Abstract
In Petunia×hybrida cv ‘Mitchell Diploid’ (MD), floral volatile benzenoid/phenylpropanoid (FVBP) biosynthesis is controlled spatially, developmentally, and daily at molecular, metabolic, and biochemical levels. Multiple genes have been shown to encode proteins that either directly catalyse a biochemical reaction yielding FVBP compounds or are involved in metabolite flux prior to the formation of FVBP compounds. It was hypothesized that multiple transcription factors are involved in the precise regulation of all necessary genes, resulting in the specific volatile signature of MD flowers. After acquiring all available petunia transcript sequences with homology to Arabidopsis thaliana R2R3-MYB transcription factors, PhMYB4 (named for its close identity to AtMYB4) was identified, cloned, and characterized. PhMYB4 transcripts accumulate to relatively high levels in floral tissues at anthesis and throughout open flower stages, which coincides with the spatial and developmental distribution of FVBP production and emission. Upon RNAi suppression of PhMYB4 (ir-PhMYB4) both petunia CINNAMATE-4-HYDROXYLASE (PhC4H1 and PhC4H2) gene transcript levels were significantly increased. In addition, ir-PhMYB4 plants emit higher levels of FVBP compounds derived from p-coumaric acid (isoeugenol and eugenol) compared with MD. Together, these results indicate that PhMYB4 functions in the repression of C4H transcription, indirectly controlling the balance of FVBP production in petunia floral tissue (i.e. fine-tunes).
Keywords: benzenoid/phenylpropanoid, flower, R2R3-MYB, petunia, transcription factor, volatiles
Introduction
Floral fragrance is typically composed of an array of volatile organic compounds. These volatile compounds are generally lipophilic liquids with high vapour pressures that putatively cross biological membranes by passive diffusion in the absence of a barrier (Pichersky et al., 2006). Many angiosperm species produce floral fragrance and each species produces a unique blend of volatile organic compounds which facilitate environmental interaction (Dudareva et al., 2006). The emission of floral volatiles can reach between 30 μg h−1 and 150 μg h−1 for some species (Knudsen et al., 2006). An established model, Petunia×hybrida cv ‘Mitchell Diploid’ (MD) has been used in numerous studies related to floral volatile synthesis. MD has large white flowers that produce copious amounts of floral volatile compounds. Volatile benzenoids and phenylpropanoids dominate the mixture of volatile compounds emitted by the MD flower (Verdonk et al., 2003; Schuurink et al., 2006). In MD, floral volatile benzenoid/phenylpropanoid (FVBP) biosynthesis is confined to the corolla limb tissue subsequent to anthesis, until senescence, and high levels of emission peak during the dark period (Kolosova et al., 2001; Underwood et al., 2005, Verdonk et al., 2005). In addition, FVBP production and emission is severely reduced after a successful pollination/fertilization event mediated by the phytohormone ethylene (Negre et al., 2003; Underwood et al., 2005; Dexter et al., 2007, 2008; Colquhoun et al., 2010a). The latter work was facilitated by an ethylene-insensitive (CaMV 35S::etr1-1) transgenic petunia line, 44568 (Wilkinson et al., 1997; Negre et al., 2003; Underwood et al., 2005), which functioned as an ethylene perception control in most experiments.
In petunia, FVBPs are derived from the aromatic amino acid phenylalanine (Boatright et al., 2004; Schuurink et al., 2006) with the production of individual FVBP compounds originating from phenylalanine directly, and the core phenylpropanoid pathway metabolites t-cinnamic acid and ferulic acid. Numerous proteins are involved in the production of FVBPs from the shikimate pathway to the end biochemical steps resulting in the direct formation of volatile compounds. To date, 12 genes have been identified and shown to encode proteins associated with the production of FVBPs in petunia (Negre et al., 2003; Boatright et al., 2004; Underwood et al., 2005; Verdonk et al., 2005; Kaminaga et al., 2006; Koeduka et al., 2006, 2008; Orlova et al., 2006; Dexter et al., 2007, 2008; Van Moerkercke et al., 2009; Colquhoun et al., 2010b; Maeda et al., 2010; Spitzer-Rimon et al., 2010). Seven of these petunia genes are involved in the direct formation of FVBP compounds, while three are associated with the production of pathway intermediates. Two are transcriptional regulators, PhODORANT1 (PhODO1, accession: AY705977) and PhEMISSION OF BENZENOIDS II (PhEOBII, accession: EU360893). Both PhODO1 and PhEOBII are R2R3-MYB transcription factors that function as positive regulators of multiple shikimate and phenylpropanoid pathway genes (Verdonk et al., 2005; Spitzer-Rimon et al., 2010). When PhODO1 and PhEOBII transcript accumulations were reduced using RNAi approaches in MD and in the Petunia×hybrida line P720, respectively, there was a partial reduction in several emitted FVBPs. Since R2R3-MYB transcription factors are known to participate in heterologous protein complexes (Quattrocchio et al., 2006) and PhODO1 and/or PhEOBII do not regulate the entire network of FVBP genes (Verdonk et al., 2005; Spitzer-Rimon et al., 2010); it is logical to hypothesize that there are other transcription factors that may be important in the regulation of FVBP biosynthesis in MD.
The R2R3-MYB transcription factor family consists of 126 genes in Arabidopsis with the R2R3 domain highly conserved throughout all 126 family members (Stracke et al., 2001). DNA binding of a target promoter is facilitated through this N-terminal R2R3 domain. Specific biological functions of the Arabidopsis R2R3-MYBs are generally conferred by the C-terminal half of the proteins, which can be extremely divergent between family members (e.g. cell fate, cell identity, phenylpropanoid metabolism, mediating phytohormone actions, and response to environmental stimuli). A pertinent example, AtMYB4 (AY070100) is a negative transcriptional regulator of its own promoter and the CINNAMATE-4-HYDROXYLASE (AtC4H, CYP73A5) promoter (Jin et al., 2000; Zhao et al., 2007). AtC4H (a cytochrome P450 protein family member) catalyses the second biochemical step in the core phenylpropanoid pathway, utilizing t-cinnamic acid and producing p-coumaric acid (Anterola et al., 2002). Conversely, AtMYB66 (AtWER: AY519623) regulates the position-dependent transcript accumulation of GLABRA2 (AtGL2: AF360294), which is required for normal epidermal cell patterning (Lee and Schiefelbein, 1999).
Regulation at the transcriptional level has a considerable role in the spatial, developmental, and hormonal control of FVBP synthesis in petunia (Verdonk et al., 2005; Colquhoun et al., 2010a). R2R3-MYB transcription factors regulate a diverse set of biological processes including genes in the shikimate and phenylpropanoid pathways (Stracke et al., 2001; Verdonk et al., 2005). Since two transcription factors have previously been associated with FVBP production in petunia, it was hypothesized that multiple transcription factors act in concert, resulting in the high level of transcriptional regulation imparted upon FVBP genes in petunia. Three petunia EST databases were searched for any homologous sequences to Arabidopsis R2R3-MYB transcription factors and, as expected, the search produced numerous petunia R2R3-MYB candidates. Based on primary sequence homology with AtMYB4 (negatively regulates AtC4H transcript accumulation, C4H is an enzyme in the core phenylpropanoid pathway) and the observation that molecular functions of a given protein are generally conserved between organisms, a single group of petunia EST sequences representing PhMYB4 were selected for further experimentation. It is shown here that Petunia×hybrida R2R3-MYB 4 (PhMYB4) functions much like AtMYB4, i.e. fine-tuning of a complex metabolic pathway to achieve a specific biochemical outcome.
