Abstract
Most plants form root symbioses with arbuscular mycorrhizal (AM) fungi, which provide them with phosphate and other nutrients. High soil phosphate levels are known to affect AM symbiosis negatively, but the underlying mechanisms are not understood. This report describes experimental conditions which triggered a novel mycorrhizal phenotype under high phosphate supply: the interaction between pea and two different AM fungi was almost completely abolished at a very early stage, prior to the formation of hyphopodia. As demonstrated by split-root experiments, down-regulation of AM symbiosis occurred at least partly in response to plant-derived signals. Early signalling events were examined with a focus on strigolactones, compounds which stimulate pre-symbiotic fungal growth and metabolism. Strigolactones were also recently identified as novel plant hormones contributing to the control of shoot branching. Root exudates of plants grown under high phosphate lost their ability to stimulate AM fungi and lacked strigolactones. In addition, a systemic down-regulation of strigolactone release by high phosphate supply was demonstrated using split-root systems. Nevertheless, supplementation with exogenous strigolactones failed to restore root colonization under high phosphate. This observation does not exclude a contribution of strigolactones to the regulation of AM symbiosis by phosphate, but indicates that they are not the only factor involved. Together, the results suggest the existence of additional early signals that may control the differentiation of hyphopodia.
Keywords: Phosphorus, arbuscular mycorrhiza, strigolactone, symbiosis, hyphopodium
Introduction
Roots of the vast majority of plant species develop symbiotic associations with arbuscular mycorrhizal (AM) soil fungi. Fungal hyphae develop in the root cortex where they form intracellular highly branched structures called arbuscules, and simultaneously in the soil where they form a dense mycelial network. Within the root the plant supplies the fungus with hexoses, at a cost of up to 20% of the carbon fixed by photosynthesis (Smith and Read, 2008). In return, it obtains water and minerals taken up from soil by the mycelial network. The main benefit of the symbiosis for the plant is an enhanced acquisition of phosphorus (P), a frequent limiting factor in plant growth due to its poor solubility and mobility in soils.
Despite the importance of AM symbiosis, cellular and molecular events underlying this interaction are only beginning to be unravelled (Parniske, 2008). Direct genetic screens to identify mycorrhizal (myc−) mutants are extremely cumbersome. As a result, most myc− mutants in fact belong to a subset of mutants initially isolated as deficient in nitrogen-fixing symbiosis, this latter interaction being easier to examine. A consequence of this bias is the relative scarcity of mutants affected in events unique to the AM symbiosis, including pre-colonization signalling and arbuscule development and function (Marsh and Schultze, 2001). Nonetheless, several specific myc− mutants have been identified in the past few years. They can be affected in different stages of the interaction as summarized in Pumplin et al. (2009): pre-symbiotic fungal growth, formation of hyphopodia (root attachment and penetration structures, formerly referred to as appressoria), epidermal penetration, and arbuscule development (see also Zhang et al., 2010).
Various physiological situations are known to affect the development of AM symbiosis. For instance, plants control the extent to which AM fungi can colonize their roots according to their own nutritional requirements. The best known example of such regulations is the control of AM symbiosis according to P availability. Roots can acquire P as inorganic orthophosphate (Pi) through different pathways (Bucher, 2007). In certain conditions the mycorrhizal uptake pathway, which involves specific Pi transporters (Rausch et al., 2001; Harrison et al., 2002; Paszkowski et al., 2002), can be the major route for P uptake (Smith et al., 2003). When P is abundant, a direct, probably less costly uptake pathway is preferred (Nagy et al., 2008), and a reduced root colonization by AM fungi is observed. This down-regulation of the symbiosis by P has been known for a long time (Graham et al., 1981; Thomson et al., 1986; Elias and Safir, 1987; Rausch et al., 2001; and many others). It seems to be a general phenomenon, although its magnitude can vary (Javot et al. 2007; Smith and Read, 2008). It has far-reaching consequences in natural ecosystems where it modulates the effect of AM fungi on plant species diversity (Collins and Foster, 2009), as well as in agriculture where strong P fertilization may in the long term decrease the presence and richness of soil AM communities (Johnson, 1993).
Little is known about mechanisms underlying the regulation of AM symbiosis by P. A recent study (Branscheid et al., 2010) has documented this down-regulation in Medicago truncatula, and investigated the identity of the internal signal that triggers suppression of the interaction under high P. Nonetheless, the downstream mechanisms that prevent or limit root colonization by AM fungi remain largely unknown. Early studies led to conflicting results and interpretations, partly due to the variety of species combinations and experimental systems. Some of these early studies interpreted the impact of high P on the fungus in terms of trophic effects: high P would decrease the root secretion of metabolites used by the fungus, such as amino acids or carbohydrates (e.g. Graham et al., 1981; Thomson et al., 1986). An alternative proposition was that qualitative rather than quantitative differences between root exudates of P-replete and P-deficient plants could account for their differential effects on the fungus (Elias and Safir, 1987). This led to the suggestion that P-deprived roots exuded important flavonoid signals that triggered pre-symbiotic fungal growth and activity (Nair et al., 1991). Advances made in the last 10 years have indeed emphasized the importance of signalling events in mycorrhizal interactions, and the recent identification of some signals may shed new light on the regulation of AM symbiosis by P.
Plants and AM fungi are known to exchange molecular signals prior to physical contact, at the so-called pre-symbiotic stage. Various lines of evidence indicate that AM fungi produce diffusible compounds able to modulate root gene expression (Kosuta et al., 2003; Weidmann et al., 2004), intracellular signalling (Navazio et al., 2007; Kosuta et al., 2008), development (Olah et al., 2005), and metabolism (Gutjahr et al., 2009). Reciprocally, plant roots secrete compounds that stimulate the fungus (Gianinazzi-Pearson et al., 1989; Siqueira et al., 1991; Tsai and Phillips, 1991; Giovannetti et al., 1996; Buée et al., 2000). A group of secondary metabolites called strigolactones were identified as major contributors to this effect (Akiyama et al., 2005; Besserer et al., 2006). Strigolactones trigger morphological and developmental responses in the fungus such as hyphal branching and spore germination, and enhance fungal mitochondrial activity and respiration (Besserer et al., 2006, 2008). Strigolactone-mediated signalling is necessary for a normal level of root colonization, as demonstrated using strigolactone-deficient mutants (Gomez-Roldan et al., 2008). Most interestingly, these root-exuded compounds also play an important role in planta, acting as hormones that contribute to the regulation of shoot branching (Gomez-Roldan et al., 2008; Umehara et al., 2008).
Prior to the discovery of their roles in AM symbiosis and plant development, strigolactones were known as germination stimulants for the seeds of the parasitic plants Striga and Orobanche (Bouwmeester et al., 2007). Damage caused to crops by these weeds is lower under strong nutrient fertilization, which led to the investigation of whether P availability influenced strigolactone release into the soil. Indeed, several studies demonstrated a strong negative effect of high P supply on strigolactone production and exudation in various species (Yoneyama et al., 2007a, b; Lopez-Raez et al., 2008). A reasonable hypothesis is that high P availability would decrease the extent of AM symbiosis by reducing strigolactone production in roots (Bouwmeester et al., 2007; Yoneyama et al., 2007b).
In this report, P fertilization conditions which lead to an almost complete arrest of the first stages of the interaction between pea (Pisum sativum L.) and two species of AM fungi are described. This strong effect is at least partly linked to regulatory events occurring in the plant partner, as shown by split-root experiments. Furthermore, it is demonstrated that like root colonization, strigolactone production is controlled in a systemic manner by P supply. Hence, strigolactones may contribute to the regulation of AM symbiosis by P, but supplementation experiments indicate that they are not the only factor involved.
