Abstract
The maximum specific hydraulic conductivity (kmax) of a plant sample is a measure of the ability of a plants’ vascular system to transport water and dissolved nutrients under optimum conditions. Precise measurements of kmax are needed in comparative studies of hydraulic conductivity, as well as for measuring the formation and repair of xylem embolisms. Unstable measurements of kmax are a common problem when measuring woody plant samples and it is commonly observed that kmax declines from initially high values, especially when positive water pressure is used to flush out embolisms. This study was designed to test five hypotheses that could potentially explain declines in kmax under positive pressure: (i) non-steady-state flow; (ii) swelling of pectin hydrogels in inter-vessel pit membranes; (iii) nucleation and coalescence of bubbles at constrictions in the xylem; (iv) physiological wounding responses; and (v) passive wounding responses, such as clogging of the xylem by debris. Prehydrated woody stems from Laurus nobilis (Lauraceae) and Encelia farinosa (Asteraceae) collected from plants grown in the Fullerton Arboretum in Southern California, were used to test these hypotheses using a xylem embolism meter (XYL'EM). Treatments included simultaneous measurements of stem inflow and outflow, enzyme inhibitors, stem-debarking, low water temperatures, different water degassing techniques, and varied concentrations of calcium, potassium, magnesium, and copper salts in aqueous measurement solutions. Stable measurements of kmax were observed at concentrations of calcium, potassium, and magnesium salts high enough to suppress bubble coalescence, as well as with deionized water that was degassed using a membrane contactor under strong vacuum. Bubble formation and coalescence under positive pressure in the xylem therefore appear to be the main cause for declining kmax values. Our findings suggest that degassing of water is essential for achieving stable and precise measurements of kmax through woody plant samples. For complete rehydration of woody samples, incubation in water under vacuum for 24 h is suggested as a reliable technique that avoids bubble problems associated with flushing under high positive pressure.
Keywords: Bubble coalescence, Encelia farinosa, Laurus nobilis, hydraulic conductivity, pectin hydrogel hypothesis, plant hydraulics, woody stems, xylem vulnerability curves
Introduction
The maximum hydraulic conductivity (kmax) is a measure of the plant vascular system's ability to transport water and solutes under optimum conditions. It is defined as the hydraulic conductivity in the absence of any reversible air embolisms in xylem conduits. Precise measurements of kmax are needed for characterizing the resistance of plants to embolism formation (Sperry and Sullivan, 1992; Alder et al., 1997), in studies of embolism repair (Salleo et al., 2004), and in comparative studies of hydraulic conductivity in different plant species (Maherali et al., 2004). Xylem traits that affect kmax include the abundance, diameters, lengths, and degree of connectedness of xylem conduits (Loepfe et al., 2007). Plants can lower kmax by filling xylem conduits with tyloses (Cochard and Tyree, 1990), gels (Bonsen and Kučera, 1990; Crews et al., 2003), or both (Sun et al., 2008), usually in response to wounding or to pathogen attack or during heartwood formation. The ionic composition of xylem sap also affects kmax (van Ieperen et al., 2000; Zwieniecki et al., 2001; Lopez-Portillo et al., 2005; Gascó et al., 2007). This process is hypothesized to involve the swelling of pectin hydrogels embedded into the pit membranes that connect xylem conduits (Zwieniecki et al., 2001), which potentially could decrease kmax.
When water or a solution with a constant ionic composition is fed through stems, roots, or petioles whose conduits are completely filled with water, one would not expect kmax to change substantially in the short term (minutes to hours), because structural features do not change over such periods and because pit membranes are thought to retain a constant hydraulic conductivity for a given ionic composition in water (Zwieniecki et al., 2001). Over longer periods (many hours to days), microbial growth and decomposition could clog or alter the structure of xylem conduits (Sperry et al., 1988) and tyloses may plug conduits as well (Sun et al., 2007). Despite the fact that short-term changes of kmax are not expected in theory, they are frequently observed. When deionized water is used as the fluid medium, kmax typically declines from initially high values, even in samples without embolized xylem conduits (Kelso et al., 1963; Sperry et al., 1988; Sperry and Tyree, 1990; van Ieperen and van Gelder, 2006; Canny et al., 2007; Cochard et al., 2010). Such short-term declines lead to unreliable measurements of kmax, which can be a serious problem for studies in plant hydraulics. This study was designed to test the hypothesis that a short-term decline in kmax was caused by one or more of the following mechanisms.
(i) Non-steady-state flow. Water forced into a cut plant sample under positive pressure may enter air-filled fibre cells, extracellular spaces, or parenchyma cells (van Ieperen et al., 2000). Lateral water flow from conduits may cause initially high rates of inflow, declining over time as cells and extracellular spaces are filled (Tyree and Yang, 1992). Hydraulic conductance measurements based on inflow, for example, using the XYL'EM apparatus (Cochard et al., 2000), could be affected by artefacts due to lateral flow.
(ii) Swelling of pectin hydrogels in pit membranes. Use of deionized water for flushing xylem could potentially deplete pit-membrane-residing pectins of cations, especially Ca2+, thereby cause hydrogel swelling, and thus decrease hydraulic conductance (Zwieniecki et al., 2001). Ca2+ ions are a structural component of some pectins, provide rigidity, and reduce pectin swelling potential (Ryden et al., 2000).
(iii) Formation and coalescence of bubbles. Gassing-out can occur when air-saturated water flows through constrictions (Kelso et al., 1963; Scardina and Edwards, 2004; Blatteau et al., 2006; Canny et al., 2007), such as xylem pit membranes or perforation plates. In a process termed ‘air binding’, small bubbles form through heterogeneous nucleation and then coalesce into larger ones that can block pit membranes or whole vessels.
(iv) Active wounding responses. Reduced water uptake by stems can be due to a physiological wounding response that may include mechanisms of damage control and repair or defences against pathogens that may take advantage of the wounded plant tissue (Cheong et al., 2002; Ramonell and Somerville, 2002). Wounding responses that have been associated with reduced water uptake in cut stems appear to involve cell wall enzyme activity, possibly related to suberin, tylose, and gel formation (van Doorn and Cruz, 2000; van Doorn and Vaslier, 2002; Loubaud and van Doorn, 2004) or in defence against pathogenic cell-wall degrading enzymes (Cheong et al., 2002; Li et al., 2003).
(v) Passive wounding effects. A decline in kmax could result if cutting a sample caused cell-wall debris to dislodge or cause gums and resins to distribute across the cut surface of the xylem (Schulte et al., 1987; Sperry et al., 1988). Flushing the samples under pressure could force such materials into vessels and clog them.
This study was designed to test for the five mechanisms described above for stem samples from two woody plant species, Laurus nobilis L. (Lauraceae), sweet bay, and Encelia farinosa Torrey & A. Gray (Asteraceae), brittlebush. Laurus, a small evergreen tree of Mediterranean origin, was chosen because it has been the subject of numerous studies in plant hydraulics (Tyree et al., 1999; Zwieniecki et al., 2001; Hacke and Sperry, 2003; Salleo et al., 2004; Gascó et al., 2006), while Encelia, a North American, drought-deciduous, desert shrub was chosen because we had previously experienced problems with unstable measurements of kmax in this species.
Materials and methods
Collection of plant samples
Branch samples were collected between July 2008 and September 2010 from a single Laurus nobilis tree and a group of Encelia farinosa shrubs growing in the Fullerton Arboretum in Fullerton, California, USA. At least 50-cm-long branches with leaves were cut off from the plants under water and, with their cut ends submerged under water, were transported to the laboratory, where they were placed in a vase filled with tap water or with an aqueous-solution, depending on treatment (see below), for 24 h to allow the stems to rehydrate. In one experiment, stems were cut under water from very well-watered plants at predawn and used as a control treatment to test for the effectiveness of the vase rehydration treatment. The stems were then submerged in a water-filled tray, where a 15-cm-long central portion was cut out with sharp anvil clippers. Stem segments were 2–6 mm in diameter (mean 3.5 mm for both species), 1–2-years-old, and did not contain any heartwood. Maximum vessel lengths in both species exceed 15 cm (Laurus 40 cm; Gascó et al., 2006; Encelia 50 cm; S Espino, unpublished data), thus, due to a few open vessels, measured kmax values may slightly overestimate true values of kmax in these species. For each treatment, 10 replicates were used for each species, but in some cases replication was reduced to 6–9 replicates, usually because leaky or damaged stems had to be discarded.
Measurements of hydraulic conductivity
A XYL'EM embolism meter (Bronkhorst, Montigny les Cormeilles, France; Cochard et al., 2000) was used for all measurements of hydraulic conductivity. Keeping stem segments submerged under water at all times, they were connected at their basal inflow end to the tubing of the apparatus. Except for treatments measured in ice water (5–11 °C), all measurements were conducted at room temperature (18.1–22.3 °C). Aqueous solutions used for conductance measurements and for flushing stems under high pressure varied between treatments. Treatments and their predicted effects are summarized in Table 1. Except where noted in Table 1, all solutions were degassed under laboratory vacuum for at least 3 h in a side-arm flask on a magnetic stirrer at approximately 30 kPa absolute pressure (=71 kPa vacuum) and passed through a 0.2 μm filter (model Polycap AS 75, Whatman Inc., Piscataway, NJ). All flow rate measurements were preceded by a measurement without a pressure differential (Δp=0) between inflow and outflow to determine baseline flow-rates (F0) into the samples. Water pressure for flushing of stems was generated with the high pressure vessel in the XYL'EM apparatus, which prevents contact between air and water during pressurization. All measurements were recorded after flow rates stabilized, typically within 1–5 min. The first measurement was conducted under a Δp of 3 kPa (FΔp). Hydraulic conductance, k, was determined as k=(FΔp–F0)/Δp and specific hydraulic conductivity, ks, for a given length, L, and wood-cross-sectional area, A, as:
| (1) |
Table 1.