Materials and methods
Plant material
Inbred Petunia×hybrida cv ‘Mitchell Diploid’ (MD) plants were used as a ‘wild-type’ control in all experiments. The homozygous ethylene-insensitive CaMV 35S:etr1-1 line 44568, generated in the MD genetic background (Wilkinson et al., 1997), was used as a negative control for ethylene sensitivity. MD, 44568, and PhMYB4 RNAi plants were grown in glass greenhouses as previously described by Dexter et al. (2007).
Selection and identification of PhMYB4
Sequences with similarity to Arabidopsis thaliana R2R3-MYB transcription factors (AtMYBs) were gathered using personal communications with collaborators; NCBI, SGN, and the 454 petunia databases (www.ncbi.nlm.nih.gov, http://solgenomics.net, and http://140.164.45.140/454petuniadb). The resulting sequences were used to construct a petunia nucleotide ‘Quasi-Contig’ representing PhMYB4. Specifically, the group included five sequences: Unigene sequences retrieved from the SGN database representing MD (SGN-U208628 and SGN-E527807: Sol Genomics Network), tentative consensus sequences from the petunia 454 database (PETIN023594: P. inflata, PETAX048797: P. axillaris), and a cDNA text file of a MD sequence named PhMYB5d8. Sequences were assembled (ContigExpress® module, Vector NTI Advance™ 11) and primers (PhMYB4 forward primer 5′-CACCAAGGCTAAACTGCATC-3′; PhMYB4 reverse primer 5′-GGGAAAAATACTCAAGGAGA-3′) were designed approximately 80–100 nt 5′ and 3′ of the deduced 777 nt coding region (GenBank accession number: PhMYB4, HM447143). Replicates of the expected, approximate 1000 nt product were amplified using Advantage® 2 Polymerase Mix (Clontech Laboratories Inc., http://www.clontech.com) from gene transcript pools of MD tissue, and purified using QIAquick™ Spin Columns (http://qiagen.com). Amplicons were ligated into pGEM®-T-easy vector (Promega Corp., http://promega.com), transformed into One Shot® Mach1™ –T1R chemically competent E. coli (Invitrogen Corp., http://invitrogen.com), multiple clones were isolated and sequenced (Big Dye V1-2; University of Florida Sequencing Core Facility) to at least a 6× coverage to check for errors.
In addition to the above method, SMART™ RACE technology was utilized to clone the full-length PhC4H1 and PhC4H2 transcripts (Colquhoun et al., 2010b), via the SMART™ RACE cDNA amplification kit (Clontech Laboratories Inc., http://www.clontech.com) according to the manufacturer's protocol. The resulting two individual full-length PhC4H cDNA sequences at 1809 nt and 1684 nt contained 1560 nt and 1515 nt coding regions (GenBank accession numbers: PhC4H1, HM447144; PhC4H2, HM447145) with predicted (Vector NTI Advance™ 11) amino acid protein sequences of 506 and 505 amino acids in length.
Generating transgenic PhMYB4 RNAi plants
To test the gene function of PhMYB4 directly, RNAi-induced gene silencing was utilized. A 287 nt sequence at the 3′ end of the PhMYB4 transcript was developed as the RNAi inducing fragment (PhMYB4 forward primers 5′-GCTCTAGATTTTGCTGCTGGAATGAAGA-3′, 5′-CGGGATCCTTTTGCTGCTGGAATGAAGA-3′; and reverse primers 5′-GGAATTCTTCCTGCTACAACTGCAACCT-3′, 5′-GGAATTCGGGAAAAATACTCAAGGAGA-3′). In planta expression of this fragment is driven by a constitutive promoter, pFMV. Further details of the technical cloning have been described previously by Dexter et al. (2007).
Sixty-five independent PhMYB4 RNAi (ir-PhMYB4) plants were generated by leaf disc transformation (Jorgensen et al., 1996) and analysed for reduced levels of PhMYB4 transcripts by semi-quantitative reverse transcriptase polymerase chain reaction (sqRT-PCR). All T0 transgenic plants with reduced levels of PhMYB4 transcripts (approximately 16), when compared to MD, were self-pollinated. The T1 generation was analysed for a 3:1 segregation of the transgene and the transcriptional phenotype using sqRT-PCR. Lines exhibiting a 3:1 segregation were self-pollinated, ultimately resulting in two independent homozygous lines: ir-PhMYB4.12-12 and ir-PhMYB4.60-7 that were used for further experimentation.
RNA isolation, tissue collection, and treatments
In all cases, total RNA was extracted as previously described (Verdonk et al., 2003) and subjected to TURBO™ DNase treatment (Ambion Inc., Austin, TX) followed by total RNA purification with RNeasy® Mini protocol for RNA cleanup (Qiagen, Valencia, CA). Total RNA was then quantified on a NanoDrop™ 1000 spectrophotometer (Thermo Scientific, Wilmington, DE) and 50 ng μl−1 dilutions were prepared and stored at –20 °C. Generation of cDNA samples used 2 μg of total RNA with SuperScript™ Reverse Transcriptase II (Invitrogen Corp., http://invitrogen.com) and was conducted more than three times for technical replications.
All petunia tissue collections were done as previously described by Colquhoun et al. (2010)a; the following is a brief description. The spatial transcript accumulation analysis consisted of total RNA isolated from petunia root, stem, stigma, anther, leaf, petal tube, petal limb, and sepal tissues from multiple greenhouse-grown MD plants harvested at 16.00 h. The developmental transcript accumulation analysis used total RNA isolated from whole petunia flowers (MD and 44568) collected at 11 consecutive stages beginning at a small bud to floral senescence from multiple greenhouse-grown plants at 16.00 h. Exogenous ethylene effects were determined using excised whole flowers from greenhouse-grown MD and 44568 plants. All flowers were treated with air or ethylene (2 μl l−1) for 0, 1, 2, 4, and 8 h beginning at 12.00 h with an experimental end time of 20.00 h. The daily time-course analysis used MD plants acclimated in a growth chamber (Environmental Growth Cambers, model TC-1, Chagrin Falls, OH, USA) set to long-day photoperiod conditions with samples collected at 06.00 h and every 3 h thereafter, for a total of 36 h. For all tissue collections, individual samples consisted of three flowers. All samples were frozen in liquid N2 and stored at –80 °C. Total RNA was then isolated from all samples, with multiple biological replicates included.
