Abstract
Background
There is lack of reported MRI studies of idiopathic acute transverse myelitis in children.
Objective
To describe the imaging features of idiopathic acute transverse myelitis in children..
Methods
We retrospectively analyzed the spinal MRI findings of children diagnosed with acute transverse myelitis . The anatomic regions, vertebral segmental length, gray or white matter involvement, cord expansion and gadolinium enhancement were examined.
Results
27 children were diagnosed with isolated monophasic acute transverse myelitis with a mean follow-up of 5.2 years. Two children later diagnosed with neuromyelitis optica were excluded from the pediatric ATM cohort. None of the patients had a subsequent diagnosis of multiple sclerosis. The mean age of onset was 9.5 years (0.5-16.9). Spinal MRIs were abnormal in 21 (78%). The mean interval between symptom onset and the MRI was 1.7 days (0-19 days). Central cord hyperintensity involving gray matter was seen in all patients. A majority (67%) of the patients demonstrated long segment lesions with a mean segment length of 6.4.
Conclusions
Central cord inflammation extending over three or more segments is the most common finding of idiopathic monophasic transverse myelitis in children. The risk of multiple sclerosis in children who experience isolated transverse myelitis as a first demyelinating event is low.
Keywords: Acute transverse myelitis, MRI, demyelinating disease, pediatric onset multiple sclerosis, spinal cord inflammation
Introduction
Acute transverse myelitis (ATM) represents a major subset of acute non-compressive myelopathies. Possible causes of acute non-compressive myelopathies include vascular, infectious, autoimmune, inflammatory, neoplastic and metastatic disorders. The term “acute transverse myelitis” denotes the inflammatory etiology excluding non-inflammatory causes.
ATM can occur as an isolated inflammatory disorder, or as part of a multifocal CNS demyelinating disease such as acute disseminated encephalomyelitis (ADEM), multiple sclerosis (MS) or neuromyelitis optica (NMO); it can also be associated with systemic rheumatological disorders. Because many features overlap, these disorders can be encompassed under the umbrella term of “acute transverse myelitis” on first presentation. After such recognizable associations are excluded, a group of patients remains in whom no causes can be identified. In this study, the term “idiopathic ATM” is used to define those patients who remain isolated and monophasic during a longitudinal follow-up and not found to be associated with another disease. Current diagnostic criteria for idiopathic ATM have been proposed by the Transverse Myelitis Consortium Working Group (TMCWG) [1].
The incidence of ATM is reported as 1400 cases per year in the United States; 20% of these cases involve individuals younger than age 18 [2]. ATM occurred in 10- 22 % of children with acute demyelination reported from pediatric demyelinating cohorts [3-5]. Long-term neurological deficits have been reported in 30-62 % of children [2, 6-7]. We retrospectively evaluated the MRI findings in children with acute transverse myelitis of the idiopathic type. Defining the MRI patterns of monophasic ATM would help to distinguish other phenotypes associated with multiphasic relapsing diseases.
PATIENTS AND METHODS
A. Patients and inclusion criteria
Patients diagnosed with acute transverse myelitis at Children's Hospital of Pittsburgh during the period from 1985-2008 and under the age of 18 at clinical presentation were studied. Patients who fulfilled the criteria proposed by the TMCWG (definite ATM) and patients who met all criteria with the exception of “objective documentation of inflammation within the spinal cord” (possible ATM) were included [1]. Excluded were patients who had symptoms outside the spinal cord (polyfocal presentation), MS-like brain lesions or symptoms suggesting meningoencephalomyelitis and initial imaging data performed beyond the 4 weeks of disease onset.
The patients were identified from the pediatric demyelinating cohort registry at Children's Hospital of Pittsburgh. This cohort has two types of patients 1) those patients identified with retrospective chart review who have then been followed yearly via phone contacts by PI and 2) those patients registered at the onset of acute demyelination and followed prospectively.
Data were collected on clinical features, neuroimaging, cerebrospinal fluid (CSF) examination and other laboratory findings. All patients underwent extensive evaluations to investigate for infectious and connective tissue disorders. As the study spans approximately 20 years time period, there was some variation of laboratory data in addition to differences determined by the individual clinical features. In general, routine laboratory tests included blood cell count, electrolytes, liver enzymes, creatinine, urea, glucose, viral serology, Lyme titer, mycoplasma pneumonia, meningoencephalitis panel, rheumatoid factor, antinuclear antibodies, anti-neutrophil cytoplasmic antibodies, Sjogren's syndrome antibody A and B, anti-cardiolipin and anti-phospholipid antibodies, angiotensin converting enzyme, compleman levels, thyroid antibodies and B12 level. NMO-IgG antibody was included in patients diagnosed after the test become available. On rare occasions paraneoplastic antibodies were sent and West Nile virus was added to infectious work-up. CSF examinations included cell count, protein, glucose, gram stain, bacterial cultures, oligoclonal bands by isoelectric focusing, IgG index, polymerase chain reaction for varicella zoster virus, Epstein-Barr virus, enterovirus, humanherpesvirus 6 and 7, cytomegalovirus and Lyme disease. CSF cytology was obtained if clinically indicated. Patients with suspected infectious myelitis had additional CSF and systemic investigations. These studies are obtained immediately after admission. Vascular investigations were performed on selected patients if CSF examination was lacking the evidence for inflammation and if there was no better explanation. Ophtalmology consults were obtained at admission and visual evoked potentials were performed in some patients. Consultation with rheumatology was obtained either as inpatient or outpatient particularly in children with significant family history of other autoimmune disorders. In addition to detailed neuroimaging analysis, we included following clinical characteristics in this study: Demographic features (age at clinical onset, gender), preceding infections or immunizations (4 weeks prior to clinical onset), family history of autoimmune disease, time to first MRI, distribution of motor deficits (mono/para/tetraparesis), CSF white cell count, protein, oligoclonal bands, IgG index, serum NMO- IgG antibody results and duration of follow-up.
