Abstract
Abscisic acid (ABA) controls plant development and regulates plant responses to environmental stresses. A role for ABA in sugar regulation of plant development has also been well documented although the molecular mechanisms connecting the hormone with sugar signal transduction pathways are not well understood. In this work it is shown that Arabidopsis thaliana mutants deficient in plastidial glycolytic glyceraldehyde-3-phosphate dehydrogenase (gapcp1gapcp2) are ABA insensitive in growth, stomatal closure, and germination assays. The ABA levels of gapcp1gapcp2 were normal, suggesting that the ABA signal transduction pathway is impaired in the mutants. ABA modified gapcp1gapcp2 gene expression, but the mutant response to the hormone differed from that observed in wild-type plants. The gene expression of the transcription factor ABI4, involved in both sugar and ABA signalling, was altered in gapcp1gapcp2, suggesting that their ABA insensitivity is mediated, at least partially, through this transcriptional regulator. Serine supplementation was able partly to restore the ABA sensitivity of gapcp1gapcp2, indicating that amino acid homeostasis and/or serine metabolism may also be important determinants in the connections of ABA with primary metabolism. Overall, these studies provide new insights into the links between plant primary metabolism and ABA signalling, and demonstrate the importance of plastidial glycolytic glyceraldehyde-3-phosphate dehydrogenase in these interactions.
Keywords: ABA, ABA signal transduction, Arabidopsis, GAPCp, glyceraldehyde-3-phosphate dehydrogenase, glycolysis, sugar signalling
Introduction
The phytohormone abscisic acid (ABA) controls the development of plants and plays a crucial role in their survival when faced with adverse environmental conditions, such as salt and water stress (Schroeder et al., 2001; Himmelbach et al., 2003). The genetic dissection of ABA signalling has now identified central players of this signal transduction pathway including ABA receptors (Shen et al., 2006; Ma et al., 2009; Melcher et al., 2009; Miyazono et al., 2009; Pandey et al., 2009; Park et al., 2009; Santiago et al., 2009; Cutler et al., 2010; Nishimura et al., 2010), the group A protein phosphatases type 2C, which negatively regulate ABA signalling (Meyer et al., 1994; Leung et al., 1997), and the SNF1-related kinase 2 (SnRK2 kinases) which are positive regulators (Yoshida et al., 2006; Vlad et al., 2009) of downstream substrates such as AREB/ABF transcription factors (Furihata et al., 2006).
A role for ABA in sugar regulation of plant development has been well documented (Arenas-Huertero et al., 2000; Leon and Sheen, 2003; Rolland et al., 2006; Rook et al., 2006). Some of the Arabidopsis sugar signalling mutants are allelic to ABA biosynthetic or signalling mutants, and many ABA biosynthetic mutants also have sugar phenotypes (Arenas-Huertero et al., 2000; Rolland et al., 2006; Ramon et al., 2008). The two main substrates for glycolysis in plants, sucrose and starch (Plaxton, 1996), are subjected to large diurnal changes in their concentrations. Thus, sugar levels and glycolysis need to be highly coordinated to provide metabolic flexibility that can respond to the differential demands of plant development and to environmental stress acclimation. In spite of considerable evidence corroborating the interactions between sugars and ABA, information about the role played by glycolysis in these interactions is lacking.
The most well known function of glycolysis is to provide energy and precursors for anabolic processes (Plaxton, 1996). In recent years, additional non-glycolytic functions, such as the regulation of transcription or apoptosis, have also been attributed to glycolytic enzymes in mammals and yeast (Kim and Dang, 2005). These additional functions of glycolytic enzymes suggest that links between metabolic sensors and development could be established directly through the enzymes that participate in metabolism (Kim and Dang, 2005). In this sense, a role for the plant glycolytic hexokinase as a glucose sensor has been reported (Moore et al., 2003; Ramon et al., 2008). On the other hand, the participation of enolase and aldolase in the plant response to salt tolerance (Barkla et al., 2009) postulates a more direct function for glycolytic enzymes in acclimation to environmental stresses.
The glycolytic glyceraldehyde-3-phosphate dehydrogenase (GAPDH) reversibly converts glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate by coupling with the reduction of NAD+ to NADH. In mammals, GAPDHs have been implicated in transcriptional regulation, DNA repair, signal transduction cascades, and apoptosis (Hara et al., 2005; Kim and Dang, 2005; Hara and Snyder, 2006; Colell et al., 2007; Min et al., 2007; Lee et al., 2009). A family of four genes encoding putative phosphorylating glycolytic GAPDHs (GAPC1, GAPC2, GAPCp1, and GAPCp2) is present in Arabidopsis thaliana (http://www.Arabidopsis.org). GAPC1 and GAPC2 are cytosolic isoforms, while GAPCp1 and GAPCp2 are located in the plastids (Muñoz-Bertomeu et al., 2009). In spite of the low gene expression level of GAPCps in Arabidopsis, gapcp double mutants (gapcp1gapcp2) show profound alterations of plant development, mainly arrested root development and sterility (Muñoz-Bertomeu et al., 2009, 2010a, b). The strategic situation of GAPCp in the glycolytic pathway could make this enzyme an important player not only for glycolysis but also for the generation of metabolites for other anabolic pathways (Plaxton, 1996; Ho and Saito, 2001; Andre et al., 2007; Baud et al., 2007). In this same vein, it was previously demonstrated that GAPCp deficiency affected the sugar and amino acid balance in Arabidopsis (Muñoz-Bertomeu et al., 2009). Another pathway that could be affected by GAPCp deficiency is ABA biosynthesis. The substrate of GAPCp, glyceraldehyde-3-phosphate, is the first precursor of the methyl-erythritol phosphate pathway responsible for ABA biosynthesis in the plastids. Here evidence is provided that a deficiency in GAPCp is able to provide insensitivity to ABA. It is demonstrated that this insensitivity is not due to a modification of the ABA content but to an impaired ABA signal transduction in the GAPCp-deficient mutant. It is shown that the gapcp1gapcp2 ABA insensitivity is caused by the sugar/amino acid imbalance displayed by the mutant. The ABA insensitivity of gapcp1gapcp2 could be mediated, at least in part, via the transcriptional regulator ABI4. A specific role for amino acid homeostasis and/or serine metabolism in the ABA–sugar interaction is also discussed. This work provides new evidence for the links between plant primary metabolism and ABA signal transduction, and demonstrates the importance of GAPCps in these interactions.
