Abstract
Rachises of grape (Vitis vinifera L.) clusters that appeared healthy or displayed symptoms of the ripening disorders berry shrivel (BS) or bunch-stem necrosis (BSN) were treated with the cellular viability stain fluorescein diacetate and examined by confocal microscopy. Clusters with BS and BSN symptoms experienced a decrease of cell viability throughout the rachis, and their berries contained 70–80% less sugar than healthy berries. The xylem-mobile dye basic fuchsin, infiltrated via the cut base of shoots with one healthy and one BS cluster, moved to all berries on the healthy cluster but generally failed to move into the peduncle of the BS cluster. Peduncle girdling did not interrupt dye movement in the xylem, but stopped solute accumulation in berries and led to berry shrinkage. In contrast, surgically destroying the peduncle xylem at the onset of ripening did not affect berry growth and solute accumulation. These results indicate that cessation of sugar and water accumulation in BS and BSN is associated with phloem death in the rachis. Although xylem flow to the berries may also cease, a functional xylem connection to the shoot may not be required for normal ripening, while water loss from berries by transpiration and xylem efflux may explain the characteristic berry shrinkage that is associated with these ripening disorders. The similarity of internal tissue breakdown in BS and BSN rachises and the correlation observed here between the proportion of shrinking berries on a cluster and the severity of rachis necrosis suggest that there may be a gradual transition between the two ripening disorders. Seeds from healthy and BS clusters showed no differences in colour, morphology, weight, viability, and ability to germinate, which indicates that the disorder may not appear until seeds are mature.
Keywords: Apoplastic dye, berry shrivel, bunch-stem necrosis, cell viability, grape berry, phloem, senescence, xylem
Introduction
Sugar accumulation in fruits is highly important for fruit quality because, in addition to imparting sweetness, sugar is the ultimate precursor for most quality-relevant components, such as acids, pigments, tannins, and aroma volatiles. In grapes (Vitis spp.), sugar is also indispensable for the process of fermentation that turns grapes into wine. Sugar (sucrose) arrives in grape berries via the phloem and is unloaded symplastically before the onset of ripening (termed veraison by viticulturists) and apoplastically thereafter (Zhang et al., 2006). Invertases then cleave sucrose into glucose and fructose which, in addition to being used for other processes, accumulate to high concentrations in the vacuoles of both mesocarp and exocarp of ripening berries (Keller, 2010). Sugar accumulation can be curtailed by two seemingly distinct ripening disorders termed ‘bunch-stem necrosis’ (BSN) and ‘berry shrivel’ (BS). Other symptoms of BS include low pH, poor colour, and visible shrivelling or shrinking of grape berries during the ripening phase (Bondada and Keller, 2007; Krasnow et al., 2009; Knoll et al., 2010). Many cultivars of Vitis vinifera L. have been observed to develop BS symptoms, including Cabernet Sauvignon, Durif, Pinot noir, Zweigelt, Sauvignon blanc, Sémillon, and possibly many others throughout the world (Bondada and Keller, 2007; Krasnow et al., 2009; Knoll et al., 2010). The cause of this syndrome is still unknown, and to date no known pathogen has been associated with its occurrence (Krasnow et al., 2009).
Although BSN may have similar effects on fruit composition (Ureta et al., 1981; Morrison and Iodi, 1990), rachis or peduncle necrosis associated with BSN but not BS is often used as the main distinction between the two disorders (Raifer and Roschatt, 2001; Krasnow et al., 2009). Nonetheless, BSN-like symptoms, including shrivelling and low sugar content, have been observed on clusters with no rachis necrosis (Stellwaag-Kittler, 1983; Morrison and Iodi, 1990), similar to BS. Thus, the distinction between BS and BSN based solely on rachis necrosis may not be as straightforward as is often asserted. The reduction in sugar and water import into BSN berries is thought to be caused by necrotic lesions penetrating and disrupting the vascular tissues in the rachis (Theiler, 1970; Hifny and Alleweldt, 1972). Similarly, BS berries experience a complete cessation of sugar and water accumulation (Krasnow et al., 2009; Knoll et al., 2010). This may suggest a decline in phloem function, because both sugar and water import by ripening berries depend on continued phloem influx (Greenspan et al., 1994; Keller et al., 2006). Field observations indicate that, like BSN, BS typically affects entire clusters or that symptomatic berries are concentrated at the distal end or on a shoulder, rather than as single berries distributed randomly throughout a cluster. Therefore, it seems likely that both disorders are triggered at the rachis level rather than at the berry level.
Water influx into ripening grape berries via the phloem usually balances berry transpiration and efflux via the xylem but, if phloem influx is limited, water loss can dominate, and berries may shrink (Keller at al., 2006; Choat et al., 2009). The grape cultivar Syrah is noted for its late-season berry shrinkage, which may be associated with transpiration and xylem efflux, combined with a loss in mesocarp cell viability (Rogiers et al., 2004; Tilbrook and Tyerman, 2008, 2009). Similarly, in the cultivar Cabernet Sauvignon affected by BS, a decrease in mesocarp cell viability coincides with visible shrivelling and weight loss (Krasnow et al., 2008). These studies indicate a possible link between shrivelling and water loss by transpiration and xylem efflux, limited phloem influx, and loss of membrane integrity in grape berries.