Predicted outcomes of Experimental treatments on the decline of kmax measurements in woody stems, assuming that the mechanism listed in the corresponding column header is operative (see Introduction for further explanations)
| Experimental treatment | Non-steady-state flow | Pectin swelling | Bubbles | Active wounding response | Passive wounding effects |
| Not degassed | o | o | o | o | o |
| Flask-degassed at 30 kPa | o | o | + | o | o |
| Degasser at 30 kPa | o | o | + | o | o |
| Degasser at 3 kPa | o | o | ++ | o | o |
| Hydrated at 3 kPa, degasser at 3 kPa | o | o | ++ | o | o |
| Ice water | o | + | + | + | o |
| Ice waterb | o | o | + | + | o |
| Bark removed before cutting | o | o | o | + | + |
| KCl, 10 mM | o | ++ | o | o | o |
| KCl, 100 mM | o | ++ | + | o | o |
| KCl, 300 mM | o | ++ | ++ | o | o |
| CaCl2, 10 mM | o | ++ | o | o | o |
| CaCl2, 40 mM | o | ++ | + | o | o |
| CaCl2, 100 mM | o | ++ | ++ | o | o |
| MgSO4, 0.5 mM | o | + | o | o | o |
| MgSO4, 20 mM | o | ++ | + | o | o |
| MgSO4, 50 mM | o | ++ | ++ | o | o |
| (NH4)2SO4, 100 mM | o | o | ++ | o | o |
| Cu(II)SO4, 0.1 mMb | o | o | o | + | o |
| Cu(II)SO4, 0.25 mM | o | o | o | + | o |
| 4-HR, 10 mMb | o | o | o | + | o |
| Hydroquinone, 10 mMb | o | o | o | + | o |
| Degasser, in- and outflow measured | +a | o | + | o | o |
Symbols: o=no alleviation predicted; + =some alleviation predicted; ++ =strong alleviation predicted.
Experimental treatment accounts for, but does not alleviate, declines in kmax.
Treatment applied during vase rehydration and during measurement.
The reported conductivities include a temperature correction to 20 °C to allow for changes of water viscosity with temperature, except for ice water, because it was impossible to control the ice-water temperature carefully during measurements and the exact water temperature inside the stems was therefore unknown. Stems were then flushed under a pressure differential of 150 kPa for 3 min, after which FΔp was determined again. In one treatment, 3 kPa ‘flushes’ were used to test for effects of high-pressure versus low-pressure flushing. This was followed by another high-pressure flush for 3 min, followed by measurement of FΔp. In a few cases indicated in the results, this sequence was repeated five times to determine kmax stability. High-pressure flushing was used in this study because it is the most commonly used method for measuring kmax in stems (Table 2).
Table 2.
Methods used in representative studies of maximum hydraulic conductivity through stem and root samples
| Reference | Sample length (cm) | Measured inflow or outflow?a | Filtered and degassed?b | Measuring pressure (kPa)b | Flushing pressure and flushing timeb | Ionic composition |
| Sperry et al. (1987) | Various | Out | 0.22 μm filter, degassed5 | ≤105 | 170 kPa, repeated flushes to kmax | 10 mM NaCl, 0.05% formaldehyde |
| Sperry et al. (1988) | 10–15 | Out* | 0.22 μm filter, degassed5 | ≤10 | 175 kPa, 20–200 min | 10 mM NaCl, (1 mM NaCl+0.5 mM CaCl2+0.2 mM KCl), ‘maple sap’, 10 mM citric acid (pH 4), 10 mM citric acid (pH <3), formaldehyde (0.05%, 0.5%), gluteraldehyde (0.05%), 10 mM oxalic acid (pH 1.3–2.4) |
| Tyree and Yang (1992) | 25–75 | In and out | 0.2 and 0.1 μm filter | 2–14 | 150 kPa, both ends, up to 200 h | 10 mM oxalic acid |
| Sperry and Sullivan (1992) | 4–15 | Out* | 0.22 μm filter, degassed5 | 40 or 70 | 175 kPa, repeated flushes to kmax | 10 mM oxalic acid |
| Sperry and Saliendra (1994) | 15–25 | Out* | 0.2 μm filter, not degassed5 | 10 | 175 kPa, no time info | HCl, pH 2 |
| Jarbeau et al. (1995) | 10 | Out* | 0.1 μm filter, degassed | 3 | 175 kPa, 1 h | 10 mM citric acid |
| Pockman and Sperry (2000) | 10 | Out* | 0.22 μm filter, not degassed5 | ≤10 | 100 kPa, repeated flushes to kmax | HCl, pH 2 |
| Hacke et al. (2000) | 14 | Out* | 0.2 μm filter, not degassed4 | –7 to –10, –3 for roots | 50–70 kPa, 30 min, kmax at –0.5 MPa | Deionized water |
| Cochard et al. (2000) | 3–4 | In** | 0.2 μm filter, degassed | 3 | 100 kPa, no time info | Water |
| van Ieperen et al. (2000) | 20 | In† | ? | –40 | N/A | Deionized water, 0.01–200 mM KCl, 6.7 mM K2SO4, 10 mM NaCl, 67 mM CaCl2, 20 mM mannitol, 20 mM melizitose |
| Zwieniecki et al. (2001) | 3–6 | Out | 0.2 μm filter, not degassed6 | 40 | 200 kPa, 10 min | Deionized water, 0.1–100 mM KCl, 10 mM sucrose, 10 mM ethanol, 10 mM NaCl, 10 mM KNO2, 10 mM CaCl2, 10 to 95% ethanol |
| Bucci et al. (2003) | 10–15 | Out8 | 0.22 μm filter, degassed | 4.9 | 200 kPa, repeated 15 min flushes to kmax | Distilled water |
| Hukin et al. (2005) | 2 | In** | 0.2 μm filter, degassed2 | 1.5 | 150 kPa, no time info | 10 mM KCl |
| Jacobsen et al. (2005) | 10–27 | Out* | 0.1 μm filter, degassed1 | 1.5–3.51 | 100 kPa, 1 h, kmax at –0.5 MPa | HCl, pH 2 |
| Gascò et al. (2006) | 1–36 | In** | 0.1 μmm filter | 9 | 190 kPa, 10 min | Deionized water, 5–150 mM KCl, 200 mM sucrose, 100 mM NaCl |
| Maherali et al. (2006) | Stems 14, roots: 27–59 | Out* | 0.2 μm filter, not degassed3 | 1.5–2 | 100 kPa, 15–20 min | Distilled water |
| van Meeteren et al. (2006) | 22 | In† | Degassed and not degassed | –40 | –40 kPa 1.5 h, then 3.4–3 kPa 30 min | 0.7 mM CaCl2, 1.5 mM NaHCO3, 50 mM CuSO4 |
| van Ieperen and van Gelder (2006) | 7–13 | In† | ? | –20 | N/A | Ultrapure deionized water, 0.1 mM CaCl2, 1 mM CaCl2, 0.1 mM CaCl2+10 mM KCl, 1 mM CaCl2+10 mM KCl, 0.1 mM CaCl2+100 mM KCl, 1 mM CaCl2+100 mM KCl, 0.1–100 mM KCl, 0.1–10 mM CaCl2 |
| Nardini et al. (2007) | 6–16 | In** | 0.1 μm filter | 9 | 190 kPa, 10 min | Deionized water, 25 mM KCl, 0.5 mM CaCl2, 1 mM CaCl2, 25 mM KCl+0.5 mM CaCl2, 25 mM KCl+1 mM CaCl2, mineral water |
| Lovisolo et al. (2008) | Petioles 1, shoots 40, roots ? | In†† | 0.1 μm filter, degassed | Petioles 40; shoots 20; roots 10 | Petioles 600 kPa7; shoots 300 kPa7; roots 300 kPa7 | 15 mM KCl |
All studies were conducted at room temperature, except for Tyree and Yang (1992), which was conducted at 1–3 °C.
Apparatus used for hydraulic conductivity measurements: Sperry apparatus (Sperry et al., 1988), **XYL'EM apparatus (Instrutec, Montigny les Cormeilles, France; Cochard et al., 2000), †van Ieperen et al. (2000). ††Hydraulic conductance flow meter (model HCFM-XP, Dynamax, Houston, TX; Tyree et al., 1995).
1Anna Jacobsen, personal communication; 2Hervé Cochard, personal communication; 3Hafiz Maherali, personal communication; 4Uwe Hacke personal communication; 5John Sperry, personal communication; 6Maciej Zwieniecki, personal communication; 7Pressure gradually increased to the maximum listed, Claudio Lovisolo personal communication; 8Sandra Bucci, personal communication.
