Abstract
The biosynthesis of S-(3-hexan-1-ol)-glutathione (3MH-S-glut) and S-(3-hexan-l-ol)-L-cysteine (3MH-S-cys), which act as flavour precursors in wines, in Vitis vinifera grapes exposed to various environmental stress conditions is reported here. Ultraviolet (UV-C) irradiation, water deficit, and biological stimulation up-regulated 3MH-S-glut and 3MH-S-cys biosynthesis in grape leaves. 3MH-S-glut and 3MH-S-cys contents in grape berries were increased by cold shock, heat shock, UV-C irradiation, and biological stimulation. The results suggest that environmental stress enhances the biosynthesis of both flavour precursors in grapevine. The transcription of VvGST1, VvGST3, VvGST4, and GGT in grapevine exposed to the stress conditions was increased markedly compared with that in control grapevine. Also, UV irradiation increased GST (glutathione S-transferase) and GGT (γ-glutamyl transferase) enzyme activities in grape berries. Recombinant VvGST3 and VvGST4, but not VvGST1, mediated the synthesis of 3MH-S-glut from reduced glutathione and trans-2-hexenal in vitro. The enzymatic mediation of flavour precursor production is a novel function of plant GSTs and may result in the detoxification of damaged grape cells under stress conditions.
Keywords: Environmental stress, flavour precursor, γ-glutamyl transferase, glutathione, glutathione S-transferase, S-(3-hexan-l-ol)-L-cysteine, S-(3-hexan-1-ol)-glutathione
Introduction
Aroma is one of the important factors affecting wine flavour. Grape berries contain an assortment of volatile organic compounds, including terpenoids, norisoprenoids (Wirth et al., 2001), and thiols (Tominaga et al., 1998b). Many aromas are formed from these compounds by enzymatic catalysis during berry development and by yeast enzymatic activities during alcoholic fermentation. The contents of aroma compounds in grape berry during development fluctuate with environmental conditions, such as solar radiation, high and/or low temperature, rainfall, biological stimulation, and soil composition (Roujou de Boubée et al., 2000; Peyrot des Gachons et al., 2005). In addition, accidental insect attack increases the amount of terpenoid compounds as a plant defence mechanism (Aharoni et al., 2003; Lee et al., 2003). However, the molecular mechanism of the induction of aroma compounds is poorly understood in grape berry.
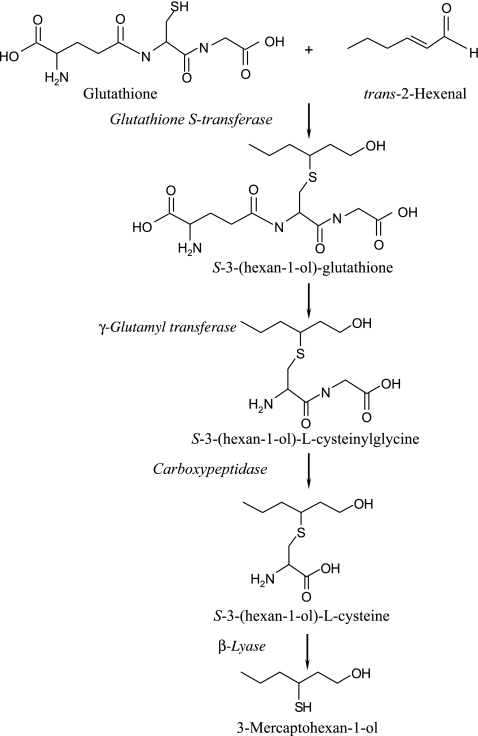
3-Mercaptohexan-1-ol (3MH) is a volatile thiol and a key contributor to the distinct odour of grapefruit or passion fruit in Sauvignon blanc wines (Tominaga et al., 1998a) as well as Colombard, Muscat, Sylvaner, Pinot blanc (Tominaga et al., 2000), Petite arvine (Fretz et al., 2005), and rose wines (Murat et al., 2001). S-(3-Hexan-l-ol)-L-cysteine (3MH-S-cys) was first identified as the precursor of 3MH in Sauvignon blanc juice (Tominaga et al., 1998b). Thereafter, S-(3-hexan-1-ol)-glutathione (3MH-S-glut) was identified as the tentative pro-precursor of 3MH-S-cys (Peyrot des Gachons et al., 2002). A pathway for the biosynthesis of glutathionylated pro-precursor 3MH and cysteinylated precursor 3MH in grapevine is proposed, as shown in Fig. 1. The 3MH contents in wines made from botrytized grapes infected by Botrytis cinerea were much higher than those in wines made from healthy grapes (Sarrazin et al., 2007). In a recent study by Thibon et al. (2009), 3MH-S-cys content was found to be much higher in botrytized grapes than in healthy grapes. These findings suggest that the 3MH-S-cys biosynthesis in grape berry may be activated by pathogen attack and, as a result, a high content of 3MH may be present in wines made from botrytized grapes. However, how the biosynthesis is activated in grape berries under stress conditions has been not clarified so far.
Fig. 1.
Proposed pathway leading to the biosynthesis of the glutathionylated pro-precursor (3MH-S-glut) and the cysteinylated precursor (3MH-S-cys) in grapevine. The production of 3-mercaptohexan-1-ol (3MH) occurred during alcoholic fermentation.
Glutathione (L-γ-glutamyl-L-cysteinyl-glycine) and glutathione S-transferases (GSTs) are indispensable for the biosynthesis of the 3MH precursor (Fig. 1). The physiological and biochemical roles of glutathione in plant include sulphur metabolism, detoxification (Noctor and Foyer 1998), and redox control (Shaul et al., 1996). Glutathione accelerates the transcription of plant defence genes and this is followed by the accumulation of phytoalexin in bean cell suspension cultures (Wingate et al., 1988). Meanwhile, glutathionylated conjugates accumulate in tobacco leaves in response to reactive electrophilic species (Davoine et al., 2006). On the other hand, plant GSTs are grouped into seven classes: phi, zeta, tau, theta, lambda, DHAR, and TCHQD (Dixon et al., 2010). The phi and tau GSTs are plant specific and can bind to a great number of xenobiotics, including pesticides (Dixon et al., 2002; Edwards and Dixon, 2005). Both GSTs are induced in response to abiotic and biotic stress. For example, co-silencing of a group of four phi GSTs in Arabidopsis resulted in altered metabolic sensitivity to oxidative stress (Sappl et al., 2009). Although the induction of Vitis vinifera GST expression was investigated using transcriptomic and proteomic studies in bud dormancy release (Halaly et al., 2008), grape berry development (Castellarin and Gaspero, 2007), and embryogenesis (Marsoni et al., 2008), little is known about the stress response of V. vinifera GSTs or the biological functions of GSTs in grapevine. Although VvGSTs may mediate the biosynthesis of 3MH-S-glut from glutathione and trans-2-hexenal (Fig. 1), there is no evidence to determine which VvGSTs are likely to be involved in the biosynthesis of 3MH-S-glut in plants. S-Glutathionylation substrates mediated by GSTs are related to herbicide detoxification and tolerance in plants (Milligan et al., 2001; Skipsey et al., 2005). In the present study, the possible reasons for the induction of specific VvGSTs in grapevine under stress conditions and the production of 3MH-S-glut from GSH and hexenal for the first time are demonstrated. Finally, a hypothetical pathway is formulated for the biosynthesis of the 3MH precursors in grapevine exposed to stress conditions.
Materials and methods
Chemicals
All chromatographic solvents were high-performance liquid chromatography (HPLC) grade. trans-2-Hexenal (98%), reduced glutathione (GSH, 99%), and S-ethyl-L-cysteine were purchased from Sigma-Aldrich (Tokyo, Japan). Sodium borohydride (98%) was obtained from Junsei Chemical (Tokyo, Japan). Abscisic acid (ABA, 98%) and Boc-L-cysteine (98%) were procured from Tokyo Chemical Industry (Tokyo, Japan) and Nova Biochem (Laufelfingen, Switzerland), respectively. The synthesis of 3MH-S-glut and 3MH-S-cys was performed as previously described (Kobayashi et al., 2010a). Commercial GST from equine liver was purchased from Sigma-Aldrich.