Transcript accumulation analysis
All transcript accumulation analyses were conducted multiple times with multiple biological replicates and equivalent results were observed. Semi-quantitative (sq)RT-PCR was performed using a Qiagen One-step RT-PCR kit (Qiagen Co., Valencia, CA, USA) with 50 ng total RNA. In all sqRT-PCR experiments the gene-specific primers were used in multiple reactions at different cycle numbers to qualify initial observations. To visualize RNA loading concentrations, samples were amplified with Ph18S primers (forward primer 5′-TTAGCAGGCTGAGGTCTCGT-3′; reverse primer 5′-AGCGGATGTTGCTTTTAGGA-3′) and analysed on an agarose gel. The following primers were designed and utilized for the visualization of the mRNA levels corresponding to PhMYB4 (forward primer 5′-CACCAAGGCTAAACTGCATC-3′; reverse primer 5′-GGGAAAAATACTCAAGGAGA-3′). ΔΔCt Quantitative (q)RT-PCR was performed and analysed using a StepOnePlus™ real-time PCR system (Applied Biosystems, Foster City, CA). Power SYBR® Green RNA-to-Ct™ 1 and 2-Step kits (Applied Biosystems, Foster City, CA) were used to amplify and detect the products according to the manufacturer's protocol. The following, qRT-PCR primers were constructed in Primer Express® software v2.0 (Applied Biosystems, Foster City, CA): PhMYB4 forward primer 5′-AACAATTTCTTTTGCTGCTGGAA-3′; PhMYB4 reverse primer 5′-TTCATCGTCCTTGATTTGTTCAA-3′; PhFBP1 forward primer 5′-TGCGCCAACTTGAGATAGCA-3′; PhFBP1 reverse primer 5′-TGCTGAAACACTTCGCCAATT-3′; PhC4H1 forward primer 5′-AGCAGGTGTAACAAACTGCAA-3′; PhC4H1 reverse primer 5′-AAACTGGGACAGGGATAGGA-3′; PhC4H2 forward primer 5′-AACTTGTCCAAACAAAAATGGA-3′; PhC4H2 reverse primer 5′-TGGCAATTTAAAACGTTTGCT-3′; PhODO1 forward primer 5′-TGCTTCAACCATGTCGAATTG-3′; PhODO1 reverse primer 5′-TCCGTGCCTGTTCTCTACGTT-3′: PhCM1 forward primer 5′-CCCTGATGAGCACCCATTC-3′; PhCM1 reverse primer 5′-ACTGCATGGGTGGCAACAC-3′; PhPAL1 forward primer 5′-GCTAGGCGGTGAGACGCTAA-3′; PhPAL1reverse primer 5′-CTCGGACAGCTGCACTGTCA-3′; PhPAL2 forward primer 5′-ACTGGCAGGCCTAATTCCAA-3′; PhPAL2 reverse primer 5′-GCGAAACGCTTCTTCAGCAT-3′. Optimization of primers was conducted and demonstrated gene specificity during melt curve analysis.
Volatile emission
For all volatile emission experiments, emitted floral volatiles from excised flowers were collected at 16.00 h and quantified as previously described (Underwood et al., 2005; Dexter et al., 2007). All samples consisted of three flowers per sample with at least three biological replicates.
Results
Isolation and cloning of PhMYB4
An in silico population of petunia nucleotide sequences enriched for R2R3-MYB transcription factors was generated by exploiting all publicly available petunia sequence information: NCBI, SGN, and the 454 petunia databases (www.ncbi.nlm.nih.gov, http://solgenomics.net, and http://140.164.45.140/454petuniadb; respectively) (Table 1). Our focus quickly narrowed (because of primary sequence homology with Arabidopsis) to a group of sequences that represented a theoretical, single R2R3-MYB gene transcript named later as PhMYB4. All of these sequences were assembled into what was called ‘Quasi-Contig 2’ (Table 1; see Supplementary Fig. S1 at JXB online) and primers were designed to amplify the deduced coding sequence. A single 1 kb product was amplified several times using a high-fidelity polymerase, and multiple clones from multiple amplifications were stringently sequenced. Close agreement between individual clones demonstrated the validity of the amplified coding sequence and was submitted to GenBank under accession number HM447143.
Table 1.
Public database search results for petunia sequences with high similarity to Arabidopsis R2R3-MYB protein sequences
| Quasi-Contig | Arabidopsis homologue | Accession | |
| SGN-U208944 | 1 | AtMYB85 | AT4G22680 |
| SGN-E529378 | |||
| PhODO1 | AY705977 | ||
| SGN-U208628 | 2 | AtMYB4 | AT4G38620 |
| SGN-E527807 | |||
| PhMYB5d8 | |||
| PETAX048797 | |||
| PETIN023594 | |||
| FTSABCA02FF646 | 3 | AtMYB57 | AT3G01530 |
| PETAX093168 | |||
| SGN-U207568 | |||
| SGN-U210146 | |||
| PETAX082985 | 4 | AtMYB24 | AT5G40350 |
| SGN-U207567 | |||
| PETIN062720 | |||
| PETAX071176 | 5 | AtMYB82 | AT5G52600 |
| PETIN004124 | |||
| EB175070 | |||
| PETIN069282 | 6 | AtMYB20 | AT1G66230 |
| PETAX086598 | |||
| PETAX063295 | |||
| PETAX039135 | 7 | AtMYB40 | AT5G14340 |
| PETIN092930 | |||
| PETAX021373 | 8 | AtMYB32 | AT4G34990 |
| PETIN001198 | |||
| PETIN016949 | 9 | AtMYB7 | AT2G16720 |
| SGN-U209055 | |||
| EB175095 | |||
| SGN-U211729 | 10 | AtMYB3 | AT1G22640 |
| PETIN076721 |
Petunia databases used are as follows: Sol Genomics Network, NCBI, and the 454 petunia databases. Assembly of petunia sequences into ‘Quasi-Contigs’ was carried out by the ContigExpress® module of Vector NTI Advance™ 11 software. Most similar Arabidopsis protein sequence was based from the predicted protein sequence of the Quasi-Contig.
The translated sequence of HM447143 was 258 amino acids in length and predicted to be nuclear localized (WoLF PSORT; http://wolfpsort.org). Subsequent to a BLAST (tBLASTn) analysis on the non-redundant nucleotide collection at NCBI; the predicted petunia protein was aligned with highly similar amino acid sequences from varying species (Fig. 1). Three main features were observed: an N-terminal R2R3 domain, C1-motif (LLSRGIDPTTHXI), and a C-terminal MYB subgroup 4 EAR-domain (PDLNLELKISPP). The petunia amino acid sequence shares 66.1% and 55.9% identity with SlTH27 (X95296) and AtMYB4, respectively. Since the predicted petunia amino acid sequence shares the highest identity to AtMYB4, compared with all of the 125 other Arabidopsis R2R3-MYB sequences, and petunia follows the Arabidopsis nomenclatural style; the petunia coding sequence was named PhMYB4 (HM447143).
Fig. 1.