The following experimental protocols were used to test the five alternative hypotheses. Some treatments potentially affect more then one of the five mechanisms. See Table 1 for a comprehensive listing of treatments and their predicted effects on the five mechanisms.
Control treatments
The two control treatments included non-degassed, deionized water, and deionized water degassed as described above.
Non-steady-state flow
To test for lateral flow from vessels into adjacent cells and extracellular spaces, the outflow of the measurement solution was measured simultaneously with the inflow by combining the XYL'EM apparatus with a Sperry apparatus (Sperry et al., 1988). In this set-up, the XYL'EM apparatus provided the water supply under gravitational pressure for measurements and high pressure for flushing. The distal outflow end of the stem segments were connected with tubing to a reservoir placed on an electronic analytical balance (model Explorer Pro EP214DC, Ohaus, Pine Brook, NJ, USA), which was connected to a computer and recorded the outflow rate. Inflow and outflow rates were measured simultaneously at 5 s intervals. To calibrate measurements using the balance against measurements with the high-precision flowmeter (model LIQUI-FLOW L1, Bronkhorst, Montigny les Cormeilles, France) that is at the core of the XYL'EM apparatus, stem samples were replaced with a 0.45 μm syringe filter and 15 cm of luer PVC tubing, which was compressed with a clamp to generate flow rates similar to those observed in Encelia stems. Flow rates were measured the same way as in all other experiments, first at Δp=0 kPa, then at Δp=3 kPa, then at Δp=3 kPa after a first 3-min flush under Δp=150 kPa, and again at Δp=3 kPa after a second 3-min flush under Δp=150 kPa.
Comparisons of inflow and outflow rates for ten replicates of Encelia stems, Laurus stems, and tubing were conducted using separate paired, two-sided t tests for the four flow rates measured. Observed differences between the four measured inflow and outflow rates (ΔF=Fin–Fout) were also compared between Encelia stems, Laurus stems, and tubing using two-sided t tests. Test results were corrected for false discovery rate in multiple comparisons (Benjamini and Hochberg, 1995).
Swelling of pectin hydrogels in pit membranes
To reduce the chance that swelling pectin hydrogels cause a decline in ks in response to flushing, aqueous solutions of 10 mM KCl and 10 mM CaCl2, were used, which are hypothesized to prevent hydrogel swelling at these low concentrations and have been shown to increase flow rates through stems compared to deionized water (Zwieniecki et al., 2001; Gascó et al., 2006; van Ieperen and van Gelder, 2006). A solution of 0.5 mM MgSO4 was also tested, because Mg2+ ions are also known to prevent pectin swelling (Zsivánovits et al., 2005). Ice water was also used as a treatment, because it can cause pectin hydrogels to shrink and become more rigid by forming hydrogen bonds (Lootens et al., 2003; Kjøniksen et al., 2004).
Formation and coalescence of bubbles
To prevent bubble formation in the stem completely, deionized water was degassed using a membrane contactor (Liqui-Cel mini-module 1.7×5.5, Membrana, Charlotte, NC, USA) in line between the external water supply and the XYL'EM apparatus. The mini-module was used in vacuum mode under 3 kPa absolute pressure, generated using a vacuum diaphragm pump (model DAA-V715A-EB, Gast, Benton Harbor, MI, USA). In addition, solutions were created that contained ionic solutes at concentrations that have been found to prevent the coalescence of small bubbles into larger ones (Craig et al., 1993). Solutions of 50 mM MgSO4, 100 mM CaCl2, 300 mM KCl, and 100 mM (NH4)2SO4 were used because they completely inhibit bubble coalescence, while solutions of 20 mM MgSO4, 40 mM CaCl2, and 100 mM KCl were used because they prevent about 50% of bubble coalescence (Craig et al., 1993). Solutions of 0.5 mM MgSO4, 10 mM CaCl2, and 10 mM KCl used to test for pectin swelling are not expected to inhibit bubble coalescence (Craig et al., 1993). Di-ammonium sulphate (NH4)2SO4 at 100 mM is expected to inhibit bubble coalescence completely (Craig et al., 1993), while only having minor osmotic effects on pectin swelling. Ice water was also used as a treatment, because it can reduce bubble coalescence in deionized water (Ribeiro and Mewes, 2006).
Active wounding responses
To slow enzyme activities at low water temperatures, branches were both rehydrated and measured in ice water for one treatment and rehydrated at room temperature, but measured in ice water for another treatment. The latter treatment was expected only to affect immediate wounding responses to cutting stems to 15 cm in length just before taking the measurements. In addition, three chemical treatments were applied that previously had been found to reduce xylem blockage and increase the water uptake into cut plant stems, including treatment with 0.25 mM Cu(II)SO4 (Loubaud and van Doorn, 2004) during measurement or 0.10 mM Cu(II)SO4 (Vaslier and van Doorn, 2003) during prehydration and measurement, 10 mM hydroquinone (Loubaud and van Doorn, 2004) during prehydration and measurement, and 10 mM 4-hexylresorcinol (4-HR) (Vaslier and van Doorn, 2003; He et al., 2006) during prehydration and measurement. Ice water as a measuring solution also was expected to slow enzyme activity and thereby inhibit active wound responses.
Passive wounding effects
The only treatment directly to address passive wounding responses was to remove the bark of stems before cutting them in order to avoid the transfer of resins from the bark to the cut surface, especially in the resin-rich Encelia.
Rehydration of partially embolized stem sections
Because most woody plant stems under natural conditions will at least be partially embolized and because flushing of stems under high pressure is time-consuming (Tyree and Yang, 1992; Yang and Tyree, 1992) and can cause a decline in ks, a prehydration treatment under vacuum (Sellin, 1991; Hietz et al., 2008) was tested to determine whether it could result in stable measurements of kmax. Ten stem segments (15 cm long) each of Encelia and Laurus were placed on a laboratory bench for 24 h, as previous studies (data not shown) had found that this treatment would induce a high degree of embolism. They were then infiltrated with deionized water under vacuum for 24 h and hydraulic conductance measured as described above.
Data analysis
The effect of flushing time and flushing pressure on specific hydraulic conductance, ks, through stems of Encelia farinosa and Laurus nobilis were tested using repeated measures ANOVA using SYSTAT (version 12.02, SYSTAT Inc., San Jose, CA, USA). Because the experiments included measurements of kmax spread out over 24 dates during a period of 8 months, for temporal effects on kmax were tested by linearly regressing the treatment means of kmax against date separately for each species using SYSTAT. To test for serial correlation between measurement dates, Durbin–Watson D statistics, calculated by SYSTAT for these regressions, were compared to critical values given in Savin and White (1977). ANOVA was used to analyse kmax as a function of species, treatments, and species×treatment interactions using SYSTAT. To identify kmax values that were significantly higher or lower than the respective overall mean kmax value for the species calculated across 22 separate experiments, post hoc tests were conducted for each species×treatment combination to test the null hypothesis that the treatment mean was equal to the overall species mean. Resulting p-values were corrected for false discovery rate in multiple comparisons (Benjamini and Hochberg, 1995). Responses of ks to repeated high-pressure flushing were characterized by calculating the mean percent change in ks after each 3 min flush for each treatment. These rates of change were analysed by ANOVA in SYSTAT with species, treatments, and species×treatment interactions as effects. To test the null hypothesis of no change in ks, post hoc tests were conducted for each species×treatment combination. Resulting p-values were corrected for false discovery rate in multiple comparisons (Benjamini and Hochberg, 1995).
Results
The overnight vase rehydration treatment used to refill gas-filled vessels worked very well in Encelia, resulting in a specific hydraulic conductivity, ks [3.29±0.55 (standard error) kg m−1 s−1 MPa−1] that was not statistically different (P=0.18) from that measured in stems harvested at predawn from the same plants after they had been well-watered (4.13±0.25 kg m−1 s−1 MPa−1). In Laurus, vase rehydration was less successful, as rehydrated stems had a lower ks (1.28±0.15 kg m−1 s−1 MPa−1) than stems from the same tree after thorough watering (1.83±0.26 kg m−1 s−1 MPa−1). The difference, which was not statistically significant (p=0.08), was alleviated by rehydrating the stems under vacuum for 20 h, which resulted in a ks of 2.40±0.44 and 1.83±0.26 kg m−1 s−1 MPa−1, which was similar (p=0.27) to that observed after thorough watering.
