels of seed dormancy within a population also result in staggered seedling emergence throughout the growing season, making removal of the weed plants far more difficult and resulting in constant replenishment of the soil seed bank. A greater understanding of the detailed mechanisms of dormancy induction and release in L. rigidum seeds is required if these processes are to be manipulated with the aim of eliminating the soil seed bank. Current physiological knowledge relates to the fact that imbibition of dormant L. rigidum seeds in the dark at warm temperatures (20–30 °C) causes a cumulative release of dormancy (Steadman, 2004; Steadman et al., 2004), and that this is related to a decrease in seed sensitivity to abscisic acid (ABA), a response which is inhibited by blue light (Goggin et al., 2008, 2009). However, other factors determining the dormancy level of seeds at maturity and the changes occurring during stratification have yet to be identified. In the current study, the biochemical basis of primary dormancy in mature L. rigidum seeds was investigated by comparing the proteomes of seed subpopulations selected from a single original population over three generations to be either low dormancy (LD) or high dormancy (HD). Based on the results of this comparison, it was decided to focus upon potential differences in the stress response and antioxidant defence capability of the selected seeds.
Previous work on seed antioxidant defence has shown that reactive oxygen species (ROS) can accumulate due to disruption of the mitochondrial electron transport chain (Leprince et al., 1994) in response to recommencement of metabolism upon imbibition, environmental stress, or during seed ageing. Excessive accumulation of ROS results in lipid peroxidation, protein and nucleic acid damage, and, eventually, a general loss of cellular function (Bailly, 2004; Halliwell, 2006), which explains the progressive decline in viability in ageing seeds. However, there is increasing evidence that ROS can also be beneficial by acting as signalling molecules in processes such as cell division, cell wall remodelling, and seed germination in a manner modulated by the soluble antioxidant molecules ascorbate and glutathione (reviewed in Foyer and Noctor, 2005). ROS such as hydrogen peroxide (H2O2) (Bailly, 2004), superoxide and quinone radicals (Oracz et al., 2009), and hydroxyl radicals (Müller et al., 2009) can all stimulate seed germination via various signalling or direct pathways. The oxidized form of glutathione (GSSG) may contribute to maintenance of seed dormancy by inhibiting protein synthesis (Kranner and Grill, 1993), whilst specific protein carbonylation during dry after-ripening has been proposed as a ROS-linked mechanism of dormancy release (Oracz et al., 2007).
Imbibed, metabolically active seeds are more vulnerable to abiotic stresses such as heat, cold, and salinity, and to pathogen attack, than dry quiescent seeds, and germination is generally inhibited under these conditions. Imbibed seeds respond to such stresses in a similar way to vegetative tissues, with accumulation of heat shock proteins (e.g. Hong and Vierling, 2001, and references therein) and osmoprotectants (e.g. Song et al., 2005), and generation of ROS. Again, the latter process can be either beneficial in stressed tissues, deterring further pathogen invasion and stimulating the antioxidant defence system, or detrimental, overwhelming the defence system and causing widespread cellular damage if the stress is too intense or prolonged (Foyer and Noctor, 2005).
To determine if selection of low or high primary dormancy in L. rigidum seeds causes differences in the antioxidant status or stress response of the seeds, a suite of antioxidant and stress-related enzymes, along with ascorbate and glutathione, were measured in subpopulations with LD and HD. Seed germination and viability under extremes of temperature or different redox environments was also assessed. By using seed populations repeatedly selected from a single original population and produced under the same environmental conditions, the effects of maternal environment on seed dormancy levels were minimized, allowing identification of selectable differences between LD and HD seeds.
Materials and methods
Chemicals
All chemicals and enzymes were obtained from Sigma-Aldrich (Sydney, Australia) unless otherwise stated.
LD and HD seed populations
Seeds were collected from a population of L. rigidum Gaud plants infesting a wheat field at Wongan Hills (30°53′S, 116°43′E) in November 2000. The germination characteristics of this seed population are detailed in Steadman (2004); the basal level of germination after 42 d under standard germination conditions (alternating 25/15 °C with a 12 h photoperiod of combined fluorescent and incandescent light at a fluence rate of 90 μmol m−2 s−1 over 400–700 nm) was 17±2%. These seeds were used as the basis repeatedly to select HD and LD seeds as described in Goggin et al. (2010). LD seeds were selected to commence germination immediately upon imbibition under standard germination conditions, whilst HD seeds were selected to require 42 d of stratification in the dark at 20 °C before being able to respond (>50% germination) to standard germination conditions. The LD and HD seeds resulting from three generations of selection (produced over 3 years during the normal growing season for L. rigidum) were used in the current study; the dormancy status of the selected populations was shown to be stable in Goggin et al. (2010).
Seed viability, as assessed by tetrazolium staining (Steadman, 2004), was close to 100% in both populations, and the moisture content at collection was 10%. Stratification and germination tests were performed on 1% (w/v) agar as described in Goggin et al. (2008), with four replicates per population and treatment.
Proteomic analysis of LD and HD seeds
Dry seeds (∼200 per replicate) were ground to powder in liquid nitrogen and extracted on ice in 50 mM KH2PO4 (pH 7.5), 1 mM Na2EDTA, 1% (v/v) Triton X-100, 5 mM dithiothreitol (DTT), 1 mM phenylmethylsulphonyl fluoride (PMSF) for 2 h. After centrifugation at 12 000 g for 30 min, the supernatant (soluble protein fraction) was precipitated for 24 h at –80 °C in 9 vols of methanol, and the pellet was further extracted in 8 M urea, 2% (v/v) Triton X-100, 5 mM DTT at room temperature for 2 h. Following centrifugation as above, the second supernatant (insoluble protein fraction) was also methanol precipitated. Soluble and insoluble proteins were collected by centrifugation, resuspended in IEF sample buffer [8 M urea, 2% (w/v) CHAPS, 60 mM DTT, 2% (v/v) IPG buffer, pH 3–10 NL (GE Life Sciences)], and stored at –80 °C for 2D-PAGE analysis. Protein concentration was measured using the modified Lowry method of Peterson (1983).