Materials and methods
Plant material and growth conditions
Several A. thaliana (ecotype Columbia 0) gapcp1 and gapcp2 single and double T-DNA mutants were previously isolated as described in Muñoz-Bertomeu et al. (2009). For this work, mutant allele gapcp1.1 (SAIL_390_G10) of the At1g79530 gene, and gapcp2.1 (SALK_137288) and gapcp2.3 (SALK_037936) alleles of the At1g16300 gene were used. Wild-type (WT) plants expressing a GAPCp1 cDNA under the control of the 35S promoter (Oex-GAPCp) were previously obtained as described in Muñoz-Bertomeu et al. (2009).
Unless otherwise stated, seeds were sterilized and sown on 0.8% agar plates containing 1/5 Murashige and Skoog (MS) with Gamborg vitamins, and MES (0.9 g l−1) adjusted to pH 5.7 with TRIS. After a 4 d treatment at 4 °C, seeds were placed in a growth chamber (SANYO, MLR-351H, Japan) at 22 °C for a 16/8 h day/night photoperiod, 100 μmol m−2 s−1. Plates were supplemented with different concentrations of ABA and serine as indicated in the figure legends. Drought stress was imposed by placing the plates under the hood for 4 h before collecting the plants. For dormancy experiments, freshly harvested seeds were sown on plates containing 0.8% agar and 0.9 g l−1 MES adjusted to pH 5.7 with TRIS. Because gapcp1gapcp2 is sterile, a population of seeds from heterozygous plants (GAPCp1gapcp1.1 gapcp2.1gapcp2.1) was used for the germination experiments. This population, named G1g1.1 g2.1g2.1, contained 25% of double mutants (gapcp1.1gapcp1.1 gapcp2.1gapcp2.1), 50% of heterozygous plants (GAPCp1gapcp1.1 gapcp2.1gapcp2.1), and 25% of single mutant plants (GAPCp1GAPCp1 gapcp2.1gapcp2.1). To score seed germination, the percentage of seeds that had germinated and developed fully green expanded cotyledons was determined. For the rest of the physiological and molecular studies, double homozygous gapcp1gapcp2 plants were identified in segregating populations of vertically grown plants by the root phenotype after 7–10 d as described (Muñoz-Bertomeu et al., 2009). Then they were subcultured on media containing different concentrations of ABA or serine for an additional 8–13 d as indicated in the figure legends and the tables. For stomata bioassay, some plantlets were grown in a Conviron growth chamber in pots filled with a (1:1, v/v) mixture of vermiculite and fertilized peat (KEKILA 50/50; kekkilä Iberia, S.L.) irrigated with demineralized water as needed.
Stomata bioassays
Stomatal responses were analysed in 3- to 4-week-old plants. Leaves were floated for 2.5 h in stomatal opening solution containing 50 mM KCl, 7 mM iminodiacetic acid, and 10 mM MES (pH 6.2). After incubation in 1 μM and 10 μM ABA for 2 h, leaf epidermal peels were obtained and the stomatal aperture was measured as described (Pei et al., 1997). Control experiments were performed in parallel with no ABA added. t-Test (one-tailed, homoscedastic) P-values were calculated.
Microarrays
Fifteen day-old seedlings (gapcp1gapcp2 and WT) vertically grown in 1/5 MS plates were used for the microarray experiments. On day 8 seedlings were subcultured on media containing 0.75 μM ABA as described above. Total RNAs from three pools of 15-day-old seedlings (gapcp1gapcp2 and WT) vertically grown in 1/5 MS plates at the same time were extracted using the RNeasy plant mini kit (Qiagen). RNA integrity was determined using RNA 6000 Nano Labchips® in an Agilent 2100 Bioanalyzer following the manufacturer's protocol. The purified RNA (8 μg) was used to generate first-strand cDNA in a reverse transcription reaction (One-Cycle Target Labeling and Control Reagents; Affymetrix). After second-strand synthesis, the double-stranded cDNAs were used to generate cRNA via an in vitro transcription reaction. The cRNA was labelled with biotin, and 20 μg of the labelled cRNA was fragmented. The size distribution of the cRNAs and fragmented cRNAs was assessed using an Eppendorf Biophotometer and electrophoresis. Fragmented cRNA (15 μg) was added to 300 μl of hybridization solution, and 200 μl of this mixture was used for hybridization on Arabidopsis ATH1 Genome Arrays for 16 h at 45 °C. The standard wash and double-stain protocols (EukGE-WS2v5-450) were applied using an Affymetrix GeneChip Fluidics Station 450. The arrays (three replicates of each line) were scanned on an Affymetrix GeneChip scanner 3000. Fluorescence images were normalized with the software GCOS from Affymetrix. Expression data of ABA-treated seedlings were compared with those obtained from the non-ABA-treated controls grown under the same conditions and at the same time (Muñoz-Bertomeu et al., 2009). Data were analysed and compared using the dChip software (Li, 2008; http://www.hsph.harvard.edu/∼cli/complab/dchip/) using a threshold criterion of 2-fold; P <0.05. All generated data were uploaded to Gene Expression Omnibus (accession no. GSE21765) at NCBI.
Q-RT-PCR
Total RNA was extracted from seedlings as for microarray experiments. RNA was treated with RNase-free DNase (Promega). Then, 0.5 μg of RNA was reverse transcribed using poly(T) primers and the first-strand cDNA synthesis Kit for RT-PCR (Roche) according to the manufacturer's instructions. Real-time quantitative PCR was performed using a 5700 sequence detector system (Applied Biosystems) with the Power SYBR Green PCR Master Mix (Applied Biosystems) according to the manufacturer's protocol. Each reaction was performed in triplicate with 1 μl of the first-strand cDNA in a total volume of 25 μl. Data are the mean of at least three biological samples. The specificity of the PCR amplification was confirmed with a heat dissociation curve (from 60 °C to 95 °C). Different internal standards were selected (Czechowski et al., 2005) depending on the efficiency of the primers. Relative mRNA abundance was calculated using the comparative Ct method according to Pfaffl (2001). Primers used for quantitative RT-PCR (Q-RT-PCR) are listed in Supplementary Table S1 available at JXB online.