The aim of the present study was to elucidate the cause of poor sugar accumulation in BS fruit and of its shrinkage. It was hypothesized that BS may appear as a consequence of an interruption of phloem transport through the peduncle and/or rachis of ripening clusters. A loss in phloem functionality would cause a decrease in water and solute transport downstream of the affected phloem portion, much like girdling. In addition, it was reasoned that shrivelling in BS might be slowed if xylem efflux could be eliminated. The cellular viability stain fluorescein diacetate (FDA) was used to determine the integrity of cell membranes in the phloem, and thus phloem functionality, in the rachis of clusters showing BS or BSN symptoms and of asymptomatic clusters. Xylem function was assessed by using the apoplastic dye basic fuchsin and by surgically destroying the xylem connection between the shoot and the peduncle. In addition, given the apparent similarities between BS and BSN it was important to clarify the relationship between the two disorders. If BS were indeed caused by a girdling effect, then its distinction from BSN would lie mostly in visible and not functional symptoms.
Materials and methods
Plant material
Two vineyards with mature, own-rooted V. vinifera cv. Cabernet Sauvignon were chosen for BS research in the 2005–2009 growing seasons due to high incidence of the disorder in previous years. The Wallula vineyard (+45°58′59.27′′, –119°2′52.20′′) was located on a southwest-facing slope south of Kennewick, Washington, USA, and the Zephyr Ridge vineyard (+45°57′56.85′′, –119°34′1.17′′) was located on a west-facing slope near Paterson, Washington, USA. Vines in both vineyards were planted with 1.83 m between plants within rows and 2.74 m between rows running southeast–northwest. Vines were trained to a bilateral cordon with vertical shoot positioning, spur-pruned in winter, and drip-irrigated during the growing season. During each growing season the vines were continually monitored for inception and progression of BS during ripening. Vine location and position of symptomatic clusters were noted, and attention was paid to any potential trends or patterns.
Test of xylem and phloem functionality
Cabernet Sauvignon shoots with two leaves and two clusters were cut in the vineyard, and the cut end was immediately submerged in a 0.1% aqueous solution of basic fuchsin (C20H19N3HCl; 338 g mol-1; Sigma-Aldrich, St Louis, MO, USA) as described by Keller et al. (2006). One of the two clusters was apparently healthy, and the other one displayed early symptoms of BS (i.e. berries had not yet visibly begun to shrink but had low sugar content, showed poor coloration, and tasted sour). After 7 h and 24 h of dye infiltration, cross-sections of shoots, peduncles, rachises, and pedicels were examined for dye movement in the xylem using a stereo dissecting microscope (EMZ-TR, Meiji Techno Co., Tokyo, Japan). Berries were removed from each cluster and the solute concentration (°Brix) recorded using a bench-top refractometer (RE40D, Mettler-Toledo, Urdorf, Switzerland). Dye infiltration of shoots was repeated six times between 2005 and 2009.
At the beginning of ripening of field-grown Cabernet Sauvignon, six clusters were cut from their shoot and hung back into the canopy near their original position. In addition, the xylem in six clusters was surgically destroyed by drilling a hole radially through each shoot at the point of cluster attachment and extending ∼10 mm into the peduncle (Fig. 1). The size of the drill bits was adapted to the peduncle diameter so as to destroy the xylem as completely as possible without injuring the phloem. Complete xylem destruction was verified on adjacent shoots using the dye infiltration technique described above. Berry samples were taken at the beginning and after 20 d to estimate changes in berry weight and solute concentration. This experiment was initiated at the early post-veraison stage (berries were pink-red and had a mean solute concentration of ∼14 °Brix) and was repeated twice in 2007.
Fig. 1.
Destruction of xylem in Cabernet Sauvignon clusters by drilling a hole across the shoot extending ∼10 mm into the peduncle.
At the beginning of ripening in 2008, five Cabernet Sauvignon clusters in the field were girdled at the peduncle by removing a 1 mm wide section of the phloem with a fine razor blade. These clusters and intact control clusters were monitored for sugar accumulation and signs of shrivelling throughout ripening. In addition, basic fuchsin was infiltrated through the cut peduncle of both girdled and intact clusters as described above to test whether phloem disruption would also interrupt xylem flow.
Cell viability staining
Clusters that were apparently healthy or displayed symptoms of BS and/or BSN were sampled twice during the 2009 ripening period. Rachises were cross-sectioned using ultra fine razor blades at the peduncle, immediately proximal to the first lateral branch, in the middle of the rachis, and ∼1–2 cm from the distal end. In addition, cross-sections were also taken from 1–2 laterals at various positions based on the incidence of disorders, but typically on the proximal 1–2 laterals. Further sections were mostly taken from locations on the main rachis distal to a cross-sectioned lateral. For instance, a healthy cluster with a BSN lateral would have cross-sections taken proximal and distal to the BSN lateral at the main rachis, at the BSN lateral, and an opposite healthy or BS lateral, if present. In a slight modification of the method by Krasnow et al. (2008), the sections were immediately submerged in 1 ml of an aqueous solution of 9.6 μM FDA (C24H16O7; 416 g mol-1; Sigma-Aldrich). Berries from each cluster were weighed and solute concentrations determined by refractometry.
To confirm that FDA stains only live cells, cross-sections of rachises were placed on a metal plate that was chilled by liquid nitrogen before staining with FDA. In addition, non-stained sections were used to detect any autofluorescence of tissues.