Immobiline pH 3–10 NL, 13 cm IPG strips (GE Life Sciences) were rehydrated for 16 h with 1 mg of sample protein and focused for a total of 17 kVh on a Multiphor II (Pharmacia) according to the manufacturer's protocol. Focused strips were equilibrated for SDS–PAGE by incubation for 15 min in equilibration buffer [50 mM TRIS-HCl (pH 8.8), 6 M urea, 30% (v/v) glycerol, 2% (w/v) SDS, 65 mM DTT], followed by a second incubation in fresh equilibration buffer containing 135 mM iodoacetamide instead of DTT. Second-dimension vertical gels [12.5% (w/v) polyacrylamide, 16×18×0.5 cm], with the strip sealed in place at the top in SDS–PAGE running buffer containing 1% (w/v) agarose, were run at 30 mA per gel at 4 °C (Laemmli, 1970). Gels were stained for 48–72 h in colloidal Coomassie Brilliant Blue G-250 (Candiano et al., 2004).
Four gels representing independent protein extractions from four replicates of 200 seeds were run for each population. Gels were imaged with a ProXPRESS 2D-Proteomic Imaging system (Perkin-Elmer, Massachusetts, USA) and spot patterns compared using Progenesis SameSpots/PG240 software (Nonlinear Dynamics, Newcastle, UK). Spots which differed in intensity by >3-fold (P <0.05) between samples were manually picked out of the gels. Matrix-assisted laser desorption ionization time of flight-time of flight (MALDI-TOF-TOF) analysis was performed on trypsin-digested protein spots using a 4800 Proteomics Analyzer mass spectrometer (Applied Biosystems, California, USA) and MS/MS spectra were analysed using the Mascot search engine (Matrix Science, London, UK: www.matrixscience.com) with either the Ludwig NR or MSDB databases (taxonomy: green plants); MOWSE scores >50 (i.e. with a <5% chance of a random hit under the parameters used) were considered to be significant. Matches with only one peptide were considered indicative.
Measurement of enzyme activities
Seeds used in enzyme assays were either dry or dark stratified for 7 d (to allow hydrated metabolism to commence without the complications of the light-requiring germinative processes in LD seeds or significant release of dormancy in HD seeds). All enzyme measurements were carried out using kinetic spectrophotometric assays. Total soluble protein concentration was measured according to Bradford (1976) using Dye Reagent Concentrate (BioRad, Hercules, California). The enzymes assayed and the methods used were: superoxide dismutase (SOD; Paoletti et al., 1986), glutathione reductase (GR; Bielawski and Joy, 1986), ascorbate peroxidase (APX; Prochazkova et al., 2001), catalase (CAT; Beers and Sizer, 1952), glutathione peroxidase (GPX; Wendel, 1981), glyoxalase I and glyoxalase II (Hoque et al., 2008), and total peroxidase (PX; Chance and Maehly, 1995).
For all enzymes except glyoxalase II, 0.25 g of seeds were extracted in 4 vols of 100 mM KH2PO4 (pH 7.5), 1 mM EDTA, 5% (v/v) glycerol, 0.002% (v/v) Triton X-100, 2 mM ascorbate, 5 mM DTT, 1 mM PMSF, and the cleared supernatant after centrifugation at 12 000 g for 7 min (1 ml) was desalted into 2 ml of 100 mM KH2PO4 (pH 7.4), 1 mM EDTA using Sephadex G-25 PD10 columns (Pharmacia). Seeds to be assayed for glyoxalase II were extracted as above except that KH2PO4 was replaced with 100 mM triethanolamine hydrochloride (pH 7.4) in the extraction and desalting buffers.
Each enzyme assay was performed in a volume of 1 ml and contained 100 μl of desalted seed extract, except for the SOD assay which contained 0, 25, 50, 100, and 200 μl for each sample. The SOD assay was started with the addition of 3.9 mM β-mercaptoethanol, the GPX assay with seed extract plus 3 U of commercial GR, the glyoxalase I assay with seed extract, and all others with the appropriate enzyme substrate. Boiled seed extracts were used as negative controls. Except for SOD, enzyme activities were calculated from the change in absorbance in the linear range of the reaction, using the following extinction coefficients in mM−1 cm−1: NADPH/NADH (for GPX and GR), 6.2; H2O2 (for CAT), 0.0436; ascorbate (for APX), 14; S-lactoylglutathione (for glyoxalase I), 3.31; 5,5'-dithiobis-(2-nitrobenzoic acid) (for glyoxalase II), 13.6; tetraguaiacol (for PX), 26.6. One unit of SOD activity was defined as the amount of protein required to inhibit NADPH oxidation by 50%.
Measurement of antioxidant molecules
Ascorbate and glutathione were measured spectrophotometrically according to Knörzer et al. (1996) except that seeds were extracted in 5% (w/v) sulphosalicylic acid. To account for the fact that LD seed extracts had twice the anthocyanin concentration of HD extracts (Goggin et al., 2010), which would interfere with the ascorbate assay (measured at 525 nm in acidic solution), blanks in which no dipyridyl was added were measured for all samples and subtracted from the absorbance of the test samples. Three independent experiments with four replicates of each sample were performed. Recovery of exogenous ascorbate, reduced glutathione (GSH), and GSSG added at the extraction stage was, respectively, 94±3, 96±6, and 84±8%.
Germination tests
The progress of dormancy release during dark stratification in LD and HD seeds was followed by incubating imbibed seeds in the dark at 20 °C for 0–42 d before transfer to standard germination conditions for a further 42 d. To compare the effects of extreme temperature on LD and HD seeds, seeds were sown on agar plates equilibrated at the appropriate temperature and incubated in the dark at 6, 20, or 35 °C for 21 d. Following stratification, the plates were placed into standard germination conditions for another 42 d. In a second experiment to determine the effects of temperature on seeds that had become less dormant via dark stratification, LD and HD seeds were incubated at 20 °C in the dark for 21 d, followed by transfer to 6, 20, or 35 °C for another 21 d in the dark before being placed in germination conditions for a final 21 d. Germination was scored weekly, and the viability of the remaining ungerminated seeds was assessed by tetrazolium staining at the end of the experiment.