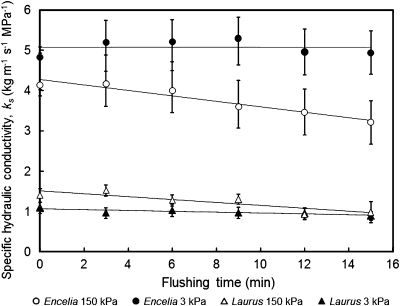
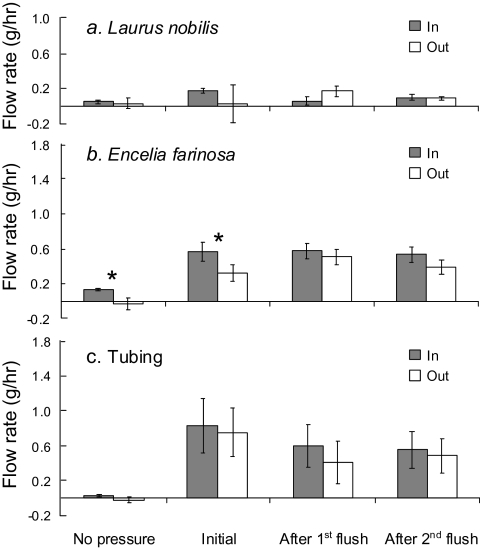
Consecutive 150 kPa flushes with deionized, non-degassed water resulted in significant declines in ks over the 15 min flushing period in both species, while 15 min of low pressure flushing at 3 kPa did not cause such a decline (Fig. 1). No significant differences were observed between inflow rates measured with the XYL'EM apparatus and outflow rates measured with a Sperry apparatus for the control (PVC tubing) and Laurus nobilis (Fig. 2a, c). Inflow rates for Encelia farinosa before flushing under high pressure were slightly higher than outflow rates (p <0.05; Fig. 2b), but the difference disappeared after flushing, and none of the differences between inflow and outflow for Laurus or Encelia were significantly different from those observed for the tubing control.
Fig. 1.
Effect of flushing time and flushing pressure on specific hydraulic conductance, ks, through stems of Encelia farinosa and Laurus nobilis when flushing with deionized, non-degassed water. Effects of species, pressure, time, and all their interactions on ks were tested with repeated measures ANOVA (Species: Sum of Squares (SS) 639.19, degrees of freedom (df) 1, F-ratio (F) 58.982, p-value (p) <0.001; pressure: SS 16.17, df 1, F 1.492, p 0.230; species×pressure: SS 35.68, df 1, F 3.292, p 0.078; time: SS 6.43, df 5, F 7.832, p <0.0001; time×species: SS 0.74, df 5, F 0.899, p 0.48313; time×pressure SS 3.486, df 5, F 4.247, p 0.001; time×species×pressure: SS 1.542, df 5, F 1.878, p 0.100). Slopes of linear trend-lines shown in the graph were significantly different from zero only in the 150 kPa treatments for both species.
Fig. 2.
Comparison of inflow rates into plant stems of Encelia farinosa, Laurus nobilis, and a tubing control, measured with a XYL'EM apparatus (Cochard et al., 2000) and simultaneous outflow rates from these samples measured with a Sperry apparatus (Sperry et al. 1988). Significant differences (p <0.05) between inflow and outflow rates are designated by an asterisk.
The overall mean kmax across 20 experiments (excluding the two ice-water treatments) for Encelia was 2.75±0.20 kg m−1 s−1 MPa−1 and for Laurus was 1.24±0.10 kg m−1 s−1 MPa−1. Means for individual experiments varied from 1.40 to 4.70 kg m−1 s−1 MPa−1 for Encelia and from 0.52 to 2.61 kg m−1 s−1 MPa−1 for Laurus. Small rates of inflow into well-hydrated stems without any driving pressure differential were observed for all treatments. No positive serial correlations among kmax measurements conducted over an 8-month period were found for either species (data not shown), suggesting that treatment effects on kmax masked any seasonal variation that may have existed. This was also evident by significant differences between treatments conducted within just a few days of each other (data not shown). For Encelia, four treatment means were significantly (p <0.05) higher than the overall species mean, including 10 mM KCl, 10 mM CaCl2, 50 mM MgSO4, and prehydration under vacuum and using a degasser at 3 kPa. Hydration and measurement in ice water, 100 mM KCl, 100 mM (NH4)2SO4, and 0.25 mM Cu(II)SO4 in the measurement solution resulted in kmax values that were significantly lower (p <0.05) than the overall mean. For Laurus, the only treatment that differed significantly from the overall mean kmax was an exceptionally high kmax measured with 0.25 mM Cu(II)SO4 solution.
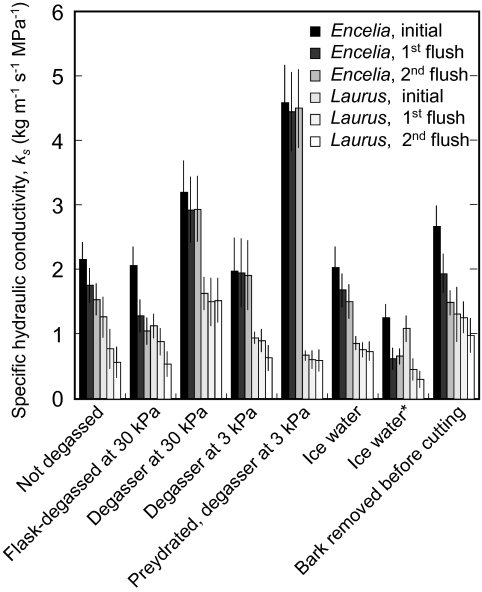
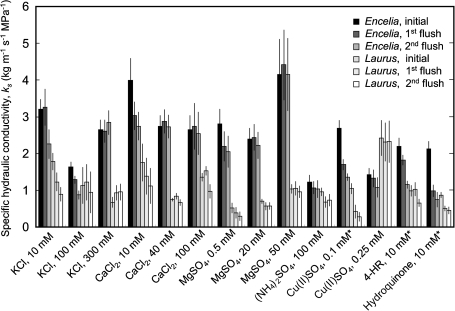
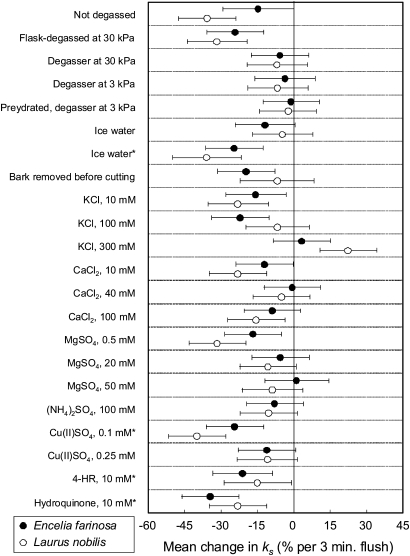
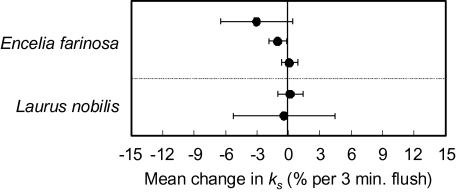
Most treatments, including the two control treatments, resulted in declines of ks after flushing at high pressure (Figs 3, 4), with slopes of decline varying from 0% to –41.4% min−1 (Fig. 5). The only significant increase in ks was observed for treatment with 300 mM KCl for Laurus (Fig. 5). Overall treatment effects on rates of decline in ks were significant, but differences between the two species were not, and the species responded similarly to the 22 experimental treatments (Fig. 5), as there was no significant species×treatment interaction. All treatments that were expected to alleviate declines of ks due to prevention of bubble formation or coalescence (Table 1) resulted in rates of change in ks that were not significantly different from zero, with the exception of 100 mM CaCl2 in Laurus. Most treatments predicted to cause at least some alleviation of decline in ks (Table 1), through the prevention of bubble formation or coalescence, also had the predicted effect, except, notably, for measurement with flask-degassed, deionized water and for treatment with ice water during prehydration and measurement for both species. Those treatments predicted to prevent a decline in ks by reducing the swelling of pectins in pit membranes (Table 1), but not by reducing bubble coalescence, such as 10 mM KCl, 10 mM CaCl2, or 0.5 mM MgSO4, did not prevent a decline in ks, but a treatment that strongly affected bubble coalescence but not pectin swelling, 100 mM (NH4)2SO4, did (Fig. 5). Treatments applied to reduce active wounding responses did not prevent a decline of ks, except for 0.25 mM Cu(II)SO4 in the measurement solution. Removing the bark before cutting the stems under water did not prevent a decline in ks, in Encelia, but produced what appeared to be an experimental artefact in Laurus, in which bark removal was difficult and required substantial handling of the stems. This resulted in low initial flow rates that increased after the first flush and then decreased after the second flush, causing an overall change in ks that was not significantly different from zero. Prehydration for 24 h under 3 kPa vacuum resulted in stable measurements of ks, whether the stems were well hydrated or embolized (Fig. 6).
Fig. 3.
Effects of physical treatments on the specific hydraulic conductivity, ks, of fully hydrated Encelia and Laurus stems before high-pressure flushing (initial), after the first 3-min high-pressure flush, and after a second 3-min high-pressure flush. *Treatment applied during vase rehydration and during measurement.
Fig. 4.
Effects of chemical treatments on the specific hydraulic conductivity, ks, of fully hydrated Encelia and Laurus stems before high-pressure flushing (initial), after the first 3-min high-pressure flush, and after a second 3-min high-pressure flush. *Treatment applied during vase rehydration and during measurement.
Fig. 5.
Effects of treatments on the change in specific hydraulic conductivity ks (±95% confidence intervals) of fully hydrated Encelia and Laurus stems in response to high-pressure flushing. Values with 95% confidence intervals overlapping the zero-line are not significantly different from zero. Overall effects of treatments on change in ks were highly significant (sum of squares, SS=5,638.4, degrees of freedom, df=21, F-ratio=6.765, p <0.001), but differences in rates of change in ks between species were not (SS=59.457, df=1, F-ratio=1.498, p=0.222), nor were species×treatment interactions (SS=1,215.037, df=21, F-ratio=1.458, p=0.089).