Confocal microscopy and image analysis
Rachis sections stained with FDA were examined with a Zeiss LSM 510 Meta Laser Scanning Microscope (Carl Zeiss Microimaging, Thornwood, NY, USA). An HBO 100 W USHIO USH-102DH mercury bulb (Meridian Instrument Company, Freeland, WA, USA) was used to emit the excitation. The excitation wavelength of the laser was set at 488 nm with a detection channel of 505–530 nm. Rachis cross-sections were placed on a microscope slide, then visualized and recorded with a built-in camera, using identical settings for detector gain, exposure time, picture size, contrast, and pinhole, to minimize variability between samples. The settings for microscope image capturing were chosen based on clear visibility of fluorescence while not overexciting and thus overestimating the presence of live cells.
Using the ImageJ software (National Institutes of Health, USA; http://rsbweb.nih.gov/ij), the percentage area of fluorescence for each cross-section was analysed. Each picture was converted into an 8-bit file then set at a constant threshold of 25 (min)–255 (max). The software's free-hand tool was used to trace the majority of the phloem region, from the vascular cambium to the endodermis, and the percentage of fluorescing area was estimated. Raw data were square-root transformed and analysed by analysis of variance (ANOVA) and Tukey's Unequal N HSD post hoc test, using Statistica 7.1 (StatSoft, Tulsa, OK, USA).
Seed germination test
Seeds were removed from berries on healthy and BS clusters, washed and placed in deionized water, and all floating seeds were counted and then discarded. Floater seeds are aborted seeds with degenerating nucellus and endosperm, and thus are unable to germinate (Ebadi et al., 1996). The seeds were then air-dried and weighed using three 10-seed replicates for each cluster. Seeds were mixed with damp sawdust, stratified at 2 °C for 5 months, then soaked in deionized water for 24 h, surface-sterilized by submergence for 5 min in 1% NaOCl, and rinsed three times with sterile deionized water. Fifty seeds per cluster were placed on moist filter paper in a Petri dish, covered and sealed with Parafilm™, and incubated at 25 °C in the light for 21 d to estimate percentage seed germination. Berry weight and solute concentration were determined on a subsample of four of the berries used for the seed germination assay from each cluster. The seed germination test was repeated four times from 2006 to 2009.
Rachis necrosis and extent of berry shrivel
In 2008 and 2009, clusters were sampled from two cultivars (Cabernet Sauvignon and Sémillon) grown in five vineyards. Berry solute concentrations were determined by refractometry. The proportion of shrivelling berries per cluster was estimated using a 0–10 scale. A cluster with a rating of 0 would be completely healthy with no shrivelling berries, while a rating of 10 indicated 100% shrivelled berries. Subsequently, all laterals were removed to leave only the main rachis, which was visually examined to estimate the degree of necrosis on a 0–10 scale, with 0 having no visible necrosis and 10 being entirely necrotic. A necrotic lesion was taken as any brown or black mark or area that was not a (raised) lenticel and instead was associated with a depression in and irregular deformation of the epidermis, unlike normal periderm formation. Necrotic lesions developed anywhere on the rachis, whereas periderm formation extends from the shoot to the peduncle and uniformly encircles the peduncle as shown in Fig. 1. Correlation analysis was used to determine the association between shrivel and necrosis and between shrivel or necrosis and berry solute concentration.
Results
Over the 5 years of this study, ripening generally began in the middle of August, and the first symptoms of BS and BSN were apparent in early to mid September. Clusters with BS or BSN symptoms were detected to varying degrees on vines throughout each vineyard, with no visual differences between vines bearing healthy, BS, or BSN clusters, or any combination of the three. No consistent patterns of either ripening disorder were apparent within or between vines in any of the vineyards. Moreover, vines that showed symptomatic clusters in one year often appeared healthy the following year, while formerly asymptomatic vines suddenly had one or several clusters displaying typical BS and/or BSN symptoms. Both BS and BSN usually affected whole clusters or all of the berries in the distal portion of a cluster. However, combinations of symptoms were also occasionally observed on one cluster, typically with a portion of the cluster (i.e. one lateral or shoulder) showing no symptoms or different symptoms from the rest of the cluster.
Xylem and phloem functionality
Basic fuchsin, infiltrated through the shoot base, was detected throughout the shoot xylem, including above the two clusters, regardless of whether the basal or the apical cluster had BS symptoms. Dye was also detected throughout the peduncle and rachis and in all examined pedicels of all healthy clusters (Fig. 2). However, dye was rarely found beyond the shoot–peduncle junction of BS clusters, except where the dye could be traced to berries on an apparently healthy shoulder. The berries of healthy clusters had a solute concentration of 25.3±0.27 °Brix (mean ±SE, n=20), which was significantly (P <0.001) higher than that of berries from BS clusters (11.8±0.37 °Brix). This indicates that the latter had essentially ceased ripening at the red-purple stage (see Fig. 5 in Keller et al., 2006).
Fig. 2.
Pedicel cross-sections of Cabernet Sauvignon berries showing the red xylem-mobile dye basic fuchsin in a healthy cluster (25.8 °Brix, n=10 berries) and absence of the dye in a cluster with symptoms of berry shrivel (BS; 12.0 °Brix, n=10 berries).
Fig. 5.
Example of image processing for a rachis cross-section stained with FDA and analysed for percentage fluorescing area by hand-tracing the phloem region. The percentage fluorescing area (37% in this example) corresponds to the percentage of live cells within the traced area. (This figure is available in colour at JXB online.)