Predicted amino acid sequence alignment of homologous R2R3-MYB proteins from various species. Sequences represented are from Arabidopsis thaliana [accession: NM_119665 (MYB32) and AY070100 (MYB4)], Eucalyptus gunnii (AJ576024), Gossypium hirsutum (AF336286), Populus trichocarpa (XM_002306144), Vitis vinifera (EF113078), Humulus lupulus (AB292244), Solenostemon scutellarioides (EF522161), Petunia×hybrida (1359067), and Solanum lycopersicum (X95296). Sequences were aligned using the AlignX program of the Vector NTI Advance™ 11 software. Conserved domains depicted above the sequences are the R2 domain (light blue), R3 domain (green), C1 motif (black), and the EAR domain (red). Residues highlighted in: blue represent consensus residues derived from a block of similar residues at a given position, green represent consensus residues derived from the occurrence of greater than 50% of a single residue at a given position, and yellow represent consensus residues derived from a completely conserved residue at a given position. Petunia sequences are highlighted in red to the left.
Isolation and cloning of two potential petunia C4Hs
AtMYB4 negatively regulates AtC4H transcription in leaf tissue (Jin et al., 2000). Therefore, there was a need to test the hypothesis that PhMYB4 functions in a similar way to AtMYB4. Because the cytochrome P450 protein family is so large (Pan et al., 2009) and one to two isoforms of C4H have been identified in various species of plants; the two most similar sequences in MD were cloned to AtC4H. The PhC4H cloning strategy was very similar to the strategy used to clone PhMYB4. However, utilizing EST libraries alone proved insufficient. SMART™ RACE technology (Colquhoun et al., 2010b) resulted in the cloning of two individual full-length cDNA sequences that are 1809 nt and 1684 nt with coding regions of 1560 nt and 1515 nt (see Supplementary Fig. S2 at JXB online). After stringent sequence analysis, these full-length cDNA sequences were submitted to GenBank under accessions: HM447144 and HM447145, respectively. The predicted amino acid sequence of both petunia cDNAs is very similar to other C4H sequences from various plant species (see Supplementary Fig. S3 at JXB online). PhC4H1 and PhC4H2 are 86.2% and 86.5% identical to AtC4H with the next most similar Arabidopsis sequence (AtC3H) 31.4% and 32.2% identical (Table 2).
Table 2.
PhC4H1 and PhC4H2 protein comparison with the four most similar expressed proteins from Arabidopsis (Align X module of Vector NTI Advance™ 11 software)
| 31.6 | 33.2 | 32.7 | ||||||
| AtC4H |
AtC3H |
AtF3H |
AtTT7 |
|||||
| Similarity | Identity | Similarity | Identity | Similarity | Identity | Similarity | Identity | |
| PhC4H1 | 92.7 | 86.2 | 48.8 | 31.4 | 48.3 | 29.2 | 44.8 | 29.0 |
| 93.5 | ||||||||
| PhC4H2 | 93.1 | 86.5 | 49.6 | 32.2 | 47.1 | 28.9 | 44.2 | 28.8 |
Numbers represent percentage of identity between adjacent proteins.
PhMYB4 transcript accumulation analysis
In order to gain a general perspective on the distribution of PhMYB4 transcript accumulation: spatial, flower development (post-organ identity), phytohormone effect (ethylene), and a daily time-course were chosen for PhMYB4 transcript accumulation analysis by sqRT-PCR (Fig. 2). Multiple sets of gene-specific primers were generated outside of the R2R3 domain (see Supplementary Fig. S4 at JXB online) and all images are representative of multiple experiments on at least two biologically replicated sets.
Fig. 2.
PhMYB4 transcript accumulation analysis (one-step sqRT-PCR). Spatial analysis used root, stem, stigma, anther, leaf, petal tube, petal limb, and sepal tissues of MD harvested at 16.00 h: shown is 24 cycles of amplification (A). Floral developmental analysis used MD and 44568 flowers from 11 sequential stages collected on one day at 16.00 h: 25 cycles (B). Ethylene treatment (2 μl l−1) analysis used excised MD and 44568 whole flowers treated for 0, 1, 2, 4, and 8 h: 24 cycles (C). Rhythmic analysis used MD plants acclimated in a growth chamber with a long-day photoperiod and samples collected every 3 h for a total of 36 h: 24 cycles (D). Ph18S was used as a loading control (16–17 cycles), and 50 ng total RNA was used per reaction in all cases. Shown are representative pictures from multiple biological and technical replications.
The spatial transcript accumulation analysis consisted of root, stem, stigma, anther, leaf, petal tube, petal limb, and sepal tissues from multiple MD plants harvested at 16.00 h (Fig. 2A). PhMYB4 transcripts were detected at relatively high levels in the petal limb, petal tube, anther, stigma, and to a lesser extent in stem tissue. In short, PhMYB4 transcripts are present in the same tissue that FVBP production is strongly localized to, the petal limb.
The MD and 44568 (ethylene-insensitive, CaMV 35S::etr1-1 transgenic MD line; Wilkinson et al., 1997) floral developmental series consisted of whole flowers collected at 11 consecutive stages beginning at a small bud to floral senescence from multiple greenhouse-grown plants at 16.00 h (for a detailed explanation; Colquhoun et al., 2010a). PhMYB4 transcripts were detected at relatively low levels throughout the closed bud stages of development in both genetic backgrounds (Fig. 2B). High levels of PhMYB4 transcripts were detected at anthesis (stage 6) and throughout all open flower stages of development examined in both MD and 44568 (stages 7–10). PhMYB4 transcripts were detected at the lowest level in observably senescing MD flower tissue (stage 11). By contrast, PhMYB4 transcripts were detected at high levels in observably senescing 44568 floral tissue (Fig. 2B). The floral developmental transcript accumulation for PhMYB4 is therefore consistent with the developmental timing of FVBP gene transcript accumulation profiles, FVBP production, and FVBP emission (Verdonk et al., 2003; Colquhoun et al., 2010a).
The ethylene study used excised whole flowers from greenhouse-grown MD and 44568 plants. All flowers were treated with air or ethylene (2 μl l−1) for 0, 1, 2, 4, and 8 h beginning at 12.00 h with an experimental end-time of 20.00 h (Fig. 2C). PhMYB4 transcript levels were not appreciably changed throughout the 8 h time-course of the ethylene experimentation in both genetic backgrounds. Therefore, PhMYB4 transcription is most likely not affected by ethylene during an initial 8 h of exposure. However, the observation of continued transcript accumulation at senescence in 44568 floral tissues (Fig 2B) suggests that PhMYB4 gene transcription is negatively affected by the phytohormone ethylene after a prolonged (>8 h) perception of ethylene, or a still to be described attribute of the floral senescence programme itself which is associated with ethylene sensitivity and perception at PhETR1.
The daily time-course analysis used MD plants acclimated in a growth chamber with a long day photoperiod, whole flower tissue was collected every 3 h for a total of 36 h (Fig. 2D). PhMYB4 transcripts were detected at relatively high levels between 15.00 h and 24.00 h, which is similar to the PhODO1 transcript accumulation profile throughout a daily time-course analysis (Verdonk et al., 2005; Colquhoun et al., 2010a).