To assess the effect of oxidants and antioxidants on seed germination, seeds were imbibed on filter paper saturated (5 ml) with 10 mM H2O2 or 10 mM sodium ascorbate (pH adjusted to 6.3, matching that of the deionized water used as a control). The filter paper was replenished with 2 ml of the appropriate solution every 7 d. Seeds were placed directly into standard germination conditions, and germination was scored weekly for 42 d. The stability of the 10 mM H2O2 solution was tested by incubating five non-dormant seeds in 1 ml of solution, under germination conditions, for 7 d (by which time most of the seeds had germinated) and then measuring the absorbance of the solution at 240 nm. The effects of the seed leachate on the absorbance were corrected for by including a deionized water control. At the end of the 7 d incubation, the absorbance of the solution was 86% of its original value, indicating that the seeds were constantly exposed to 8.6–10 mM H2O2 for the entire 42 d of the experiment. The effect of exogenous compounds on the endogenous glutathione and ascorbate pools was measured by assaying seeds that had been imbibed on 10 mM H2O2 or 10 mM ascorbate for 2 d in the dark. This short incubation time was designed to allow full imbibition but minimize interference from dormancy release processes occurring over longer stratification times. Seeds were thoroughly rinsed before extraction of the endogenous antioxidant molecules.
Statistical analysis
Each parameter was measured in three or four independent biological replicates, and data were analysed using single-factor analysis of variance (ANOVA) and the least significant difference test at the 5% level of significance. If experiments were carried out more than once and there was no significant difference between experiments, the data were pooled.
Results
Proteomic analysis of LD and HD seeds
Figure 1 shows representative 2D-polyacrylamide gels run with soluble and insoluble proteins from LD and HD seeds, with circles around protein spots that were differentially expressed by at least 3-fold (P <0.05). One soluble protein was expressed more highly in LD seeds, and six in HD seeds (Fig. 1A, B). There were 22 differentially expressed insoluble proteins, all expressed more highly in HD seeds (Fig. 1C, D). Of these 29 spots, the seven soluble proteins and one of the insoluble proteins were identified by the Mascot (www.matrixscience.com) search engine from their MS/MS spectra (Table 1). MOWSE scores >50 were considered to be significant, as, under the search parameters used, a score of 50 roughly corresponds to a 5% chance of obtaining a random hit, and this chance rapidly decreases as the MOWSE score increases (www.matrixscience.com). Identifications in which only one peptide was matched to a protein were considered to be tentative, with close similarities between observed and expected molecular mass being used as further confirmation of a positive hit. Four of the identified soluble proteins were involved in plant responses to stress [small heat shock proteins (sHSPs) 17.2 and 17.9] or toxic metabolites (GPX and glyoxalase I) and two were glycolytic enzymes [cytosolic triosephosphate isomerase (TPI) and enolase], whilst the other two spots (one soluble, one insoluble) matched to proteins of unknown function (Table 1).
Fig. 1.
Proteomic analysis of low- and high-dormancy seeds. Soluble (A, B) and insoluble (C, D) proteins extracted from low-dormancy (A, C) and high-dormancy (B, D) seeds were separated by 2D-PAGE and their spot patterns compared. Representative gels from four independent replicates are shown. Protein spots with a differential intensity of >3-fold (P <0.05), as determined by scanning densitometry, are circled.
Table 1.
Identification of differentially expressed low-dormancy (LD) and high-dormancy (HD) seed proteins using MALDI-TOF-TOF
| Spot no. | Expression ratio HD:LD | Observed Mr (kDa); pI | Closest match (accession no.) | Match predicted Mr; pI | MOWSE score | No. of peptides matched |
| S-1 | 0.14 | 20.9; 5.6 | Triticum aestivum GPX-like fragment (AAP80645) | 13.2; 9.8 | 83 | 1 |
| S-2 | 4.7 | 61.6; 5.6 | Oryza sativa enolase (ABL74573) | 45.9; 5.2 | 220 | 3 |
| S-3 | 4.1 | 64.5; 6.0 | Arabidopsis thaliana protein F23M19 (AAD39604) | 67.0; 9.2 | 56 | 1 |
| S-4 | 3.4 | 38.7; 5.6 | Sporobolus stapfianus hypothetical protein (CAA71754)a | 31.9; 5.7 | 113 | 3 |
| S-5 | 4.6 | 28.0; 5.6 | Solanum chacoense cytosolic TPI (AAR11379) | 27.0; 5.7 | 74 | 1 |
| S-6 | 4.0 | 18.9; 5.7 | Pennisetum glaucum heat shock protein 17.9 (CAA63903) | 17.9; 5.8 | 145 | 2 |
| S-7 | 3.1 | 16.3; 5.7 | Dactylis glomerata heat shock protein 17.2 (ABA60374) | 17.2; 5.4 | 202 | 5 |
| I-11 | 3.1 | 20.6; 5.4 | Oryza sativa hypothetical protein OSJNBa0060P14 (CAD41037) | 17.6; 6.1 | 52 | 4 |
Selected MS/MS spectra of the other insoluble proteins were used to deduce the peptide sequences manually, and these were analysed by BLAST searching with molecular mass limits set at 10 kDa on either side of the observed molecular mass. As the protein identifications were low confidence at best, and did not lead to further investigation, these results are not presented. The two identifications with the highest confidence level (e <1) were both seed storage proteins (identified as a 12S globulin and γ-gliadin).