Metabolite determination
Starch and total soluble glucose were determined with the ENZYTEC starch kit (ATOM). ABA content was determined by liquid chromatography-electrospray tandem mass spectrometry (Durgbanshi et al., 2005). Total glutathione was extracted and subsequently quantified by reverse-phase HPLC after derivatization with monobromobimane (Molecular Probes) following previously described methods (Dominguez-Solis et al., 2001)
Results
gapcp1gapcp2 are ABA insensitivity
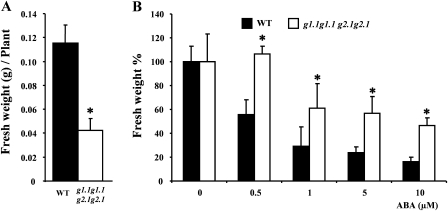
At the seedling stage, gapcp1gapcp2 are smaller than WT plants when grown in a medium without ABA (Fig. 1A). The fresh weight of 3-week-old gapcp1gapcp2 plants grown in MS medium was almost three times lower than that of the WT (Fig. 1A). However, gapcp1gapcp2 showed less sensitivity to growth inhibition by ABA. While WT growth was inhibited by ∼50% by 0.5 μM ABA, the growth of gapcp1gapcp2 was not significantly affected by this concentration (Fig. 1B). A 2-fold higher ABA concentration (1 μM) was necessary to achieve the same level of growth inhibition in gapcp1gapcp2 when compared with the WT (Fig. 1B).
Fig. 1.
The gapcp1gapcp2 mutant shows ABA insensitivity to growth. (A) Fresh weight of the aerial part of 3-week-old gapcp1gapcp2 seedlings (g1.1g1.1 g2.1g2.1) as compared with the wild type (WT). (B) Fresh weight (%) of the aerial part of 3-week-old gapcp1gapcp2 seedlings (g1.1g1.1 g2.1g2.1) as compared with the WT under different ABA concentrations. Seedlings were treated for 13 d with the ABA concentrations indicated in the figure. Data are the mean ±SD, n ≥5 plates, each plate containing four (WT) and six (g1.1g1.1 g2.1g2.1) plants. *Significant at a P-value <0.05 as compared with the WT. For simplicity, g stands for gapcp.
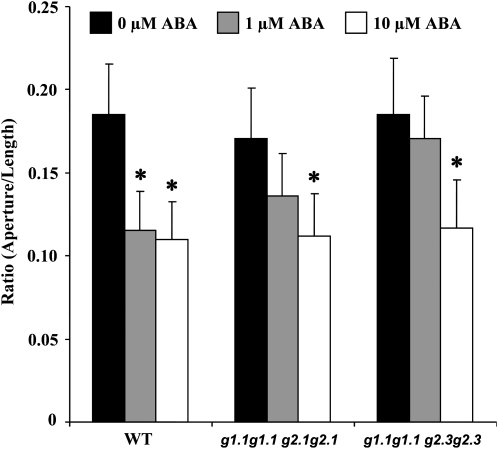
It may be argued that gapcp1gapcp2 ABA growth insensitivity could be associated with the slower growth rate of the mutant under non-ABA conditions as compared with the WT. To rule out this possibility other typical ABA responses were examined. Stomata bioassay experiments revealed that gapcp1gapcp2 responds to ABA by closing stomata, but the response needs a higher ABA concentration if compared with the response of the WT (Fig. 2).
Fig. 2.
The gapcp1gapcp2 mutant shows ABA insensitivity to stomatal closing. ABA-induced stomatal closing in the wild type (WT) and in two different alleles of gapcp1gapcp2 (g1.1g1.1 g2.1g2.1, g1.1g1.1 g2.3g2.3) plants. Data are the mean ±SD, n=30–40 stomata per experiment. *Significant at a P-value <0.05 as compared with non-ABA-treated plants. For simplicity, g stands for gapcp.
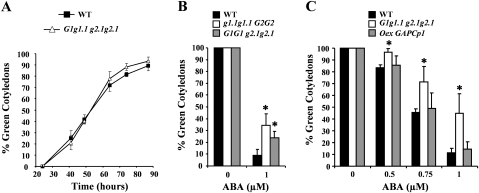
Seed germination assays were also performed. Since gapcp1gapcp2 seeds are sterile, single gapcp mutants (gapcp1 and gapcp2) and a mixed population of GAPCp-deficient seeds (single mutant, double mutant, and heterozygous), named G1g1.1 g2.1g2.1, originating from heterozygous mother plants (GAPCp1gapcp1gapcp2gapcp2), were used in these experiments. Seed dormancy was not altered by GAPCp deficiency as compared with the WT (Fig. 3A). However, germination of GAPCp-deficient seeds was insensitive to ABA. The ABA insensitivity phenotype was already observed in gapcp single mutants (Fig. 3B) and this phenotype was also observed in G1g1.1 g2.1g2.1 (Fig. 3C). Plants overexpressing GAPCp1 (Oex-GAPCp1) did not show hypersensitivity to ABA (Fig. 3C), which suggests that either only loss of GAPCp1 function affects the ABA response or that other compensatory and/or regulatory mechanisms are responsible for this lack of phenotype.
Fig. 3.
The gapcp1gapcp2 mutant shows ABA insensitivity during germination. (A) Germination of the wild type (WT) and a mixed population of GAPCp-deficient (double mutant, heterozygous and single mutant plants; G1g1.1 g2.1g2.1) seeds. Fresh seeds without a cold pre-treatment were plated in agar plates containing 0.2 g l−1 MES. (B) Germination of 10-day-old seeds from the WT and single mutants of GAPCp1 (g1.1g1.1 G2G2) and GAPCp2 (G1G1 g2.1g2.1) with and without 1 μM ABA. (C) Germination of 10-day-old WT seeds, a mixed population of GAPCp-deficient seeds (double mutant, heterozygous, and single mutant; G1g1.1 g2.1g2.1), and GAPCp1-overexpressing (Oex-GAPCp1) seeds under different ABA concentrations. Data are the mean ±SD; each experiment consisted of at least four plates with 60 seeds each. Data from Oex-GAPCp1 are from a representative line, but similar results were obtained with two other T3 overexpressing lines. The experiment was repeated several times with different pools of seeds. *Significant at a P-value <0.05 as compared with WT seeds. For simplicity, g stands for gapcp and G for GAPCp.