Materials and methods
Plant and fungal materials
Seeds of garden pea (Pisum sativum L., cv Terese) were surface sterilized with 3.2% sodium hypochlorite for 10 min and 95% ethanol for 2 min, and washed four times with sterile distilled water. Seeds were germinated on agar–water [0.8% (w/v)] solid medium for 4 d at 25 °C.
Gigaspora rosea spores (DAOM 194757) were produced and surface sterilized as described in Besserer et al. (2006). Sterile Glomus intraradices spores (DAOM 197198) were purchased from Premier Tech Ltée (Rivière du loup, Québec, Canada) or produced according to St-Arnaud et al. (1996).
Growth and inoculation of plants
Plants were grown in a growth chamber under a 16 h photoperiod (22 °C day, 20 °C night), in pots containing sterilized charred clay (Oil-Dri, Klasmann, France) as substrate. They were fertilized daily with half-strength Long Ashton Nutrient Solution (LANS; Hewitt, 1966) containing a final concentration of 7.5 μM (low P; LP), 75 μM (medium P; MP), or 750 μM (high P; HP) sodium dihydrogen phosphate (NaH2PO4). Phosphate was supplied as KH2PO4 instead of NaH2PO4 in two experiments.
For the determination of mycorrhizal ability, germinated seedlings were transferred to 250 ml pots. They were inoculated with 150 or 600 spores of Gl. intraradices, or 100 spores of Gi. rosea. Two-thirds of the spores were mixed with the substrate and one-third was added close to the seedling. The percentage of root length colonized by the fungus (i.e. showing arbuscules, vesicles, or both) was determined by the gridline intersection method (Giovannetti and Mosse, 1980) using a dissecting microscope after staining with Schaeffer black ink (Vierheilig et al., 1998). For the quantification of early symbiotic structures, roots were handled very carefully during rinses and staining to avoid tearing off hyphae from the roots. For each plant, 60 randomly picked 1 cm long root fragments mounted on glass slides were examined under a microscope at ×40 magnification, and hyphopodia were counted.
For split-root experiments, sterile seedlings were grown on solid medium (nutrient solution solidified with 0.4% phytagel) until radicles were ∼2 cm long. The root apex was cut off and the primary root was divided lengthwise into two equal parts. Seedlings were kept on solid medium for another week during which lateral roots developed on both sides of the split root. They were then transferred to two-compartment pot systems with 150 ml of substrate per compartment. Each compartment was inoculated with 90 spores of Gl. intraradices mixed with the substrate. Following plant harvest, root fresh weight was determined and plants for which one side of the root system was >2-fold heavier than the other side were excluded from the analysis.
Preparation of root exudate extracts
Three-week-old non-inoculated plants fertilized with LP or HP nutrient solution were removed from the substrate. Their roots were rinsed and immersed in the same nutrient solution for 24 h. Exudates were extracted with 1 vol. of ethyl acetate, then the organic phase was treated with 1 vol. of 0.2 M K2HPO4. Residual water was removed with anhydrous MgSO4, and ethyl acetate extracts were filtered and dried under vacuum. Exudate extracts were resuspended in the appropriate solvents for branching bioassays or mass spectrometry analysis, and their concentration was adjusted on a root dry weight basis.
Determination of P contents
Inorganic phosphate (Pi) content was measured using the colorimetric method based on molybdenum blue described in Nanamori et al. (2004). The only modification was that plant tissues were ground in perchloric acid using a FASTPREP® system (MP Biomedicals) with lysing matrix A.
Gigaspora rosea hyphal branching bioassay
Spores of Gi. rosea were germinated on solid M medium (Bécard and Fortin, 1988) as described in Besserer et al. (2006). Root exudate extracts produced by the equivalent of 120 mg of root dry weight were resuspended in 1 ml of 10% (v:v) acetonitrile. Samples of 5 μl were applied on both sides of the main hypha of a 6-day-old germinated spore. Newly formed apices were counted 48 h after treatment. The experiment included spores treated with 10% acetonitrile (negative control) and with 100 nM GR24 in 10% acetonitrile (positive control).
For the experiment described in Supplementary Fig. S1 available at JXB online, spores were germinated on LP or HP half-strength LANS solidified with 0.7% high gel strength agar, and treated either with 100 nM GR24 or with the solvent only.
Glomus intraradices germination assays
The assays were carried out in 25-compartment plates. Four compartments were used for each treatment. In each compartment, 1 ml of a sterile suspension of Gl. intraradices spores at 30 spores ml−1 was added to 1 ml of sterile test solution. The test solutions corresponded to full-strength LP or HP LANS, so the final concentrations of nutrients were equivalent to those of the watering solutions. The nutrient solutions alone were tested, as well as the same solutions containing root exudate extracts (1 ml of test solution then contained root exudates produced by the equivalent of 1 mg of root dry weight). Plates were incubated at 30 °C under 2% CO2 in the dark. Spore germination rates were determined 5 d after treatment.
Chromatography and mass spectrometry analyses
Root exudate extracts were dissolved in acetonitrile:water [1:1 (v/v)]. Strigolactone detection was performed using a 4000 Q Trap mass spectrometer with a Turbo V ESI source in the positive mode, coupled to an Agilent 1100 series HPLC system as described in Gomez-Roldan et al. (2008), except for the following modifications. HPLC separation was performed using a C18 column (5 μm, 2.1×250 mm, ACCLAIM 120C18, Dionex). Solutions of formic acid:water [1:103 (v/v); A] and formic acid:acetonitrile [1:103 (v/v); B] were pumped at 0.2 ml min−1. The gradient was: 50% B for 5 min, 50–70% B in 5 min, 70% B for 10 min, 70–100% B in 10 min, and 100% B for 5 min. The reported peak intensities correspond to extracts obtained with the equivalent of 75 mg of root dry weight. GR24 was added as an external standard to all samples at a final concentration of 100 nM. The two major pea strigolactones and GR24 were detected in the MRM mode by monitoring the transitions 405>97 m/z and 405>345 m/z for fabacyl acetate, 389>233 m/z and 411>254 m/z for orobanchyl acetate, and 299>202 m/z for GR24.
Statistical analyses
Results were analysed by analysis of variance (ANOVA) followed by Tukey's HSD test or Student's t-test using Statgraphics Centurion software (Sigma Plus). Data in Figs 2, 3D, and 5 were subjected to logarithmic, cosine, and arc sine transformation, respectively, prior to analysis. Data in Supplementary Fig. S1 at JXB online were analysed by a Kruskal–Wallis test.
Fig. 2.
Gigaspora rosea hyphal branching in response to GR24, LP or HP root exudates. Germinated spores of Gi. rosea were treated with GR24 and/or root exudates of low (LP) or high (HP) phosphate-grown plants, or with the solvent alone as negative control (10% acetonitrile; AcN). Newly formed hyphal apices were counted 48 h after treatment. White bars, controls; grey bars, root exudates alone; black bars, root exudates+GR24. Error bars show the SEM; n=24–26 treated spores for each condition. Different letters indicate statistically significant differences according to one-way ANOVA followed by Tukey's test (P <0.05).
Fig. 3.