Destroying the peduncle xylem at the onset of ripening (Fig. 1) had no apparent effects on fruit ripening. The berries completely changed colour from pink-red to blue, and berry growth and solute accumulation over the subsequent 20 d were similar to the rates on control clusters (Table 1). In contrast, removing the cluster from the shoot not only stopped solute accumulation but also led to significant (P <0.001) berry shrinkage, while the berries turned purple but not blue. Similar to cluster removal, girdling completely stopped sugar accumulation (Table 1), whereas control berries accumulated solutes at a rate of 3.1±0.52 mg d−1 over the subsequent 56 d. Over the same period, the control berries grew (+4.4±1.75 mg d−1), while the berries on girdled clusters shrank (Table 1). Coloration on girdled clusters was also extremely reduced and often only appeared once the berries had begun to shrink. Although girdling did not interrupt xylem flow through the peduncle, as demonstrated by the continuation of basic fuchsin movement across the girdle (data not shown), this result is strictly qualitative and does not permit any conclusions regarding girdling effects on rates of water flow in the xylem.
Table 1.
Effect of peduncle xylem destruction, cluster detachment, and phloem girdling at the onset of ripening on growth and solute accumulation of Cabernet Sauvignon berries over 20 d
| Final berry weight (g) | Final solute concentration (°Brix) | Berry growth rate (mg d−1) | Solute accumulation rate (mg d−1) | |
| Intact control | 0.73±0.016 a | 18.4±0.56 a | 6.5±0.81 a | 2.51±0.153 a |
| No xylem | 0.69±0.050 a | 18.5±0.24 a | 6.5±1.33 a | 2.60±0.325 a |
| Detached | 0.47±0.022 b | 18.1±0.25 a | -6.8±0.41 b | –0.01±0.002 b |
| Girdled | 0.59±0.032 | 12.1±1.13 | -3.9±0.79 | –0.10±0.199 |
Data are means ±SE (n=6 clusters). Means followed by the same letter do not differ significantly at P <0.05 (note: girdling was tested in a separate experiment and is included here for comparison only).
Rachis cell viability
Among the clusters sampled for viability staining, healthy berries had much higher (P <0.001) solute concentrations than BSN and, especially, BS berries (Table 2). Owing to the berry shrinkage that had occurred prior to sample collection, the weight of BSN berries was far lower than that of healthy berries, while BS berries were intermediate. Accordingly, the amount of sugar per berry was highest (P <0.001) for healthy clusters and equally low for BS and BSN clusters (Table 2). Despite pronounced differences in berry solute concentration and content, the number of seeds per berry, seed weight, colour, shape, percentage of floating seeds, and percentage of seeds germinated were similar between healthy and BS clusters (Table 3).
Table 2.
Berry weight, solute concentration, and solute content of Cabernet Sauvignon berries sampled before harvest from healthy clusters and clusters with symptoms of berry shrivel (BS) or bunch-stem necrosis (BSN)
| Berry weight (g) | Solute concentration (°Brix) | Solute content (mg berry−1) | |
| Healthy | 1.09±0.026 a | 25.0±0.26 a | 274±7.7 a |
| BS | 0.69±0.028 b | 13.1±0.27 c | 92±5.2 b |
| BSN | 0.53±0.034 c | 18.2±1.46 b | 99±8.9 b |
Data are means ±SE. (n=28–40 clusters). Means followed by the same letter do not differ significantly at P <0.05.
Table 3.
Fruit and seed characteristics of Cabernet Sauvignon berries sampled before harvest from healthy clusters and clusters with symptoms of berry shrivel (BS)
| Berry weight (g) | Solute concentration (°Brix) | Solute content (mg berry−1) | Seeds per berry | Seed weight (mg) | Floating seeds (%) | Seed germination (%) | |
| Healthy | 1.12±0.038 a | 25.2±0.39 a | 284±11.8 a | 1.4±0.05 a | 37±0.9 a | 2.6±0.64 a | 30±3.7 a |
| BS | 0.65±0.050 b | 13.5±0.85 b | 90±10.7 b | 1.4±0.05 a | 36±0.6 a | 2.0±0.70 a | 26±5.7 a |
Data are means ±SE. Fruit characteristics (n=28–32 berries); seed weight and floating seeds (n=210–240 seeds); seed germination (n=350–400 seeds). Means followed by the same letter do not differ significantly at P <0.05.
Although no comparable studies have been published that describe FDA use in grape rachises, rachis cross-sections stained here with FDA showed fluorescence similar to studies that used the same stain in grape berries (Krasnow et al., 2008; Tilbrook and Tyerman, 2008). In intact cells, bright-green fluorescence was restricted within the cytoplasm, indicating cell membrane integrity. Frozen then thawed portions of a cross-section did not show any fluorescence, and unstained cross-sections did not produce any autofluorescence (data not shown). This indicates that only live cells were capable of fluorescing upon FDA staining. The intensity of fluorescence was fairly consistent throughout the study; however, occasionally fluorescence decreased slightly when the working solution was used all day, presumably due to photobleaching and the stain's inherent instability. This effect was limited by using new working solution every day, along with maintaining identical conditions and working with a healthy, BS, and BSN cluster in series. This ensured that any differences between the rachises were indicative of cellular viability and not the state of the dye, permitting consistent and accurate image analysis.