ABA and glutathione levels are not modified in gapcp1gapcp2
The ABA-insensitive phenotype of gapcp1gapcp2 may indicate that this mutant is ABA deficient. The methyl-erythritol phosphate pathway responsible for ABA biosynthesis is located in the plastids. The first precursor in this pathway is glyceraldeyde-3-phosphate, the substrate of GAPCp. The ABA content in the aerial part of gapcp1gapcp2 did not differ significantly from that of the WT under non-drought stress conditions. In addition, the mutant was able to respond to imposed drought stress by increasing the ABA content similarly to the WT (Table 1).
Table 1.
ABA (ng g−1 DW) and glutathione (nmol g−1 DW) content in the wild-type (WT) and gapcp1gapcp2 (g1.1g1.1 g2.1g2.1) with or without drought stress
| Lines | ABA | Glutathione |
|
| Aerial part | Aerial part | Roots | |
| WT (control) | 26.6±1.4 | 2454±169 | 1586±57 |
| g1.1g1.1 g2.1g2.1 (control) | 24.1±1.7 | 2513±128 | 1627±81 |
| WT (drought stress) | 179.8±5.3 | – | – |
| g1.1g1.1 g2.1g2.1 (drought stress) | 173.8±4.6 | – | – |
Three-week-old plants were grown in plates. For drought stress, plates were left open under the hood for 4 h before harvesting. Results are the mean ±SD, n ≥3.
gapcp1gapcp2 roots display serine deficiency (Muñoz-Bertomeu et al., 2009). Since serine is the precursor of glycine and cysteine, both of which form part of the gluthatione molecule, the levels of this metabolite were also measured. The glutathione contents in both the aerial part and the roots of gapcp1gapcp2 did not differ significantly from those of the WT (Table 1).
gapcp1gapcp2 plants display an altered gene expression in response to ABA
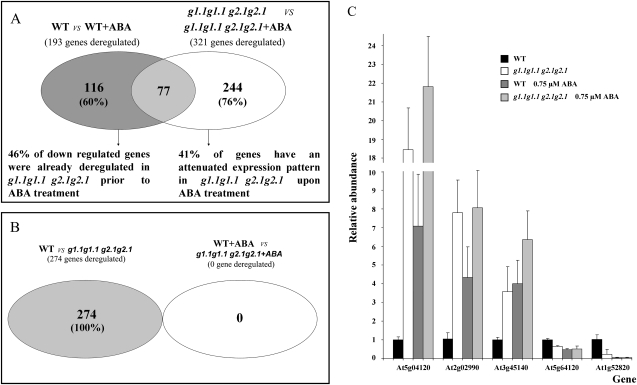
To assess the effect of ABA on the gene expression of gapcp1gapcp2, a microarray analysis comparing gapcp1gapcp2 and the WT after ABA treatment was performed. These data were compared with those obtained under identical conditions with non-ABA-treated plants (Muñoz-Bertomeu et al., 2009). Full details of the microarray analysis are provided in Supplementary Table S2 at JXB online.
ABA treatment deregulated 321 genes (a >2-fold difference relative to the WT; P <0.05) in gapcp1gapcp2 as compared with the non-ABA-treated mutants and 193 genes in the WT as compared with the non-ABA-treated WT (Fig. 4A, and Supplementary Table S2 at JXB online). ABA-treated WT and gapcp1gapcp2 only shared 24% of the deregulated genes. Thus, the gene expression response of gapcp1gapcp2 to ABA differed from that of the WT, and could be related to the insensitivity phenotypes observed in the mutant.
Fig. 4.
ABA gene expression is deregulated in gapcp1gapcp2. (A) Comparison of the wild type (WT) versus WT+ABA and gapcp1gapcp2 (g1.1g1.1 g2.1g2.1) versus g1.1g1.1 g2.1g2.1+ABA. (B) Comparison of the WT versus g1.1g1.1 g2.1g2.1 and WT+ABA versus g1.1g1.1 g2.1g2.1+ABA. (C) Quantification of transcript level changes in g1.1g1.1 g2.1g2.1 upon ABA treatment. RNA was extracted from 18-day-old gapcp1gapcp2 and WT seedlings grown in the presence or absence of 0.75 μM ABA for 10 d. The expression of a selection of up- and down-regulated genes in gapcp1gapcp2 was quantified using real-time PCR. Data are the mean ±SD; n=3. For simplicity, g stands for gapcp.
More than 270 genes were deregulated in gapcp1gapcp2 as compared with the WT (Fig. 4B, and Supplementary Table S2). Surprisingly, after long-term ABA treatment there were no significant differences in gene expression between gapcp1gapcp2 and the WT (Fig. 4B). Some of the genes deregulated by ABA in the WT, especially the down-regulated genes (46%), were already deregulated in gapcp1gapcp2 prior to ABA treatment (Fig. 4A). Furthermore, in 41% of genes deregulated in gapcp1gapcp2, differences in gene expression between gapcp1gapcp2 and the WT were attenuated by the hormone treatment (Fig. 4A). The effect of ABA on several genes deregulated in gapcp1gapcp2 was reconfirmed by real-time Q-RT-PCR. As shown in Fig. 4C, the gene expression differences between gapcp1gapcp2 and the WT were attenuated upon ABA treatment.