Mycorrhizal root colonization in split-root systems. (A) Experimental design. Each root system was divided into two parts placed in different pots to allow differential phosphate fertilization. Both sides were inoculated with 90 spores of Gl. intraradices and plants were grown for 6 weeks. Control plants were fertilized with the same solution on both sides. Results in B, C, and D correspond to the same plants. (B) Root colonization levels determined by observation of stained root samples. For control plants, colonization levels measured on both sides were averaged. (C, D) Inorganic orthophosphate (Pi) content in leaves (C) and roots (D). Error bars show the SEM; n=5–7 plants for each condition. Different letters indicate statistically significant differences according to one-way ANOVA followed by Tukey's test (P <0.05).
Fig. 5.
Effect of strigolactone supplementation on root colonization. Plants inoculated with 150 spores of Gl. intraradices were fertilized daily with LP or HP nutrient solution, supplemented or not with 10 nM GR24. The extent of root colonization was determined by observation of stained root samples. Error bars show the SEM; n=7–8 plants for each condition. Different letters indicate statistically significant differences according to one-way ANOVA followed by Tukey's test (P <0.05).
Results
Experimental system
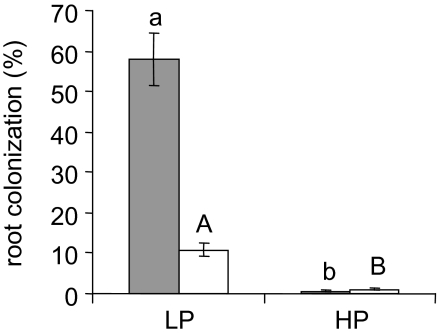
Pea plants in interaction with Gl. intraradices have been used previously to determine the importance of strigolactones in AM symbiosis (Gomez-Roldan et al., 2008). For the present study the main advantage of pea was that the strigolactones produced by this species are characterized (Yoneyama et al., 2008; Xie et al., 2009) and readily detectable. To evaluate the effect of P supply on AM interactions, plants were fertilized with half-strength LANS (Hewitt, 1966) containing different concentrations of P. The HP solution contained the normal concentration of phosphate of half-strength LANS—that is, 750 μM P. MP and LP corresponded to 10- and 100-fold lower phosphate concentrations, respectively. Plant growth evaluated by fresh weight was the lowest under LP, intermediate under MP, and maximal under HP (Supplementary Table S1 at JXB online). Therefore, HP fertilization provides sufficient but not excess P, while LP corresponds to P starvation conditions. Pea plants exhibited a remarkably strong mycorrhizal response to P fertilization (Fig. 1): when inoculated with Gl. intraradices, plants grown under LP exhibited colonization levels of ∼60%, while hardly any fungal structures could be observed in roots under HP (<1% root length colonized). The effect of HP was also tested in the interaction between P. sativum and Gi. rosea, a fungus phylogenetically distant from Gl. intraradices. Root colonization levels were lower with Gi. rosea than with Gl. intraradices (Fig. 1). Similar observations have been reported in another legume, Medicago sativa (Douds et al. 1998) and may reflect host preferences in AM interactions. Nonetheless, high P exerted a similarly strong negative effect on root colonization with both fungal species, suggesting that the regulation mechanisms involved are not fungus specific.
Fig. 1.
Effect of phosphate fertilization on mycorrhizal root colonization. Plants inoculated with 600 spores of Gl. intraradices (grey bars) or 100 spores of Gi. rosea (white bars) were grown under low (LP) or high (HP) phosphate fertilization. The extent of root colonization was determined after observation of stained root samples as the fraction of root length showing arbuscules, vesicles, or both in the case of Gl. intraradices, and arbuscules in the case of Gi. rosea. Error bars show the SEM; n=5–6 plants when inoculated with Gl. intraradices and n=7–8 plants when inoculated with Gi. rosea. Different letters indicate statistically significant differences according to Student's t-test (P <0.05).
Mycorrhizal phenotype of plants grown under HP
Plants seem to possess multiple checkpoints for mycorrhizal invasion, and the interaction can be stopped at distinct stages in various mutant backgrounds (Pumplin et al., 2009). To determine at what stage the interaction was arrested under HP, inoculated roots were subjected to closer microscopic examination. This allows the observation of all fungal structures including hyphopodia, which are visible as flattened, lenticular hyphal tips attached to the root epidermal surface (Garriock et al., 1989). Like the frequency of arbuscules and vesicles reported in Fig. 1, the number of hyphopodia per unit of root length was markedly reduced under HP as compared with LP, in plants inoculated either with Gl. intraradices or with Gi. rosea (Table 1). Similar observations were made when P was supplied as NaH2PO4 or KH2PO4, indicating that the observed effect was not due to the phosphate counterion. It has to be noted that when present, hyphopodia, arbuscules, and vesicles in HP-grown roots could not be distinguished morphologically from those observed in LP-grown roots (results not shown). In an experiment with Gl. intraradices, roots were also observed at an earlier time point, 4 weeks post-inoculation (wpi). Again, the frequency of hyphopodia was much lower under HP than under LP. Under the present conditions, this time point corresponds to the very first stages of root colonization. (At 3 wpi no fungal structures can be observed on roots, and at 4 wpi the root colonization level is <5%; data not shown. This slow progression of AM symbiosis establishment is most probably related to the inoculation with spores rather than with more infectious sources of inoculum.) Together, these results indicate that under HP the interaction was arrested prior to the formation of hyphopodia on the root epidermis.
Table 1.
Frequency of hyphopodia on inoculated roots
| Weeks post-inoculation | No. of hyphopodia m−1 root | ||
| Gl. intraradices KH2PO4 | 6 | LP | 112±42.4 a |
| HP | 6.67±1.67 b | ||
| Gl. intraradices NaH2PO4 | 4 | LP | 38.8±5.42 a |
| HP | 1.33±0.83 b | ||
| 6 | LP | 42.9±2.67 a | |
| HP | 0.42±0.42 b | ||
| Gi. rosea KH2PO4 | 7 | LP | 61.7±10.63 a |
| HP | 10.2±3.94 b |
Plants inoculated with 150 spores of Gl. intraradices or 100 spores of Gi. rosea were grown under LP or HP with KH2PO4 or NaH2PO4 as phosphorus source. Data correspond to three independent experiments: one with Gl. intraradices and KH2PO4, one with Gl. intraradices and NaH2PO4, and one with Gi. rosea and KH2PO4. Root samples were examined microscopically 4, 6, or 7 weeks post-inoculation for the presence of hyphopodia. Values indicate the average number of hyphopodia per metre of root length, ±SEM. n=3–4 plants when inoculated with Gl. intraradices and n=7–8 plants when inoculated with Gi. rosea (60 cm of roots analysed per plant). Data were analysed separately for each experiment and time point. Different letters indicate statistically significant differences according to Student's t-test (P <0.05).
Effect of P supply on pre-symbiotic signalling
The possible involvement of diffusible signals acting at the pre-symbiotic stage was considered. Such signals could include activators or inhibitors of early fungal development. Germination rates of Gl. intraradices spores exceeded 96% within 5 d in LP and HP nutrient solutions alone, and in these solutions supplemented with extracts of root exudates prepared from LP- or HP-grown plants (data not shown). This suggests the absence of a negative impact of HP conditions on this process.
Hyphal branching activities of root exudates from HP- and LP-grown plants were then evaluated. This experiment was carried out on germinated spores of Gi. rosea, for which hyphal branching can more easily be observed. Root exudate extracts of LP-grown plants, as well as GR24, stimulated hyphal branching (Fig. 2). The combination of both treatments showed an additive effect. Root exudate extracts of HP-grown plants did not enhance hyphal branching relative to the control, which could be due either to the lack of stimulants or to the presence of inhibitors. The addition of GR24 to exudate extracts of HP-grown plants resulted in an activity similar to that of GR24 alone, suggesting that these extracts do not contain inhibitors of the strigolactone effect. Rather, they may lack important stimulants of hyphal branching.