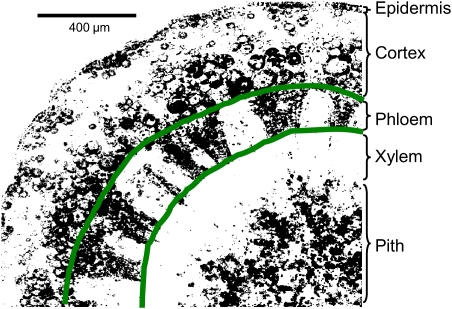
Cross-sections taken from different positions in the rachis varied in terms of fluorescing cell types and quantity of fluorescence. In healthy clusters, this was mainly due to differences in anatomical maturity between positions and a corresponding change in types and numbers of live cells. Peduncle cross-sections typically had well-developed periderm and lignified xylem, and fluorescence was mostly restricted to cells in the phloem region and the cortex (data not shown). Proximal to the first lateral or shoulder the extent of fluorescing tissues increased to include more cells in the vascular cylinder (except xylem conduits) and cortex, including the epidermis, and occasionally the rays and pith (Fig. 3). In the middle of the rachis the extent of fluorescence typically increased to include more cells in the epidermis, cortex, and rays, as well as in the phloem and pith. The distal end of the rachis lagged in development in comparison with the peduncle; thus there was generally more fluorescence towards the tip of a cluster, especially in the pith area (Fig. 3A). Rachis laterals showed types of fluorescing cells comparable with a similar-sized section of main rachis.
Fig. 3.
Cross-sections of Cabernet Sauvignon rachises stained with FDA and examined for cell viability (green fluorescence) by confocal microscopy. A healthy cluster (A) and a cluster with symptoms of berry shrivel (B). Photos on the left in A and B show the appearance of the rachis without lateral branches (top) and of the whole cluster (bottom). Arrows in (B) indicate typical necrotic lesions similar to those analysed for Fig. 7. Micrographs on the right in A and B labelled 1–4 show cross-sections corresponding to positions 1–4 on the rachis; e, epidermis; c, cortex; p, phloem; r, ray; x, xylem; pi, pith. Fruit characteristics for key sections are: (A2) 24.9 °Brix, 1.0 g berry weight, 257 mg of solutes per berry; (A4) 25.0 °Brix, 1.0 g berry weight, 250 mg of solutes per berry; (B2) 11.9 °Brix, 0.63 g berry weight, 74 mg of solutes per berry; (B4) 15.3 °Brix, 0.47 g berry weight, 71 mg of solutes per berry.
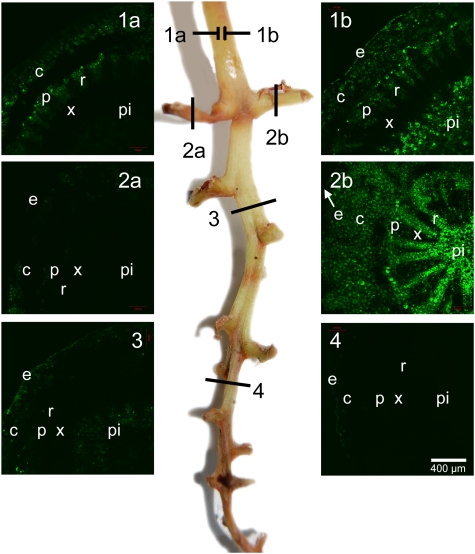
Healthy clusters typically showed bright fluorescence at any location of the rachis (Fig. 3A), whereas BS clusters were highly variable but usually showed much less fluorescence, indicating a loss of cell viability (Fig. 3B). Clusters showing symptoms of BSN usually showed little or no fluorescence, depending on the severity of the necrosis. In stark contrast to the case in healthy clusters, the intensity of fluorescence generally declined towards the distal end of BS clusters, often drastically so (Fig. 3B). At the tip, some residual fluorescence was sometimes detected in the epidermis. Lower fluorescence was associated with lower berry weight, solute concentration, and sugar per berry (Table 2). By sectioning the main rachis proximal and distal to symptomatic laterals, it was possible to examine the intensity of fluorescence proximal and distal to a branch point to a lateral showing healthy, BS, or BSN symptoms. For instance, in Fig. 4, the first section was divided into 1a and 1b to represent two sides of the cross-section. Side 1b was proximal to a healthy lateral (section 2b). In comparison, side 1a showed much lower fluorescence and was proximal to a lateral with BSN symptoms that had no fluorescence (section 2a). Fluorescence also declined towards the distal end, with BS symptoms in the middle (section 3) and BSN symptoms at the tip (section 4).
Fig. 4.
Cross-sections of a Cabernet Sauvignon rachis with multiple symptoms stained with FDA and examined for cell viability (green fluorescence) by confocal microscopy. Micrographs on either side labelled 1–4 (a and b) show cross-sections corresponding to positions 1–4 (a and b) in the centre photo of the rachis with symptoms of bunch-stem necrosis (2a, 4), healthy berries (2b), and berry shrivel (3); e, epidermis; c, cortex; p, phloem; r, ray; x, xylem; pi, pith. Fruit characteristics for each section are: (2a) 12.6 °Brix, 0.61 g berry weight, 77 mg of solutes per berry; (2b) 27.3 °Brix, 1.0 g berry weight, 285 mg of solutes per berry; (3) 11.7 °Brix, 0.62 g per berry, 72 mg of solutes per berry; (4) 13.3 °Brix, 0.43 g berry weight, 56 mg of solutes per berry.