Plant materials
Seedlings of grapevines (V. vinifera cvs. Chardonnay, Koshu, and Merlot) were grafted onto 101-14 rootstock and cultivated for ∼10 weeks in a greenhouse under solar radiation (average 16 MJ m−2) and 55% (average) relative humidity (Supplementary Fig. S1A available at JXB online).
Sauvignon blanc, Chardonnay, Koshu, and Merlot grape berries, grown in vineyards located in Yamanashi Prefecture, were collected at 16 weeks post-flowering in the 2009 growing season. Berries infected with downy mildew were also collected between 10 and 14 weeks post-flowering in the 2009 growing season.
Stress treatment of grape leaves
Seedlings with 6–7 expanding leaves were used for stress treatment. For cold shock and heat shock treatments, the seedlings were exposed to 0 °C and 40 °C, respectively, for 1 h. UV-C irradiation of the seedlings was performed at 253.7 nm for 10 min in a ventilated room (SANYO, Tokyo, Japan). For the water deficit treatment, the seedlings were maintained under the water deficit condition for 2 weeks. Biological stimulation was carried out by inoculating each leaf with ∼1 ml of Botryotinia fuckeliana (NCRB5911) spore suspension (1×105 cells ml−1). The stressed seedlings were incubated at 20 °C in a clean room for the indicated periods. After incubation, the leaves were collected and subjected to further analyses.
Stress treatment of grape berries
Grape berries were collected at 16 weeks post-flowering and subjected to the same stress treatments as those for grape leaves, but with slight modification. Briefly, for the cold and heat shock treatments, the berries were exposed to 0 °C and 40 °C, respectively, for 1 h every day. The berries were exposed to UV-C at 253.7 nm for 15 min.
Analysis of water leaf potential
Water leaf potential (Ψ pd) was measured according to a previously reported method (Mccown and Wall, 1979; Turner et al., 1988) using the pressure chamber technique (Pressure Chamber Model 600, PMS Instrument Company, USA).
Extraction of 3MH-S-glut, 3MH-S-cys, ABA, and GSH from grape leaves and berry skins
Grape leaves and berry skins were pulverized in liquid nitrogen. A 1 g aliquot of the powder was measured and added to 9 ml of extraction buffer [10% (v/v) methanol containing 0.1% (v/v) formic acid]. To prevent GSH oxidation, the extraction buffer containing 0.1% (v/v) potassium metabisulphite was used for the analysis of GSH. The mixture was shaken at room temperature for 16 h in the dark and the supernatant was filtered through a 0.45 μm cellulose acetate filter (Advantec Toyo, Tokyo, Japan). The filtrate was subjected to quantitative analysis of 3MH-S-glut, 3MH-S-cys, ABA, and GSH by liquid chromatography–tandem mass spectrometry (LC-MS/MS) as described below.
Quantitative analysis of 3MH-S-glut, 3MH-S-cys, ABA, and GSH using LC-MS/MS
Quantitative analysis of 3MH-S-glut, 3MH-S-cys, ABA, and GSH was performed using an LC-MS/MS system (MS/MS; API 3200 QTRAP; Applied Biosystems Japan Ltd, Tokyo, Japan). Purification and concentration analysis of synthesized 3MH-S-glut and 3MH-S-cys were accomplished by proton (1H) nuclear magnetic resonance (NMR) measurements according to a previously described procedure (Kobayashi et al., 2010a). Although the analysis of the diastereoisomers of 3MH-S-cys was performed by Thibon et al. (2008), the gross weight of the R- and S-configuration products was determined in the present study.
The conditions for HPLC (CBM-20A, Shimadzu, Kyoto, Japan) were as follows: Atlantis T3, 3.0 μm, 2.1 mm×150 mm column (Waters, Massachusetts, USA); column oven temperature, 40 °C (5 min); mobile phase A [distilled water containing 0.1% (v/v) formic acid]; mobile phase B [acetonitrile containing 0.1% (v/v) formic acid]; and flow rate, 0.2 ml min−1. Linear gradient programs were set from 10% of mobile phase B to 100% of mobile phase B over a 10 min period.
Mass spectrometry conditions for 3MH-S-glut and 3MH-S-cys were determined as described previously (Kobayashi et al., 2010a). Briefly, the optimized conditions for 3MH-S-glut analysis were as follows: curtain gas, 15 psi; ion spray voltage, 5500 V; temperature, 600 °C; nebulizing gas (GS1), 70 psi; drying gas (GS2), 70 psi; and collision gas, 3. For the enhanced product ion (EPI) experiment, the scan rate was set at 4000 ams s−1, the fixed fill time was selected for Q0 trapping (250 ms), and mass spectra were recorded between m/z 100 and 500. The parent ion was detected at m/z 408.2 by HPLC-MS/MS and liquid secondary ion mass spectrometry (LSIMS), as reported previously (Peyrot des Gachons et al., 2002; Davoine et al., 2006). Multiple reaction monitoring (MRM) parameters were m/z 408.2→162.1 among some ion pairs, with a dwell time of 150 ms each. In addition, the curtain gas, ion spray voltage, temperature, GS1, GS2, collision gas, and EPI experiment for GSH were the same as those for 3MH-S-glut. MRM parent ion was detected at m/z 308.1 using LC-MS (Davoine et al., 2006; Toit et al., 2007). MRM parameters were m/z 308.1→179.2.
The optimized conditions for 3MH-S-cys analysis were set as follows: curtain gas, 20 psi; ion spray voltage, 5500 V; temperature, 700 °C; GS1, 70 psi; GS2, 50 psi; and collision gas, 5. The EPI experiment was carried out under the same conditions as those for 3MH-S-glut. The MRM parent ion was detected at m/z 222.2 using LC-MS (Luisier et al., 2008; Thibon et al., 2008). MRM parameters were m/z 222.2→83.2.
The optimized conditions for ABA analysis were determined according to the method reported by Forcat et al. (2008) with slight modification. The conditions were set as follows: curtain gas, 40 psi; ion spray voltage, −4500 temperature, 400 °C; GS1, 50 psi; GS2, 60 psi; and collision gas, medium. The parent ion was detected at m/z 263.0 for ABA using the LC-ESI-MS/MS method described previously (Forcat et al., 2008). MRM parameters were m/z 263.0→153.1 for ABA.
Analysis of grape characteristics
Healthy and downy mildew-infected grape berries were collected from the same clusters and fresh berry weight was measured. Grape berries were pressed to 50% of the total weight using a micropress (Quick juicer, Chibakogyosyo, Chiba, Japan). Total soluble solids (TSS) in the juice were measured with a refractometer (Pocket PaL-1, Atago, Tokyo, Japan) and expressed as Brix. Juice pH was measured with a pH meter (MH-60S, Toakogyo, Tokyo, Japan). Titratable acidity (TA) in the juice was determined by adding 10 ml of distilled water to 10 ml of the juice and subjecting the solution to neutralization titration to pH 7.0 using 0.1 M NaOH. TA is expressed as g of tartaric acid l−1. For the quantitative analysis of 3MH-S-glut, 3MH-S-cys, and GSH contents in the juice, the juice was diluted with an equal volume of the extraction buffer [with 0.1% (v/v) potassium metabisulphite for GSH]. The mixture was filtered through a 0.45 μm cellulose acetate filter and the concentrated filtrate was subjected to LC-MS/MS as described above.
RNA isolation and real-time quantitative RT-PCR
Total RNA from grape leaves and skins was extracted as previously described (Tesniere and Vayda, 1991) and the pellet was purified using an RNeasy Plant Mini Kit (Qiagen, Hilden, Germany).