Systemic control of AM symbiosis by P
The effect of HP fertilization on AM symbiosis reported above is unusually strong. This raises the possibility that, although HP conditions do not correspond to a very high phosphate concentration, they somehow disturb early fungal development (the reduced root colonization would then be a secondary effect of these perturbations). Indeed, AM fungi can sense and react to P availability (Requena et al., 2003). As shown above, HP does not prevent germination of Gl. intraradices spores. Moreover, whether HP could prevent fungal responsiveness to GR24 was tested with the branching bioassay. Gigaspora rosea spores grown in LP or HP conditions responded equally well to GR24 (Supplementary Fig. S1 at JXB online), suggesting that P fertilization should not affect fungal ability to respond to strigolactones. To investigate further whether the early arrest of AM symbiosis could be due to a post-germination direct effect of HP on the fungus, split-root experiments were carried out in which two halves of a root system were fertilized independently. In the experimental set-up described in Fig. 3A, all compartments were inoculated with spores of Gl. intraradices. Test plants (denoted LP/HP) were watered with LP on one side and HP on the other. Both sides of control plants (denoted LP/LP and HP/HP) were watered with the same solution. In these control plants, colonization rates were comparable with those observed in intact plants—that is, high in LP and very low in HP (<0.1% root length colonized; Fig 3B). The HP-watered roots of test LP/HP plants behaved like those of HP/HP plants, showing hardly any colonization. The most striking observation was that LP-watered roots of LP/HP plants were markedly less colonized than LP/LP plants (2% of root length versus 60%). In other words, root colonization on the LP side of LP/HP plants did not respond to local fertilization conditions but to the fertilization of a distant part of the plant. This systemic regulation indicates that the effects of HP are mediated by the plant in split LP/HP plants. This does not exclude the possibility that additional direct effects on the fungus contribute to the reduced root colonization in HP/HP or intact HP plants.
Pi contents were determined in roots and leaves of the same inoculated split-root plants (Fig. 3C, D). The results are expressed in micromol Pi g−1 fresh weight to reflect the actual availability of Pi in the different tissues [NB: a high P supply resulted in increased root and leaf biomass in both LP/HP and HP/HP plants (results not shown), so the total amount of P taken up by these plants was higher than in LP/LP plants]. The results in Fig. 3C and D show that LP/HP plants accumulated Pi in leaves rather than in roots, indicating that in these conditions leaves acted as a stronger sink than roots. Roots on the LP side displayed Pi contents similar to those of LP/LP plants, yet their colonization rates were much lower (Fig. 3B). Therefore, down-regulation of AM symbiosis is not triggered by root Pi content. In contrast, leaves of LP/HP plants accumulated Pi at levels comparable with HP/HP plants. It can thus be hypothesized that the low root colonization levels observed in both types of plants may be related to high leaf Pi contents.
Effect of P supply on strigolactone production in split-root systems
Given that previous results point towards an effect of HP on early events in the AM interaction, that strigolactones are important pre-symbiotic signals (Gomez-Roldan et al., 2008), and that their production is regulated by P supply (Yoneyama et al., 2007a, b; Lopez-Raez et al., 2008), it is reasonable to envisage that these compounds mediate the effect of HP. If such is the case, one would expect strigolactone synthesis to be systemically regulated by P, like AM symbiosis.
Root exudates obtained with split-root plants were analysed to address this question. The experimental set-up was similar to that described in Fig. 3, except that the plants were not inoculated. Figure 4 shows LC-MS/MS chromatograms obtained with extracts of root exudates. In the MRM detection mode used, each line corresponds to an MS/MS mass transition characteristic of one of the two major strigolactones produced by pea: fabacyl acetate (Xie et al., 2009) and orobanchyl acetate (Yoneyama et al., 2008). Two mass transitions were monitored for each strigolactone and gave a signal at the same retention time; for clarity only one transition is shown. Synthetic standards of these two strigolactones also eluted at the same retention times (data not shown), demonstrating that the monitored signals truly corresponded to strigolactones. GR24 was added in equal quantities to all samples as an external standard to visualize any possible artefacts due to sample loading or matrix effects. The signal obtained with GR24 was similar between samples, indicating that the amounts of other strigolactones in the samples could be appropriately compared. Chromatograms obtained with control LP/LP and HP/HP plants (Fig. 4) were similar to those obtained with intact plants grown under LP or HP (data not shown), and confirm the previously reported inhibitory effect of P supply on strigolactone production (Yoneyama et al., 2007a, b; Lopez-Raez et al., 2008). The analysis of root extracts rather than exudates led to similar observations (data not shown), indicating that the regulation occurs at the level of strigolactone biosynthesis rather than exudation. Furthermore, the HP side of LP/HP split-root plants barely produced detectable strigolactones, and therefore behaved as an intact HP root system. In contrast, the LP side of LP/HP plants produced much less strigolactone than control LP/LP plants (Fig. 4). This indicates that the HP side of these split-root plants negatively regulated strigolactone production on the LP side through systemic signalling.
Fig. 4.
Systemic control of strigolactone production. Split-root plants were fertilized with low phosphorus (LP) on one side and high phosphorus (HP) on the other (LP/HP plants), or with the same solution on both sides (LP/LP and HP/HP plants). Root exudate extracts were analysed by LC-MS/MS in the MRM mode. The synthetic strigolactone analogue GR24 was added in equal quantity to all samples as an external standard. Chromatograms show the most abundant mass transition for each of the two major pea strigolactones, fabacyl acetate and orobanchyl acetate. Insets show the mass transition corresponding to the external standard GR24 (299>202 m/z).
Supplementation of HP-grown plants with exogenous strigolactones
The strong down-regulation of strigolactone synthesis by HP (Fig. 4) could account for the reduced root colonization (Fig. 1) and the absence of hyphal branching activity of root exudates (Fig. 2) observed under these conditions. To address this hypothesis, HP-grown plants were supplemented with exogenous strigolactones. The experimental conditions used (treatment with the synthetic strigolactone GR24, concentration and frequency of application) were previously demonstrated to be effective since they could rescue the mycorrhizal phenotype of strigolactone-deficient mutants (Gomez-Roldan et al., 2008). This strigolactone treatment was also sufficient to enhance mycorrhizal symbiosis establishment in LP-grown plants inoculated with Gl. intraradices (Fig. 5). Surprisingly, root colonization of HP-grown plants was not improved by strigolactone supplementation. Similar observations were made for HP-grown plants inoculated with Gi. rosea (data not shown). Strigolactone treatment did not stimulate the formation of hyphopodia on HP-grown roots either: 5.0±1.7 hyphopodia m−1 of root were observed in treated roots versus 6.7±1.7 in untreated roots in plants inoculated with Gl. intraradices, and 6.04±1.31 in treated roots versus 10.2±3.94 in untreated roots in plants inoculated with Gi. rosea.
Discussion
HP supply can strongly inhibit AM symbiosis
The regulation of AM symbiosis by P supply has been observed repeatedly and is considered a general phenomenon. In contrast to most previous studies, however, the experimental conditions described in the present report lead to a clear-cut mycorrhizal phenotype under HP supply, since hardly any symbiotic structures are observed (Fig. 1). Similar effects of HP were observed with 150 and 600 Glomus spores per plant (Fig. 5 and Fig. 1, respectively), indicating that a higher inoculum density was not able to circumvent the regulatory mechanisms. The discrepancy between the strong mycorrhizal phenotype reported here and the more moderate effects of P reported previously may relate to the plant species used, and/or to the experimental conditions: in this study, P was supplied daily in the nutrient solution, and plants were inoculated with spores rather than fragments of infected roots containing different kinds of propagules. Inoculation with spores, often regarded as less virulent, probably helps to reveal moderate phenotypes that could be masked with stronger sources of inoculum. For example, the pmi1 mutant of tomato exhibits a severe phenotype when inoculated with spores, but is colonized normally when inoculated with mycorrhizal nurse plants (David-Schwartz et al., 2001).