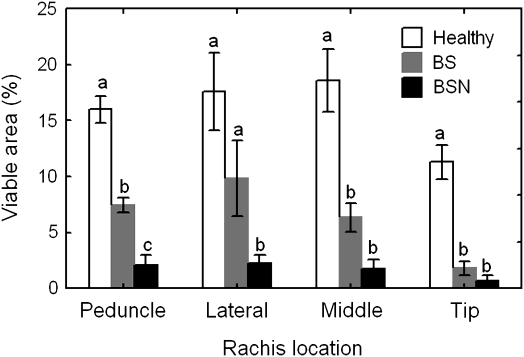
Image processing was able to quantify the observations made above by giving a relative percentage area of fluorescence for each cross-section. Because the fluorescing cell types varied among rachis locations and because the cross-sections were of varying size and rarely fit completely into the frame of the camera lens, the tissue that is most important for sugar and water influx into the berries (i.e. the phloem) was isolated by free-hand outlining (Fig. 5). Image processing revealed significant differences (P <0.001) in rachis cell viability among cluster types. The percentage viable phloem area in the peduncle was highest in healthy clusters, intermediate in BS clusters, and lowest in BSN clusters (Fig. 6). While the laterals showed more viable phloem area in healthy and BS clusters than in BSN clusters, BS and BSN rachises had less viable phloem than healthy rachises in both the middle and tip cross-sections. Fluorescence was essentially absent from many of the sections taken from BS and BSN clusters, especially near the distal end.
Fig. 6.
Cell viability in the phloem region of rachis cross-sections taken at various positions of healthy Cabernet Sauvignon clusters and clusters with symptoms of berry shrivel (BS) and/or bunch-stem necrosis (BSN). Data are means ±SE (n=8–76). Means followed by the same letter within locations do not differ significantly at P <0.05.
Association between rachis necrosis and berry shrivel
The solute concentration of berries from clusters with a shrivel rating of 0 ranged from 21.0 °Brix to 30.5 °Brix (mean 26.5), while that of clusters with a shrivel rating ≥1 was significantly lower (P <0.001), ranging from 10.4 °Brix to 21.5 °Brix (mean 15.9). Although solute concentration was inversely correlated with both the necrosis rating (r= –0.64, P <0.001) and the shrivel rating (r= –0.78, P <0.001), there was no significant difference in solute concentration among clusters with either a necrosis or shrivel rating ≥1. Regardless of cultivar, vineyard location, and year, the proportion of shrivelled berries on a cluster increased as the severity and number of necrotic lesions on the rachis increased (Fig. 7A). Many of these lesions were small and not always visible to the naked eye. Some clusters showed shrivel at the distal end with only slight necrosis on the rachis, and it was common for the berries at the tip of clusters to shrivel first. Only three of the 149 clusters that were assessed showed symptoms of shrivel in the complete absence of visible necrosis. Although the extent of shrivel varied widely within each necrosis class, it was evident that as berry shrivelling became more severe, necrotic lesions generally became more prominent. In some instances necrosis was confined to individual pedicels, but necrotic lesions were particularly frequent in the axil (distal side) of laterals branching from the main axis of the rachis of both BS and BSN clusters (Fig. 7B). This location was confirmed to have fewer viable cells by using the FDA technique (data not shown). Moreover, low cell viability was also observed in BS clusters that showed little to no necrosis on the rachis (Fig. 3B).
Fig. 7.
Correlation between the extent of rachis necrosis and the severity of berry shrivel (A), and necrotic lesions in a rachis axil or on a pedicel (B). Each data point represents a single cluster (n ≥62). Data for two cultivars (Cabernet Sauvignon and Semillon) and five vineyard locations were pooled. Curves were fitted by negative exponential smoothing. Correlations were also significant within cultivars and within vineyard locations; differences between years were not significant. Note that lenticels in B are raised, whereas necrotic lesions are depressed.
Discussion
The combination of confocal microscopy with FDA staining proved to be an effective technique for testing the viability of grapevine rachis tissues. The present data indicate that both the BS and BSN ripening disorders of grapevines are associated with cell death in the rachis supporting the berries. While this result was expected for BSN, which is defined by necrotic lesions on the rachis, the finding that cell death also occurs with BS is novel and at first surprising, because external symptoms on the rachis have thus far not been associated with BS (Raifer and Roschatt, 2001; Bondada and Keller, 2007; Krasnow et al., 2009; Knoll et al., 2010). Although BS clusters assayed in the present study also often had some, even if little and confined, rachis necrosis, visible necrosis was not necessary for a loss in cell viability to occur. Thus, BS clusters could experience a significant loss in phloem function without any visual symptoms on the rachis. Girdling also often, but not always, led to the development of rachis necrosis distal to the girdle, and these symptoms resembled those associated with BSN. The correlation that was found between the severity of BS on a cluster and the extent of necrotic lesions on its rachis also suggests the two disorders might not be as distinct as previously thought. Interestingly, in their anatomical investigations of BSN, Hifny and Alleweldt (1972) and Jähnl (1975) observed cell plasmolysis in internal rachis tissues before external symptoms became apparent. Work is currently in progress in the authors’ laboratory to determine the timing of cell death associated with both BS and BSN.
