Abstract
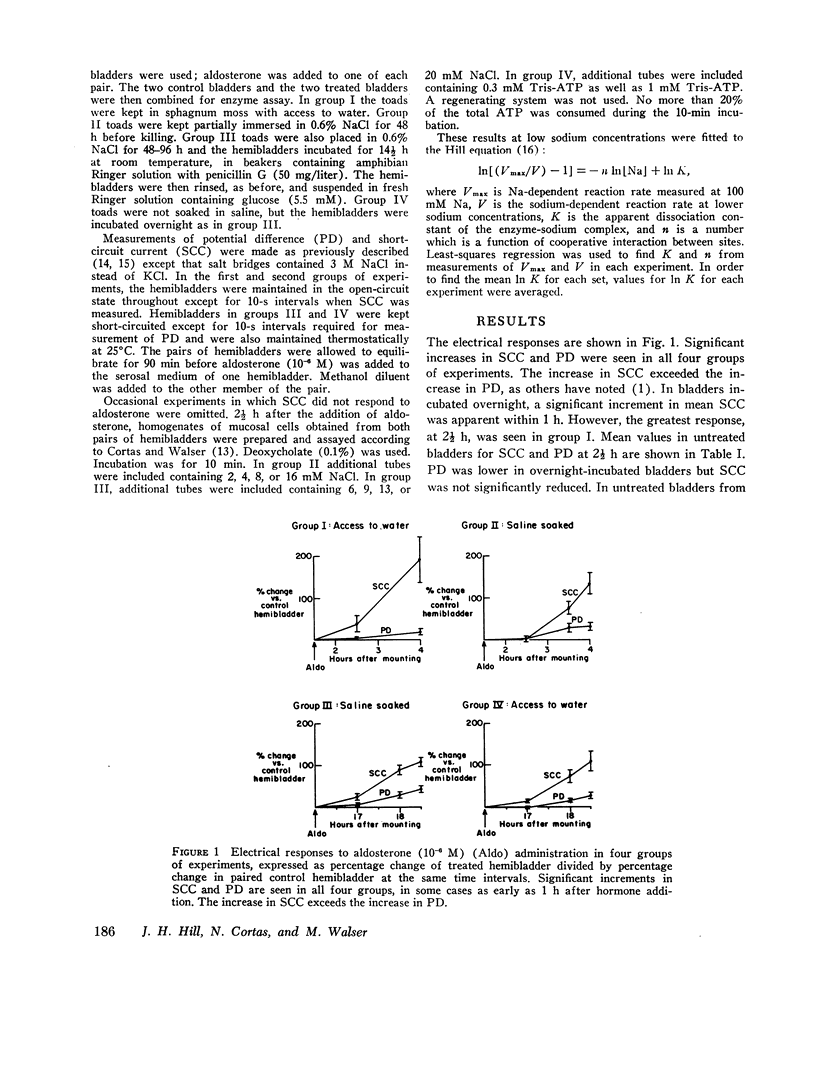
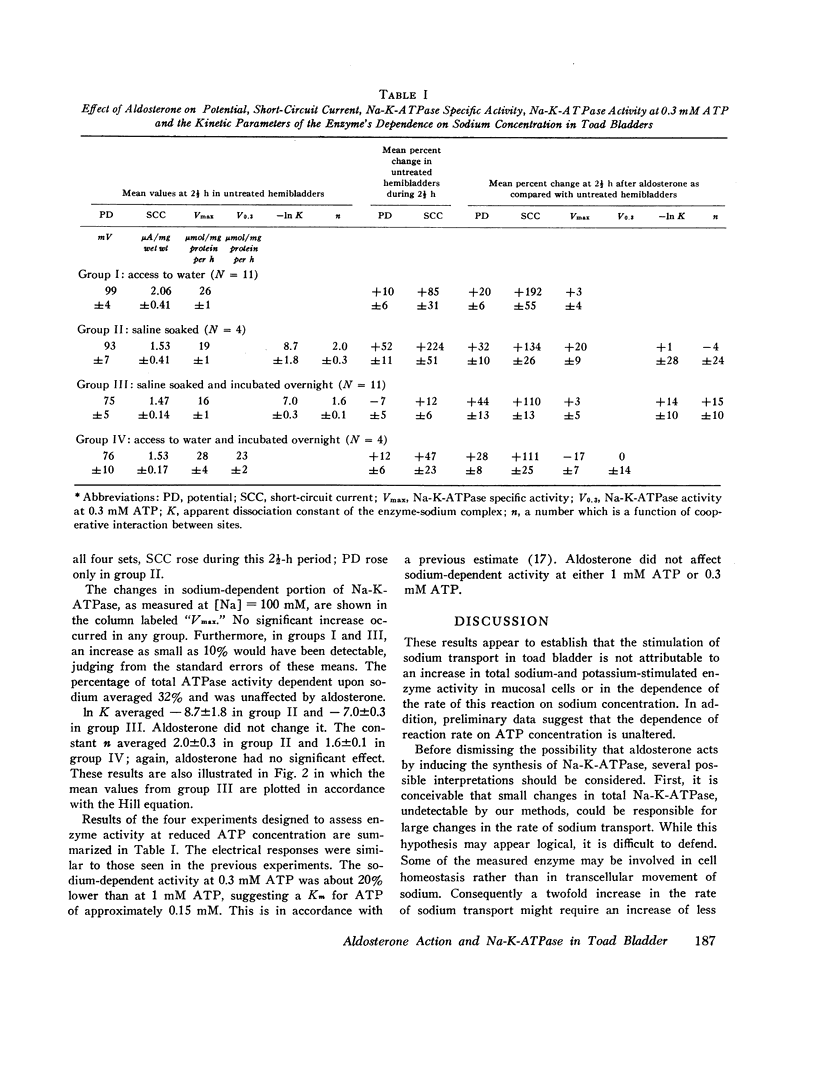
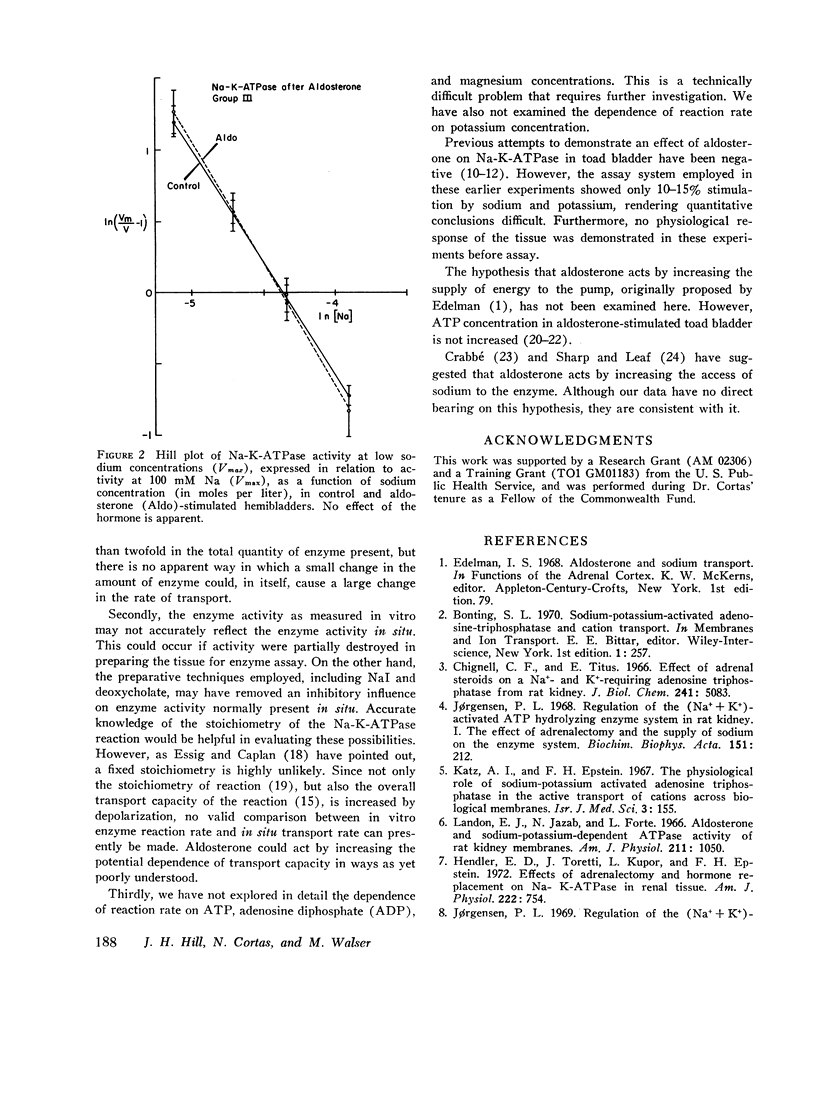
Urinary hemibladders obtained from toads soaked in water or saline were treated with aldosterone, 10-6 M, either 1½ or 16 h after mounting. After 2½ h exposure to the hormone, short-circuit current was increased by 110-192% and open-circuit potential by 20-44% as compared with untreated paired hemibladders. Mucosal cells were then assayed for sodium-potassium-stimulated adenosine triphosphatase (ATPase). No increase occurred in activity per milligram protein or in the portion of total activity dependent on sodium. Activity at low sodium concentrations was also measured and analyzed by means of the Hill equation in terms of K, the apparent dissociation constant of the enzyme-sodium complex, and n, a number that expresses the degree of interaction between binding sites. Neither K nor n was significantly altered by aldosterone. A few experiments were also carried out at low ATP concentrations (0.3 mM); again no change in sodium-dependent activity was noted. The results indicate that aldosterone does not stimulate sodium transport by increasing the quantity of sodium-potassium adenosine triphosphatase in mucosal cells or the dependence of this activity on sodium or ATP concentrations.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., HATHAWAY J. A., SMITH E. C. KINETICS OF REGULATORY ENZYMES. KINETIC ORDER OF THE YEAST DIPHOSPHOPYRIDINE NUCLEOTIDE ISOCITRATE DEHYDROGENASE REACTION AND A MODEL FOR THE REACTION. J Biol Chem. 1965 Jun;240:2682–2690. [PubMed] [Google Scholar]
- BONTING S. L., CANADY M. R. NA-K ACTIVATED ADENOSINE TRIPHOSPHATASE AND SODIUM TRANSPORT IN TOAD BLADDER. Am J Physiol. 1964 Nov;207:1005–1009. doi: 10.1152/ajplegacy.1964.207.5.1005. [DOI] [PubMed] [Google Scholar]