ABA affects carbohydrate metabolism and deregulates ABI4 expression in gapcp1gapcp2
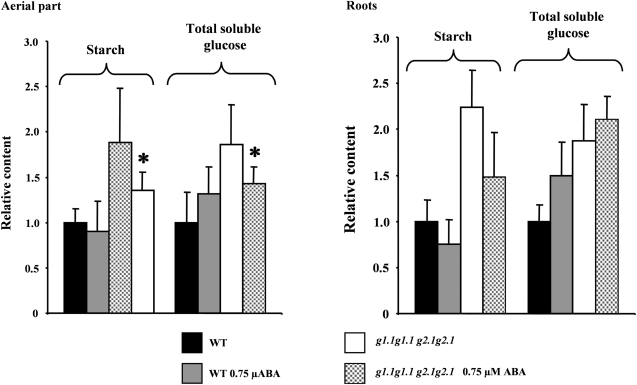
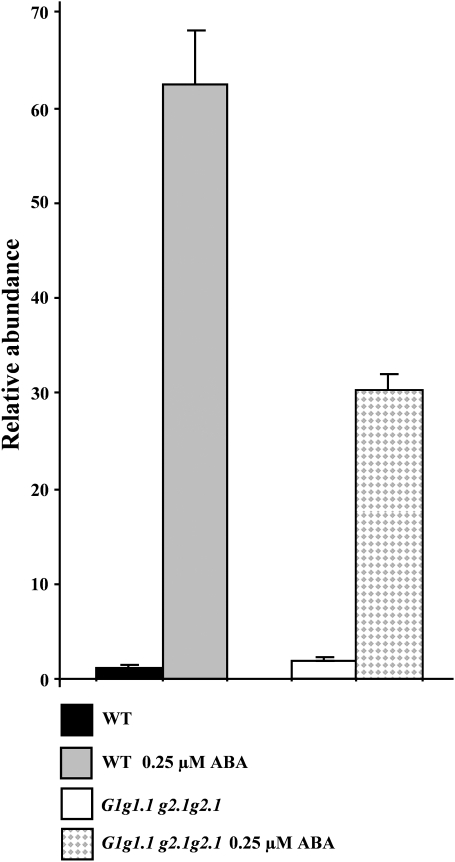
gapcp1gapcp2 present high levels of carbohydrates in both the aerial part and roots as compared with the WT (Muñoz-Bertomeu et al., 2009). ABA treatment reduced both starch and total soluble glucose levels in the aerial part of gapcp1gapcp2 (Fig. 5). The effect of ABA on the root carbohydrate level of gapcp1gapcp2 was not as clear. Although there was a trend to a reduction in the starch and an increase in the glucose content, these changes were not significant (Fig. 5). As the ABA-INSENSITIVE transcription factor ABI4 has been described as a central transcriptional regulator connecting ABA with sugar signalling, the gene expression of ABI4 was investigated in 48 h imbibed seeds (±0.25 μM ABA) from the WT and from a mixed population of GAPCp-deficient seeds (G1g1.1 g2.1g2.1). ABI4 induction by ABA was reduced by GAPCp inactivation as compared with the WT (Fig. 6).
Fig. 5.
ABA reduces sugar content in gapcp1gapcp2. Starch and total soluble glucose in the aerial part and roots of 3-week-old wild-type (WT) and gapcp1gapcp2 (g1.1g1.1 g2.1g2.1) seedlings grown in the presence or absence of 0.75 μM ABA for 10 d. Values were normalized to the mean response of the WT in mg g fresh weight−1 (starch, 1.75±0.33 in the aerial part of the WT, 0.19±0.05 in roots of the WT; soluble glucose, 0.31±0.10 in the aerial part of the WT, 0.87±0.15 in roots of the WT). Data are the mean ±SD, n ≥3;. *Significant at a P-value <0.05 as compared with non-ABA-treated plants. For simplicity, g stands for gapcp.
Fig. 6.
ABI4 expression is deregulated in gapcp1gapcp2. Quantification of changes in ABI4 expression in seeds from the wild type (WT) and from a mixed population deficient in GAPCp (double mutant, heterozygous, and single mutant; G1g1.1 g2.1g2.1). RNA was extracted from seeds which had been imbibed for 48 h and germinated in the presence or absence of 0.25 μM ABA. ABI4 expression was quantified using real-time PCR. Data are the mean ±SD; n=3. For simplicity, g stands for gapcp and G for GAPCp.
ABA insensitivity of gapcp1gapcp2 is partially dependent on serine supply
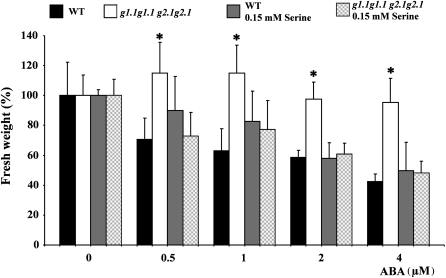
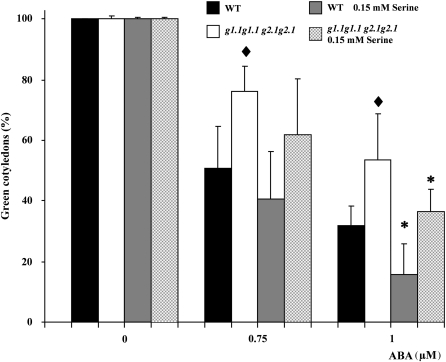
It was previously demonstrated that serine supplementation to the growing medium restored normal carbohydrate levels in gapcp1gapcp2 (Muñoz-Bertomeu et al., 2009). In order to investigate whether the gapcp1gapcp2 ABA insensitivity phenotype may be related to only its impaired carbohydrate metabolism, the ABA phenotypes of gapcp1gapcp2 were investigated in those mutants supplemented with serine. The presence of serine in the growing medium abolished gapcp1gapcp2 insensitivity to growth inhibition by ABA at all the concentrations used (Fig. 7). Serine also significantly reduced the ABA insensitivity of gapcp1gapcp2 during germination (Fig. 8). In the presence of serine, the inhibition by ABA of gapcp1gapcp2 germination did not differ from that of the WT not supplied with serine (Fig. 8). Interestingly, serine supplementation produced hypersensitivity to ABA in both the WT and gapcp1gapcp2 (Fig. 8).
Fig. 7.
gapcp1gapcp2 ABA insensitivity to growth is abolished by serine supplementation. Fresh weight (%) of the aerial part of 18-day-old gapcp1gapcp2 seedlings (g1.1g1.1 g2.1g2.1) as compared with the wild type (WT) under different concentrations of ABA and serine. Seedlings were treated for 9 d with the concentrations of ABA and serine indicated in the figure. Data are the mean ±SD, n ≥5 plates; each plate contained 4 (WT) and six (g1.1g1.1 g2.1g2.1) plants. *Significant at a P-value <0.05 as compared with the WT. For simplicity, g stands for gapcp.
Fig. 8.
Serine supplementation confers hypersensitivity to ABA during germination. Germination of 10-day-old seeds from wild-type (WT) and heterozygous gapcp (G1g1.1 g2.1g2.1) plants under different concentrations of ABA and serine. Data are the mean ±SD; each experiment consisted of at least four plates with 60 seeds each. The experiment was repeated several times with different pools of seeds. •♦Significant at a P-value <0.05 as compared with the non-serine-treated WT seeds. *Significant at a P-value <0.05 as compared with the non-serine-treated seeds within each genotype. For simplicity, g stands for gapcp and G for GAPCp.