Fig. 6.
Effects of 24 h prehydration of submerged woody plant stems under 3 kPa (absolute pressure) vacuum on the change in specific hydraulic conductivity ks (±95% confidence intervals) in response to repeated high pressure flushing. Values with 95% confidence intervals overlapping the zero-line are not significantly different from zero. Multiple points for Laurus and Encelia stand for separate experiments.
Discussion
Stems of Laurus nobilis proved to be very difficult to rehydrate. Most flushing treatments did not result in specific hydraulic conductivities, ks, as high as those measured in stems taken at predawn after thorough watering over two nights. The failure of vase rehydration was most probably due to stomatal closure, observed for leaves of cut branches (data not shown), and rehydrating the stems under vacuum for 20 h restored ks to the levels seen in well-watered plants. This suggests that vase storage overnight did not induce rapid gel or tylose formation in vessels. Encelia farinosa branches rehydrated well in the vase, and their stomata did not close. Thus, for Laurus, measurements of ks probably remained below kmax in this study, as Laurus stems remained partially embolized. This does not affect the conclusions to be drawn from this study, which addressed the problem of declines in ks rather than the question of how to achieve true measurements of kmax, but it raises the question of how reliable measurements of kmax can be achieved in different species. Our results show that flushing stems under high pressure can be problematic. Rehydration under vacuum (Hietz et al., 2008) may affect xylem structure and function, so it may not be suitable for all studies.
Our comparison of methods between a XYL'EM apparatus (Cochard et al., 2000) that measures inflow into a stem and a Sperry apparatus (Sperry et al., 1988) that measures outflow from a stem showed no significant differences between flow rates, suggesting that both methods lead to similar measurements of maximum hydraulic conductivity (kmax). Initial inflow into Encelia stems slightly exceeded outflow, but did not significantly exceed inflow and outflow comparisons for a tubing control. Observations of higher inflow than outflow had been previously reported by Tyree and Yang (1992). As the differences observed in this study were minor, it appears that there is little concern about potential unpredictable secondary responses to lateral water flow, such as the movement of ions into vessels, that may affect hydraulic conductance (van Ieperen, 2007). Interestingly, inflow into well-hydrated stems was consistently observed, even without any driving pressure differential. Interestingly, this no-pressure flow declined to zero in Laurus after repeated flushes, but not in Encelia (data not shown). Initial flow through stems without a driving pressure differential is commonly observed in plant hydraulics studies (John Sperry, personal communication) and is routinely deducted from measured flow rates under pressure. The mechanisms responsible for such no-pressure flow would certainly seem to warrant some attention.
Declines in hydraulic conductance have been attributed to the swelling of pectin hydrogels in pit membranes as Ca2+ ions are flushed from the pectins by deionized water (Zwieniecki et al., 2001). Cations, such as K+, Na+, and Ca2+, can reduce hydration of pectin gels by associating with negatively-charged galacturonic acid groups (Zwieniecki et al., 2001; van Ieperen, 2007). In addition, Ca2+ ions crosslink demthylesterized pectin molecules (Jarvis, 1984; Willats et al., 2001) and thereby increase gel rigidity and decrease hydration. Mg2+ ions appear to have a similar effect (Zsivánovits et al., 2005). In our experiments, low concentrations of K+, Mg2+, and Ca2+ salts caused high values of kmax in Encelia, but did not prevent decline in ks in either species. Observed alleviations of declines at high salt concentrations were probably due to the property of these salts to inhibit bubble coalescence almost completely at these higher concentrations (Marrucci and Nicodemo, 1967; Lessard and Zieminski, 1971; Craig et al., 1993; Zahradník et al., 1995). This interpretation is strongly supported by the finding that (NH4)2SO4, a very effective bubble coalescence inhibitor (Craig et al., 1993), alleviated the decline in ks, even though ammonium ions are unlikely to affect pectin hydration. The stem segments used in this study were shorter than the maximum vessel length, which means that at least some water could pass through open vessels without crossing pit membranes. Other studies conducted to date to test the pectin hydrogel hypotheses have used stem lengths ranging from 3 cm to 16 cm (Zwieniecki et al., 2001; Boyce et al., 2004; Nardini et al., 2007), with only one study examining stem lengths of up to 36 cm (Gascó et al., 2006). Flow through open vessels did not obscure membrane effects in any of these studies.
Four chemical and two physical treatments that were predicted to alleviate declines in ks by preventing either the formation or coalescence of gas bubbles in the xylem all had the predicted effect, providing very strong support for the conclusion that declines in ks are caused by the blockage of vessels by bubbles. Similarly, cut flowers have been found to take up degassed water more rapidly than non-degassed water (van Ieperen et al., 2002; van Meeteren et al., 2006). Our findings confirm the conclusions of Kelso et al. (1963) and Canny et al. (2007), who proposed that a thorough degassing of the measurement solution is essential for stable and precise measurements of hydraulic conductivity. Our findings show that thorough degassing, as provided by the membrane contactors used in this study, is needed to obtain stable kmax. It is important to note that the common degassing method of stirring water under laboratory vacuum was not sufficient to prevent a decline of kmax (Fig. 5).
Our findings of bubbles as the main cause for declining hydraulic conductivity through woody stems while under positive pressure raise the question whether similar effects occur in the transpiration stream under natural conditions, as proposed by Canny et al. (2007). This could indeed be the case if xylem sap is air-saturated, as normally assumed (Lybeck, 1959; Hammel, 1967; Sperry and Sullivan, 1992; Yang and Tyree, 1992; McCully et al., 2000; Cobb et al., 2007). Passage of air-saturated water through pit membranes could cause the formation of bubbles through heterogeneous nucleation, followed by bubble coalescence, and the air blockage of vessels. It should be noted, however, that contrary to the suggestion by Canny et al. (2007), this would not occur due to the water pressure drop that occurs as water flows through constrictions, because decreasing water pressure actually increases air solubility (Mercury and Tardy, 2001; Mercury, 2006). The familiar gassing-out of water under vacuum only occurs when water is in contact with a gas phase under partial vacuum (Mercury et al., 2003), which is not the case in xylem because it is in contact with a gas phase under atmospheric pressure. Gassing-out of xylem sap could still occur due to heterogeneous nucleation or in response to lower air solubility with rising temperatures as the xylem sap emerges from cool roots into warmer stems and branches.
Physiological wounding responses are a concern in hydraulic studies of cut plant samples. Responses to wounding could be induced by a rapid increase in water potential, which sets off hydraulic and/or electrical signals (Stahlberg and Cosgrove, 1995; Stahlberg et al., 2005; Fromm and Lautner, 2007). Cell-wall proteins, some of which reside in pit membranes (Harrak et al., 1999), can react within minutes to wounding (Bradley et al., 1992). Experiments with cut flowers have shown that physiological wounding responses can be reduced by keeping stems at low temperatures (van Meeteren, 1992; van Doorn and Cruz, 2000). In this study, measurement in deionized water at 4–6 °C reduced the decline in ks, but this effect may have been due to several causes, including increasing air solubility that reduced bubble formation, reduced bubble coalescence (Ribeiro and Mewes, 2006), shrinking of pectin hydrogels (Kjøniksen et al., 2004; Lootens et al., 2003), or reduced physiological activity.
A more specific inhibition of active wounding responses was expected from chemical treatments that had been found previously to reduce active wounding responses, reduce xylem blockage, and increase water uptake into cut plant stems (van Meeteren et al., 2000; van Doorn and Vaslier, 2002; Vaslier and van Doorn, 2003; Loubaud and van Doorn, 2004; He et al., 2006). In this study, Cu2+ ions, applied at a concentration of 0.25 mM for measurement and flushing, reduced the decline in hydraulic conductance, as found in previous studies (van Doorn and Vaslier, 2002; Vaslier and van Doorn, 2003; Loubaud and van Doorn, 2004). Copper is an essential micronutrient, is toxic at super-optimal levels, and plays a wide variety of roles in plant biochemistry (Maksymiec, 1997). Its functions in xylem may include effects on peroxidases, which are among the most common enzymes in xylem sap (Buhtz et al., 2004; Kehr et al., 2005), and whose activities Cu2+ ions have been found to increase (Chen et al., 2002) or inhibit (Zancani et al., 1995), probably depending on the nature of specific peroxidases. Cu2+ ions also bind strongly to cell wall pectins (Dronnet et al., 1996; Wehr et al., 2004) and may thereby inhibit enzymatic attack (Wehr et al., 2004) and/or contribute to cell wall loosening by causing non-enzymatic scission of other wall polysaccharides (Fry et al., 2002). In our study, 0.25 mM Cu(II)SO4 caused exceptionally high values of kmax in Laurus and exceptionally low values of kmax in Encelia, which means that this is probably not a treatment to be recommended for a standardized protocol of kmax measurements.