- Chignell C. F., Titus E. Effect of adrenal steroids on a Na+- and K+-requiring adenosine triphosphatase from rat kidney. J Biol Chem. 1966 Nov 10;241(21):5083–5089. [PubMed] [Google Scholar]
- Cortas N., Walser M. (Na + -K + )-activated ATPase in isolated mucosal cells of toad bladder. Biochim Biophys Acta. 1971 Oct 12;249(1):181–187. doi: 10.1016/0005-2736(71)90095-2. [DOI] [PubMed] [Google Scholar]
- Dalton T., Snart R. S. Respiratory and enzymic changes in response to steroid hormones. J Endocrinol. 1970 Jun;47(2):159–166. doi: 10.1677/joe.0.0470159. [DOI] [PubMed] [Google Scholar]
- Essig A., Caplan S. R. Energetics of active transport processes. Biophys J. 1968 Dec;8(12):1434–1457. doi: 10.1016/S0006-3495(68)86565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D. B., Allen J. E., Rasmussen H. On the mechanism of action of aldosterone. Proc Natl Acad Sci U S A. 1969 Sep;64(1):330–337. doi: 10.1073/pnas.64.1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler J. S., Preston A. S., Orloff J. The effect of aldosterone of glycolysis in the urinary bladder of the toad. J Biol Chem. 1969 Jun 25;244(12):3194–3199. [PubMed] [Google Scholar]
- Hendler E. D., Torretti J., Kupor L., Epstein F. H. Effects of adrenalectomy and hormone replacement on Na- K-ATPase in renal tissue. Am J Physiol. 1972 Mar;222(3):754–760. doi: 10.1152/ajplegacy.1972.222.3.754. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L. Regulation of the (Na+ + K+)-activated ATP hydrolyzing enzyme system in rat kidney. I. The effect of adrenalectomy and the supply of sodium on the enzyme system. Biochim Biophys Acta. 1968 Jan 8;151(1):212–224. doi: 10.1016/0005-2744(68)90176-9. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L. Regulation of the (Na+ equals K+)-activated ATP hydrolyzing enzyme system in rat kidney. II. The effect of aldosterone on the activity in kidneys of adrenalectomized rats. Biochim Biophys Acta. 1969 Nov 18;192(2):326–334. [PubMed] [Google Scholar]