Although cell death was not confined to the phloem alone, the present results clearly show a loss in phloem viability in the rachis of BS and BSN clusters. This was associated with low solute content and shrinkage of grape berries downstream of rachis portions experiencing cell death, even in the absence of external symptoms. Because death of phloem cells is necessarily associated with a loss of phloem function, these data indicate a girdling effect from within the vascular tissues. Although the evidence presented here is preliminary, because samples were collected only after the appearance of visible symptoms, it is posited that sugar accumulation in the berries may be restricted by the loss of viability and thus functionality of the rachis phloem. Given that BS symptoms typically appeared simultaneously on entire clusters or at least on all distal berries (rather than on only individual berries), the observed loss of membrane integrity in the affected berries (Krasnow et al., 2008, 2009) may be a consequence of the interruption of phloem flow through the rachis rather than the cause of it. Simultaneous triggering of BS symptoms within each berry on a cluster also seems improbable, because the onset of ripening differs between berries on a cluster and between clusters (Coombe, 1992).
In addition to the loss of phloem function, clusters with BS symptoms were also unable to transport xylem-mobile dye through the rachis. The latter may be due to an increase in hydraulic resistance or a change in driving force, although at present it is only possible to speculate about likely causes. It could be imagined that vessels might become plugged by tyloses or by gel-like substances originating from the senescing phloem and parenchyma cells, as may occur in grapevine shoots near pruning wounds (Sun et al., 2008). Yet microscopic examination thus far has not revealed any obvious changes in the vessels of clusters showing symptoms of BS (this study; BRB, unpublished data) or BSN (Theiler, 1970; Hifny and Alleweldt, 1972). Xylem flow from the shoot to the peduncle could also decline or even stop if death of phloem and parenchyma cells surrounding the rachis xylem led to a reduction in or cessation of water transfer to the phloem and evaporation from the surface. Such transfer and evaporation have been postulated as the likely driving force for xylem water flow from the shoot towards ripening grape berries (Keller et al., 2006). This interpretation is not at odds with the finding that peduncle girdling did not prevent basic fuchsin movement into the rachis, because girdling would still permit water evaporation downstream of the girdle. An alternative or additional explanation for the cessation of dye movement into BS clusters might be backpressure caused by xylem efflux from the shrinking berries. However, this seems unlikely, because if phloem influx normally provides the driving force for xylem efflux (Keller et al., 2006; Choat et al., 2009), then one should expect phloem death to be associated with a decrease in efflux rather than an increase.
Visible berry shrinkage in BS-affected clusters, presumably caused by water loss from the berries, generally began at least 1–2 weeks after sugar accumulation had ceased, consistent with other studies on BS (Krasnow et al., 2009; Knoll et al., 2010). In addition, Krasnow et al. (2008, 2009) observed that sugar accumulation in BS fruit was followed by a decline in berry cell viability, which in turn coincided with shrivelling. Water loss from intact berries is likely to be limited due to high vacuolar solute concentration and closure of aquaporins in response to turgor loss (Chrispeels et al., 1999; Keller et al., 2006). However, following disruption of membrane integrity, water may follow hydrostatic pressure gradients and exit the berry by surface evaporation as well as through the pedicel (Tilbrook and Tyerman, 2008, 2009). It seems likely that berries shrink because phloem death in the rachis prevents further water influx to balance water loss.
The present results show that a functional xylem connection between the shoot and the peduncle is not necessary for normal berry ripening; hence, cessation of xylem flow can be ruled out as a cause of BS. In contrast to the destruction of peduncle xylem, girdling of the peduncle or rachis was found to result in berry shrinkage, increased deformability, cessation of sugar accumulation and pigment development, and often varying degrees of rachis necrosis (Creasy and Lombard, 1993; Rogiers et al., 2006; this study). Similarly, berries affected by BS contain low sugar and anthocyanins (Krasnow et al., 2009; Knoll et al., 2010), and BSN clusters experience a girdling effect leading to flaccid and wrinkled berries with a soft texture and poor colour (Theiler, 1970; Hifny and Alleweldt, 1972; Morrison and Iodi, 1990). Thus, loss in rachis viability in BSN causes similar symptoms to BS (except for the absence of visible necrosis in some cases of BS), suggesting that impeded phloem import plays a role in both disorders. The correlation between the proportion of shrivelling berries and the extent of rachis necrosis further supports this conclusion.
The present data demonstrate that the seeds of BS berries are fully mature, viable, and may germinate at the same rate as do seeds from healthy berries whose sugar content may be several fold higher. Because no known pathogen has been identified as the causal agent of BS (Krasnow et al., 2009), the authors’ laboratory is currently testing whether the disorder is a stress response. It is conceivable that ‘surplus’ grapes with mature seeds may be aborted during stress periods to ensure long-term survival of the plant (Keller et al., 2001; Keller, 2010).
Acknowledgments
Financial support was provided by the Wine Advisory Committee, the Washington Wine Commission, and the WSU Agricultural Research Center. We thank Dr Valerie Lynch-Holm and Dr Christine Davitt of the WSU Franceschi Microscopy and Imaging Center and Lynn Mills for technical assistance. We also thank Dr. Bill Riley of Ste. Michelle Wine Estates, Rick Hamman of Hogue Ranches, Bill and Andy Den Hoed of Vigneron Management LLC, and Tedd Wildman of Stone Tree Vineyard for allowing us to sample fruit from their vineyards.