Response of LD and HD seeds to temperature extremes
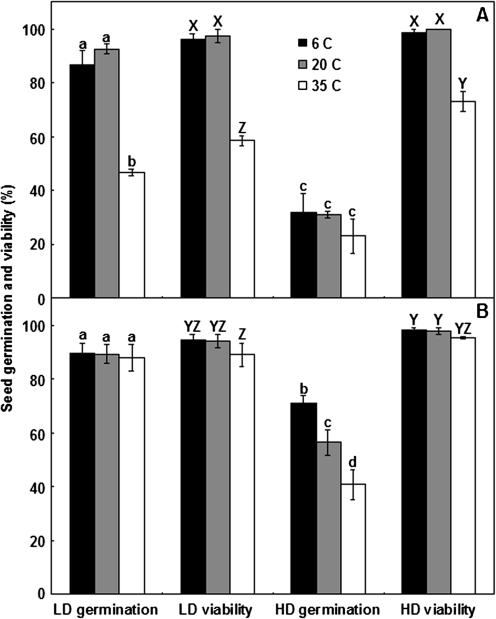
As the proteomic analysis indicated that HD seeds showed higher constitutive expression of two sHSPs, the response of seeds to 21 d dark stratification at 6, 20, or 35 °C was assessed. Dark stratification for 21 d at 6 °C or 20 °C removed residual dormancy in LD seeds, allowing germination to >80% when subsequently moved to standard germination conditions (25/15 °C with a 12 h photoperiod) (Fig. 2A). In contrast, stratification at 35 °C resulted in significantly lower germination due to a 40% loss of viability (Fig. 2A). HD seeds reached ∼30% germination after transfer from 21 d dark stratification, irrespective of temperature, due to their deep dormancy; only 25% loss of viability after stratification at 35 °C occurred in these seeds (Fig. 2A). When seeds were pre-treated by dark stratification at 20 °C for 21 d to reduce their dormancy level before applying the temperature stress, the viability of both the LD and HD seeds was no longer reduced by 21 d at 35 °C (Fig. 2B). Interestingly, incubation at 6 °C for the final 21 d was a slightly more effective dormancy release treatment in HD seeds than incubation at 20 °C for the entire 42 d, whilst final incubation at 35 °C was less effective than at 20 °C (Fig. 2B).
Fig. 2.
Effect of extreme stratification temperatures on germination and viability of low-dormancy (LD) and high-dormancy (HD) seeds. LD and HD seeds were (A) dark stratified for 21 d at 6, 20, or 35 °C and then transferred to standard germination conditions (25/15 °C, 12 h photoperiod) for a further 42 d, or (B) dark stratified for 21 d at 20 °C, then for 21 d at 6, 20, or 35 °C, and finally transferred to germination conditions for a final 21 d, after which time, germination (as a percentage of the total number of seeds) was recorded. The viability of ungerminated seeds was assessed by tetrazolium staining and the number of viable seeds was added to the number of germinated seeds to give total seed viability. Values are means ±SE (n=4). Different letters (lower case for germination, upper case for viability) above columns within each panel indicate significant differences between means; pooled LSD values were 12.7 for germination and 6.3 for viability.
Activity of detoxification enzymes in LD and HD seeds
The activity of a suite of antioxidant/detoxification enzymes was measured in LD and HD seeds to determine if the latter are potentially better equipped to cope with stress, as suggested by the results of the proteomic analysis. Production of ROS is increased under most biotic and abiotic stresses (Szalai et al., 2009), and can also lead to stimulation of seed germination (Bailly, 2004), so it was hypothesized that the HD seeds may have a more active antioxidant defence system to (i) prevent ROS-mediated stimulation of germination and (ii) protect the imbibed, non-germinating seeds from oxidative and ultrastructural damage arising from environmental stresses.
Measurement of APX, CAT, glyoxalase II, GPX, GR, and SOD activities in dry and 7 d dark stratified LD and HD seeds revealed that only glyoxalase II showed a difference between populations (higher in HD), and this difference was only evident in dry seeds (Table 2). The two enzyme activities of greatest interest, with consistent differences between LD and HD seeds, were glyoxalase I and PX, so these were studied in more detail during dormancy release over the course of 42 d dark stratification. LD seeds, which were already able to germinate to 80% when imbibed under standard germination conditions, reached >90% germination as a result of dark stratification at 20 °C for 7–42 d prior to transfer to germination conditions (Fig. 3A). Germination of HD seeds gradually increased from 14% after 0 d dark stratification to 70% after 42 d, with 50% of seeds responding to ≤14 d of dark stratification, but the remainder requiring >35 d to reduce dormancy sufficiently for germination to be initiated by alternating light and temperature conditions (Fig. 3A).
Table 2.
Activity of antioxidant defence enzymes in dry and 7 d dark stratified low-dormancy (LD) and high-dormancy (HD) seeds
| Dry seeds |
7 d Dark stratified seeds |
|||
| LD | HD | LD | HD | |
| APX | 0.02±0.01 a | 0.01±0.00 b,c | 0.02±0.00 a,b | 0.00±0.00 c |
| CAT | 14.1±1.7 b | 13.7±4.9 b | 31.1±3.0 a | 30.7±1.0 a |
| Glyox. II | 0.43±0.03 b,c | 0.52±0.01 a | 0.47±0.004 a,c | 0.50±0.01 a |
| GPX | 0.02±0.01 a | 0.02±0.01 a | 0.01±0.00 a | 0.02±0.00 a |
| GR | 0.11±0.01 a | 0.12±0.01 a | 0.13±0.01 a | 0.12±0.01 a |
| SOD | 50±7 a | 44±7 a | 49±2 a | 41±6 a |
APX, ascorbate peroxidase; CAT, catalase; Glyox. II, glyoxalase II, GPX, gluthatione peroxidase; GR, glutathione reductase; SOD, superoxide dismutase.
Values are means ±SE (n=4). Activities are expressed as μmol product formed min−1 mg−1 protein, except that of SOD, which is the amount of enzyme required to inhibit oxidation of NADPH by 50%. Different letters across rows indicate significantly (P <0.05) different values.
Fig. 3.
Changes during dark stratification of low-dormancy (LD) and high-dormancy (HD) seeds. Seeds were stratified at 20 °C in the dark for 0–42 d and assessed for (A) final germination percentage at 42 d after transfer of stratified seeds to germination conditions, (B) glyoxalase I activity, (C) total peroxidase activity, and (D) the ratio of reduced (GSH) to total glutathione. Parameters in B, C, and D were measured immediately after 0, 7, 21, or 42 d dark stratification. Values are means ±SE (n=4).
Glyoxalase I activity increased slightly in LD seeds over the first 21 d of dark stratification, before returning to its original level at 42 d. Activity in HD seeds showed a similar pattern over time, but was consistently (1.3- to 1.8-fold) higher than in LD seeds (Fig. 3B). PX activity in LD seeds increased by 2.5-fold in the first 7 d of dark stratification and then remained relatively stable. In the HD seeds, PX activity took longer to peak, reaching its maximum at 21 d, and then decreased again between 21 d and 42 d. PX activity was always 1.5- to 3-fold higher in the LD seeds (Fig. 3C).