PhC4H1 and PhC4H2 transcript accumulation analysis
Primary sequence features of PhC4H1 and PhC4H2 suggested that two distinct transcripts for CINNAMATE-4-HYDROXYLASE were cloned from petunia. Assuming the possible C4H transcripts produced functional proteins involved in FVBP synthesis (i.e. metabolite flux through the phenylpropanoid pathway by producing p-coumaric acid), it was hypothesized that the transcript accumulation profile for one or both petunia C4Hs was similar to those described for qualified FVBP transcripts over the course of flower development (Colquhoun et al., 2010a). Therefore, a flower developmental transcript accumulation analysis for PhC4H1 and PhC4H2 was conducted as described above except that qRT-PCR was used (Fig. 3). Both PhC4H1 and PhC4H2 transcripts were detected in MD whole flower tissue throughout development with the highest abundance detected during the open flower stages (7–10), and the lowest abundance in observably senescing flower tissue (stage 11). Compared with the floral bud stages (1–5), PhC4H1 transcript accumulation increased 3–4-fold during the fully open flower stages (7–10), while PhC4H2 transcript accumulation increased 6–10-fold during these stages. These results indicate both PhC4H1 and PhC4H2 transcript accumulation profiles are similar to FVBP transcript profiles over the course of flower development in MD.
Fig. 3.
PhC4H1 and PhC4H2 floral developmental transcript accumulation analysis (one-step qRT-PCR). Floral developmental analysis used MD flowers from 11 sequential stages collected on one day at 16.00 h. Gene-specific primers were designed and optimized as well as template concentration, which was identified as 50 ng total RNA per reaction. PhFBP1 and Ph18S were used as endogenous control transcripts. Shown are representative histograms from multiple biological replications using ΔΔCt method (mean ±se; n=3).
Creation of transgenic PhMYB4 RNAi lines
Since the most homologous Arabidopsis protein (AtMYB4) to PhMYB4 is a negative regulator (EAR-domain) of AtC4H transcription, and the PhMYB4 transcriptional profile (Fig. 2) is similar to FVBP genes (i.e. volatile production); it was hypothesized that in MD flower tissue, PhMYB4 functions to fine-tune the carbon flux of the phenylpropanoid pathway via mediating the precise level of C4H protein through transcriptional repression. The choice was made to utilize a reverse genetic approach (RNAi-mediated gene silencing) to test PhMYB4 gene function. This transgenic approach ultimately (see Supplementary Fig. S5 at JXB online) produced two independent T2 homozygous lines with reduced PhMYB4 transcript levels, ir-PhMYB4.12-12 and ir-PhMYB4.60-7.
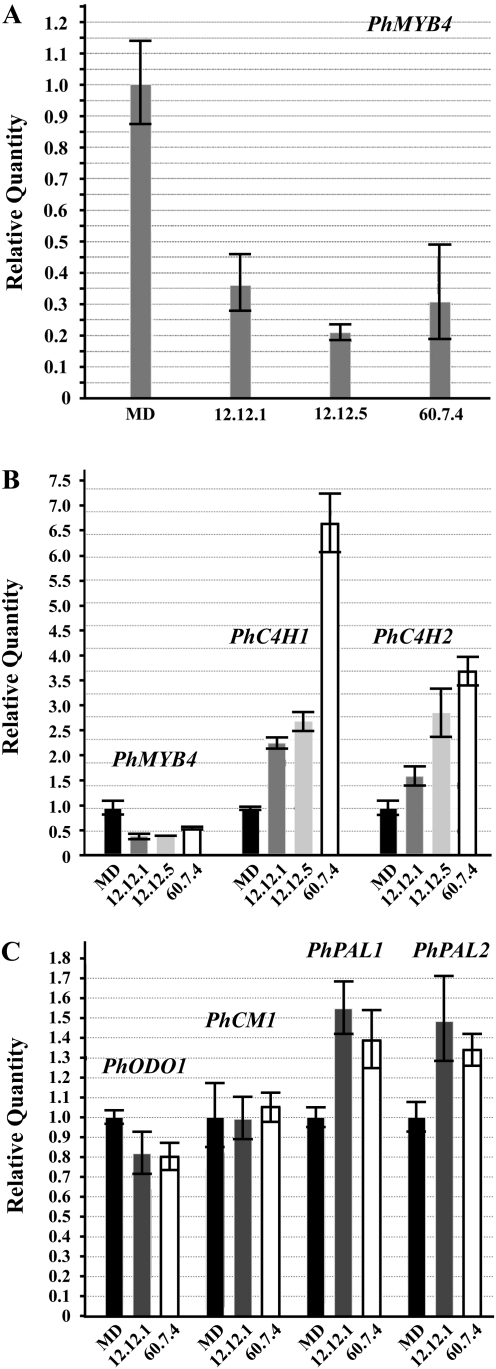
Two-step qRT-PCR (to subtract any RNA product from the transgene) was chosen for a targeted, comparative transcript accumulation analysis between the homozygous ir-PhMYB4 lines (12-12 and 60-7) and MD (Fig. 4). Compared with MD, PhMYB4 transcript accumulation was reduced in whole flowers of 12-12 and 60-7 by approximately 75% (Fig. 4A). To test if PhMYB4 functions similarly to AtMYB4, PhC4H1 and PhC4H2 transcripts were measured. Both transcripts were detected at significantly higher levels in the ir-PhMYB4 flowers compared with MD (Fig. 4B). Since it was apparent that the transcriptional profile of genes theoretically responsible for the second step in the phenylpropanoid pathway had been perturbed, the transcript levels of specific published genes associated with the phenylpropanoid pathway in petunia were analysed (Fig. 4C). The first step of the phenylpropanoid pathway, PHENYLALANINE AMMONIA-LYASE [PhPAL1 (AY705976)and PhPAL2 (CO805160)] transcripts were detected at modestly higher levels in the ir-PhMYB4 lines, but not to the same magnitude as PhC4Hs. CHORISMATE MUTASE1 [PhCM1 (EU751616)] transcript accumulation, a gene responsible for the initial commitment of metabolites into the phenylpropanoid pathway (Colquhoun et al., 2010b), was unaltered between MD and the ir-PhMYB4 lines. PhODO1 transcripts were detected at slightly reduced levels in the ir-PhMYB4 lines compared with MD, but the reduction was marginal.
Fig. 4.
Comparative transcript accumulation analysis between MD and the homozygous ir-PhMYB4 lines (two-step qRT-PCR). All cDNA templates were 1/10 dilutions of cDNA stock samples generated from 2 μg total RNA isolated from stage 8 flowers at 16.00 h. Histograms are representative of multiple experiments and multiple biological replicates, and analysed by ΔΔCt method with PhFBP1 and Ph18S as the internal references. Individual petunia transcripts analysed are PhMYB4 (A, B); PhC4H1 and PhC4H2 (B); PhODO1, PhCM1, PhPAL1, and PhPAL2 (C).