First-strand cDNA was synthesized from 500 ng of total RNA using a PrimeScript RT Reagent Kit (Takara-Bio Inc., Shiga, Japan) according to the manufacturer's instructions. Nucleotide sequences of the primers used in this study were as follows: C-repeat-binding transcription factor (CBF4) primers (5′-TTCGCACAAACGGAAAACTG-3′ and 5′-GCTCGCGCACTTCACTCA-3′, corresponding to bases 142–161 and 251–234, respectively, GenBank accession no. AY706986), hypothetical protein highly similar to heat shock protein 21 (CAN67481) primers (5′-TCCGGCGACATCAAGGTT-3′ and 5′-ACTTGCGCCTTCCTTCTCTTC-3′, corresponding to bases 3399–3416 and 3484–3469, respectively, GenBank accession no. AM445422), phenylalanine ammonium-lyase (PAL) primers (5′-GCGCCACATATCCACTGATG-3′ and 5′-CCTCGAATGCCCCTATTTTTT-3′, corresponding to bases 1733–1752 and 1852–1832, respectively, GenBank accession no. EF192469), non-expressor of PR1 (NPR1) primers (5′-TCGTCAGCGCATTCAGAAAC-3′ and 5′-GGAGATTGCCAAGGATTACGAA-3′, corresponding to bases 343–362 and 456–435, respectively, GenBank accession no. EU404483), glutathione S-transferase 1 (VvGST1) primers (5′-CCCGTATGCGAGTCCTTGAT-3′ and 5′-GAACTTGGCTTTTGCTCTTTGG-3′, corresponding to bases 282–301 and 386–365, respectively, GenBank accession no. AY156048), glutathione S-transferase 2 (VvvGST2) primers (5′-TTCTTCCCAATCCAGCAGTCA-3′ and 5′-CGTAGAGCGCAGCGACAAC-3′, corresponding to bases 26–46 and 115–97, respectively, GenBank accession no. EF088687), glutathione S-transferase 3 (VvGST3) primers (5′-TGCAAAGGTGTTGGACATCTATG-3′ and 5′-TGTGAATGGAAGGTGGCTAAGA-3′, corresponding to bases 997–1019 and 1096–1075, respectively, GenBank accession no. EF469244), glutathione S-transferase 4 (VvGST4) primers (5′-AGTCATCTTCCGGCCATCAG-3′ and 5′-CTCCCACCATGCACTCACACT-3′, corresponding to bases 502–521 and 591–571, respectively, GenBank accession no. AY971515), glutathione S-transferase 5 (VvGST5) primers (5′-TCCTTGTGCTCTCCTTTGATGATAT-3′ and 5′-CGTGTCCTTATTTGCCTTGTTG-3′, corresponding to bases 1 412 896–1 412 920 and 1 412 976–1 412 954, respectively, GenBank accession no. NC_012018), γ-glutamyl transferase (GGT) primers (5′-TGGCAACAGCTTAGAGGCAGTA-3′ and 5′-CCCACCTGCCTTTCTCACAT-3′, corresponding to bases 2 248 444–2 248 465 and 2 248 543–2 248 524, respectively, GenBank accession no. NC_012017), and 18S rRNA primers (5′-CGAAAGCATTTGCCAAGGAT-3′ and 5′-CCTGGTCGGCATCGTTTATG-3′, corresponding to bases 522–541 and 625–606, respectively, GenBank accession no. AF207053). Real-time quantitative reverse transcription-PCR (real-time RT-PCR) was performed using an ABI PRISM 7300 Real-Time PCR System (Applied Biosystems) with SYBR Green detection modules as previously described (Kobayashi et al., 2009). Negative control and 18S rRNA primers were used for background analysis and normalization, respectively (Downey et al., 2003; Fujita et al., 2005). The dissociation curves for each sample were analysed to verify the specificity of the amplification reaction. Data were analysed using the 7300 system software SDS 1.3.0 (Applied Biosystems). Expression levels were determined as the number of amplification cycles needed to reach a fixed threshold, as described previously (Pfaffl, 2001; Reid et al., 2006). Triplicate experiments using three independent samples were performed in a 96-well reaction plate.
GST and GGT enzyme activities in grape berries
To evaluate GST and GGT activities in grape berry exposed to UV-C irradiation, the berries were exposed to UV-C at 253.7 nm for 15 min and incubated for the indicated periods. Grape juices were obtained from the berries pressed to 50% of the total weight using a micropress. The juices were passed through PD MiniTrap G-25 columns (GE Healthcare, Tokyo, Japan), and the extracts were used for the measurement of GST and GGT enzyme activities.
GST enzyme activity was measured using a GST assay kit (Sigma-Aldrich) according to the manufacturer's instructions. Briefly, 50 μl of the extracts was added to 1 ml of the reaction mixture comprising Dulbecco's phosphate-buffered saline, 2 mM L- reduced glutathione, and 1 mM 1-chloro-2,4-dinitrobenzene. Enzymatic activity was measured at 340 nm over a 1 min interval. GST specific activity was defined as the amount of enzyme catalysing the formation of 1 nmol S-(2,4-dinitrophenyl)glutathione min−1 by 1 ml of the grape juice.
GGT enzyme activity was measured using the protocol reported by Ohkama-Ohtsu et al. (2007). Briefly, the reaction mixture comprising 100 mM TRIS-HCl (pH 8.0), 5 mM γ-glutamyl p-nitroanilide, 100 mM glycylglycine, and 50 μl of the extract was prepared. Enzymatic activity was measured at 410 nm over a 10 min interval. GGT specific activity was defined as the amount of enzyme catalysing the formation of 1 nmol p-nitroanilide min−1 by 1 ml of the grape juice.
Purification of recombinant VvGST proteins from Escherichia coli
To express 6×His-tagged recombinant VvGST1, VvGST3, and VvGST4 proteins in E. coli, the coding region of the VvGSTs was amplified from grape cDNA using PCR with primer sets, 5′-CTGCTCGAGATGGCAAACAGTGACCAC-3′ containing an XhoI site (underlined) and 5′-GCGCTGCAGCTATATCCCCATTTTCTT-3′ containing a PstI site (underlined) for VvGST1, 5′-CTACTCGAGATGGTGGTGAAGGTG-3′ containing an XhoI site (underlined) and 5′-AAACTGCAGTCACTCCAAGAGGGG-3′ containing a PstI site (underlined) for VvGST3, and 5′-CTGCTCGAGATGGTGATGAAGGTG-3′ containing an XhoI site (underlined) and 5′-AATCTGCAGTCAAGCAGCGAGCTCC-3′ containing a PstI site (underlined) for VvGST4, respectively. The amplified cDNAs were digested with XhoI and PstI followed by ligation into a pCold I vector (Takara) digested by XhoI and PstI. The constructs were transformed into an E. coli JM109 strain. Recombinant His-tagged VvGSTs were purified from the transformant using a Protino Ni pre-packed column (Macherey-Nagel, Duren, Germany) in accordance with the manufacturer's instructions. Purification efficiency was confirmed using SDS–polyacrylamide gel electrophoresis (SDS–PAGE).
Activity of recombinant VvGSTs in in vitro synthetic analysis of 3MH-S-glut
The activities of recombinant VvGSTs were measured with a DetextX glutathione S-transferase fluorescent activity kit (Cosmo bio Co., Ltd, Tokyo, Japan). Briefly, 50 μl of each recombinant VvGST were put into 96-well microtitre plates, and then 25 μl of the detection regent and GSH was added in the plates. The mixtures were incubated at room temperature for 30 min. After incubation, the kinetic studies were performed by monitoring fluorescent signals at 460 nm with excitation at 370–410 nm. Each GST activity was expressed as mU ml−1 based on the standard curve obtained. To evaluate the activity of recombinant VvGSTs in 3MH-S-glut synthesis, 1 mM GSH, 1 mM trans-2-hexenal, and recombinant VvGSTs were added into a microtube. The reaction mixture was incubated at 25 °C for 48 h in the dark. Commercial GST from equine liver was used as the control protein having GST activity. After reaction, the amount of 3MH-S-glut in the reaction mixture was measured using LC-MS/MS as described above and expressed as μM per mU of GST activity.
Statistical analysis
Data are expressed as means ±SDs. Statistical analysis was performed by the t-test.
Results
3MH-S-glut and 3MH-S-cys contents in grape leaves
3MH-S-glut and 3MH-S-cys contents were measured in leaves of Chardonnay, Koshu, and Merlot seedlings, and the results are shown in Supplementary Fig. S1 at JXB online. The difference in 3MH-S-glut content among the leaves of the same plant was ∼2.8-fold (Chardonnay: 15.5–42.7 ng g−1), 4.5-fold (Koshu: 3.72–16.9 ng g−1), and 4.0-fold (Merlot: 5.22–22.6 ng g−1) (Supplementary Fig. S1B), whereas the difference in 3MH-S-cys content was ∼1.7-fold (Chardonnay: 4.39–7.46 ng g−1), 4.5-fold (Koshu: 0.57–3.43 ng g−1), and 4.0-fold (Merlot: 0.46–3.38 ng g−1) (Supplementary Fig. S1C). Young leaves had the highest contents of both flavour precursors in the grape seedlings tested, suggesting that 1 g of grape leaf produced 3MH-S-glut and 3MH-S-cys constitutively in the order of nanograms. To evaluate the induction of 3MH-S-glut and 3MH-S-cys production in the stress-treated leaves, five leaves were collected from each seedling and used for extraction.