The other two chemicals applied to reduce active wounding responses, hydroquinone and 4-hexylresorcinol (4-HR), had no effect on preventing the decline in kmax. These chemicals were chosen because they have been found to reduce xylem blockage and delay wilting in cut flowers (van Doorn and Vaslier, 2002; Vaslier and van Doorn, 2003; Loubaud and van Doorn, 2004). Hydroquinone, a peroxidase substrate (Bagirova et al., 2001) and inhibitor (Martinez et al., 2001) is a powerful phytotoxin at the concentration used in this study (10 mM) that strongly affects cell membrane integrity (Pandey et al., 2005). The polyphenol oxidase inhibitor 4-hexylresorcinol (Dawley and Flurkey, 1993) had no effect in our study. Polyphenol oxidases play roles in lignifying xylem and in wounding responses (Richardson et al., 2000; Mayer, 2006). They do not appear to occur normally in mature xylem (Buhtz et al., 2004; Kehr et al., 2005), so it is not clear how they could affect water uptake through cut stems.
Cutting of plant stems could decrease hydraulic conductivity by clogging of vessels with gums, resins, or debris from the xylem and the bark. To reduce the transfer of substances from the bark to the xylem, bark was removed from submerged stems before cutting them. This treatment had no effect on alleviating the decline of ks in Encelia, and produced experimental artefacts in Laurus that lead to inconclusive results. Examination of stems for evidence of debris in the xylem was initially planned for this study, but the plans were dropped as too labour-intensive and unnecessary after it was found that the declines in ks could be alleviated with methods that affect bubble formation and coalescence.
Arguing that a lack of method standardization in plant hydraulics could negatively affect progress in the field, Pratt et al. (2008) recently called for a common toolbox of methods. A comparison of methods used by different plant hydraulics research laboratories to measure kmax through stems (Table 2) shows that methods vary widely in the length of samples, pressure differentials and flushing times, chemical composition of measurements solutions, and in whether or not solutions are degassed. As a result, measurements of kmax and the percentage loss of conductance (PLC) in xylem vulnerability curves from different published studies are strictly comparable only within laboratories and among collaborating researchers. Two conclusions from this study would seem to merit incorporation into future protocols to measure kmax: (i) Thorough degassing of measurement solutions using a degassing unit, such as the Liqui-Cel mini-module used in this study, and complete air removal from the measuring apparatus. (ii) Hydration of submerged samples under vacuum instead of flushing at high pressure. The latter is recommended based on the findings of this study for angiosperm stems, based on a previous study of conifer stems (Hietz et al., 2008), and, most importantly, based on theoretical considerations and measurements which showed that complete air removal from a severely embolized stems by high-pressure flushing takes many hours (Yang and Tyree, 1992). Eliminating high-pressure flushing from protocols to measure native embolisms and kmax has the added advantage of allowing increased sample sizes by shifting measurements of kmax to the next day after the samples have been incubated for 20–24 h under vacuum. Subsequent to the experiments described in this paper, it was found that vacuum hydration lead to stable measurements of kmax for eight out of ten species tested, with relatively small declines for the remaining two species (1–3% min−1; data not shown). A detailed protocol for measurements of native embolisms and kmax,, especially for XYL'EM users, is available from the authors upon request.
Vacuum hydration was found to lead to stable measurements of kmax in this study, but further comparative research on the effects of vacuum versus high pressure infiltration of angiosperm wood seems warranted. Both techniques could cause artefacts involving non-conducting xylem cells and could potentially damage xylem structures. Several hours of flushing with thoroughly degassed water under very low pressure may be the optimal solution. Another technique that reduces the chance of bubble formation during flushing is to measure and flush very short stem segments (2–4 cm). This technique used by Hervé Cochard and colleagues (e.g. Hukin et al., 2005) greatly reduces the number of vessel endings at which bubbles could form or get entrapped within a stem segment. By allowing water to flow through a stem without crossing vessel ends, this technique would tend to overestimate kmax, but this may be of little concern for measurements of PLC within a species.
Obviously, the choice of methods used for measuring kmax will depend on the study system and the questions asked. Adding chemicals to the measurement solution provided no clear advantage in this study over the use of thoroughly degassed, deionized water, which is why the latter is suggested as the easiest choice for a standard solution. Responses of kmax to salts vary widely between species and seasonally (Gascó et al., 2007; Trifilò et al., 2008), which means that there is no recipe for a chemical composition of the measuring solution that could be a standard choice for all studies. Deionized and thoroughly degassed water therefore appears to be the most parsimonious choice for a standard measurement solution.
Acknowledgments
We thank Judy Quang, Tracy Vo, and Daisha Ortega for help with measurements, Ernesto Cassillas, Vickie Nguyen, and Matthew Sutton for help with sampling. Anna Jacobsen, Sandra Bucci, Hervé Cochard, Hafiz Maherali, Uwe Hacke, John Sperry, Maciej Zwieniecki, Claudio Lovisolo, and Mel Tyree generously shared details about their experimental protocols summarized in Table 2. Comments from three anonymous reviewers greatly helped to improve the manuscript.
This work was supported by the Andrew W Mellon Foundation and the National Science Foundation (IOS-0641765).
References
- Alder NN, Pockman WT, Sperry JS, Nuismer SM. Use of centrifugal force in the study of xylem cavitation. Journal of Experimental Botany. 1997;48:665–674. [Google Scholar]
- Bagirova NA, Shekhovtsova TN, van Huystee RB. Enzymatic determination of phenols using peanut peroxidase. Talanta. 2001;55:1151–1164. doi: 10.1016/s0039-9140(01)00544-6. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B. 1995;57:289–300. [Google Scholar]
- Blatteau J-E, Souraud J-B, Gempp E, Boussuges A. Gas nuclei, their origin, and their role in bubble formation. Aviation, Space, and Environmental Medicine. 2006;77:1068–1076. [PubMed] [Google Scholar]
- Bonsen KJM, Kučera LJ. Vessel occlusions in plants - morphological, functional and evolutionary aspects. Iawa Bulletin. 1990;11:393–399. [Google Scholar]
- Boyce CK, Zwieniecki MA, Cody GD, Jacobsen C, Wirick S, Knoll AH, Holbrook NM. Evolution of xylem lignification and hydrogel transport regulation. Proceedings of the National Academy of Sciences (USA) 2004;101:17555–17558. doi: 10.1073/pnas.0408024101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley DJ, Kjellbom P, Lamb CJ. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: A novel, rapid defense response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Bucci SJ, Scholz FG, Goldstein G, Meinzer FC, Sternberg LDSL. Dynamic changes in hydraulic conductivity in petioles of two savanna tree species: factors and mechanisms contributing to the refilling of embolized vessels. Plant, Cell and Environment. 2003;26:1633–1645. [Google Scholar]
- Buhtz A, Kolasa A, Arlt K, Walz C, Kehr J. Xylem sap protein composition is conserved among different plant species. Planta. 2004;219:610–618. doi: 10.1007/s00425-004-1259-9. [DOI] [PubMed] [Google Scholar]
- Canny MJ, Sparks JP, Huang CX, Roderick ML. Air embolisms exsolving in the transpiration water: the effect of constrictions in the xylem pipes. Functional Plant Biology. 2007;34:95–111. doi: 10.1071/FP06210. [DOI] [PubMed] [Google Scholar]
- Chen EL, Chen YA, Chen LM, Liu ZH. Effect of copper on peroxidase activity and lignin content in Raphanus sativus. Plant Physiology and Biochemistry. 2002;40:439–444. [Google Scholar]
- Cheong YH, Chang HS, Gupta R, Wang X, Zhu T, Luan S. Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiology. 2002;129:661–677. doi: 10.1104/pp.002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb AR, Choat B, Holbrook NM. Dynamics of freeze–thaw embolism in Smilax rotundifolia (Smilacaceae) American Journal of Botany. 2007;94:640–649. doi: 10.3732/ajb.94.4.640. [DOI] [PubMed] [Google Scholar]