The effect of HP supply on AM symbiosis is partly mediated by the plant
Among the conditions tested, plant growth was maximal under HP conditions (Supplementary Table S1 at JXB online), which correspond to a moderate P supply (750 μM): in studies on P starvation responses, the P-replete condition usually falls in the 1–3 mM range [e.g. Bonser et al. (1996) on pea; Valdes-Lopez et al. (2008) on bean; Pant et al. (2008) on Arabidopsis]. The HP nutrient solution does not exhibit toxicity towards the fungal partner, as evaluated by spore germination tests. It also does not seem to modify the ability of the fungus to respond to strigolactones (Supplementary Fig. S1). In addition, in split-root experiments the inhibition of root colonization can be observed in a compartment where the fungus is only exposed to LP (Fig. 3). This regulation of AM symbiosis through systemic signalling is consistent with previous reports (e.g. Thomson et al., 1991; Rausch et al., 2001). It shows that the very strong inhibition of root colonization triggered by HP in the present report involves plant-driven processes and is not only due to a direct effect of local P concentration on the fungus. Yet, the existence of such direct effects cannot be excluded.
HP supply arrests AM symbiosis in its first stages
Microscopic examination of root samples revealed that HP fertilization reduced the number of hyphopodia formed on the root epidermis. This represents a novel HP-related mycorrhizal phenotype. A straightforward interpretation is that HP prevents hyphopodium formation per se. Alternatively, one could imagine that defects in later symbiotic stages could also lead to a reduced number of hyphopodia; for example, an impaired progression of the fungus within roots could delay or reduce the number of secondary infection events, which would in turn result in a smaller number of attached external hyphae. Several arguments lead us to conclude that the block in AM symbiosis triggered by HP occurs prior to primary hyphopodium formation, rather than later in the symbiotic process. First, the steps of clearing and staining the roots prior to microscopic observation were performed with particular care to prevent possible stripping and loss of hyphopodia (particularly those that did not lead to root colonization). Second, similar observations were made at 4 and 6 wpi (Table 1). The first time point (4 wpi) corresponds to the very beginning of the infection process, when the first arbuscules become visible (<5% root length colonized). Hyphopodia observed at this time point therefore most probably derived from primary hyphae of germinated spores, rather than from secondary infections. At this time point, a very strong effect of HP fertilization was already noted. Third, mutants affected in later stages of the interaction typically exhibit a normal (sometimes even higher) number of hyphopodia (Bradbury et al., 1991; Bonfante et al., 2000). Therefore, the present observations strongly suggest that HP conditions prevent either pre-symbiotic fungal development or attachment to roots.
In contrast to the formation of appressoria by pathogenic fungi, the differentiation of these attachment and penetration structures by AM fungi is still poorly understood. Plants grown under HP are reminiscent of tomato pmi1 and pmi2 (David-Schwartz et al., 2001, 2003), and maize nope1 and taci1 mutants (Paszkowski et al., 2006), in which a reduced frequency of hyphopodia was observed. Unfortunately the genes affected by these mutations have not been identified yet. Nonetheless these mutants, together with the HP conditions described in the present report, should be useful to decipher the mechanisms involved in hyphopodium differentiation.
Different kinds of mechanisms could regulate the formation of hyphopodia under HP. One of them is the production by plant roots of stimulatory or inhibitory diffusible compounds. Candidate compounds include flavonoids, some of which have been reported to stimulate AM root colonization by enhancing the number of fungal entry points (Scervino et al., 2007). Polyamines have also been proposed to favour the formation of hyphopodia (El Ghachtouli et al., 1995). P availability also affects the production of compounds known to affect fungal development more generally (reviewed in Vierheilig, 2004), but in most instances their contribution to the regulation of AM symbiosis by P has not been tested functionally. An exception is the report by Akiyama et al. (2002) that a C-glycosylflavonoid accumulated in melon roots upon P starvation, and that supplementation with this compound restored normal mycorrhizal rates under HP. The reduced accumulation of this compound may therefore account for the decreased root colonization under HP. In contrast to the present report, however, the effects of HP were not observed in the first visible stages of the interaction. Two time points were examined by Akiyama et al. (2002): 25 d and 45 d post-inoculation (dpi). At 25 dpi, the root colonization levels were similar under LP and HP. HP triggered down-regulation of AM symbiosis only at 45 dpi. In agreement with this, an AM-stimulating effect of the C-glycosylflavonoid on HP-grown plants was only observed at 45 dpi. In contrast, in the present conditions the negative impact of HP on root colonization could be observed as soon as the control roots became colonized (28 dpi, Table 1). Therefore, the mechanisms underlying suppression of AM symbiosis by HP may be different in the two systems. In addition, different plant species produce distinct arrays of flavonoids, making it difficult to extrapolate results from one species to another. Still, flavonoids remain interesting candidates as mediators of the P effect.
In the present experimental system, branching bioassays supported the hypothesis of an effect of P on pre-symbiotic fungal development, since root exudate extracts of HP-grown plants failed to stimulate hyphal branching (Fig. 2). These extracts did not inhibit the effect of GR24 on the fungus, and therefore appeared to lack branching stimulants that are present in exudates of LP-grown plants. It must be noted, however, that in these experiments ethyl acetate extracts of root exudates were used in order to allow an adequate concentration of the samples. Therefore, it cannot be excluded that in addition to the lack of stimulants in the organic fraction, fungal inhibitors could be found in the aqueous fraction of HP root exudates.
Another possible type of regulatory process is the display of signals on the root epidermal surface. For example, AM fungal hyphae can recognize specific patterns displayed by epidermal cells and differentiate hyphopodia on cell wall fragments of the epidermis, but not of other root tissues (Nagahashi and Douds, 1997). In addition, hyphopodia are formed on grooves between adjacent epidermal cells rather than on the outer cell wall. Interestingly, cell walls in these grooves appear thinner, looser, and richer in non-esterified pectin as compared with the tangential walls of epidermal cells (Bonfante et al., 2000). Whether such changes in cell wall composition contribute to the effect of HP on the formation of hyphopodia deserves further investigation.
P supply affects strigolactone production in a systemic manner
Strigolactones, identified as important contributors to the effect of host roots on pre-symbiotic fungal growth and metabolism (Akiyama et al., 2005; Besserer et al., 2006, 2008), were obvious candidates to mediate the effect of P supply because their synthesis is known to correlate inversely with P supply (Yoneyama et al., 2007a, b; Lopez-Raez et al., 2008). In agreement with this, strigolactones were undetectable in root exudates of plants grown under HP. Furthermore, HP supply was able to down-regulate strigolactone production in a systemic manner, as evidenced by the analysis of split-root plants (Fig. 4). This novel finding is particularly interesting in the context of the hormonal function of strigolactones. Indeed, a recent study has proposed that strigolactones mediate the tillering response to P starvation in rice (Umehara et al., 2010). In addition to the effect of strigolactones on lateral bud outgrowth, a role in the control of root architecture has recently been suggested (Koltai et al., 2009). This raises the possibility that P supply on one side of a plant affects development of a distant part of the root system through a modulation of strigolactone synthesis. It is already known that modifications of root architecture in response to P availability are integrated at the whole-plant level (Williamson et al., 2001), and it would be worth investigating the contribution of strigolactones to this phenomenon.