Soluble antioxidant status in LD and HD seeds
There was a high concentration of (highly reduced) ascorbate in the dry seeds of both populations (>300 nmol g−1 dry weight), in contrast to previous studies (reviewed in Tommasi et al., 1999), and this was maintained after 7 d dark stratification (data not shown). As there were no major differences between populations, the ascorbate pool was not studied further.
The glutathione pool was studied over the course of 42 d dark stratification because initial measurements in dry and imbibed seeds indicated stable differences between the LD and HD populations. The size of the glutathione pool increased upon imbibition but was generally the same in LD and HD seeds (Table 3). The redox potential of glutathione, calculated as the half-cell reduction potential EGSSG/2GSH (Schafer and Buettner 2001), was less negative (i.e. more oxidizing) in dry LD compared with dry HD seeds, but upon imbibition there was no further difference between populations (Table 3). However, when expressed as the ratio of GSH to total glutathione, the LD seeds were more oxidized than the HD seeds at all times except 21 d (Fig. 3D).
Table 3.
Concentration and half-cell reduction potential (EGSSG/2GSH) of the glutathione pool in low-dormancy (LD) and high-dormancy (HD) seeds over 42 d dark stratification at 20 °C
| [GSH] (nmol g−1 DW) |
[GSSG] (nmol g−1 DW) |
EGSSG/2GSH (mV) |
||||
| Time (d) | LD | HD | LD | HD | LD | HD |
| 0 | 25±3 c | 31±2 d | 37±2 b* | 29±2 a | –156±4 a | –169±4 b* |
| 7 | 101±5 a | 116±8 a | 58±3 a* | 20±2 a | –163±4 a | –173±4 b |
| 21 | 64±9 b | 56±3 c | 31±5 b | 19±3 a | –147±7 a | –144±3 a |
| 42 | 52±14 b,c | 84±8 b | 39±7 b | 28±3 a | –142±8 a | –155±5 a |
The concentration of GSSG is expressed as GSH equivalents. Values are means ±SE (n=4); different letters within columns denote a significant difference (P <0.05) between time points. An asterisk denotes a significant difference between LD and HD populations for the appropriate parameter.
Across the time course of dark stratification, there were weak to moderate positive correlations between final seed germination and PX activity during stratification (Fig. 4A), and between final germination and GSSG concentration (Fig. 4B). Weak to moderate negative correlations were observed between PX activity and EGSSG/2GSH (Fig. 4C) (i.e. a lower PX activity corresponds to a more negative EGSSG/2GSH), and between glyoxalase I activity and GSSG concentration (Fig. 4D). Overall, this suggests that low dormancy is associated with a more oxidized glutathione pool, higher PX activity, and lower glyoxalase I activity.
Fig. 4.
Correlation between germination and stress defence parameters in seeds. The linear correlation (r2) between (A) germination and total peroxidase (PX) activity, (B) germination and GSSG concentration, (C) PX activity and glutathione reduction state (EGSSG/2GSH), or (D) GSSG concentration and glyoxalase I activity was assessed using the data from the 0–42 d time course of dark stratification presented in Fig. 3 and Table 3. Enzyme activities are expressed as μmol min−1 mg−1 protein, GSSG concentration as nmol g−1 dry weight, germination as % viable seeds germinated at 42 d, and EGSSG/2GSH as mV.
The effect of exogenous oxidant or antioxidant molecules on germination was therefore assessed. Methyl viologen, which generates superoxide radicals, had no or negative effects on L. rigidum seed germination, depending on the concentration and duration of application (data not shown). H2O2 at 10 mM stimulated germination in both LD and HD seeds, whilst 10 mM ascorbate inhibited germination of LD seeds but had no effect on the HD seeds (Fig. 5A). It was expected that the effects of ascorbate and H2O2 on the germination of LD seeds would be due to changes in their glutathione status, so this was measured in LD and HD seeds imbibed on 10 mM ascorbate or H2O2 at 20 °C for 2 d. The size and EGSSG/2GSH of the total glutathione pool were not affected by ascorbate in either the LD or HD seeds, and H2O2 did not affect the pool size (Fig. 5B). Whilst the EGSSG/2GSH of the glutathione pool was made less negative (more oxidizing) by H2O2 in HD seeds, it unexpectedly became more negative (reducing) in the LD seeds (Fig. 5C). Similarly, the ratio of GSH to total glutathione was increased from 0.66 to >0.8 by H2O2 in LD seeds. The ascorbate pool was not significantly affected by exogenous ascorbate or H2O2 (data not shown).
Fig. 5.
Effect of hydrogen peroxide and ascorbate on seed germination and glutathione status. Low-dormancy (LD) and high-dormancy (HD) seeds were treated with 10 mM H2O2 or ascorbate (pH 6.3) and their (A) germination after 42 d in standard germination conditions, (B) glutathione concentration, and (C) glutathione half-cell reduction potential were assessed. Parameters in B and C were measured in seeds that had been imbibed on the appropriate chemical for 2 d in the dark at 20 °C. Deionized water was used as a control. Values are means ±SE (n=4). Pooled LSD values were: (A) 9.3; (B) 25.1; (C) 12.1.
Discussion
Differential expression of proteins involved in thermotolerance in LD and HD seeds
The proteomics study on L. rigidum seeds selected for LD or HD resulted in the identification of three proteins potentially related to thermotolerance as being at least 3-fold more highly expressed in HD seeds. Two of these were sHSPs (sHSP17.2 and sHSP17.9), a group of polypeptides thought to be involved in thermotolerance in higher plants by maintaining proteins in a folding-competent state until the stress has been removed (Smýkal et al., 2000). Higher expression of sHSP17.6 and sHSP26.5 was also observed in dormant seeds of Arabidopsis (Cadman et al., 2006). sHSP17.6 is known to be under the control of the ABA-responsive transcription factor ABI3 (Cadman et al., 2006), which is linked with dormancy by being involved in the inhibition of endosperm rupture during germination (Piskurewicz et al., 2009).