- Cochard H, Bodet C, Améglio T, Cruiziat P. Cryo-scanning electron microscopy observations of vessel content during transpiration in walnut petioles. Facts or artifacts? Plant Physiology. 2000;124:1191–1202. doi: 10.1104/pp.124.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Herbette S, Hernández E, Hölttä T, Mencuccini M. The effects of sap ionic composition on xylem vulnerability to cavitation. Journal of Experimental Botany. 2010;61:275–285. doi: 10.1093/jxb/erp298. [DOI] [PubMed] [Google Scholar]
- Cochard H, Tyree MT. Xylem dysfunction in Quercus: vessel sizes, tyloses, cavitation and seasonal changes in embolism. Tree Physiology. 1990;6:393–407. doi: 10.1093/treephys/6.4.393. [DOI] [PubMed] [Google Scholar]
- Craig VSJ, Ninham BW, Pashley RM. The effect of electrolytes on bubble coalescence in water. Journal of Physical Chemistry. 1993;97:10192–10197. [Google Scholar]
- Crews LJ, McCully ME, Canny MJ. Mucilage production by wounded xylem tissue of maize roots – time course and stimulus. Functional Plant Biology. 2003;30:755–766. doi: 10.1071/FP03052. [DOI] [PubMed] [Google Scholar]
- Dawley RM, Flurkey WH. Differentiation of tyrosinase and laccase using 4-hexyl-resorcinol, a tyrosinase inhibitor. Phytochemistry. 1993;33:281–284. [Google Scholar]
- Dronnet VM, Renard CMGC, Axelos MAV, Thibault JF. Characterisation and selectivity of divalent metal ions binding by citrus and sugar-beet pectins. Carbohydrate Polymers. 1996;30:253–263. [Google Scholar]
- Fromm J, Lautner S. Electrical signals and their physiological significance in plants. Plant, Cell and Environment. 2007;30:249–257. doi: 10.1111/j.1365-3040.2006.01614.x. [DOI] [PubMed] [Google Scholar]
- Fry SC, Miller JG, Dumville JC. A proposed role for copper ions in cell wall loosening. Plant and Soil. 2002;247:57–67. [Google Scholar]
- Gascó A, Nardini A, Gortan E, Salleo S. Ion-mediated increase in the hydraulic conductivity of Laurel stems: role of pits and consequences for the impact of cavitation on water transport. Plant, Cell and Environment. 2006;29:1946–1955. doi: 10.1111/j.1365-3040.2006.01570.x. [DOI] [PubMed] [Google Scholar]
- Gascó A, Salleo S, Gortan E, Nardini A. Seasonal changes in the ion-mediated increase of xylem hydraulic conductivity in stems of three evergreens: any functional role? Physiologia Plantarum. 2007;129:597–606. [Google Scholar]
- Hacke UG, Sperry JS. Limits to xylem refilling under negative pressure in Laurus nobilis and Acer negundo. Plant, Cell and Environment. 2003;26:303–311. [Google Scholar]
- Hacke UG, Sperry JS, Pittermann J. Drought experience and cavitation resistance in six shrubs from the Great Basin, Utah. Basic and Applied Ecology. 2000;1:31–41. [Google Scholar]
- Hammel HT. Freezing of xylem sap without cavitation. Plant Physiology. 1967;42:55–66. doi: 10.1104/pp.42.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrak H, Chamberland H, Plante M, Bellemare C, Lafontaine JG, Tabaeizadeh Z. A proline-, threonine-, and glycine-rich protein down-regulated by drought is localized in the cell wall of xylem elements. Plant Physiology. 1999;121:557–564. doi: 10.1104/pp.121.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Joyce DC, Irving DE, Faragher JD. Stem end blockage in cut Grevillea 'Crimson Yul-lo' inflorescences. Postharvest Biology and Technology. 2006;41:78–84. [Google Scholar]
- Hietz P, Rosner S, Sorz J, Mayr S. Comparison of methods to quantify loss of hydraulic conductivity in Norway spruce. Annals of Forest Science. 2008;65:502. [Google Scholar]
- Hukin D, Cochard H, Dreyer E, Thiec DL, Bogeat-Triboulot MB. Cavitation vulnerability in roots and shoots: does Populus euphratica Oliv., a poplar from arid areas of Central Asia, differ from other poplar species? Journal of Experimental Botany. 2005;56:2003–2010. doi: 10.1093/jxb/eri198. [DOI] [PubMed] [Google Scholar]
- Jacobsen AL, Ewers FW, Pratt RB, Paddock WA, III, Davis SD. Do xylem fibers affect vessel cavitation resistance? Plant Physiology. 2005;139:546–556. doi: 10.1104/pp.104.058404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbeau JA, Ewers FW, Davis SD. The mechanism of water stress-induced embolism in two species of chaparral shrubs. Plant, Cell and Environment. 1995;18:189–196. [Google Scholar]
- Jarvis MC. Structure and properties of pectin gels in plant cell walls. Plant, Cell and Environment. 1984;7:153–164. [Google Scholar]
- Kehr J, Buhtz A, Giavalisco P. Analysis of xylem sap proteins from Brassica napus. BMC Plant Biology. 2005;5:11. doi: 10.1186/1471-2229-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso WC, Jr, Gertjejansen RO, Hossfeld RL. The effect of air blockage upon the permeability of wood to liquids. University of Minnesota Agricultural Experiment Station Technical Bulletin. 1963;242:1–40. [Google Scholar]
- Kjøniksen A-L, Hiorth M, Nyström B. Temperature-induced association and gelation of aqueous solutions of pectin. A dynamic light scattering study. European Polymer Journal. 2004;40:2427–2435. [Google Scholar]
- Lessard RR, Zieminski SA. Bubble coalescence and gas transfer in aqueous electrolytic solutions. Industrial and Engineering Chemistry Fundamentals. 1971;10:260–269. [Google Scholar]
- Li R, Rimmer R, Yu M, Sharpe AG, Séguin-Swartz G, Lydiate D, Hegedus DD. Two Brassica napus polygalacturonase inhibitory protein genes are expressed at different levels in response to biotic and abiotic stresses. Planta. 2003;217:299–308. doi: 10.1007/s00425-003-0988-5. [DOI] [PubMed] [Google Scholar]
- Loepfe L, Martienez-Vilalta J, Piñol J, Mencuccini M. The relevance of xylem network structure for plant hydraulic efficiency and safety. Journal of Theoretical Biology. 2007;247:788–803. doi: 10.1016/j.jtbi.2007.03.036. [DOI] [PubMed] [Google Scholar]
- Lootens D, Capel F, Durand D, Nicolai T, Boulenguer P, Langendorff V. Influence of pH, Ca concentration, temperature and amidation on the gelation of low methoxyl pectin. Food Hydrocolloids. 2003;17:237–244. [Google Scholar]
- Lopez-Portillo J, Ewers FW, Angeles G. Sap salinity effects on xylem conductivity in two mangrove species. Plant, Cell and Environment. 2005;28:1285–1292. [Google Scholar]
- Loubaud M, van Doorn WG. Wound-induced and bacteria-induced xylem blockage in roses, Astilbe, and Viburnum. Postharvest Biology and Technology. 2004;32:281–288. [Google Scholar]
- Lovisolo C, Perrone I, Hartung W, Schubert A. An abscisic acid-related reduced transpiration promotes gradual embolism repair when grapevines are rehydrated after drought. New Phytologist. 2008;180:642–651. doi: 10.1111/j.1469-8137.2008.02592.x. [DOI] [PubMed] [Google Scholar]
- Lybeck BR. Winter freezing in relation to the rise of sap in tall trees. Plant Physiology. 1959;34:482–486. doi: 10.1104/pp.34.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali H, Moura CF, Caldeira MC, Willson CJ, Jackson RB. Functional coordination between leaf gas exchange and vulnerability to xylem cavitation in temperate forest trees. Plant, Cell and Environment. 2006;29:571–583. doi: 10.1111/j.1365-3040.2005.01433.x. [DOI] [PubMed] [Google Scholar]
- Maherali H, Pockman WT, Jackson RB. Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology. 2004;85:2184–2199. [Google Scholar]
- Maksymiec W. Effect of copper on cellular processes in higher plants. Photosynthetica. 1997;34:321–342. [Google Scholar]
- Marrucci G, Nicodemo L. Coalescence of gas bubbles in aqueous solutions of inorganic electrolytes. Chemical Engineering Science. 1967;22:1257–1265. [Google Scholar]
- Martinez GA, Civello PM, Chaves AR, Añón MC. Characterization of peroxidase-mediated chlorophyll bleaching in strawberry fruit. Phytochemistry. 2001;58:379–387. doi: 10.1016/s0031-9422(01)00266-7. [DOI] [PubMed] [Google Scholar]
- Mayer AM. Polyphenol oxidases in plants and fungi: going places? A review. Phytochemistry. 2006;67:2318–2331. doi: 10.1016/j.phytochem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- McCully ME, Shane MW, Baker AN, Huang CX, Ling LEC, Canny MJ. The reliability of cryoSEM for the observation and quantification of xylem embolisms and quantitative analysis of xylem sap in situ. Journal of Microscopy. 2000;198:24–33. doi: 10.1046/j.1365-2818.2000.00679.x. [DOI] [PubMed] [Google Scholar]
- Mercury L. Gas solubilities in capillary water confined in finely dispersed systems. In: Somasundaran P, editor. Encyclopedia of surface and colloid science. Vol. 4. New York: Taylor & Francis; 2006. pp. 2665–2677. [Google Scholar]
- Mercury L, Azaroual M, Zeyen H, Tardy Y. Thermodynamic properties of solutions in metastable systems under negative or positive pressures. Geochimica et Cosmochimica Acta. 2003;67:1769–1785. [Google Scholar]
- Mercury L, Tardy Y. Negative pressure of stretched liquid water. geochemistry of soil capillaries. Geochimica et Cosmochimica Acta. 2001;65:3391–3408. [Google Scholar]
- Nardini A, Gascò A, Trifilò P, Lo Gullo MA, Salleo S. Ion-mediated enhancement of xylem hydraulic conductivity is not always suppressed by the presence of Ca2+in the sap. Journal of Experimental Botany. 2007;58:2609–2615. doi: 10.1093/jxb/erm105. [DOI] [PubMed] [Google Scholar]