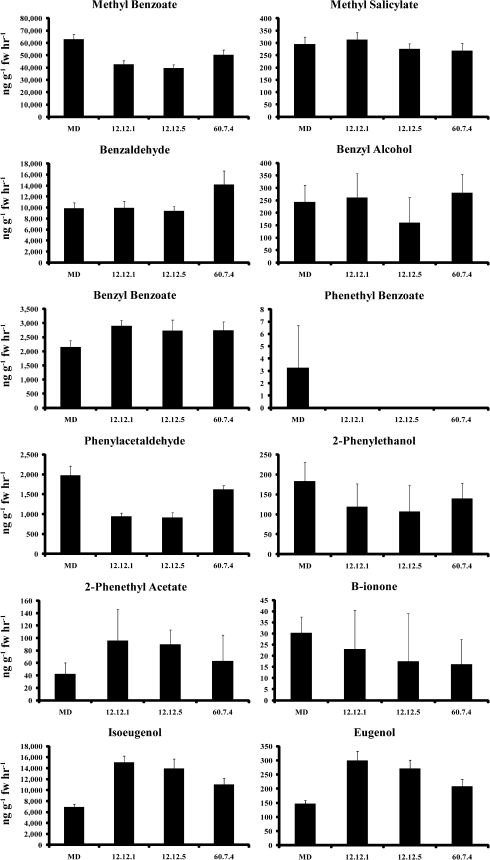
In transgenic ir-PhMYB4 petunia flowers, a reduction of PhMYB4 transcript level is accompanied by an increase in PhC4H1 and PhC4H2 transcript levels. However, PhCM1 transcripts are unaltered (Fig. 4). If the overall metabolite flux into the phenylpropanoid pathway (CM) is consistent between MD and the ir-PhMYB4 lines, and the protein levels of a specific enzymatic step separating the simple benzenoids from phenylpropanoids (C4H) is elevated; the overall FVBP signature may be manipulated in a way which does not simply decrease total substrate pools or remove a specific volatile compound. Instead, a comparable shift in volatile compounds derived from t-cinnamic acid to volatile compounds ultimately derived from p-coumaric acid may be observed. To test this hypothesis, emitted FVBPs were collected and analyzed from MD and the two ir-PhMYB4 lines 12-12 and 60-7 (Fig. 5). Focusing on the major compounds from each branch on the FVBP pathway (Fig. 6): phenylacetaldehyde, methyl benzoate, isoeugenol, the hypothesis appears supported. The ir-PhMYB4 lines emit less phenylacetaldehyde and methyl benzoate compared with MD, while emission of isoeugenol is elevated by approximately 2-fold (Fig. 5). Taken together, these data suggest that in the ir-PhMYB4 lines, increased levels of PhC4H1 and PhC4H2 transcripts result in elevated emission of volatile compounds after the C4H enzymatic step at the expense of volatile compounds before the C4H step (Fig. 6).
Fig. 5.
Floral volatile emission analysis of representative plants from two independent T2 ir-PhMYB4 lines (12-12 and 60-7). Developmentally staged flowers (stage 8) were used to collect FVBP emission at 18.00 h (mean ±se; n=3). Twelve major FVBP compounds were identified and quantified with all measurements ng g−1 fresh weight h−1.
Fig. 6.
A condensed, schematic representation of the FVBP pathway beginning at phenylalanine in petunia. FVBP production consists of three main branch-points; phenylalanine, t-cinnamic acid, and ferulic acid. Floral volatile compounds derived from each branch-point are highlighted in pink and proteins are in red. Multiple arrows indicate multiple biochemical steps.
Discussion
In MD, FVBP biosynthesis is both complex and co-ordinately controlled. Regulation of gene transcription is a key aspect of control (Verdonk et al., 2005), which appears conserved and can be utilized to screen candidate genes involved in the production of FVBPs in petunia (Colquhoun et al., 2010a, b). To date, a few transcription factors have been identified and empirically shown to be associated with the production of FVBPs in petunia. In Arabidopsis, the R2R3-MYB transcription factor family consists of 126 genes with functions of these individual gene products varying from phenylpropanoid metabolism to cell shape (Stracke et al., 2001). It was originally hypothesized that multiple transcription factors, namely R2R3-MYBs, would be involved in the transcriptional regulation of the petunia FVBP genes from the shikimate pathway to the end of the FVBP biosynthetic pathway. Through the work presented here, it is shown that there is at least one more R2R3-MYB transcription factor involved in the molecular regulation imparted upon the FVBP gene network in MD flowers, PhMYB4.
Every resource at hand was searched for sequence information from species of petunia which demonstrated similarity to any of the 126 Arabidopsis R2R3-MYB transcription factors. Next, all the available sequences were basically assembled (Vector NTI) into Contigs (Table 1). A specific contig representing PhMYB4 was then validated experimentally.
AtMYB4 indirectly regulates the accumulation of hydroxycinnamate esters (function as UV protectants) in Arabidopsis leaf tissue by negatively affecting transcription of AtC4H and itself (Jin et al., 2000; Zhao et al., 2007). C4H is a P450 monooxygenase family member that catalyses an oxidative reaction fundamental to the core of the phenylpropanoid pathway that uses t-cinnamic acid as a substrate and produces p-coumaric acid (Mizutani et al., 1997; Anterola et al., 2002). In petunia, FVBPs are all derived from an initial metabolite in the phenylpropanoid pathway, phenylalanine (Boatright et al., 2004; Schuurink et al., 2006) (Fig. 6). It was initially hypothesized that PhMYB4 may repress all or part of the FVBP gene network transcription throughout the bud stages of flower development or during the morning hours of a daily time-course (Colquhoun et al., 2010a). However, our hypothesis was not supported when the PhMYB4 expression profile was examined. PhMYB4 transcript accumulation was similar to that of qualified FVBP genes through multiple categories with the highest relative accumulation in petal limb tissue during the open flower stages (Fig. 2). Interestingly, PhMYB4 transcripts were also detected at high levels in the sexual organs (Fig. 2A); however, the sexual organs of the petunia flower do not emit appreciable levels of FVBP compounds (Underwood et al., 2005). Focusing on the context of AtMYB4 function enables us to refine the original hypothesis. AtMYB4 impedes AtC4H transcription. The Arabidopsis leaf cell that requires the accumulation of UV-B protectants reduces the level of AtMYB4 transcripts and AtC4H transcription is elevated ultimately to provide high amounts of hydroxycinnamate esters derived from p-coumaric acid (Jin et al., 2000). These results and concepts led us to hypothesize that PhMYB4 partially represses PhC4H transcription, modulating the balance of FVBP emission between compounds derived from t-cinnamic acid and p-coumaric acid in MD.