3MH-S-glut and 3MH-S-cys contents in grape leaves exposed to stress conditions
To evaluate the effects of cold shock, heat shock, UV-C irradiation, biological stimulation, and water deficit, the expression of stress markers was analysed on day 3 after the stress treatment (Supplementary Fig. S2 at JXB online). The results suggest that the experimental systems that evaluate the biosynthesis of 3MH-S-glut and 3MH-S-cys in grape leaves under stress conditions are correctly designed.
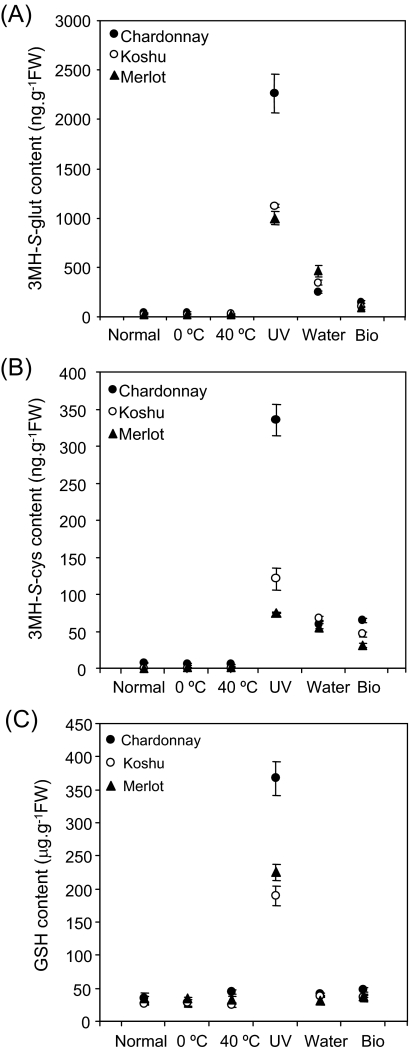
3MH-S-glut, 3MH-S-cys, and GSH contents in grape leaves exposed to various stress conditions for 72 h are shown in Fig. 2A–D. The biosynthesis of 3MH-S-glut in grape leaves was up-regulated by UV-C irradiation, water deficit, and biological stimulation regardless of the grape cultivar (Fig. 2A). Of all the stress conditions examined, UV-C irradiation of grape leaves induced the biosynthesis of 3MH-S-glut most markedly: Chardonnay, Koshu, and Merlot accumulated 2260, 1125, and 1001 ng of 3MH-S-glut g−1 fresh weight of grape leaf. UV-C irradiation, water deficit, and biological stimulation also increased 3MH-S-cys content in grape leaves (Fig. 2B). Chardonnay, Koshu, and Merlot accumulated 335, 121, and 75 ng of 3MH-S-cys g−1 fresh weight of grape leaf when exposed to UV-C irradiation. Meanwhile, cold and heat shock did not affect the biosynthesis of 3MH-S-glut and 3MH-S-cys in grape leaves. Taken together, the biosynthesis of 3MH-S-glut and 3MH-S-cys was up-regulated in grape leaves exposed to UV-C irradiation, water deficit, and biological stimulation. On the other hand, GSH content was increased only in grape leaves exposed to UV-C irradiation (Fig. 2D).
Fig. 2.
Measurement of 3MH-S-glut, 3MH-S-cys, and GSH contents in grape leaves of seedlings exposed to stress conditions. (A) 3MH-S-glut, (B) 3MH-S-cys, (C) GSH. Chardonnay, Koshu, and Merlot seedlings were used and incubated under stress conditions as described in the Materials and methods. Data are shown as means ±SDs of triplicate experiments.
Correlation between expression of GST and GGT, and production of 3MH-S-glut and 3MH-S-cys in grape leaves and berries
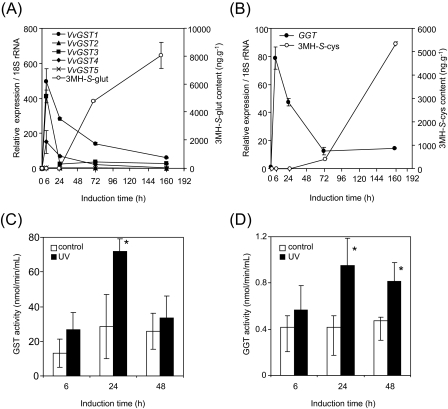
VvGST1, VvGST3, and VvGST4 expression was up-regulated in grape leaves exposed to UV-C irradiation and peaked at 6 h (Fig. 3A). VvGST1 and VvGST4 expression gradually decreased thereafter, whereas VvGST3 expression showed a rapid decrease 24 h after UV-C irradiation. VvGST2 and VvGST5 expression was not induced in UV-C-irradiated grape leaves. After VvGST genes were transcribed, 3MH-S-glut was synthesized and accumulated in grape leaves. Meanwhile, the expression of GGT, which regulates the synthesis of S-(3-hexan-l-ol)-L-cysteinylglycine (3MH-S-cysgly), an intermediate between 3MH-S-glut and 3MH-S-cys (Fig. 1), peaked at 6 h and gradually decreased thereafter (Fig. 3B). After GGT was transcribed, 3MH-S-cys was synthesized and accumulated in grape leaves.
Fig. 3.
Induction of GST and GGT in UV-C-irradiated grape leaves and berries. (A) The expression of VvGST genes (VvGST1, VvGST2, VvGST3, VvGST4, and VvGST5) and 3MH-S-glut content. (B) GGT gene expression and 3MH-S-cys content. Chardonnay seedlings were exposed to UV-C irradiation and cultivated for the indicated periods as described in the Materials and methods. For gene expression analyses, 18S rRNA was used as the internal control. Data were calculated as gene expression relative to 18S rRNA gene expression. Data are shown as means ±SDs of triplicate experiments. (C) GST enzyme activity. (D) GGT enzyme activity. Sauvignon blanc bunches were exposed to UV-C irradiation and incubated for the indicated periods. GST and GGT enzyme activities were measured and calculated as described in the Materials and methods. Data are shown as means ±SDs of triplicate experiments. Control, no treatment; UV, UV irradiation. *P <0.05 as compared with control.
On the other hand, UV-C irradiation increased GST activity 200% in grape berries by 24 h post-treatment (Fig. 3C). GGT enzymatic activity in grape berries exposed to UV-C irradiation was up-regulated by 24 h and 48 h post-treatment (Fig. 3D). In this experiment 3MH-S-glut in grape berries was increased from 33.2±3.2 nM to 52.4±4.7 nM by UV-C irradiation by 24 h post-treatment, while 3MH-S-cys contents in grape berries exposed to UV-C irradiation were also increased from 16.3±0.5 nM to 23.0±1.6 nM by 24 h post-treatment. Taken together, the results suggest that the proposed pathway in Fig. 1 may be appropriate and that VvGST genes and GGT may be the key enzymes for the biosynthesis of flavour precursors, 3MH-S-glut and 3MH-S-cys, in grape.