The analysis of Pi contents in root and shoot tissues of split-root plants (Fig. 3C, D) revealed that root colonization levels and strigolactone production were linked to shoot Pi rather than to external P availability or local Pi concentrations in roots. This is consistent with the fact that HP exerts a dominant effect over LP in LP/HP split-root plant with regards to mycorrhizal and strigolactone exudation responses, and also with regards to Pi content (in LP/HP plants shoot Pi contents are similar to those of HP/HP plants). However, the signal underlying this systemic signalling remains unknown. Branscheid et al. (2010) proposed that the microRNA miR399 could act as a P starvation-induced signal to stimulate AM symbiosis under low P. MiR399 is known to accumulate in shoots under P deprivation, and to be transported to roots where it targets PHO2, a negative regulator of several P starvation responses (Lin et al., 2008; Pant et al., 2008). Interestingly, miR399 expression responded to AM root colonization, but overexpression of miR399 was not sufficient to improve AM root colonization under HP (Branscheid et al., 2010), suggesting that additional internal signals are required. Other microRNAs expressed in response to AM colonization and/or P supply (Gu et al. 2010) may be alternative candidates as systemic signals.
Strigolactones are not solely responsible for P-triggered down-regulation of AM symbiosis
The putative role of strigolactones as mediators of the P effect on AM symbiosis was supported by the good correlation between mycorrhizal colonization and strigolactone exudation in split-root plants (Figs 3, 4). Supplementation with exogenous GR24, however, failed to restore AM symbiosis in HP-grown plants (Fig. 5). These novel results rule out the proposed hypothesis that HP-grown plants are poorly colonized by AM fungi simply because they do not produce strigolactones (Yoneyama et al., 2007b; Lopez-Raez et al., 2008). Although a role for strigolactones in the process is still possible and indeed likely, additional mechanisms remain to be discovered. This is consistent with the proposition that hyphal branching is a complex response involving several classes of compounds (Nagahashi and Douds, 2007). The present observations do not imply, however, that the absence of additional stimulatory compounds in HP root exudates is the only explanation for the lack of root colonization under HP.
An additional possibility is that the hormonal function of strigolactones (rather than their role as rhizospheric signals) is involved in the regulation of AM symbiosis, for example by influencing root development or the ability of root cells to accommodate AM fungi. This question was not addressed in the present study, and the concentration of GR24 necessary to restore the putative hormonal function(s) of strigolactones in roots is not known. Nevertheless, the hypothesis of a strigolactone requirement at the plant hormonal level is not supported by previous observations that strigolactone-deficient mutants could still be slightly colonized by AM fungi (Gomez-Roldan et al., 2008).
As determined by hyphal branching bioassays, HP conditions do not seem to prevent the stimulation of the fungus by strigolactones. The combination of HP root exudates with GR24 results in an activity similar to that of LP root exudates (Fig. 2). This suggests that hyphal branching and associated metabolic processes are restored in the supplementation experiment (HP+GR24) described in Fig. 5. The observation that this is not sufficient to allow root colonization by the fungus or the formation of hyphopodia points towards an effect of HP on steps other than hyphal branching, possibly including the differentiation of hyphopodia.
Conclusion
Collectively, the various reports on the down-regulation of AM symbiosis by P suggest that several successive layers of control operate in roots grown under HP. The experimental conditions used by different authors shed light on one or the other of these control mechanisms. Those described in this report allow the manipulatation of mycorrhizal symbiosis by targeting some of the first events in the interaction, and the testing of a number of hypotheses related to these events. It is demonstrated for the first time that the regulation of AM symbiosis by P is accompanied by a systemic regulation of strigolactone production, an important observation with regards to the hormonal function of these compounds. The decreased strigolactone content under HP, however, does not solely account for the strong mycorrhizal phenotype. The results therefore suggest the existence of additional early signalling events, some of which probably affect the differentiation of hyphopodia. A better understanding of this regulation should reveal important mechanisms required for the symbiosis under favourable conditions, and help circumvent the limitations for this symbiosis associated with the extensive use of P fertilizers in agriculture.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Effect of phosphate concentration on Gi. rosea hyphal branching responsiveness to GR24.
Table S1. Effect of phosphate fertilization on shoot and root fresh weight.
Acknowledgments
The authors would like to thank Dr Koichi Yoneyama (Utsunomiya University, Japan) for the gift of synthetic standards of orobanchyl acetate and fabacyl acetate, and Dr Christian Brière (CNRS, Toulouse, France) for advice on statistical analyses. This study was partly funded by ASEDIS-SO, Toulouse, France. The Q Trap mass spectrometer was made available to us by the Metabolomics and Fluxomics platform of Toulouse (MetaToul).
References
- Akiyama K, Matsuoka H, Hayashi H. Isolation and identification of a phosphate deficiency-induced C-glycosylflavonoid that stimulates arbuscular mycorrhiza formation in melon roots. Molecular Plant-Microbe Interactions. 2002;15:334–340. doi: 10.1094/MPMI.2002.15.4.334. [DOI] [PubMed] [Google Scholar]
- Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- Bécard G, Fortin JA. Early events of vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytologist. 1988;108:211–218. doi: 10.1111/j.1469-8137.1988.tb03698.x. [DOI] [PubMed] [Google Scholar]
- Besserer A, Bécard G, Jauneau A, Roux C, Séjalon-Delmas N. GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism. Plant Physiology. 2008;148:402–413. doi: 10.1104/pp.108.121400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besserer A, Puech-Pages V, Kiefer P, Gomez-Roldan V, Jauneau A, Roy S, Portais JC, Roux C, Bécard G, Séjalon-Delmas N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biology. 2006;4:e226. doi: 10.1371/journal.pbio.0040226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfante P, Genre A, Faccio A, Martini I, Schauser L, Stougaard J, Webb J, Parniske M. The Lotus japonicus LjSym4 gene is required for the successful symbiotic infection of root epidermal cells. Molecular Plant-Microbe Interactions. 2000;13:1109–1120. doi: 10.1094/MPMI.2000.13.10.1109. [DOI] [PubMed] [Google Scholar]