In order to examine this hypothesis, complete cDNA sequences of PhC4H1 and PhC4H2 were isolated from MD flower tissue (see Supplementary Fig. S2 at JXB online). The predicted protein sequences for both PhC4Hs are highly conserved with published C4H sequences from numerous plant species (see Supplementary Fig. S3 at JXB online; Table 2). Moreover, both petunia C4H transcripts accumulate throughout flower development consistent with the increased requirement for phenylpropanoid metabolites after anthesis (Fig. 3).
To test the function of PhMYB4 directly, multiple independent, homozygous transgenic PhMYB4 RNAi lines (ir-PhMYB4 12-12 and 60-7) were generated in the MD genetic background. The PhMYB4 3' sequence used to create these lines was quite divergent from any other petunia R2R3-MYB sequence that was recovered in all the public petunia EST databases. Transcript accumulation of multiple other R2R3-MYB sequences throughout the generation of the homozygous ir-PhMYB4 lines by was analysed sqRT-PCR and a reduction of transcript accumulation was not detected except what is shown for PhODO1 (Fig. 4C). Also, the corresponding RNAi fragment sequence of AtMYB4 is 53.6% identical to the next most similar sequence from Arabidopsis, contained in AtMYB7. Together, these findings suggest only PhMYB4 transcript accumulation was directly affected by the introduction of the ir-PhMYB4 RNAi fragment.
PhMYB4 transcript levels are reduced by approximately 80% in the ir-PhMYB4 12-12 line (Fig. 4A) with a concomitant 2–3-fold increase in PhC4H1 and PhC4H2 transcript levels (Fig. 4B). These straightforward molecular data suggest that, in flower tissue of MD plants, PhMYB4 functions much like AtMYB4 in a basic negative association to C4H transcript accumulation. PhPAL1 and PhPAL2 transcripts were also detected at an elevated level in the ir-PhMYB4 lines compared with MD, but not to the same extent (Fig. 4C). Various possibilities could explain the increase in PAL transcript in the transgenic plants such as: PhMYB4 also represses petunia PALs, or the petunia PALs and C4Hs are involved in a positive linear relationship as is the case in Arabidopsis (Borevitz et al., 2000). In either case, this work and multiple recent publications (Colquhoun et al., 2010b; Maeda et al., 2010) suggest that the enzymatic properties of the individual petunia PAL and C4H isoforms may dictate the general metabolic status of FVBP production in MD.
A demonstrated increase in gene transcript accumulation is not enough to conclude the corresponding protein and/or activity level is elevated. MD flowers present a unique environment to deduce protein activities and/or contributions through commonly derived or ‘downstream’ products, emitted FVBPs. If a reduction in PhMYB4 transcript equates to increases in PhC4H1 and PhC4H2 protein levels with an accessory elevation in activity, increases in down-stream FVBP emissions could be a plausible result of such molecular and biochemical alterations, pending the capacity of the metabolite flux through the specific section of the FVBP pathway is not a limiting factor. Indeed, the phenylpropanoids isoeugenol and eugenol are emitted at higher levels in the ir-PhMYB4 lines compared with MD (Fig. 5), while methyl benzoate (a large quantity benzenoid derived from t-cinnamic acid) emission is reduced in the ir-PhMYB4 lines (Fig. 5). Together, these data not only indicate that an increase in PhC4H transcript accumulation leads to an increase in PhC4H activity (note: individual contributions of PhC4H1 or PhC4H2 cannot be discerned), but that an increase in PhC4H activity results in elevated quantities of metabolites derived from the product of the C4H reaction at the expense of metabolites derived from the substrate and product of the PAL reaction (Figs 5, 6). Thus, PhMYB4 function (Fig. 6) is necessary for the characteristic FVBP signature emitted by MD, and PhMYB4 fine-tunes the FVBP signature at the level of C4H.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Fig. S1. A schematic representation of individual EST consensus sequences from divergent databases and petunia species to generate ‘Quasi-Contigs’ using the ContigExpress® module of Vector NTI Advance™ 11 software.
Supplementary Fig. S2. A nucleotide alignment of PhC4H1 and PhC4H2 using the Align X module of Vector NTI Advance™ 11 software.
Supplementary Fig. S3. Predicted amino acid sequence alignment of homologous CINNAMATE-4-HYDROXYLASE proteins from various species.
Supplementary Fig. S4. Schematic representation of the PhMYB4 gene model as shown in Vector NTI Advance™ 11.
Supplementary Fig. S5. sqRT-PCR transcript accumulation analysis in floral tissues of independent T0 PhMYB4-RNAi lines and MD plants.
Acknowledgments
This work was supported by grants from the USDA Nursery and Floral Crops Initiative, the Fred C Gloeckner Foundation, and the Florida Agricultural Experiment Station.
References
- Anterola AM, Jeon JH, Davin LB, Lewis NG. Transcriptional control of monolignol biosynthesis in Pinus taeda: factors affecting monolignol ratios and carbon allocation in phenylpropanoid metabolism. Journal of Biological Chemistry. 2002;277:18272–18280. doi: 10.1074/jbc.M112051200. [DOI] [PubMed] [Google Scholar]
- Boatright J, Negre F, Chen X, Kish CM, Wood B, Peel G, Orlova I, Gang D, Rhodes D, Dudareva N. Understanding in vivo benzenoid metabolism in petunia petal tissue. Plant Physiology. 2004;135:1993–2011. doi: 10.1104/pp.104.045468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C. Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. The Plant Cell. 2000;12:2383–2394. doi: 10.1105/tpc.12.12.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun TA, Schimmel BC, Kim JY, Reinhardt D, Cline K, Clark DG. A petunia chorismate mutase specialized for the production of floral volatiles. The Plant Journal. 2010b;61:145–155. doi: 10.1111/j.1365-313X.2009.04042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun TA, Verdonk JC, Schimmel BC, Tieman DM, Underwood BA, Clark DG. Petunia floral volatile benzenoid/phenylpropanoid genes are regulated in a similar manner. Phytochemistry. 2010a;71:158–167. doi: 10.1016/j.phytochem.2009.09.036. [DOI] [PubMed] [Google Scholar]
- Dexter R, Qualley A, Kish CM, Ma CJ, Koeduka T, Nagegowda DA, Dudareva N, Pichersky E, Clark D. Characterization of a petunia acetyltransferase involved in the biosynthesis of the floral volatile isoeugenol. The Plant Journal. 2007;49:265–275. doi: 10.1111/j.1365-313X.2006.02954.x. [DOI] [PubMed] [Google Scholar]
- Dexter RJ, Verdonk JC, Underwood BA, Shibuya K, Schmelz EA, Clark DG. Tissue-specific PhBPBT expression is differentially regulated in response to endogenous ethylene. Journal of Experimental Botany. 2008;59:609–618. doi: 10.1093/jxb/erm337. [DOI] [PubMed] [Google Scholar]