3MH-S-glut, 3MH-S-cys, and GSH contents in grape berries exposed to stress conditions
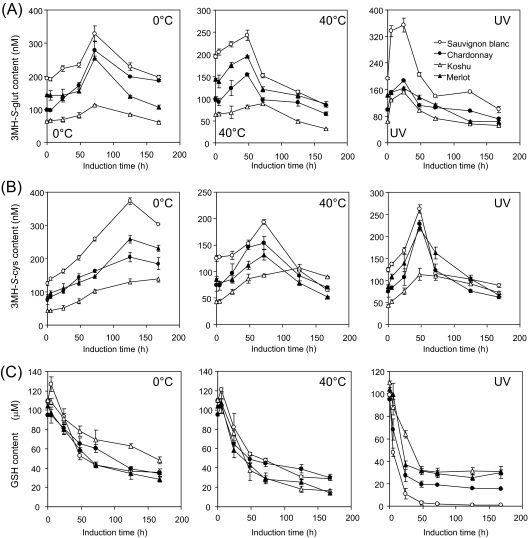
3MH-S-glut, 3MH-S-cys, and GSH contents in grape berries exposed to cold shock, heat shock, and UV-C irradiation are shown in Fig. 4. 3MH-S-glut content in grape berries exposed to cold shock continued to increase up to 72 h and decreased thereafter (Fig. 4A). 3MH-S-glut contents in grape berries exposed to heat shock and UV-C irradiation continued to increase up to 48 h and 24 h, respectively, and rapidly decreased thereafter (Fig. 4A). 3MH-S-cys accumulation in grape berries was also induced for as long as 120 h by cold shock and decreased thereafter (Fig. 4B), whereas 3MH-S-cys contents in grape berries exposed to heat shock and UV-C irradiation continued to increase up to 72 h and 48 h, respectively, and decreased thereafter (Fig. 4B). In contrast, GSH contents in grape berries exposed to the stress conditions decreased, although GSH contents showed a slight increase up to 6 h in grape berries that were exposed to cold shock and heat shock (Fig. 4D). UV-C irradiation decreased GSH content in grape berries within 48 h. Taken together, 3MH-S-glut and 3MH-S-cys biosynthesis was up-regulated in grape berries exposed to cold shock, heat shock, and UV-C irradiation, and these up-regulations were accompanied by a reduction in GSH.
Fig. 4.
Measurement of 3MH-S-glut, 3MH-S-cys, and GSH contents in grape berries exposed to stress conditions. (A) 3MH-S-glut, (B) 3MH-S-cys, (C) GSH. Grape berries were collected at 16 weeks post-flowering and exposed to each stress. Data are shown as means ±SDs of triplicate experiments.
Correlation between expression of VvGST genes and GGT, and production of 3MH-S-glut and 3MH-S-cys in downy mildew infected grape berries
Table 1 lists the variations in fruit characteristics, such as berry weight, TSS, TA, pH, and 3MH-S-glut, 3MH-S-cys, and GSH contents, between healthy and downy mildew-infected grape berries from the same grape clusters. The weight of downy mildew-infected berries was lower than that of healthy berries, and TSS and TA in the juice from downy mildew-infected grape berries were lower than those from healthy grape berries. 3MH-S-glut contents in the juice from downy mildew-infected Sauvignon blanc, Chardonnay, Koshu, and Merlot berries were increased by 24-, 57-, 23-, and 35-fold relative to those in healthy berries. Correspondingly, 3MH-S-cys contents in the juice from downy mildew-infected Sauvignon blanc, Chardonnay, Koshu, and Merlot berries increased by 16-, 34-, 19-, and 51-fold relative to those in healthy berries.
Table 1.
Comparison of grape characteristics of healthy and downy mildew infected-grape juice
| Grape characteristic | Sauvignon blanc |
Chardonnay |
Koshu |
Merlot |
||||
| Healthy | Downy mildew | Healthy | Downy mildew | Healthy | Downy mildew | Healthy | Downy mildew | |
| Berry weight (g)a | 1.39±0.2b | 0.94±0.3 | 1.60±0.3 | 1.02±0.2 | 1.57±0.2 | 0.81±0.3 | 1.60±0.2 | 1.02±0.3 |
| TSS | 11.4±0.2 | 10.4±0.1 | 12.5±0.2 | 11.2±0.1 | 5.7±0.1 | 6.9±0.1 | 12.2±0.1 | 13.3±0.2 |
| TA (g l−1) | 24.9±0.7 | 22.9±1.3 | 22.0±0.4 | 21.4±0.7 | 77.3±2.3 | 69.5±3.2 | 22.8±1.3 | 21.0±1.4 |
| pH | 2.60±0.02 | 2.73±0.03 | 2.79±0.04 | 2.84±0.03 | 2.34±0.02 | 2.40±0.02 | 2.85±1.3 | 2.95±0.03 |
| 3MH-S-glut (nM) | 33.9±1.4 | 829.7±14.2 | 21.6±0.2 | 1227.3±44.7 | 17.1±1.9 | 393.4±35.1 | 27.9±3.8 | 997.1±63.9 |
| 3MH-S-cys (nM) | 51.1±3.5 | 854.3±6.3 | 26.6±2.5 | 908.8±22.3 | 18.3±1.6 | 349.8±11.5 | 12.5±1.3 | 640.0±6.3 |
| GSH (nM) | 5603±62 | 122±4 | 2080±32 | 390±22.3 | 1084±12 | 497±11.5 | 2766±1.3 | 387±29 |
Berry weight and standard deviations were calculated from 10 berries.
Data are shown as means ±SDs of triplicate experiments.
VvGST1 transcription in downy mildew-infected Sauvignon blanc, Chardonnay, Koshu, and Merlot berry skins was up-regulated by 52.4-, 67.5-, 54.1-, and 23.2-fold, respectively (Table 2). The transcription of VvGST3 and VvGST4 was also increased in downy mildew-infected berry skins relative to healthy berry skins, irrespective of the cultivar examined. In contrast, the transcription of VvGST2 and VvGST5 was not induced in downy mildew-infected grape berry skins. GGT transcription was also up-regulated in downy mildew-infected Sauvignon blanc, Chardonnay, Koshu, and Merlot berry skins by 1.7-, 4.9-, 2.6-, and 2.9-fold, respectively. The accumulation of 3MH-S-glut and 3MH-S-cys was also up-regulated in downy mildew-infected grape berry skins and juice (Table 1).
Table 2.
VvGST and GGT transcripts, and 3MH-S-glut and 3MH-S-cys contents in healthy and downy mildew-infected grape skins
| Sauvignon blanc |
Chardonnay |
Koshu |
Merlot |
|||||
| Healthy | Downy mildew | Healthy | Downy mildew | Healthy | Downy mildew | Healthy | Downy mildew | |
| VvGST1a | 1.0±0.34b | 52.4±8.03 | 1.0±0.21 | 67.5±1.71 | 1.0±0.28 | 54.1±1.38 | 1.0±0.14 | 23.2±1.87 |
| VvGST2 | 1.0±0.34 | 0.39±0.14 | 1.0±0.21 | 0.99±0.02 | 1.0±0.05 | 1.3±0.06 | 1.0±0.06 | 0.96±0.14 |
| VvGST3 | 1.0±0.08 | 3.9±0.28 | 1.0±0.11 | 5.0±0.32 | 1.0±0.08 | 2.7±0.38 | 1.0±0.09 | 2.9±0.03 |
| VvGST4 | 1.0±0.11 | 18.9±1.47 | 1.0±0.07 | 1.8±0.18 | 1.0±0.27 | 5.5±1.02 | 1.0±0.08 | 2.4±0.21 |
| VvGST5 | 1.0±0.39 | 1.1±0.01 | 1.0±0.31 | 1.2±0.18 | 1.0±0.37 | 0.47±0.21 | 1.0±0.44 | 0.53±0.15 |
| GGT | 1.0±0.05 | 1.7±0.11 | 1.0±0.12 | 4.9±0.24 | 1.0±0.10 | 2.6±0.15 | 1.0±0.01 | 2.9±0.27 |
| 3MH-S-glut (nM) | 200.8±15.3 | 842.7±44.3 | 53.2±17.7 | 531.8±20.4 | 40.7±4.3 | 471.9±43.1 | 141.1±5.7 | 592.4±20.6 |
| 3MH-S-cys (nM) | 32.1±1.9 | 149.1±12.7 | 10.9±1.0 | 226.8±10.4 | 22.6±0.3 | 109.6±1.8 | 31.7±1.4 | 133.8±12.5 |
Transcriptional levels of VvGST genes and GGT in downy mildew skins are expressed as gene expression relative to healthy skins.
Data are shown as means ±SDs of triplicate experiments.