- Bonser AM, Lynch J, Snapp S. Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris. New Phytologist. 1996;132:281–288. doi: 10.1111/j.1469-8137.1996.tb01847.x. [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Roux C, Lopez-Raez JA, Bécard G. Rhizosphere communication of plants, parasitic plants and AM fungi. Trends in Plant Science. 2007;12:224–230. doi: 10.1016/j.tplants.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Bradbury SM, Peterson RL, Bowley SR. Interactions between three alfalfa nodulation genotypes and two Glomus species. New Phytologist. 1991;119:115–120. doi: 10.1111/j.1469-8137.1991.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Branscheid A, Sieh D, Pant BD, May P, Devers EA, Elkrog A, Schauser L, Scheible WR, Krajinski F. Expression pattern suggests a role of MiR399 in the regulation of the cellular response to local Pi increase during arbuscular mycorrhizal symbiosis. Molecular Plant-Microbe Interactions. 2010;23:915–926. doi: 10.1094/MPMI-23-7-0915. [DOI] [PubMed] [Google Scholar]
- Bucher M. Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytologist. 2007;173:11–26. doi: 10.1111/j.1469-8137.2006.01935.x. [DOI] [PubMed] [Google Scholar]
- Buée M, Rossignol M, Jauneau A, Ranjeva R, Bécard G. The pre-symbiotic growth of arbuscular mycorrhizal fungi is induced by a branching factor partially purified from root exudates. Molecular Plant-Microbe Interactions. 2000;13:693–698. doi: 10.1094/MPMI.2000.13.6.693. [DOI] [PubMed] [Google Scholar]
- Collins CD, Foster BL. Community-level consequences of mycorrhizae depend on phosphorus availability. Ecology. 2009;90:2567–2576. doi: 10.1890/08-1560.1. [DOI] [PubMed] [Google Scholar]
- David-Schwartz R, Badani H, Smadar W, Levy AA, Galili G, Kapulnik Y. Identification of a novel genetically controlled step in mycorrhizal colonization: plant resistance to infection by fungal spores but not extra-radical hyphae. The Plant Journal. 2001;27:561–569. doi: 10.1046/j.1365-313x.2001.01113.x. [DOI] [PubMed] [Google Scholar]
- David-Schwartz R, Gadkar V, Wininger S, Bendov R, Galili G, Levy AA, Kapulnik Y. Isolation of a premycorrhizal infection (pmi2) mutant of tomato, resistant to arbuscular mycorrhizal fungal colonization. Molecular Plant-Microbe Interactions. 2003;16:382–388. doi: 10.1094/MPMI.2003.16.5.382. [DOI] [PubMed] [Google Scholar]
- Douds DD, Galvez L, Bécard G, Kapulnik Y. Regulation of arbuscular mycorrhizal development by plant host and fungus species in alfalfa. New Phytologist. 1998;138:27–35. [Google Scholar]
- El Ghachtouli N, Paynot M, Morandi D, Martin-Tanguy J, Gianinazzi S. The effect of polyamines on endomycorrhizal infection of wild-type Pisum sativum, cv. Frisson (nod+myc+) and two mutants (nod−myc+and nod−myc−) Mycorrhiza. 1995;5:189–192. [Google Scholar]
- Elias KS, Safir GR. Hyphal elongation of Glomus fasciculatus in response to root exudates. Applied and Environmental Microbiology. 1987;53:1928–1933. doi: 10.1128/aem.53.8.1928-1933.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriock ML, Peterson RL, Ackerley CA. Early stages in colonization of Allium porum (leek) roots by the vesicular-arbuscular mycorrhizal fungus, Glomus versiforme. New Phytologist. 1989;112:85–92. [Google Scholar]
- Gianinazzi-Pearson V, Branzanti B, Gianinazzi S. In vitro enhancement of spore germination and early hyphal growth of a vesicular-arbuscular mycorrhizal fungus by root exudates and plant flavonoids. Symbiosis. 1989;7:243–255. [Google Scholar]
- Giovannetti M, Mosse B. An evaluation of techniques for measuring vesicular-arbuscular infection in roots. New Phytologist. 1980;84:489–500. [Google Scholar]
- Giovannetti M, Sbrana C, Citernesi AS, Avio L. Analysis of factors involved in fungal recognition responses to host-derived signals by arbuscular mycorrhizal fungi. New Phytologist. 1996;133:65–71. [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- Graham JH, Leonard RT, Menge JA. Membrane-mediated decrease in root exudation responsible for phosphorus inhibition of vesicular-arbuscular mycorrhiza formation. Plant Physiology. 1981;68:548–552. doi: 10.1104/pp.68.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M, Xu K, Chen A, Zhu Y, Tang G, Xu G. Expression analysis suggests potential roles of microRNAs for phosphate and arbuscular mycorrhizal signaling in Solanum lycopersicum. Physiologia Plantarum. 2010;138:226–237. doi: 10.1111/j.1399-3054.2009.01320.x. [DOI] [PubMed] [Google Scholar]
- Gutjahr C, Novero M, Guether M, Montanari O, Udvardi M, Bonfante P. Presymbiotic factors released by the arbuscular mycorrhizal fungus Gigaspora margarita induce starch accumulation in Lotus japonicus roots. New Phytologist. 2009;183:53–61. doi: 10.1111/j.1469-8137.2009.02871.x. [DOI] [PubMed] [Google Scholar]
- Harrison MJ, Dewbre GR, Liu J. A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. The Plant Cell. 2002;14:2413–2429. doi: 10.1105/tpc.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt EJ. Sand and water culture methods used in the study of plant nutrition. 2nd edn. London: Commonwealth Agricultural Bureau; 1966. [Google Scholar]
- Javot H, Pumplin N, Harrison MJ. Phosphate in the arbuscular mycorrhizal symbiosis: transport properties and regulatory roles. Plant, Cell and Environment. 2007;30:310–322. doi: 10.1111/j.1365-3040.2006.01617.x. [DOI] [PubMed] [Google Scholar]
- Johnson NC. Can fertilization of soil select less mutualistic mycorrhizae? Ecological Applications. 1993;3:749–757. doi: 10.2307/1942106. [DOI] [PubMed] [Google Scholar]
- Koltai H, Dor E, Hershenhorn J, et al. Strigolactones’ effect on root growth and root-hair elongation may be mediated by auxin-efflux carriers. Journal of Plant Growth Regulation. 2009;26:129–136. [Google Scholar]
- Kosuta S, Chabaud M, Lougnon G, Gough C, Dénarié J, Barker DG, Bécard G. A diffusible factor from arbuscular mycorrhizal fungi induces symbiosis-specific MtENOD11 expression in roots of Medicago truncatula. Plant Physiology. 2003;131:952–962. doi: 10.1104/pp.011882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosuta S, Hazledine S, Sun J, Miwa H, Morris RJ, Downie JA, Oldroyd GE. Differential and chaotic calcium signatures in the symbiosis signaling pathway of legumes. Proceedings of the National Academy of Sciences, USA. 2008;105:9823–9828. doi: 10.1073/pnas.0803499105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-I, Chiang SF, Lin W-Y, Chen J-W, Tseng CY, Wu PC, Chiou T-J. Regulatory network of microRNA399 and PHO2 by systemic signaling. Plant Physiology. 2008;147:732–746. doi: 10.1104/pp.108.116269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Ráez JA, Charnikhova T, Gómez-Roldán V, et al. Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytologist. 2008;178:863–874. doi: 10.1111/j.1469-8137.2008.02406.x. [DOI] [PubMed] [Google Scholar]
- Marsh JF, Schultze M. Analysis of arbuscular mycorrhizas using symbiosis-defective plant mutants. New Phytologist. 2001;150:525–532. [Google Scholar]
- Nagahashi G, Douds DD. Appressorium formation by AM fungi on isolated cell walls of carrot roots. New Phytologist. 1997;136:299–304. [Google Scholar]
- Nagahashi G, Douds D., Jr Separated components of root exudate and cytosol stimulate different morphologically identifiable types of branching responses by arbuscular mycorrhizal fungi. Mycological Research. 2007;111:487–492. doi: 10.1016/j.mycres.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Nagy R, Drissner D, Amrhein N, Jakobsen I, Bucher M. Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytologist. 2008;181:950–959. doi: 10.1111/j.1469-8137.2008.02721.x. [DOI] [PubMed] [Google Scholar]