Discussion
gapcp1gapcp2 has an impaired ABA signal transduction pathway
gapcp1gapcp2 was insensitive to ABA, but the mutant's dormancy response was not altered as compared with the WT, indicating that the ABA phenotype observed in the mutants is hormone specific. The impaired gapcp1gapcp2 ABA response could indicate an alteration either of ABA biosynthesis or of the hormone signalling. The ABA levels in gapcp1gapcp2 were normal, suggesting that GAPCp affects ABA signal transduction. In this sense, the gene expression response of gapcp1gapcp2 to ABA differs from that of the WT. Thus, GAPCp inactivation does not eliminate gene responses to ABA but modifies them. The altered gene expression upon ABA treatment can be related to the ABA insensitivity of gapcp1gapcp2 and suggests that ABA signal transduction in the mutant is impaired.
The ABA response of gapcp1gapcp2 interferes with carbohydrate metabolism
The gapcp1gapcp2 ABA insensitivity phenotype may relate to its impaired carbohydrate metabolism. Starch levels have been seen to decrease upon stress, such as cold (Nagao et al., 2005), salt (Kempa et al., 2007), or ABA treatment (Akihiro et al., 2005). It could be argued that gapcp1gapcp2 display more ABA insensitivity than the WT because they have a higher starch content than the WT under non-stress conditions. ABA would induce the mobilization of starch to obtain compatible solutes under stress conditions. In fact, the ABA treatment of gapcp1gapcp2 was associated with a reduction in the starch and soluble glucose levels in the aerial part. This result indicates that gapcp1gapcp2 respond to ABA. Furthermore, the results are a direct proof that ABA interferes with starch metabolism.
The transcription factor ABI4 has been implicated in both ABA and sugar signal transduction cascades during early seedling development and could be one of the links between these two pathways (Acevedo-Hernandez et al., 2005; Rook et al., 2006; Bossi et al., 2009). ABI4 has also been implicated in the retrograde signalling between plastids and the nucleus (Koussevitzky, 2007). The induction of the ABI4 gene by ABA in GAPCp-deficient seeds was attenuated as compared with that observed in the WT. These results indicate that the ABA insensitivity of gapcp1gapcp2 may be mediated, at least at the initial developmental stages, through ABI4. Since these expression experiments were performed in a mixed population of single mutants, double mutants, and heterozygous seeds, much higher differences in ABI4 expression should be expected between a homogeneous population of gapcp1gapcp2 and WT seeds. Finally, the deregulation of ABI4 expression in gapcp1gapcp2 supports the microarray conclusions indicating that GAPCp inactivation modifies the ABA-induced gene response.
Involvement of serine metabolism in ABA signal transduction
As ABI4 expression is narrowed down to the initial stages of seedling development, it is possible that other signalling molecules participate in the connection between sugar and ABA signal transduction. Serine supply restored normal ABA growth sensitivity in gapcp1gapcp2, which could be due to its effect on carbohydrate metabolism, as already discussed. However, it could also be related, at least in part, to changes in the amino acid pools. A recent metabolomic study has shown a correlation among drought stress, ABA production, and amino acid accumulation in Arabidopsis (Urano et al., 2009). The total free amino acids in gapcp1gapcp2 increased as compared with the control plants (Muñoz-Bertomeu et al., 2009). It is reasonable to think that an altered amino acid homeostasis provokes an impaired ABA transduction as a feedback.
In addition, a more specific effect of serine and/or derivatives on ABA signalling may also be considered. Serine was the only amino acid whose content was significantly lowered by 16% in the gapcp1gapcp2 roots as compared with the WT (Muñoz-Bertomeu et al., 2009). In this work, it was shown how serine supply increased the germination sensitivity of both the WT and gapcp1gapcp2 to ABA, which suggests that this amino acid could be a link connecting ABA signal transduction and primary metabolism. Reactive oxygen species (ROS) participate in ABA signal transduction (Guan et al., 2000; Pei et al., 2000; Kwak et al., 2003; Suhita et al., 2004). As a result of serine deficiency in the mutant, the levels of gluthathione, a central player in the scavenging of ROS, could be reduced. Thus, the ABA responses of gapcp1gapcp2 could be affected by a compromised antioxidant capacity as compared with the WT. However, the glutathione levels were normal in both the aerial part and roots of gapcp1gapcp2, making this possibility improbable.
There is considerable evidence to suggest that serine may be involved in the responses of plants to various environmental stresses. For instance, an accumulation of serine in plants grown at low temperatures and elevated salinity has been reported (Ho and Saito, 2001, and references therein). In addition, the overexpression of the gene encoding 3-phosphoglycerate dehydrogenase, which catalyses the first step of the phosphorylated pathway of serine biosynthesis, improved the tolerance of Arabidopsis to salt and cold stresses, although the molecular mechanism involved remains unclear to date (Waditee et al., 2007). It is suggested that the participation of serine in the responses of plants to salt and cold stresses could be mediated through the ABA signal transduction pathway.
In contrast to mutants, Oex-GAPCp1 lines did not show an ABA phenotype. Although high levels of GAPCp1 transcript are observed in Oex-GAPCp1 lines (Supplementary Fig. S1 at JXB online), their carbohydrate and serine contents did not change significantly as compared with the WT (Muñoz-Bertomeu et al., 2009). This could be due to a metabolic readjustment achieved either indirectly or directly through a specific regulation of GAPCp activity (post-transcriptional and/or post-translational regulation). Therefore, the lack of ABA phenotype of Oex-GAPCp1 lines would support the conclusion that it is the alteration of the metabolic homeostasis observed in gapcp1gapcp2 which impairs the ABA response.
Given the autotrophic nature of plants, the regulation of chloroplast/plastid functions is crucial for plant survival. The present results not only indicate that altered plastidial glycolysis impairs the signalling network of C, N, and ABA operating in Arabidopsis seedling development, but also provide evidence of the importance of GAPCps in these interactions. Evidence has also been provided for a link between the glycolytic pathway and the ABI4 transcription factor. Finally, the results suggest that serine (directly or indirectly) may play a role in connecting primary metabolism and ABA signal transduction.
Accession numbers
Arabidopsis Genome Initiative locus identifiers of Arabidopsis genes used in this article are as follows: GAPCp1 (At1g79530), GAPCp2 (At1g16300).
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Quantification of GAPCp1 transcript level in the roots and aerial part of GAPCp1-overexpressing (Oex-GAPCp1) lines as compared with the wild type (WT). Data from a representative overexpressing line are presented.