VvGST3 and VvGST4 mediate 3MH-S-glut synthesis in vitro
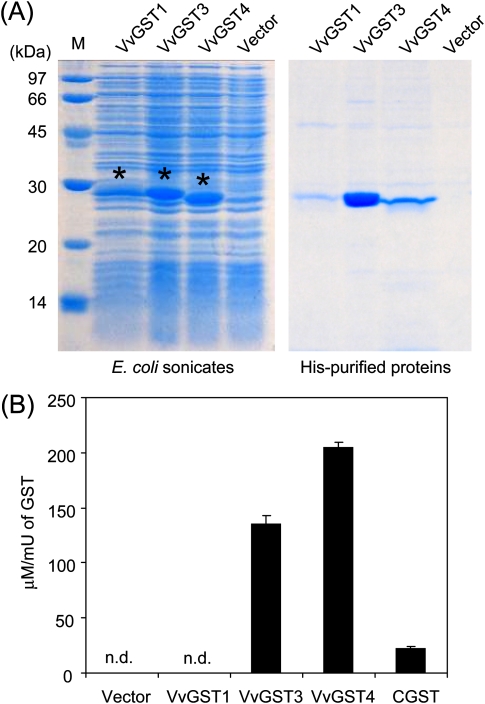
His-tagged recombinant VvGST1, VvGST3, and VvGST4 proteins were expressed in E. coli cells and purified from the cells using Ni columns (Fig. 5A). The GST activity of recombinant VvGST1, VvGST3, and VvGST4 was confirmed, whereas the purified protein from E. coli transfected with vector only did not express GST activity (data not shown). In the in vitro experiment to evaluate the activity of recombinant VvGST in 3MH-S-glut synthesis, GSH and trans-2-hexenal were reacted in the presence of each recombinant VvGST. Since the reaction mixture used for the in vitro experiment involved neither reductase nor NADHP, which acts as the coenzyme, here 3MH-S-glut production was evaluated from the content of 3-S-(hexenal)-glut produced in this reaction mixture. VvGST3 and VvGST4 mediated the production of 3MH-S-glut under the conditions tested (Fig. 5B), whereas VvGST1 showed no activity, similar to the purified protein from E. coli transfected with vector only. Interestingly, commercial GST from equine liver also showed 3MH-S-glut-producing activity, but the activity was less than those of VvGST3 and VvGST4 (Fig. 5B, CGST). Taken together, the results suggest that 3MH-S-glut production in grape may be mediated by VvGST3 and VvGST4, but not by non-specific proteins having GST activity.
Fig. 5.
Activity of recombinant VvGSTs in the in vitro synthesis of 3MH-S-glut. (A) The recombinant proteins VvGST1, VvGST3, and VvGST4, in SDS–PAGE. Escherichia coli sonicates are shown in the left panel. His-purified proteins (15 μl of purified solution) electrophoresed on a 12.5% SDS–polyacrylamide gel (right). M, protein marker. Asterisks indicate recombinant VvGST proteins. Vector, pCold I vector as negative control. (B) VvGST3 and VvGST4 mediate 3MH-S-glut synthesis in vitro. Data are shown as means ±SDs of triplicate experiments. n.d., not detected. CGST, commercial GST from equine liver.
Discussion
Characterization of 3MH-S-glut and 3MH-S-cys biosynthesis in grapevine exposed to stress conditions
The present study accomplished the molecular characterization of the biosynthetic pathway of two flavour precursors, 3MH-S-glut and 3MH-S-cys, in grapevine and the response of the pathway under stress conditions. The expression of VvGST1, VvGST3, and VvGST4 was induced in grape leaves by UV-C irradiation (Fig. 3A) and in grape berry skins by downy mildew infection (Table 2), and this was followed by 3MH-S-glut accumulation. Although VvGST1 and VvGST4 may function to transport anthocyanin into the vacuoles of grape cells (Conn et al., 2008), the up-regulation of their transcription was observed in grape berry skins of white cultivars that are deficient in anthocyanin accumulation (Table 2). Therefore, it is plausible that the VvGST genes up-regulated by UV-C irradiation play important roles in a number of physiological responses, including both enzymatic activity, such as the biosynthesis of 3MH-S-glut, and non-enzymatic activity, such as the intercellular transport of substrates. In this study, it was demonstrated that the in vitro production of 3MH-S-glut from GSH and hexenal was mediated by recombinant VvGST3 and VvGST4 proteins (Fig. 5). This is a novel function of plant GSTs. The enzymatic activities of VvGST3 and VvGST4 in 3MH-S-glut production were higher than that of commercial GST protein, suggesting that 3MH-S-glut production from GSH and hexenal needs specific plant GSTs. It could not be determined why, similarly to commercial GST, VvGST1 did not mediate the reaction (Fig. 5). Future study involving the X-ray structure analysis of VvGST3 and VvGST4 as well as VvGST1 may provide more information, including the active centres, substrate specificities, and unique binding sites of these GSTs. On the other hand, the up-regulation of GGT gene expression was also detected in grape leaves (Fig. 3B) and berry skins (Table 2). GGT can cleave the γ-peptide bond between glutamate and cysteine in glutathione (Ohkama-Ohtsu et al., 2007). This report supports that GGT might be a key enzyme in 3MH-S-glut degradation. In the present study, recombinant GGT protein could not be obtained with the same method as that for VvGSTs. Most animal and plant GGTs are heterodimeric enzymes (Suzuki et al., 2002; Shaw et al., 2005). By means of predicted amino acid sequence analysis, grape GGT also appears to be heterodimeric (data not shown). Therefore, a direct link between GGT induction and 3MH-S-cysgly production could not be demonstrated in grape, although UV-C irradiation increased GGT enzyme activity in grape berries (Fig. 3D). In addition, 3MH-S-cysgly may be converted immediately by carboxypeptidase, and there is no method to detect and identify 3MH-S-cysgly at present. The establishment of a method for the measurement of 3MH-S-cysgly by LC-MS/MS is required to complete the determination of the biosynthetic pathway degrading 3MH-S-glut by GGT in grapevine.
Hypothetical pathway for biosynthesis of 3MH-S-glut and 3MH-S-cys in grapevine
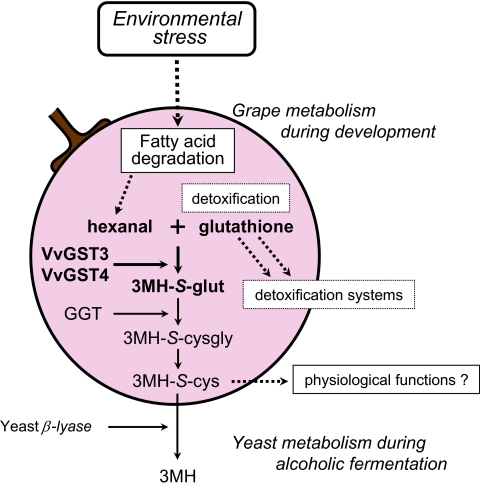
A hypothetical pathway for the biosynthesis of 3MH-S-glut and 3MH-S-cys in grapevine exposed to environmental stress has been formulated (Fig. 6). The degradation of polyunsaturated fatty acids (PUFAs) to form fatty acid derivatives is catalysed by lipoxygenase (LOX), which plays important roles as a defence signal in plants through the complex network (Weber, 2002). For example, the LOX-mediated peroxidation of membrane fatty acids plays a vital role in cell death signalling against pathogens (Rustérucci et al., 1999). Bleé (2002) and Howe and Schilmiller (2002) reported that, as a response to environmental stress by plants, 9-LOX and 13-LOX derivatives were produced from linoleic acid (C18:2) and linolenic acid (C18:3), which are the major PUFAs in plants, via reactions catalysed by LOX, the CYP 74 family, peroxygenase, or an uncharacterized reductase. The enzymatic reaction catalysed by hydroperoxide lyase yields C6 carbons including hexanal as one of the 13-LOX derivatives. As GSH interacts with other stress response distributors in GSH-mediated detoxification systems (Dixon et al., 1998), GSH may conjugate to hexenal, which is a stress response distributor (Howe and Schilmiller, 2002), as one of the detoxification reactions in grapevine exposed to stress conditions. The present study directly demonstrated that GSH conjugates to hexenal via the activity of VvGST3 and VvGST4, resulting in 3MH-S-glut production. UV-C irradiation may induce the immediate production of various derivative compounds, excluding C6 aldehyde, in plants. For the detoxification and/or antioxidation of these compounds, plants need a lot of GSH. Therefore, UV-C irradiation treatment induced GSH accumulation to complement the supply of GSH (Fig. 2D). Finally, 3MH-S-glut may be converted into 3MH-S-cysgly via the enzymatic activity of GGT, and then 3MH-S-cysgly is transformed into 3MH-S-cys by carboxypeptidase (Dubourdieu and Tominaga 2009).
Fig. 6.