- Nair MG, Safir GR, Siqueira JO. Isolation and identification of vesicular-arbuscular mycorrhiza-stimulatory compounds from clover (Trifolium repens) roots. Applied and Environmental Microbiology. 1991;57:434–439. doi: 10.1128/aem.57.2.434-439.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanamori M, Shinano T, Wasaki J, Yamamura T, Rao IM, Osaki M. Low phosphorus tolerance mechanisms: phosphorus recycling and photosynthate partitioning in the tropical forage grass, Brachiaria hybrid cultivar Mulato compared with rice. Plant and Cell Physiology. 2004;45:460–469. doi: 10.1093/pcp/pch056. [DOI] [PubMed] [Google Scholar]
- Navazio L, Moscatiello R, Genre A, Novero M, Baldan B, Bonfante P, Mariani P. A diffusible signal from arbuscular mycorrhizal fungi elicits a transient cytosolic calcium elevation in host plant cells. Plant Physiology. 2007;144:673–681. doi: 10.1104/pp.106.086959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oláh B, Brière C, Bécard G, Dénarié J, Gough C. Nod factors and a diffusible factor from arbuscular mycorrhizal fungi stimulate lateral root formation in Medicago truncatula via the DMI1/DMI2 signalling pathway. The Plant Journal. 2005;44:195–207. doi: 10.1111/j.1365-313X.2005.02522.x. [DOI] [PubMed] [Google Scholar]
- Pant BD, Buhtz A, Kehr J, Scheible WR. MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. The Plant Journal. 2008;53:731–738. doi: 10.1111/j.1365-313X.2007.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parniske M. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nature Reviews Microbiology. 2008;6:763–775. doi: 10.1038/nrmicro1987. [DOI] [PubMed] [Google Scholar]
- Paszkowski U, Jakovleva L, Boller T. Maize mutants affected at distinct stages of the arbuscular mycorrhizal symbiosis. The Plant Journal. 2006;47:165–173. doi: 10.1111/j.1365-313X.2006.02785.x. [DOI] [PubMed] [Google Scholar]
- Paszkowski U, Kroken S, Roux C, Briggs SP. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proceedings of the National Academy of Sciences, USA. 2002;99:13324–13329. doi: 10.1073/pnas.202474599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin N, Mondo SJ, Topp S, Starker CG, Gantt JS, Harrison MJ. Medicago truncatula Vapyrin is a novel protein required for arbuscular mycorrhizal symbiosis. The Plant Journal. 2009;61:482–494. doi: 10.1111/j.1365-313X.2009.04072.x. [DOI] [PubMed] [Google Scholar]
- Rausch C, Daram P, Brunner S, Jansa J, Laloi M, Leggewie G, Amrhein N, Bucher M. A phosphate transporter expressed in arbuscule-containing cells in potato. Nature. 2001;414:462–470. doi: 10.1038/35106601. [DOI] [PubMed] [Google Scholar]
- Requena N, Breuninger M, Franken P, Ocón A. Symbiotic status, phosphate, and sucrose regulate the expression of two plasma membrane H+-ATPase genes from the mycorrhizal fungus Glomus mosseae. Plant Physiology. 2003;132:1540–1549. doi: 10.1104/pp.102.019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scervino JM, Ponce MA, Erra-Bassells R, Bompadre J, Vierheilig H, Ocampo JA, Godeas A. The effect of flavones and flavonols on colonization of tomato plants by arbuscular mycorrhizal fungi of the genera Gigaspora and Glomus. Canadian Journal of Microbiology. 2007;53:702–709. doi: 10.1139/W07-036. [DOI] [PubMed] [Google Scholar]
- Siqueira JO, Safir GR, Nair MG. Stimulation of vesicular-arbuscular mycorrhiza formation and growth of white clover by flavonoid compounds. New Phytologist. 1991;118:87–93. [Google Scholar]
- Smith SE, Read D. Mycorrhizal symbiosis. 3rd edn. London: Academic Press; 2008. [Google Scholar]
- Smith SE, Smith FA, Jakobsen I. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiology. 2003;133:16–20. doi: 10.1104/pp.103.024380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Arnaud M, Hamel C, Vimard B, Caron M, Fortin JA. Enhanced hyphal growth and spore production of the arbuscular mycorrhizal fungus Glomus intraradices in an in vitro system in the absence of host roots. Mycological Research. 1996;100:328–332. [Google Scholar]
- Thomson BD, Robson AD, Abbott LK. Effects of phsophorus on the formation of mycorrhizas by Gigaspora calospora and Glomus fasciculatum in relation to root carbohydrates. New Phytologist. 1986;103:751–765. [Google Scholar]
- Thomson BD, Robson AD, Abbott LK. Soil mediated effects of phosphorus supply on the formation of mycorrhizas by Scutellispora calospora (Nicol. & Gerd.) Walker & Sanders on subterranean clover. New Phytologist. 1991;118:463–469. [Google Scholar]
- Tsai SM, Phillips DA. Flavonoids released naturally from alfalfa promote development of symbiotic Glomus spores in vitro. Applied and Environmental Microbiology. 1991;57:1485–1488. doi: 10.1128/aem.57.5.1485-1488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Magome H, Takeda-Kamiya N, Yamaguchi S. Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant and Cell Physiology. 2010;51:1118–1126. doi: 10.1093/pcp/pcq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- Valdés-López O, Arenas-Huertero C, Ramírez M, Girard L, Sánchez F, Vance CP, Luis Reyes J, Hernández G. Essential role of MYB transcription factor: PvPHR1 and microRNA: PvmiR399 in phosphorus-deficiency signalling in common bean roots. Plant, Cell and Environment. 2008;31:1834–1843. doi: 10.1111/j.1365-3040.2008.01883.x. [DOI] [PubMed] [Google Scholar]
- Vierheilig H. Regulatory mechanisms during the plant–arbuscular mycorrhizal fungus interaction. Canadian Journal of Botany. 2004;82:1166–1176. [Google Scholar]
- Vierheilig H, Coughlan AP, Wyss U, Piche Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Applied and Environmental Microbiology. 1998;64:5004–5007. doi: 10.1128/aem.64.12.5004-5007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann S, Sanchez L, Descombin J, Chatagnier O, Gianinazzi S, Gianinazzi-Pearson V. Fungal elicitation of signal transduction-related plant genes precedes mycorrhiza establishment and requires the dmi3 gene in Medicago truncatula. Molecular Plant-Microbe Interactions. 2004;17:1385–1393. doi: 10.1094/MPMI.2004.17.12.1385. [DOI] [PubMed] [Google Scholar]
- Williamson LC, Ribrioux SP, Fitter AH, Leyser HM. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiology. 2001;126:875–882. doi: 10.1104/pp.126.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Yoneyama K, Harada Y, Fusegi N, Yamada Y, Ito S, Yokota T, Takeuchi Y, Yoneyama K. Fabacyl acetate, a germination stimulant for root parasitic plants from Pisum sativum. Phytochemistry. 2009;70:211–215. doi: 10.1016/j.phytochem.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Yoneyama K, Xie X, Kusumoto D, Sekimoto H, Sugimoto Y, Takeuchi Y, Yoneyama K. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta. 2007a;227:125–132. doi: 10.1007/s00425-007-0600-5. [DOI] [PubMed] [Google Scholar]
- Yoneyama K, Xie X, Sekimoto H, Takeuchi Y, Ogasawara S, Akiyama K, Hayashi H, Yoneyama K. Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytologist. 2008;179:484–494. doi: 10.1111/j.1469-8137.2008.02462.x. [DOI] [PubMed] [Google Scholar]
- Yoneyama K, Yoneyama K, Takeuchi Y, Sekimoto H. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta. 2007b;225:1031–1038. doi: 10.1007/s00425-006-0410-1. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Blaylock LA, Harrison MJ. Two Medicago truncatula half-ABC transporters are essential for arbuscule development in arbuscular mycorrhizal symbiosis. The Plant Cell. 2010;22:1483–1497. doi: 10.1105/tpc.110.074955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.