Table S1. Primers used in this work
Table S2. Microarray data. The data were compared using the dChip software.
Acknowledgments
This work was funded by the Spanish Government (Ministerio de Ciencia e Innovación BFU2009-07020) and by the Regional Valencian Government (ACOMP/2009/328, PROMETEO/2009/075). We thank Julian Schroeder (UCSD) for supporting initial research on this project and support from NIH (R01GM060396) and NSF (MCB0918220) grants to JS. We thank Servei Central de Support a la Investigació Experimental (SCIE) of the Universitat de Valencia for technical assistance in microarrays and Q-RT-PCR. We also thank the Salk Institute Genomic Analysis Laboratory for providing the sequence-indexed Arabidopsis T-DNA insertion mutants. We finally wish to thank Aurelio Gomez-Cadenas (UJI Castelló) and Carlos C. Romero (CSIC and University of Seville) for helping in the design and the determination of ABA and glutathione, respectively.
Glossary
Abbreviations
- ABA
abscisic acid
- gapcp1 gapcp2
Arabidopsis plastidial glyceraldehyde-3-phosphate dehydrogenase double mutant
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GAPC
cytosolic glycolytic glyceraldehyde-3-phosphate dehydrogenase
- G1g1.1 g2.1g2.1
population of seeds from heterozygous plants (GAPCp1gapcp1.1 gapcp2.1gapcp2.1)
- GAPCp
plastidial glycolytic glyceraldehyde-3-phosphate dehydrogenase
- MS
Murashige and Skoog
- Oex-GAPCp
plants overexpressing GAPCp
- WT
wild type
References
- Acevedo-Hernandez GJ, Leon P, Herrera-Estrella LR. Sugar and ABA responsiveness of a minimal RBCS light-responsive unit is mediated by direct binding of ABI4. The Plant Journal. 2005;43:506–519. doi: 10.1111/j.1365-313X.2005.02468.x. [DOI] [PubMed] [Google Scholar]
- Akihiro T, Mizuno K, Fujimura T. Gene expression of ADP-glucose pyrophosphorylase and starch contents in rice cultured cells are cooperatively regulated by sucrose and ABA. Plant and Cell Physiology. 2005;46:937–946. doi: 10.1093/pcp/pci101. [DOI] [PubMed] [Google Scholar]
- Andre C, Froehlich JE, Moll MR, Benning C. A heteromeric plastidic pyruvate kinase complex involved in seed oil biosynthesis in Arabidopsis. The Plant Cell. 2007;19:2006–2022. doi: 10.1105/tpc.106.048629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, Leon P. Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes and Development. 2000;14:2085–2096. [PMC free article] [PubMed] [Google Scholar]
- Barkla BJ, Vera-Estrella R, Hernandez-Coronado M, Pantoja O. Quantitative proteomics of the tonoplast reveals a role for glycolytic enzymes in salt tolerance. The Plant Cell. 2009;21:4044–4058. doi: 10.1105/tpc.109.069211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud S, Wuilleme S, Dubreucq B, de Almeida A, Vuagnat C, Lepiniec L, Miquel M, Rochat C. Function of plastidial pyruvate kinases in seeds of Arabidopsis thaliana. The Plant Journal. 2007;52:405–419. doi: 10.1111/j.1365-313X.2007.03232.x. [DOI] [PubMed] [Google Scholar]
- Bossi F, Cordoba E, Dupre P, Mendoza MS, Roman CS, Leon P. The Arabidopsis ABA-INSENSITIVE (ABI) 4 factor acts as a central transcription activator of the expression of its own gene, and for the induction of ABI5 and SBE2.2 genes during sugar signaling. The Plant Journal. 2009;59:359–374. doi: 10.1111/j.1365-313X.2009.03877.x. [DOI] [PubMed] [Google Scholar]
- Colell A, Ricci JE, Tait S, et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: emergence of a core signaling network. Annual Review of Plant Biology. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiology. 2005;139:5–17. doi: 10.1104/pp.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Solis JR, Gutierrez-Alcala G, Vega JM, Romero LC, Gotor C. The cytosolic O-acetylserine(thiol)lyase gene is regulated by heavy metals and can function in cadmium tolerance. Journal of Biological Chemistry. 2001;276:9297–9302. doi: 10.1074/jbc.M009574200. [DOI] [PubMed] [Google Scholar]
- Durgbanshi A, Arbona V, Pozo O, Miersch O, Sancho JV, Gomez-Cadenas A. Simultaneous determination of multiple phytohormones in plant extracts by liquid chromatography-electrospray tandem mass spectrometry. Journal of Agricultural and Food Chemistry. 2005;53:8437–8442. doi: 10.1021/jf050884b. [DOI] [PubMed] [Google Scholar]
- Furihata T, Maruyama K, Fujita Y, Umezawa T, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K. Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proceedings of the National Academy of Sciences, USA. 2006;103:1988–1993. doi: 10.1073/pnas.0505667103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan LM, Zhao J, Scandalios JG. Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. The Plant Journal. 2000;22:87–95. doi: 10.1046/j.1365-313x.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- Hara MR, Agrawal N, Kim SF, et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nature Cell Biology. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- Hara MR, Snyder SH. Nitric oxide–GAPDH–Siah: a novel cell death cascade. Cellular and Molecular Neurobiology. 2006;26:527–538. doi: 10.1007/s10571-006-9011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelbach A, Yang Y, Grill E. Relay and control of abscisic acid signaling. Current Opinion in Plant Biology. 2003;6:470–479. doi: 10.1016/s1369-5266(03)00090-6. [DOI] [PubMed] [Google Scholar]
- Ho CL, Saito K. Molecular biology of the plastidic phosphorylated serine biosynthetic pathway in Arabidopsis thaliana. Amino Acids. 2001;20:243–259. doi: 10.1007/s007260170042. [DOI] [PubMed] [Google Scholar]
- Kempa S, Rozhon W, Samaj J, et al. A plastid-localized glycogen synthase kinase 3 modulates stress tolerance and carbohydrate metabolism. The Plant Journal. 2007;49:1076–1090. doi: 10.1111/j.1365-313X.2006.03025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Dang CV. Multifaceted roles of glycolytic enzymes. Trends in Biochemical Sciences. 2005;30:142–150. doi: 10.1016/j.tibs.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Koussevitzky S. Signals from chloroplasts converge to regulate nuclear gene expression. Science. 2007;316:1698–1698. [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. NADPH oxidase Atrbohd and Atrbohf genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO Journal. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MN, Ha SH, Kim J, Koh A, Lee CS, Kim JH, Jeon H, Kim DH, Suh PG, Ryu SH. Glycolytic flux signals to mTOR through glyceraldehyde-3-phosphate dehydrogenase-mediated regulation of Rheb. Molecular andl Cellular Biology. 2009;29:3991–4001. doi: 10.1128/MCB.00165-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon P, Sheen J. Sugar and hormone connections. Trends in Plant Science. 2003;8:110–116. doi: 10.1016/S1360-1385(03)00011-6. [DOI] [PubMed] [Google Scholar]