Hypothetical pathway for the biosynthesis of 3MH-S-glut and 3MH-S-cys in grapevine exposed to environmental stress conditions.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. An experimental model plant. (A) Chardonnay, Koshu, and Merlot seedlings were used in this study. Numbers indicate leaf positions on the stem of a seedling. (B) 3MH-S-glut content and (C) 3MH-S-cys content were measured in the leaves at each leaf position. Data are shown as means ±SDs of triplicate experiments.
Figure S2. Response of grape leaves of seedlings under stress conditions. Chardonnay seedlings were exposed to stress conditions as described in Materials and methods. (A) CBF-like transcription factor (CBF4; Xiao et al., 2008). (B) Hypothetical protein highly similar to heat shock protein 21 (CAN67481; Kobayashi et al., 2010b). (C) Phenylalanine ammonia lyase (PAL; Bonomelli et al., 2004). (D) Non-expressor of PR1 (NPR1; Mukhtar et al., 2009). Transcriptional analysis was performed at 72 h after stress treatment using real-time RT-PCR. 18S rRNA was used as the internal control. Data were calculated as gene expression relative to 18S rRNA gene expression. Normal, no treatment; 0 °C, cold shock treatment; 40 °C, heat shock treatment; UV, UV-C irradiation; water, water deficit treatment; and Bio, biological stimulation. (E) ABA content (Bray, 2002) and (F) water status in Chardonnay leaves exposed to stress conditions were also measured. Data are shown as means ±SDs of triplicate experiments. Statistical analyses of ABA contents and water status were performed by the t-test. *P <0.05, compared with ‘Normal’ leaves.
Acknowledgments
The authors would like to thank Dr Cécile Thibon and Professor Denis Dubourdieu, University of Bordeaux, UMR Oenology, INRA, ISVV, for scientific suggestions.
References
- Aharoni A, Giri AP, Deuerlein S, Griepink F, De Kogel WJ, Verstappen FWA, Verhoeven HA, Jongsma MA, Schwab W, Bouwmeester HJ. Terpenoid metabolism in wild-type and transgenic plants. The Plant Cell. 2003;15:2866–2884. doi: 10.1105/tpc.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleé E. Impact of phyto-oxylipins in plant defense. Trends in Plant Science. 2002;7:315–321. doi: 10.1016/s1360-1385(02)02290-2. [DOI] [PubMed] [Google Scholar]
- Bonomelli A, Mercier L, Franchel J, Baillieul F, Benizri E, Mauro MC. Response of grapevine defenses to UV-C exposure. American Journal of Enology and Viticulture. 2004;55:51–59. [Google Scholar]
- Bray EA. Abscisic acid regulation of gene expression during water-deficit stress in the era of the Arabidopsis genome. Plant, Cell and Environment. 2002;25:153–161. doi: 10.1046/j.1365-3040.2002.00746.x. [DOI] [PubMed] [Google Scholar]
- Castellarin SD, Gaspero GD. Transcriptional control of anthocyanin biosynthetic genes in extreme phenotypes for berry pigmentation of naturally occurring grapevines. BMC Plant Biology. 2007;7:46. doi: 10.1186/1471-2229-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn S, Curtin C, Bézier A, Franco C, Zhang W. Purification, molecular cloning, and characterization of glutathione S-transferase (GSTs) from pigmented Vitis vinifera L. cell suspension cultures as putative anthocyanin transport proteins. Journal of Experimental Botany. 2008;59:3621–3634. doi: 10.1093/jxb/ern217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoine C, Falletti O, Douki T, Iacazio G, Ennar N, Montillet JL, Triantaphylidès MC. Adducts of oxylipin electrophiles to glutathione reflect a 13 specificity of downstream lipoxygenase pathway in the tobacco hypersensitive response. Plant Physiology. 2006;140:1484–1493. doi: 10.1104/pp.105.074690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DP, Cummins I, Cole DJ, Edwards R. Glutathione-mediated detoxification systems in plants. Current Opinion in Plant Biology. 1998;1:258–266. doi: 10.1016/s1369-5266(98)80114-3. [DOI] [PubMed] [Google Scholar]
- Dixon DP, Lapthorn A, Edwards R. Plant glutathione transferases. Genome Biology. 2002:1–304. doi: 10.1186/gb-2002-3-3-reviews3004. 3, reviews 3004.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DP, Skipsey M, Edwards R. Role of glutathione transferases in plant secondary metabolism. Phytochemistry. 2010;71:338–350. doi: 10.1016/j.phytochem.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Downey MO, Harvey JS, Robinson SP. Synthesis of flavonols and expression of flavonol synthase genes in the developing grape berries of Shiraz and Chardonnay (Vitis vinifera L.) Australian Journal of Grape and Wine Research. 2003;9:110–121. [Google Scholar]
- Dubourdieu D, Tominaga T. Polyfunctional thiol compounds. In: Moreno-Arribas MV, Polo MC, editors. Wine chemistry and biochemistry. New York: Springer Science +Business Media; 2009. pp. 275–293. [Google Scholar]
- Edwards R, Dixon DP. Plant glutathione transferases. Methods in Enzymology. 2005;401:169–186. doi: 10.1016/S0076-6879(05)01011-6. [DOI] [PubMed] [Google Scholar]
- Forcat S, Bennett MH, Mansfield JW, Grant MR. A rapid and robust method for simultaneously measuring changes in the phytohormones ABA, JA and SA in plants following biotic abiotic stress. Plant Methods. 2008;4:16. doi: 10.1186/1746-4811-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fretz CB, Luisier JL, Tominaga T, Amadò R. 3-Mercaptohexanol: an aroma impact compound of Petite Arvine wine. American Journal of Enology and Viticulture. 2005;56:407–410. [Google Scholar]
- Fujita A, Soma N, Goto-Yamamoto N, Shindo H, Kakuta T, Koizumi T, Hashizume K. Anthocyanidin reductase gene expression and accumulation of flavan-3-ols in grape berry. American Journal of Enology and Viticulture. 2005;56:336–342. [Google Scholar]
- Halaly T, Pang X, Batikoff T, Crane O, Keren A, Venkateswari J, Ogrodovitch A, Sadka A, Lavee S, Or E. Similar mechanisms might be triggered by alternative external stimuli that induce dormancy release in grape buds. Planta. 2008;228:79–88. doi: 10.1007/s00425-008-0720-6. [DOI] [PubMed] [Google Scholar]
- Howe GA, Schilmiller AL. Oxylipin metabolism in response to stress. Current Opinion in Plant Biology. 2002;5:230–236. doi: 10.1016/s1369-5266(02)00250-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Katoh H, Takayanagi T, Suzuki S. Characterization of thermotolerance-related genes in grapevine (Vitis vinifera) Journal of Plant Physiology. 2010b;167:812–819. doi: 10.1016/j.jplph.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Suzuki S, Tanzawa F, Takayanagi T. Low expression of flavonoid 3′,5′-hydroxylase (F3′,5′H) associated with cyanidin-based anthocyanins in grape leaf. American Journal of Enology and Viticulture. 2009;60:362–367. [Google Scholar]
- Kobayashi H, Takase H, Kaneko K, Tahzawa F, Takata R, Suzuki S, Konno T. Analysis of S-3-(hexan-1-ol)-glutathione and S-3-(hexan-1-ol)-l-cysteine in Vitis vinifera L. cv. Koshu for aromatic wines. American Journal of Enology and Viticulture. 2010a;61:176–185. [Google Scholar]
- Lee S, Peterson CJ, Coats JR. Fumigation toxicity of monoterpenoids to several stored product insects. Journal of Stored Products Research. 2003;39:77–85. [Google Scholar]
- Luisier JM, Buettner H, Volker S, Rausis T, Frey U. Quantification of cysteine S-conjugate of 3-sulfanylhexan-1-ol in must and wine of Petite Arvine vine by stable isotope dilution analysis. Journal of Agricultural and Food Chemistry. 2008;56:2883–2887. doi: 10.1021/jf072963o. [DOI] [PubMed] [Google Scholar]
- Marsoni M, Bracale M, Espen L, Prinsi B, Negri AS, Vannini C. Proteomic analysis of somatic embryogenesis in Vitis vinifera. Plant Cell Reports. 2008;27:347–356. doi: 10.1007/s00299-007-0438-0. [DOI] [PubMed] [Google Scholar]