- Dudareva N, Negre F, Nagegowda D, Orlova I. Plant volatiles: recent advances and future perspectives. Critical Reviews in Plant Science. 2006;25:417–440. [Google Scholar]
- Jin H, Cominelli E, Bailey P, Parr A, Mehrtens F, Jones J, Tonelli C, Weisshaar B, Martin C. Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO Journal. 2000;19:6150–6161. doi: 10.1093/emboj/19.22.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen RA, Cluster PD, English J, Que Q, Napoli CA. Chalcone synthase cosuppression phenotypes in petunia flowers: comparison of sense vs. antisense constructs and single-copy vs. complex T-DNA sequences. Plant Molecular Biology. 1996;31:957–973. doi: 10.1007/BF00040715. [DOI] [PubMed] [Google Scholar]
- Kaminaga Y, Schnepp J, Peel G, et al. Plant phenylacetaldehyde synthase is a bifunctional homotetrameric enzyme that catalyzes phenylalanine decarboxylation and oxidation. Journal of Biological Chemistry. 2006;281:23357–23366. doi: 10.1074/jbc.M602708200. [DOI] [PubMed] [Google Scholar]
- Knudsen JT, Eriksson R, Gershenzon J, Stahl B. Diversity and distribution of floral scent. Botanical Reviews. 2006;72:1–120. [Google Scholar]
- Koeduka T, Fridman E, Gang DR, et al. Eugenol and isoeugenol, characteristic aromatic constituents of spices, are biosynthesized via reduction of a coniferyl alcohol ester. Proceedings of the National Academy of Sciences, USA. 2006;103:10128–10133. doi: 10.1073/pnas.0603732103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeduka T, Louie GV, Orlova I, et al. The multiple phenylpropene synthases in both Clarkia breweri and Petunia hybrida represent two distinct protein lineages. The Plant Journal. 2008;54:362–374. doi: 10.1111/j.1365-313X.2008.03412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosova N, Gorenstein N, Kish CM, Dudareva N. Regulation of circadian methyl benzoate emission in diurnally and nocturnally emitting plants. The Plant Cell. 2001;13:2333–2347. doi: 10.1105/tpc.010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MM, Schiefelbein J. WEREWOLF, a MYB-related protein in Arabidopsis, is a position-dependent regulator of epidermal cell patterning. Cell. 1999;99:473–483. doi: 10.1016/s0092-8674(00)81536-6. [DOI] [PubMed] [Google Scholar]
- Maeda H, Shasany AK, Schnepp J, Orlova I, Taguchi G, Cooper BR, Rhodes D, Pichersky E, Dudareva N. RNAi suppression of Arogenate Dehydratase1 reveals that phenylalanine is synthesized predominantly via the arogenate pathway in petunia petals. The Plant Cell. 2010;22:832–849. doi: 10.1105/tpc.109.073247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M, Ohta D, Sato R. Isolation of a cDNA and a genomic clone encoding cinnamate 4-hydroxylase from Arabidopsis and its expression manner in planta. Plant Physiology. 1997;113:755–763. doi: 10.1104/pp.113.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A, Melnyk CW, Bassett A, Hardcastle TJ, Dunn R, Baulcombe DC. Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science. 2010;328:872–875. doi: 10.1126/science.1187959. [DOI] [PubMed] [Google Scholar]
- Negre F, Kish CM, Boatright J, Underwood B, Shibuya K, Wagner C, Clark DG, Dudareva N. Regulation of methylbenzoate emission after pollination in snapdragon and petunia flowers. The Plant Cell. 2003;15:2992–3006. doi: 10.1105/tpc.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova I, Marshall-Colon A, Schnepp J, et al. Reduction of benzenoid synthesis in petunia flowers reveals multiple pathways to benzoic acid and enhancement in auxin transport. The Plant Cell. 2006;18:3458–3475. doi: 10.1105/tpc.106.046227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Fujioka S, Peng J, Chen J, Li G, Chen R. The E3 ubiquitin ligase SCFTIR1/AFB and membrane sterols play key roles in auxin regulation of endocytosis, recycling, and plasma membrane accumulation of the auxin efflux transporter PIN2 in Arabidopsis thaliana. The Plant Cell. 2009;21:568–580. doi: 10.1105/tpc.108.061465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E, Noel JP, Dudareva N. Biosynthesis of plant volatiles: nature's diversity and ingenuity. Science. 2006;311:808–811. doi: 10.1126/science.1118510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocchio F, Verweij W, Kroon A, Spelt C, Mol J, Koes R. PH4 of petunia is an R2R3 MYB protein that activates vacuolar acidification through interactions with basic-helix-loop-helix transcription factors of the anthocyanin pathway. The Plant Cell. 2006;18:1274–1291. doi: 10.1105/tpc.105.034041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurink RC, Haring MA, Clark DG. Regulation of volatile benzenoid biosynthesis in petunia flowers. Trends in Plant Science. 2006;11:20–25. doi: 10.1016/j.tplants.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Spitzer-Rimon B, Marhevka E, Barkai O, Marton I, Edelbaum O, Masci T, Prathapani NK, Shklarman E, Ovadis M, Vainstein A. EOBII, a gene encoding a flower-specific regulator of phenylpropanoid volatiles' biosynthesis in petunia. The Plant Cell. 2010;22:1961–1976. doi: 10.1105/tpc.109.067280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshaar B. The R2R3-MYB gene family in Arabidopsis thaliana. Current Opinion in Plant Biology. 2001;4:447–456. doi: 10.1016/s1369-5266(00)00199-0. [DOI] [PubMed] [Google Scholar]
- Underwood BA, Tieman DM, Shibuya K, Dexter RJ, Loucas HM, Simkin AJ, Sims CA, Schmelz EA, Klee HJ, Clark DG. Ethylene-regulated floral volatile synthesis in petunia corollas. Plant Physiology. 2005;138:255–266. doi: 10.1104/pp.104.051144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Moerkercke A, Schauvinhold I, Pichersky E, Haring MA, Schuurink RC. A plant thiolase involved in benzoic acid biosynthesis and volatile benzenoid production. The Plant Journal. 2009;60:292–302. doi: 10.1111/j.1365-313X.2009.03953.x. [DOI] [PubMed] [Google Scholar]
- Verdonk JC, Haring MA, van Tunen AJ, Schuurink RC. ODORANT1 regulates fragrance biosynthesis in Petunia flowers. The Plant Cell. 2005;17:1612–1624. doi: 10.1105/tpc.104.028837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdonk JC, Ric de Vos CH, Verhoeven HA, Haring MA, van Tunen AJ, Schuurink RC. Regulation of floral scent production in petunia revealed by targeted metabolomics. Phytochemistry. 2003;62:997–1008. doi: 10.1016/s0031-9422(02)00707-0. [DOI] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Clark DG, Bleecker AB, Chang C, Meyerowitz EM, Klee HJ. A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nature Biotechnology. 1997;15:444–447. doi: 10.1038/nbt0597-444. [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhang W, Zhao Y, Gong X, Guo L, Zhu G, Wang X, Gong Z, Schumaker KS, Guo Y. SAD2, an importin-like protein, is required for UV-B response in Arabidopsis by mediating MYB4 nuclear trafficking. The Plant Cell. 2007;19:3805–3818. doi: 10.1105/tpc.106.048900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.