Bettey and Finch-Savage (1998) found that cabbage seeds that contained higher levels of sHSP17.6 at maturity had greater vigour (measured by their germination performance following exposure to high temperatures or to water stress) and were able to withstand ageing to a greater extent than seeds with lower expression of sHSP17.6. Similarly, in the current study, the higher expression of two sHSPs in the HD seeds corresponded to better maintenance of seed viability following imbibition under high temperature (35 °C) than was observed in the LD seeds. Although the cabbage seed populations were produced under different environmental conditions, thus eliciting differential expression of sHSP17.6 (Bettey and Finch-Savage, 1998), the L. rigidum seed populations were produced under the same (non-stressed) conditions, with the major difference between them being their level of dormancy. There is also a link between dormancy and accumulation of sHSPs in non-stressed vegetative tissues, where the sHSPs are associated with storage proteins and may play a role in protecting these from premature hydrolysis (Lubaretz and zur Nieden, 2002).
The fact that the LD seeds acquired comparable heat tolerance to the HD seeds after pre-incubation at 20 °C for 21 d suggests that once metabolism has commenced under non-stressed conditions, the LD seeds become better equipped to prepare for less favourable conditions. The apparent selection of higher constitutive expression of sHSPs as a consequence of deliberate selection for high dormancy in the HD seeds may, in contrast, represent preparation for future unfavourable conditions even before imbibition.
The third protein related to thermotolerance that was differentially expressed in LD and HD seeds is enolase, a bifunctional protein which not only catalyses the penultimate step of glycolysis but is also a transcription factor specifically involved in cold tolerance in Arabidopsis and in mammalian cells (Lee et al., 2002). Higher levels of enolase protein have also been observed in chilled rice roots (Lee et al., 2009) and in cold-tolerant gentian (Gentiana triflora) plants (Takahashi et al., 2006), demonstrating that this function of enolase is not confined to Arabidopsis. The higher expression of enolase in the HD seeds could potentially be linked to its different behaviour under cold stratification. Although higher stratification temperatures (up to 30 °C) resulted in faster rates of dormancy release in the original L. rigidum population from which the LD and HD seeds were selected (Steadman, 2004) as would be expected in a winter annual species (e.g. Milberg and Andersson, 1998, and references therein), dormancy release in the HD seeds appeared to be accelerated by incubation at 6 °C (but only when this was preceded by a 20 °C pre-treatment). The reason for this can only be speculated upon at this stage. For example, it is possible that selection for HD has resulted in the seeds responding to low temperatures as a ‘last-chance’ opportunity for germination during the winter before conditions become too hot and dry to support plant growth, flowering, and seed production.
Overall, the selection of higher expression of proteins related to heat and cold tolerance in seeds that were selected for their slow rate of dormancy release in response to dark stratification suggests that the HD seeds have become prepared for long periods of imbibition without germination, when seeds are more vulnerable to extremes of temperature than when they are dry. This development of thermotolerance occurred in the absence of temperature stress in the mother plants, and can only be linked with the dormancy status of the seeds.
Differential expression of proteins involved in detoxification in LD and HD seeds
The higher expression of a putative GPX in LD seeds, and of glyoxalase I in HD seeds, suggested that selection for LD and HD resulted in different capabilities for coping with toxic metabolites such as ROS and methylglyoxal. Interestingly, methylglyoxal is a by-product of the TPI reaction (Kalapos, 2008), and both (cytosolic) TPI and glyoxalase I, which is the major enzyme of methylglyoxal detoxification, were more highly expressed in HD seeds. There was no difference between LD and HD seeds in terms of TPI enzyme activity in vitro (Goggin et al., 2010), but there could nevertheless be a functional relevance in the differential expression of TPI. Cytosolic TPI from the fern Pteris vittata confers tolerance to arsenic via a mechanism which appears to be unrelated to its glycolytic activity (Rathinasabapathi et al., 2006). Additionally, TPI activity, which can be induced under various stresses such as salinity, drought, high temperature, and pathogen attack (summarized in Del Buono et al., 2009), is regulated by glutathionylation and phosphorylation, so the amount of protein may not correspond to enzyme activity. HD seeds may thus produce a large amount of TPI protein but keep a proportion of it in an inactive form until stress conditions are encountered. Rice TPI has been shown to be dephosphorylated in response to increased ABA levels, again suggesting a link with stress responses, but the physiological significance of this is currently unknown (He and Li, 2008).
The higher expression of glyoxalase I in HD seeds was reflected functionally by a higher enzyme activity in dry and dark stratified seeds than was observed in the LD seeds. Previous studies have shown that plant glyoxalase I expression is often increased in response to abiotic stresses such as salinity (e.g. Hoque et al., 2008) and cold (Lee et al., 2009), and so the constitutively higher expression of glyoxalase I could be another means by which dry HD seeds are already prepared to encounter stressful conditions after imbibition. Glyoxalase II, the enzyme following glyoxalase I in the methylglyoxal detoxification pathway, also had slightly higher activity in dry HD seeds. This difference was abolished upon imbibition but, as glyoxalase I appears to be the rate-limiting enzyme (Ferguson et al., 1998), differences in activity of glyoxalase II may not be as important.
Potential role for PX in dormancy mediation
Total PX activity was measured as one of the suite of antioxidant defence enzymes examined in this study, but it appears that its potential role in mediating the dormancy level may be related to the generation of ROS via the hydroxylic cycle rather than ROS detoxification via the peroxidative cycle. Plant apoplastic PXs are involved in a multitude of functions including cell wall remodelling and resistance to abiotic and biotic stresses through the generation of ROS (reviewed in Passardi et al., 2005), and the hydroxyl radicals produced by the PX-catalysed reaction of H2O2 with superoxide are thought to be involved in the cell wall loosening that is required for rupture of the endosperm and testa during initiation of germination (Müller et al., 2009). In LD seeds, total PX activity was consistently higher than in HD seeds and increased steadily over 42 d dark stratification. The HD seeds, which gradually lost dormancy over 42 d dark stratification, showed a concomitant increase in PX activity up until 21 d. The relatively strong correlation between PX activity at the end of stratification and the subsequent germination percentage suggests a role for PX in determining the dormancy level of L. rigidum seeds.