- Mccown RL, Wall BH. Improvement of pressure chamber measurements of two legumes by constriction of stems. Plant and Soil. 1979;51:447–451. [Google Scholar]
- Milligan AS, Daly A, Parry MAJ, Lazzeri PA, Jepson I. The expression of a maize glutathione S-transferase gene in transgenic wheat confers herbicide tolerance, both in planta and in vitro. Molecular Breeding. 2001;7:301–315. [Google Scholar]
- Mukhtar MS, Nishimura MT, Dangl J. NPR1 in plant defense: it's not over ‘til it's turned over. Cell. 2009;137:804–806. doi: 10.1016/j.cell.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Murat ML, Tominaga T, Dubourdieu D. Assessing the aromatic potential of Cabernet Sauvignon and Merlot musts used to produce rose wine by assaying the cysteinylated precursor of 3-mercaptohexan-1-ol. Journal of Agricultural and Food Chemistry. 2001;49:5412–5417. doi: 10.1021/jf0103119. [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Ohkama-Ohtsu N, Radwan S, Peterson A, Zhao P, Bodr AF, Xiang C, Oliver DJ. Characterization of the extracellular γ-glutamyl transpeptidase transpeptidases, GGT1 and GGT2, in Arabidopsis. The Plant Journal. 2007;49:865–877. doi: 10.1111/j.1365-313X.2006.03004.x. [DOI] [PubMed] [Google Scholar]
- Peyrot des Gachons C, Tominaga T, Dubourdieu D. Sulfur aroma precursor present in S-glutathione conjugate form: identification of S-3-(hexan-1-ol)-glutathione in must from Vitis vinifera L. cv. Sauvignon blanc. Journal of Agricultural and Food Chemistry. 2002;50:4076–4079. doi: 10.1021/jf020002y. [DOI] [PubMed] [Google Scholar]
- Peyrot des Gachons C, van Leeuwen C, Tominaga T, Soyer JP, Gaudillère JP, Dubourdieu D. Influence of water and nitrogen deficit on fruit ripening and aroma potential of Vitis vinifera L cv Sauvignon blanc in field conditions. Journal of the Science of Food and Agriculture. 2005;85:73–85. [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29:2003–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid KE, Olsson N, Schlosser J, Peng F, Lund ST. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biology. 2006;6:27–37. doi: 10.1186/1471-2229-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roujou de Boubée D, van Leeuwen C, Dubourdieu D. Organoleptic impact of 2-methoxy-3-isobutylpyrazine on red Bordeaux and Loire wines. Effect of environmental conditions on concentrations in grapes during ripening. Journal of Agricultural and Food Chemistry. 2000;55:10281–10288. doi: 10.1021/jf000181o. [DOI] [PubMed] [Google Scholar]
- Rustérucci C, Montillet JL, Agnel JP, et al. Involvement of lipoxygenase-dependent production of fatty acid hydroperoxides in the development of the hypersensitive cell death induced by cryptogein on tobacco leaves. Journal of Biological Chemistry. 1999;274:36446–36455. doi: 10.1074/jbc.274.51.36446. [DOI] [PubMed] [Google Scholar]
- Sappl PG, Carroll AJ, Clifton R, Lister R, Whelan J, Millar AH, Singh K. The Arabidopsis glutathione transferase gene family displays complex stress regulation and co-silencing multiple genes results in altered metabolic sensitivity to oxidative stress. The Plant Journal. 2009;58:53–68. doi: 10.1111/j.1365-313X.2008.03761.x. [DOI] [PubMed] [Google Scholar]
- Sarrazin E, Shinkaruk S, Tominaga T, Bennetau B, Frrot E, Dubourdieu D. Odorous impact of volatile thiols on the aroma of young botrytised sweet wines: identification and quantification of new sulfanyl alcohols. Journal of Agricultural and Food Chemistry. 2007;55:1437–1444. doi: 10.1021/jf062582v. [DOI] [PubMed] [Google Scholar]
- Shaul O, Mironov V, Burssens S, Montagu MV, Inzé D. Two Arabidopsis cyclin promoters mediate distinctive transcriptional oscillation in synchronized tobacco BY-2 cells. Proceedings of the National Academy of Sciences, USA. 1996;93:4868–4872. doi: 10.1073/pnas.93.10.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw ML, Pither-Joyce MD, McCallum JA. Purification and cloning of a γ-glutamyl transpeptidase from onion (Allium cepa) Phytochemistry. 2005;66:515–522. doi: 10.1016/j.phytochem.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Skipsey M, Cummins I, Andrews CJ, Jepson I, Edwards R. Manipulation of plant tolerance to herbicides through co-ordinated metabolic engineering of a detoxifying glutathione transferase and thiol cosubstrate. Plant Biotechnology Journal. 2005;3:409–420. doi: 10.1111/j.1467-7652.2005.00134.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kumagai H. Autocatalytic processing of gamma-glutamyltranspeptidase. Journal of Biological Chemistry. 2002;277:43536–43543. doi: 10.1074/jbc.M207680200. [DOI] [PubMed] [Google Scholar]
- Tesniere C, Vayda ME. Method for the isolation of high-quality RNA from grape berry tissues without contaminating tannins or carbohydrates. Plant Molecular Biology Reporter. 1991;9:242–251. [Google Scholar]
- Thibon C, Dubourdieu D, Darriet P, Tominaga T. Impact of noble rot on the aroma precursor of 3-sulfanylhexanol content in Vitis vinifera L. cv Sauvignon blanc and Semillon grape juice. Food Chemistry. 2009;114:1359–1364. [Google Scholar]
- Thibon C, Shinkaruk S, Tominaga T, Bennetau B, Dubourdieu D. Analysis of the diastereoisomers of the cysteinylated aroma precursor of 3-sulfanylhexanol in Vitis vinifera grape must by gas chromatography coupled with ion trap tandem mass spectrometry. Journal of Chromatography A. 2008;1183:150–157. doi: 10.1016/j.chroma.2007.12.082. [DOI] [PubMed] [Google Scholar]
- Toit WJ, Lisjak K, Stander M, Prevoo D. Using LC-MSMS to assess glutathione levels in South African white grape juice and wines made with different levels of oxygen. Journal of Agricultural and Food Chemistry. 2007;55:2765–2769. doi: 10.1021/jf062804p. [DOI] [PubMed] [Google Scholar]
- Tominaga T, Baltenweck-Guyot R, Peyrot des Gachons C, Dubourdieu D. Contribution of volatile thiols to the aromas of white wines made from several Vitis vinifera grape varieties. American Journal of Enology and Viticulture. 2000;51:178–181. [Google Scholar]
- Tominaga T, Murat ML, Dubourdieu D. Development of a method for analyzing the volatile thiols involved in the characteristic aroma of wines made from Vitis vinifera L. cv. Sauvignon blanc. Journal of Agricultural and Food Chemistry. 1998a;46:1044–1048. [Google Scholar]
- Tominaga T, Peyrot des Gachons C, Dubourdieu D. A new type of flavor precursors in Vitis vinifera L. cv. Sauvignon Blanc: S-cysteine conjugates. Journal of Agricultural and Food Chemistry. 1998b;46:5215–5219. doi: 10.1021/jf990979b. [DOI] [PubMed] [Google Scholar]
- Turner NC. Measurement of plant water status by the pressure chamber technique. Irrigation Science. 1988;9:289–308. [Google Scholar]
- Weber H. Fatty acid-derived signals in plants. Trends in Plant Science. 2002;7:217–224. doi: 10.1016/s1360-1385(02)02250-1. [DOI] [PubMed] [Google Scholar]
- Wingate VPM, Lawton MA, Lamb CJ. Glutathione causes a massive and selective induction of plant defense genes. Plant Physiology. 1988;87:206–210. doi: 10.1104/pp.87.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth J, Guo W, Baumes R, Günata Z. Volatile compounds released by enzymatic hydrolysis of glycoconjugates of leaves and grape berries from Vitis vinifera Muscat of Alexandria and Shiraz cultivars. Journal of Agricultural and Food Chemistry. 2001;49:2917–2923. doi: 10.1021/jf001398l. [DOI] [PubMed] [Google Scholar]
- Xiao H, Tattersall EA, Siddiqua MK, Crmer GR, Nassuth A. CBF4 is a unique member of the CBF transcription factor family of Vitis vinifera and Vitis riparia. Plant, Cell and Environment. 2008;31:1–10. doi: 10.1111/j.1365-3040.2007.01741.x. [DOI] [PubMed] [Google Scholar]