- Katz A. I., Epstein F. H. The physiological role of sodium-potassium activated adenosine triphosphatase in the active transport of cations across biological membranes. Isr J Med Sci. 1967 Jan-Feb;3(1):155–166. [PubMed] [Google Scholar]
- Landon E. J., Jazab N., Forte L. Aldosterone and sodium-potassium-dependent ATPase activity of rat kidney membranes. Am J Physiol. 1966 Oct;211(4):1050–1056. doi: 10.1152/ajplegacy.1966.211.4.1050. [DOI] [PubMed] [Google Scholar]
- Manitius A., Bensch K., Epstein F. H. (Na+K+)-activated ATPase in kidney cell membranes of normal and methyprednisolone-treated rats. Biochim Biophys Acta. 1968 Jun 11;150(4):563–571. doi: 10.1016/0005-2736(68)90045-x. [DOI] [PubMed] [Google Scholar]
- Sharp G. W., Coggins C. H., Lichtenstein N. S., Leaf A. Evidence for a mucosal effect of aldosterone on sodium transport in the toad bladder. J Clin Invest. 1966 Oct;45(10):1640–1647. doi: 10.1172/JCI105471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. W., Leaf A. Mechanism of action of aldosterone. Physiol Rev. 1966 Oct;46(4):593–633. doi: 10.1152/physrev.1966.46.4.593. [DOI] [PubMed] [Google Scholar]
- Snart R. S. The two stage nature of the aldosterone response. J Steroid Biochem. 1972 Feb;3(2):129–136. doi: 10.1016/0022-4731(72)90042-8. [DOI] [PubMed] [Google Scholar]
- Vieira F. L., Caplan S. R., Essig A. Energetics of sodium transport in frog skin. II. The effects of electrical potential on oxygen consumption. J Gen Physiol. 1972 Jan;59(1):77–91. doi: 10.1085/jgp.59.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walser M., Butler S. E., Hammond V. Reversible stimulation of sodium transport in the toad bladder by stretch. J Clin Invest. 1969 Sep;48(9):1714–1723. doi: 10.1172/JCI106137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walser M. Components of sodium and chloride flux across toad bladder. Biophys J. 1972 Apr;12(4):351–368. doi: 10.1016/S0006-3495(72)86089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]