Glossary
Abbreviations
- BS
berry shrivel
- BSN
bunch-stem necrosis
- FDA
fluorescein diacetate
References
- Bondada B, Keller M. Grape berry shrivel: symptoms, probable causes, and effect on fruit quality. Proceedings of the XV International GESCO Symposium. Poreč, Croatia. 2007:582–586. [Google Scholar]
- Choat B, Gambetta GA, Shackel KA, Matthews MA. Vascular function in grape berries across development and its relevance to apparent hydraulic isolation. Plant Physiology. 2009;151:1677–1687. doi: 10.1104/pp.109.143172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels MJ, Crawford NM, Schroeder JI. Proteins for transport of water and mineral nutrients across the membranes of plant cells. The Plant Cell. 1999;11:661–675. doi: 10.1105/tpc.11.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombe BG. Research on development and ripening of the grape berry. American Journal of Enology and Viticulture. 1992;43:101–110. [Google Scholar]
- Creasy GL, Lombard PB. Vine water stress and peduncle girdling effects on pre- and post-veraison grape berry growth and deformability. American Journal of Enology and Viticulture. 1993;44:193–197. [Google Scholar]
- Ebadi A, Sedgley M, May P, Coombe BG. Seed development and abortion in Vitis vinifera L. cv. Chardonnay. International Journal of Plant Science. 1996;157:703–712. [Google Scholar]
- Greenspan MD, Shackel KA, Matthews MA. Developmental changes in the diurnal water budget of the grape berry exposed to water deficits. Plant, Cell and Environment. 1994;17:811–820. [Google Scholar]
- Hifny HAA, Alleweldt G. Untersuchungen zur Stiellähme der Reben. I. Die Symptomatologie der Krankheit. Vitis. 1972;10:298–313. [Google Scholar]
- Jähnl G. Anatomische Veränderungen bei Stiellähme. Mitteilungen Klosterneuburg. 1975;25:57–62. [Google Scholar]
- Keller M. The science of grapevines—anatomy and physiology. Burlington, MA: Academic Press; 2010. [Google Scholar]
- Keller M, Kummer M, Vasconcelos MC. Reproductive growth of grapevines in response to nitrogen supply and rootstock. Australian Journal of Grape and Wine Research. 2001;7:12–18. [Google Scholar]
- Keller M, Smith JP, Bondada BR. Ripening grape berries remain hydraulically connected to the shoot. Journal of Experimental Botany. 2006;57:2577–2587. doi: 10.1093/jxb/erl020. [DOI] [PubMed] [Google Scholar]
- Knoll M, Achleitner D, Redl H. Sugar accumulation in ‘Zweigelt’ grapes as affected by ‘Traubenwelke’. Vitis. 2010;49:101–106. [Google Scholar]
- Krasnow M, Matthews M, Shackel K. Evidence for substantial maintenance of membrane integrity and cell viability in normally developing grape (Vitis vinifera) berries throughout development. Journal of Experimental Botany. 2008;59:849–859. doi: 10.1093/jxb/erm372. [DOI] [PubMed] [Google Scholar]
- Krasnow M, Weis N, Smith RJ, Benz MJ, Matthews M, Shackel K. Inception, progression, and compositional consequences of a berry shrivel disorder. American Journal of Enology and Viticulture. 2009;60:24–34. [Google Scholar]
- Morrison JC, Iodi M. The influence of waterberry on the development and composition of Thompson seedless grapes. American Journal of Enology and Viticulture. 1990;41:301–305. [Google Scholar]
- Raifer B, Roschatt C. Welkekrankheit bei Weintrauben. Das Deutsche Weinmagazin. 2001;38:143–145. [Google Scholar]
- Rogiers SY, Greer DH, Hatfield JM, Orchard BA, Keller M. Solute transport into Shiraz berries during development and late-ripening shrinkage. American Journal of Enology and Viticulture. 2006;57:73–80. [Google Scholar]
- Rogiers SY, Hatfield JM, Jaudzems VG, White RG, Keller M. Grape berry cv. Shiraz epicuticular wax and transpiration during ripening and preharvest weight loss. American Journal of Enology and Viticulture. 2004;55:121–127. [Google Scholar]
- Stellwaag-Kittler F. Äussere Symptomatik der Stiellähme an Trauben. Mitteilungen Klosterneuburg. 1983;33:94–99. [Google Scholar]
- Sun Q, Rost TL, Matthews MA. Wound-induced vascular occlusions in Vitis vinifera (Vitaceae): tyloses in summer and gels in winter. American Journal of Botany. 2008;95:1498–1505. doi: 10.3732/ajb.0800061. [DOI] [PubMed] [Google Scholar]
- Theiler R. Anatomische Untersuchungen an Traubenstielen im Zusammenhang mit der Stiellähme. Die Wein-Wissenschaft. 1970;25:381–417. [Google Scholar]
- Tilbrook J, Tyerman SD. Cell death in grape berries: varietal differences linked to xylem pressure and berry weight loss. Functional Plant Biology. 2008;35:173–184. doi: 10.1071/FP07278. [DOI] [PubMed] [Google Scholar]
- Tilbrook J, Tyerman SD. Hydraulic connection of grape berries to the vine: varietal differences in water conductance into and out of berries, and potential for backflow. Functional Plant Biology. 2009;36:541–550. doi: 10.1071/FP09019. [DOI] [PubMed] [Google Scholar]
- Ureta F, Boidron JN, Bouard J. Influence of desséchement de la rafle on grape quality. American Journal of Enology and Viticulture. 1981;32:90–92. [Google Scholar]
- Zhang XY, Wang XL, Wang XF, Xia GH, Pan QH, Fan RC, Wu FQ, Yu XC, Zhang DP. A shift of phloem unloading from symplasmic to apoplasmic pathway is involved in developmental onset of ripening in grape berry. Plant Physiology. 2006;142:220–232. doi: 10.1104/pp.106.081430. [DOI] [PMC free article] [PubMed] [Google Scholar]