The antioxidant defence system in LD and HD seeds
ROS have been suggested to break dormancy and/or stimulate germination in the seeds of a number of species (e.g. Oracz et al., 2007; Sarath et al., 2007); additionally, generation of ROS often accompanies the stress response of plants (Bailly, 2004). These are two reasons for hypothesizing that HD seeds may possess a more effective antioxidant defence system than LD seeds—that is, to prevent stimulation of germination by ROS, and because they appear to be constitutively prepared for environmental stress. However, this hypothesis was not supported with respect to seed ascorbate concentration or a range of antioxidant enzyme activities, which showed no major differences between LD and HD seeds.
The glutathione pool did show some differences between populations, but the link between the glutathione reduction state and dormancy level is tenuous. The selection of LD seeds led to selection of a higher initial concentration of GSSG, and there was a moderate positive correlation between GSSG and germination percentage over 42 d dark stratification, suggesting that the GSSG concentration may be linked to lower seed dormancy in L. rigidum. Direct measurements of ROS in the embryos, as was done by Oracz et al. (2009) in sunflower seeds, would help to clarify the role of ROS and glutathione in L. rigidum seed dormancy.
Conclusions
Stable selection of low and high seed dormancy in L. rigidum resulted in the HD seeds showing higher expression of sHSPs and enolase, a greater tolerance to imbibition under high temperatures, and higher constitutive expression and activity of glyoxalase I, which may all be linked to a greater preparedness for tolerating the stressful conditions potentially encountered after imbibition. In contrast, the LD seeds had higher constitutive PX activity, which suggests that the ability to initiate germination rapidly in these seeds may be linked to a greater capacity for the cell wall weakening required for the radicle to emerge. The moderate correlation between germinability and GSSG concentration points to a possible role for glutathione redox signalling as another heritable mediator of seed dormancy.
Acknowledgments
This work was funded by the Australian Research Council. The 2D gel spot pattern analysis was performed by Proteomics International, and the MS/MS analyses by the Lotterywest State Biomedical Facility, Proteomics Node, located at the Western Australian Institute for Medical Research (WAIMR), WA. The authors thank the anonymous reviewers of this and a previous version of the manuscript for their helpful comments and suggestions.
Glossary
Abbreviations
- APX
ascorbate peroxidase
- CAT
catalase
- GPX
glutathione peroxidase
- GR
glutathione reductase
- HD
high dormancy
- LD
low dormancy
- PX
total peroxidase
- ROS
reactive oxygen species
- sHSP
small heat shock protein
- SOD
superoxide dismutase
References
- Bailly C. Active oxygen species and antioxidants in seed biology. Seed Science Research. 2004;14:93–107. [Google Scholar]
- Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. Journal of Biological Chemistry. 1952;195:133–140. [PubMed] [Google Scholar]
- Bettey M, Finch-Savage WE. Stress protein content of mature Brassica seeds and their germination performance. Seed Science Research. 1998;8:347–355. [Google Scholar]
- Bielawski W, Joy KW. Properties of glutathione reductase from chloroplasts and roots of pea. Phytochemistry. 1986;25:2261–2265. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cadman CSC, Toorop PE, Hilhorst HWM, Finch-Savage WE. Gene expression profiles of Arabidopsis Cvi seeds during dormancy cycling indicate a common underlying dormancy control mechanism. The Plant Journal. 2006;46:805–822. doi: 10.1111/j.1365-313X.2006.02738.x. [DOI] [PubMed] [Google Scholar]
- Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG. Blue-silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 2004;25:1327–1333. doi: 10.1002/elps.200305844. [DOI] [PubMed] [Google Scholar]
- Chance B, Maehly AC. Assay of catalases and peroxidases. Methods in Enzymology. 1955;2:765–775. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- Chauhan BS, Gill G, Preston C. Influence of environmental factors on seed germination and seedling emergence of rigid ryegrass (Lolium rigidum) Weed Science. 2006;54:1004–1012. [Google Scholar]
- Del Buono D, Prinsi B, Espen L, Scarponi L. Triosephosphate isomerases in Italian ryegrass (Lolium multiflorum): characterization and susceptibility to herbicides. Journal of Agricultural and Food Chemistry. 2009;57:7924–7930. doi: 10.1021/jf901681q. [DOI] [PubMed] [Google Scholar]
- Ferguson GP, Tötemeyer S, MacLean MJ, Booth IR. Methylglyoxal production in bacteria: suicide or survival? Archives of Microbiology. 1998;170:209–219. doi: 10.1007/s002030050635. [DOI] [PubMed] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytologist. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant, Cell and Environment. 2005;28:1056–1071. [Google Scholar]
- Goggin DE, Emery RJN, Powles SB, Steadman KJ. Initial characterisation of low and high seed dormancy populations of Lolium rigidum produced by repeated selection. Journal of Plant Physiology. 2010;167:1282–1288. doi: 10.1016/j.jplph.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Goggin DE, Steadman KJ, Emery RJN, Farrow SC, Benech-Arnold RL, Powles SB. ABA inhibits germination but not dormancy release in mature imbibed seeds of Lolium rigidum Gaud. Journal of Experimental Botany. 2009;60:3387–3396. doi: 10.1093/jxb/erp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggin DE, Steadman KJ, Powles SB. Green and blue light photoreceptors are involved in maintenance of dormancy in imbibed annual ryegrass (Lolium rigidum) seeds. New Phytologist. 2008;180:81–89. doi: 10.1111/j.1469-8137.2008.02570.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiology. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Li J. Proteomic analysis of phosphoproteins regulated by abscisic acid in rice leaves. Biochemical and Biophysical Research Communications. 2008;371:883–888. doi: 10.1016/j.bbrc.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Hong S-W, Vierling E. Hsp101 is necessary for heat tolerance but dispensable for development and germination in the absence of stress. The Plant Journal. 2001;27:25–35. doi: 10.1046/j.1365-313x.2001.01066.x. [DOI] [PubMed] [Google Scholar]