- Pandey DK, Mishra N, Singh P. Relative phytotoxicity of hydroquinone on rice (Oryza sativa L.) and associated aquatic weed green musk chary (Chary zeylanica Willd.) Pesticide Biochemistry and Physiology. 2005;83:82–96. [Google Scholar]
- Pockman WT, Sperry JS. Vulnerability to cavitation and the distribution of Sonoran Desert vegetation. American Journal of Botany. 2000;87:1287–1299. [PubMed] [Google Scholar]
- Pratt RB, Jacobsen AL, North GB, Sack L, Schenk HJ. Plant hydraulics: new discoveries in the pipeline. New Phytologist. 2008;179:590–593. doi: 10.1111/j.1469-8137.2008.02566.x. [DOI] [PubMed] [Google Scholar]
- Ramonell KM, Somerville S. The genomics parade of defense responses: to infinity and beyond. Current Opinion in Plant Biology. 2002;5:1–4. doi: 10.1016/s1369-5266(02)00266-2. [DOI] [PubMed] [Google Scholar]
- Ribeiro CP, Mewes D. On the effect of liquid temperature upon bubble coalescence. Chemical Engineering Science. 2006;61:5704–5716. [Google Scholar]
- Richardson A, Duncan J, McDougall GJ. Oxidase activity in lignifying xylem of a taxonomically diverse range of trees: identification of a conifer laccase. Tree Physiology. 2000;20:1039–1047. doi: 10.1093/treephys/20.15.1039. [DOI] [PubMed] [Google Scholar]
- Ryden P, MacDougall AJ, Tibbits CW, Ring SG. Hydration of pectic polysaccharides. Biopolymers. 2000;54:398–405. doi: 10.1002/1097-0282(200011)54:6<398::AID-BIP40>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Salleo S, Lo Gullo MA, Trifilò P, Nardini A. New evidence for a role of vessel-associated cells and phloem in the rapid xylem refilling of cavitated stems of Laurus nobilis L. Plant, Cell and Environment. 2004;27:1065–1076. [Google Scholar]
- Savin NE, White KJ. The Durbin–Watson test for serial correlation with extreme sample sizes or many regressors. Econometrica. 1977;45:1989–1996. [Google Scholar]
- Scardina P, Edwards M. Air binding of granular media filters. Journal of Environmental Engineering. 2004;130:1126–1138. [Google Scholar]
- Schulte PJ, Gibson AC, Nobel PS. Xylem anatomy and hydraulic conductance of Psilotum nudum. American Journal of Botany. 1987;74:1438–1445. [Google Scholar]
- Sellin A. Hydraulic conductivity of xylem depending on water saturation level in Norway spruce (Picea abies (L.) Karst) Journal of Plant Physiology. 1991;138:466–469. [Google Scholar]
- Sperry JS, Donnelly JR, Tyree MT. A method for measuring hydraulic conductivity and embolism in xylem. Plant, Cell and Environment. 1988;11:35–40. [Google Scholar]
- Sperry JS, Holbrook NM, Zimmermann MH, Tyree MT. Spring filling of xylem vessels in wild grapevine. Plant Physiology. 1987;83:414–417. doi: 10.1104/pp.83.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JS, Saliendra NZ. Intra- and inter-plant variation in xylem cavitation in Betula occidentalis. Plant, Cell and Environment. 1994;17:1233–1241. [Google Scholar]
- Sperry JS, Sullivan JEM. Xylem embolism in response to freeze–thaw cycles and water stress in ring-porous, diffuse-porous, and conifer species. Plant Physiology. 1992;100:605–613. doi: 10.1104/pp.100.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JS, Tyree MT. Water-stress-induced xylem embolism in three species of conifers. Plant, Cell and Environment. 1990;13:427–436. [Google Scholar]
- Stahlberg R, Cosgrove DJ. Comparison of electric and growth responses to excision in cucumber and pea-seedlings. 2. Long-distance effects are caused by the release of xylem pressure. Plant, Cell and Environment. 1995;18:33–41. doi: 10.1111/j.1365-3040.1995.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Stahlberg R, Cleland RE, Van Volkenburgh E. Decrement and amplification of slow wave potentials during their propagation in Helianthus annuus L. shoots. Planta. 2005;220:550–558. doi: 10.1007/s00425-004-1363-x. [DOI] [PubMed] [Google Scholar]
- Sun Q, Rost TL, Matthews MA. Wound-induced vascular occlusions in Vitis vinifera (Vitaceae): tyloses in summer and gels in winter. American Journal of Botany. 2008;95:1498–1505. doi: 10.3732/ajb.0800061. [DOI] [PubMed] [Google Scholar]
- Sun Q, Rost TL, Reid MS, Matthews MA. Ethylene and not embolism is required for wound-induced tylose development in stems of grapevines. Plant Physiology. 2007;145:1629–1636. doi: 10.1104/pp.107.100537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilò P, Lo Gullo MA, Salleo S, Callea K, Nardini A. Xylem embolism alleviated by ion-mediated increase in hydraulic conductivity of functional xylem: insights from field measurements. Tree Physiology. 2008;28:1505–1512. doi: 10.1093/treephys/28.10.1505. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Patiño S, Bennink J, Alexander J. Dynamic measurements of root hydraulic conductance using a high-pressure flowmeter in the laboratory and field. Journal of Experimental Botany. 1995;46:83–94. [Google Scholar]
- Tyree MT, Salleo S, Nardini A, Lo Gullo MA, Mosca R. Refilling of embolized vessels in young stems of laurel. Do we need a new paradigm? Plant Physiology. 1999;120:11–21. doi: 10.1104/pp.120.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Yang S. Hydraulic conductivity recovery versus water pressure in xylem of Acer saccharum. Plant Physiology. 1992;100:669–676. doi: 10.1104/pp.100.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn WG, Cruz P. Evidence for a wounding-induced xylem occlusion in stems of cut chrysanthemum flowers. Postharvest Biology and Technology. 2000;19:73–83. [Google Scholar]
- van Doorn WG, Vaslier N. Wounding-induced xylem occlusion in stems of cut chrysanthemum flowers: roles of peroxidase and cathechol oxidase. Postharvest Biology and Technology. 2002;26:275–284. [Google Scholar]
- van Ieperen W. Ion-mediated changes of xylem hydraulic resistance in planta: fact or fiction? Trends in Plant Science. 2007;12:137–142. doi: 10.1016/j.tplants.2007.03.001. [DOI] [PubMed] [Google Scholar]
- van Ieperen W, van Gelder A. Ion-mediated flow changes suppressed by minimal calcium presence in xylem sap in Chrysanthemum and Prunus laurocerasus. Journal of Experimental Botany. 2006;57:2743–2750. doi: 10.1093/jxb/erl039. [DOI] [PubMed] [Google Scholar]
- van Ieperen W, van Meeteren U, Nijsse J. Embolism repair in cut flower stems: a physical approach. Postharvest Biology and Technology. 2002;25:1–14. [Google Scholar]
- van Ieperen W, van Meeteren U, van Gelder H. Fluid ionic composition influences hydraulic conductance of xylem conduits. Journal of Experimental Botany. 2000;51:769–776. doi: 10.1093/jexbot/51.345.769. [DOI] [PubMed] [Google Scholar]
- van Meeteren U. Role of air embolism and low water temperature in water balance of cut chrysanthemum flowers. Scientia Horticulturae. 1992;51:275–284. [Google Scholar]
- van Meeteren U, Arevalo-Galarza L, van Doorn WG. Inhibition of water uptake after dry storage of cut flowers: role of aspired air and wound-induced processes in Chrysanthemum. Postharvest Biology and Technology. 2006;41:70–77. [Google Scholar]
- van Meeteren U, van Gelder H, van Ieperen W. Reconsideration of the use of deionized water as vase water in postharvest experiments on cut flowers. Postharvest Biology and Technology. 2000;18:169–181. [Google Scholar]
- Vaslier N, van Doorn WG. Xylem occlusion in bouvardia flowers: evidence for a role of peroxidase and cathechol oxidase. Postharvest Biology and Technology. 2003;28:231–237. [Google Scholar]
- Wehr JB, Menzies NW, Blamey FPC. Inhibition of cell-wall autolysis and pectin degradation by cations. Plant Physiology and Biochemistry. 2004;42:485–492. doi: 10.1016/j.plaphy.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Willats WGT, McCartney L, Mackie W, Knox JP. Pectin: cell biology and prospects for functional analysis. Plant Molecular Biology. 2001;47:9–27. [PubMed] [Google Scholar]
- Yang S, Tyree MT. A theoretical-model of hydraulic conductivity recovery from embolism with comparison to experimental data on Acer saccharum. Plant, Cell and Environment. 1992;15:633–643. [Google Scholar]
- Zahradník J, Fialová M, Kastanek F, Green KD, Thomas NH. The effect of electrolytes on bubble coalescence and gas holdup in bubble-column reactors. Chemical Engineering Research and Design. 1995;73:341–346. [Google Scholar]
- Zancani M, Nagy G, Vianello A, Macri F. Copper-inhibited NADH-dependent peroxidase-activity of purified soya bean plasma-membranes. Phytochemistry. 1995;40:367–371. [Google Scholar]
- Zsivánovits G, Marudova M, Ring S. Influence of mechanical properties of pectin films on charge density and charge density distribution in pectin macromolecule. Colloid and Polymer Science. 2005;284:301–308. [Google Scholar]
- Zwieniecki MA, Melcher PJ, Holbrook NM. Hydrogel control of xylem hydraulic resistance in plants. Science. 2001;291:1059–1062. doi: 10.1126/science.1057175. [DOI] [PubMed] [Google Scholar]