- Leung J, Merlot S, Giraudat J. The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. The Plant Cell. 1997;9:759–771. doi: 10.1105/tpc.9.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Automating dChip: toward reproducible sharing of microarray data analysis. BMC Bioinformatics. 2008;9:231. doi: 10.1186/1471-2105-9-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- Melcher K, Ng LM, Zhou XE, et al. A gate–latch–lock mechanism for hormone signalling by abscisic acid receptors. Nature. 2009;462:602–608. doi: 10.1038/nature08613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Leube MP, Grill E. A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science. 1994;264:1452–1455. doi: 10.1126/science.8197457. [DOI] [PubMed] [Google Scholar]
- Min J, Kim YK, Cipriani PG, et al. Forward chemical genetic approach identifies new role for GAPDH in insulin signaling. Nature Chemical Biology. 2007;3:55–59. doi: 10.1038/nchembio833. [DOI] [PubMed] [Google Scholar]
- Miyazono KI, Miyakawa T, Sawano Y, et al. Structural basis of abscisic acid signalling. Nature. 2009;462:609–614. doi: 10.1038/nature08583. [DOI] [PubMed] [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science. 2003;300:332–336. doi: 10.1126/science.1080585. [DOI] [PubMed] [Google Scholar]
- Muñoz-Bertomeu J, Cascales-Minana B, Alaiz M, Segura J, Ros R. A critical role of plastidial glycolytic glyceraldehyde-3-phosphate dehydrogenase in the control of plant metabolism and development. Plant Signaling and Behavior. 2010a;5:67–69. doi: 10.4161/psb.5.1.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Bertomeu J, Cascales-Minana B, Irles-Segura A, Mateu I, Nunes-Nesi A, Fernie AR, Segura J, Ros R. The plastidial glyceraldehyde-3-phosphate dehydrogenase is critical for viable pollen development in Arabidopsis. Plant Physiology. 2010b;152:1830–1841. doi: 10.1104/pp.109.150458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Bertomeu J, Cascales-Miñana B, Mulet JM, Baroja-Fernandez E, Pozueta-Romero J, Kuhn JM, Segura J, Ros R. Plastidial glyceraldehyde-3-phosphate dehydrogenase deficiency leads to altered root development and affects the sugar and amino acid balance in Arabidopsis. Plant Physiology. 2009;151:541–558. doi: 10.1104/pp.109.143701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao M, Minami A, Arakawa K, Fujikawa S, Takezawa D. Rapid degradation of starch in chloroplasts and concomitant accumulation of soluble sugars associated with ABA-induced freezing tolerance in the moss Physcomitrella patens. Journal of Plant Physiology. 2005;162:169–180. doi: 10.1016/j.jplph.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Sarkeshik A, Nito K, et al. PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis. The Plant Journal. 2010;61:290–299. doi: 10.1111/j.1365-313X.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Nelson DC, Assmann SM. Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis. Cell. 2009;136:136–148. doi: 10.1016/j.cell.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. The Plant Cell. 1997;9:409–423. doi: 10.1105/tpc.9.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton WC. The organization and regulation of plant glycolysis. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47:185–214. doi: 10.1146/annurev.arplant.47.1.185. [DOI] [PubMed] [Google Scholar]
- Ramon M, Rolland F, Sheen J. Sugar sensing and signaling. In: Somerville C, Meyerowitz M, editors. The Arabidopsis book. Rockville, MD: American Society of Plant Biologists; 2008. doi: 10.1199/tab.0117, http://www.aspb.org/publications/arabidopsis/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annual Review of Plant Biology. 2006;57:675–709. doi: 10.1146/annurev.arplant.57.032905.105441. [DOI] [PubMed] [Google Scholar]
- Rook F, Hadingham SA, Li Y, Bevan MW. Sugar and ABA response pathways and the control of gene expression. Plant, Cell and Environment. 2006;29:426–434. doi: 10.1111/j.1365-3040.2005.01477.x. [DOI] [PubMed] [Google Scholar]
- Santiago J, Dupeux F, Round A, Antoni R, Park SY, Jamin M, Cutler SR, Rodriguez PL, Marquez JA. The abscisic acid receptor PYR1 in complex with abscisic acid. Nature. 2009;462:665–668. doi: 10.1038/nature08591. [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Kwak JM, Allen GJ. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature. 2001;410:327–330. doi: 10.1038/35066500. [DOI] [PubMed] [Google Scholar]
- Shen YY, Wang XF, Wu FQ, et al. The Mg-chelatase H subunit is an abscisic acid receptor. Nature. 2006;443:823–826. doi: 10.1038/nature05176. [DOI] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiology. 2004;134:1536–1545. doi: 10.1104/pp.103.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano K, Maruyama K, Ogata Y, et al. Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. The Plant Journal. 2009;57:1065–1078. doi: 10.1111/j.1365-313X.2008.03748.x. [DOI] [PubMed] [Google Scholar]
- Vlad F, Rubio S, Rodrigues A, Sirichandra C, Belin C, Robert N, Leung J, Rodriguez PL, Lauriere C, Merlot S. Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. The Plant Cell. 2009;21:3170–3184. doi: 10.1105/tpc.109.069179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waditee R, Bhuiyan NH, Hirata E, Hibino T, Tanaka Y, Shikata M, Takabe T. Metabolic engineering for betaine accumulation in microbes and plants. Journal of Biological Chemistry. 2007;282:34185–34193. doi: 10.1074/jbc.M704939200. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K. The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. Journal of Biological Chemistry. 2006;281:5310–5318. doi: 10.1074/jbc.M509820200. [DOI] [PubMed] [Google Scholar]