- Hoque MA, Banu MNA, Nakamura Y, Shimoishi Y, Murata Y. Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. Journal of Plant Physiology. 2008;165:813–824. doi: 10.1016/j.jplph.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Kalapos MP. The tandem of free radicals and methylglyoxal. Chemico-Biological Interactions. 2008;171:251–271. doi: 10.1016/j.cbi.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Knörzer OC, Durner J, Böger P. Alterations in the antioxidative system of suspension-cultured soybean cells (Glycine max) induced by oxidative stress. Physiologia Plantarum. 1996;97:388–396. [Google Scholar]
- Kranner I, Grill D. Content of low-molecular-weight thiols during the imbibition of pea seeds. Physiologia Plantarum. 1993;88:557–562. doi: 10.1111/j.1399-3054.1993.tb01371.x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee D-G, Ahsan N, Lee S-H, Lee JJ, Bahk JD, Kang KY, Lee B-H. Chilling stress-induced proteomic changes in rice roots. Journal of Plant Physiology. 2009;166:1–11. doi: 10.1016/j.jplph.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Lee H, Guo Y, Ohta M, Xiong L, Stevenson B, Zhu J-K. LOS2, a genetic locus required for cold-responsive gene transcription encodes a bi-functional enolase. EMBO Journal. 2002;21:2692–2702. doi: 10.1093/emboj/21.11.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leprince O, Atherton NM, Deltour R, Hendry GAF. The involvement of respiration in free radical processes during loss of desiccation tolerance in germinating Zea mays L. Plant Physiology. 1994;104:1333–1339. doi: 10.1104/pp.104.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubaretz O, zur Nieden U. Accumulation of plant small heat-stress proteins in storage organs. Planta. 2002;215:220–228. doi: 10.1007/s00425-002-0745-1. [DOI] [PubMed] [Google Scholar]
- Milberg P, Andersson L. Does cold stratification level out differences in seed germinability between populations? Plant Ecology. 1998;134:225–234. [Google Scholar]
- Müller K, Linkies A, Vreeburg RAM, Fry SC, Krieger-Liszkay A, Leubner-Metzger G. In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiology. 2009;150:1855–1865. doi: 10.1104/pp.109.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oracz K, El-Maarouf-Bouteau H, Farrant JM, Cooper K, Belghazi M, Job C, Job D, Corbineau F, Bailly C. ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. The Plant Journal. 2007;50:452–465. doi: 10.1111/j.1365-313X.2007.03063.x. [DOI] [PubMed] [Google Scholar]
- Oracz K, El-Maarouf-Bouteau H, Kranner I, Bogatek R, Corbineau F, Bailly C. The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiology. 2009;150:494–505. doi: 10.1104/pp.109.138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti F, Aldinucci D, Mocali A, Caparrini A. A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Analytical Biochemistry. 1986;154:536–541. doi: 10.1016/0003-2697(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Passardi F, Cosio C, Penel C, Dunand C. Peroxidases have more functions than a Swiss army knife. Plant Cell Reports. 2005;24:255–265. doi: 10.1007/s00299-005-0972-6. [DOI] [PubMed] [Google Scholar]
- Peterson GL. Determination of total protein. Methods in Enzymology. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Piskurewicz U, Turečková V, Lacombe E, Lopez-Molina L. Far-red light inhibits germination through DELLA-dependent stimulation of ABA synthesis and ABI3 activity. EMBO Journal. 2009;28:2259–2271. doi: 10.1038/emboj.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazkova D, Sairam RK, Srivastava GC, Singh DV. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Science. 2001;161:765–771. [Google Scholar]
- Rathinasabapathi B, Wu S, Sundaram S, Rivoal J, Srivastava M, Ma LQ. Arsenic resistance in Pteris vittata L.: identification of a cytosolic triosephosphate isomerase based on cDNA expression cloning in. Escherichia coli. Plant Molecular Biology. 2006;62:845–857. doi: 10.1007/s11103-006-9060-8. [DOI] [PubMed] [Google Scholar]
- Sarath G, Hou G, Baird LM, Mitchell RB. Reactive oxygen species, ABA and nitric oxide interactions on the germination of warm-season C4-grasses. Planta. 2007;226:697–708. doi: 10.1007/s00425-007-0517-z. [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radical Biology and Medicine. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Smýkal P, Mašín J, Hrdý I, Konopásek I, Žárský V. Chaperone activity of tobacco HSP18, a small heat shock protein, is inhibited by ATP. The Plant Journal. 2000;23:703–713. doi: 10.1046/j.1365-313x.2000.00837.x. [DOI] [PubMed] [Google Scholar]
- Song SQ, Lei YB, Tian XR. Proline metabolism and cross-tolerance to salinity and heat stress in germinating wheat seeds. Russian Journal of Plant Physiology. 2005;52:793–800. [Google Scholar]
- Steadman KJ. Dormancy release during hydrated storage in Lolium rigidum seeds is dependent on temperature, light quality, and hydration status. Journal of Experimental Botany. 2004;55:929–937. doi: 10.1093/jxb/erh099. [DOI] [PubMed] [Google Scholar]
- Steadman KJ, Bignell GP, Michael PJ. Stimulating dormancy release and emergence of annual ryegrass (Lolium rigidum) seeds using short-term hydrated storage in darkness. Australian Journal of Agricultural Research. 2004;55:787–795. [Google Scholar]
- Szalai G, Kellős T, Galiba G, Kocsy G. Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. Journal of Plant Growth Regulation. 2009;28:66–80. [Google Scholar]
- Takahashi M, Hikage T, Yamashita T, Saitoh Y, Endou M, Tsutsumi K. Stress-related proteins are specifically expressed under non-stress conditions in the overwinter buds of the gentian plant. Gentiana triflora. Breeding Science. 2006;56:39–46. [Google Scholar]
- Tommasi F, Paciolla C, Arrigoni O. The ascorbate system in recalcitrant and orthodox seeds. Physiologia Plantarum. 1999;105:193–198. [Google Scholar]
- Wendel A. Glutathione peroxidase. Methods in Enzymology. 1981;77:325–333. doi: 10.1016/s0076-6879(81)77046-0. [DOI] [PubMed] [Google Scholar]