The study was approved by University of Pittsburgh Institutional Review Board.
B) Imaging Analysis
Spinal and brain MRIs obtained at clinical onset were reviewed. Two experienced neuroradiologists (KAP, CRF), retrospectively reviewed the studies while blinded to the specific clinical findings at onset. Any differences in assessment were resolved by consensus.
All MRIs were performed on 1.5T magnets. Since this was a retrospective analysis, the patients had not been imaged under a standardized protocol; however, all spinal MRIs included the minimum of sagittal and axial T1-weighted (T1W), sagittal and axial fast spin echo, or spin echo T2-weighted (T2W), as well as post contrast sagittal and axial T1W sequences.
The following imaging characteristics were recorded on spinal MRIs: 1. Anatomic region(s) were classified as cervical, thoracic and lumbar spine (distal cord/ conus). A lesion involving more than one anatomic region was defined as multiregional. If more than one discrete lesion were found, it was defined as multifocal. 2. Vertebral segmental length (one segment= one vertebra body height); the cephalocaudal extension of each abnormality estimated on sagittal T2W sequences were expressed in a number of segments for each involved region. 3. Cord expansion was determined on sagittal T2W images. 4. Gray or white matter involvement was assessed on axial T2W images. 5. Enhancement was assessed on both the sagittal and axial planes. Presence of hemorrhage and syrinx assessed on T1W and T2W sequences were recorded.
Statistical analysis
Statistical analyses were performed with SAS, version 9.1 under the alpha level of 0.05. Descriptive statistics were presented as counts and percentages for categorical variables, and mean ± standard deviation or median with range for continuous variables.
RESULTS
Thirty-five patients had isolated transverse myelitis at first presentation that included the cases with definite and possible ATM by TMCWG criteria. Six patients were excluded from this analysis as initial MRIs were not obtained within the 4 weeks of disease onset or imaging data were not available to review. Two patients who later developed multiple relapses of both myelitis and optic neuritis had positive NMO-IgG antibodies and were diagnosed with NMO; they were excluded from the idiopathic ATM cohort.
None of the other patients had relapses during the follow-up. None had a subsequent diagnosis of MS or systemic rheumatological disorder. 27 patients with isolated monophasic ATM and with available imaging data at clinical onset were analyzed in this study. The mean follow-up period was 5.2 years (SD; 4.6, range; 0.04-13.1). 14 patients fulfilled the TMSCG criteria for definite ATM and 13 had possible ATM. In possible ATM patients, clinical course was not suggestive for vascular myelopathy, and extensive evaluations did not reveal any other etiologies. The mean age of onset was 9.5 years (SD; 5.7, range; 0.5-16.9). Male to female ratio was 1.07 (14/13). Most of the patients (74%) presented with paraparesis. Table 1 summarizes the clinical and CSF characteristics of patients. Only 5 subjects were tested for NMO-IgG antibody since test has become available in recent years.
Table 1.
Clinical and laboratory characteristics in ATM
| Characteristics | n/Total | % |
|---|---|---|
| Preceding infection | 10/26 | 38.5 |
| Preceding immunization | 2/25 | 8.0 |
| FH of autoimmune disease | 7/27 | 25.9 |
| Monoparesis | 4/27 | 14.8 |
| Paraparesis | 20/27 | 74.1 |
| Tetraparesis | 3/27 | 11.1 |
| CSF pleocytosis (median: 52 cells/mm3; range:6–205) | 10/27 | 37.0 |
| Elevated protein (median: 57 mg/dl; range: 45–77) | 12/27 | 44 |
| Elevated IgG index | 2/21 | 9.5 |
| Oligoclonal bands positive | 0/22 | 0.0 |
| NMO-IgG antibody | 0/5 | 0.0 |
Spinal MRI results
MRIs were performed on admission or shortly after admission on most of the patients. The mean interval from symptom onset to initial MRI study was 1.7 days (SD; 4.3, range; 0-19 days). Spinal MRIs were abnormal in 21 (78%) patients, while 6 patients had normal results. A total of 30 lesions were analyzed. Seven patients (33%) had multifocal lesions. The cervico-thoracic cord was the most frequently involved region. Characteristics of abnormal MRI are shown on Table 2.
Table 2.
Characteristics of abnormal MRI findings in childhood ATM (n = 21)
| n | % | Total | ||
|---|---|---|---|---|
| Region | Only cervical | 3 | 14.3 | 21 |
| Only thoracic | 6 | 28.6 | ||
| Only lumbar | 1 | 4.8 | ||
| Multiregional | 11 | 52.3 | ||
| Multiregional | Cervical and thoracic | 7 | 63.6 | 11 |
| Thoracic and lumbar | 1 | 9.1 | ||
| Cervical, thoracic and lumbar | 1 | 9.1 | ||
| Cervical, thoracic, lumbar and conus | 2 | 18.2 | ||
| Involvement at axial plane | Gray matter only | 14 | 66.7 | 21 |
| White matter only | 0 | 0.0 | ||
| Both gray and white matter | 7 | 33.3 | ||
| Segment length | <3 | 7 | 33.3 | 21 |
| ≥3 | 14 | 66.7 | ||
| Gadolinium Enhancement | Yes | 4 | 19.1 | 21 |
| No | 17 | 80.9 | ||
| Swelling/cord expansion | Yes | 9 | 42.9 | 21 |
| No | 12 | 57.1 |
In all patients, centrally located high signal intensity involving gray matter was seen on axial T2-weighted images (Figure 1). This central cord abnormality involved gray matter and nearby surrounding white matter in seven patients. No patient, including those with short segment lesions, had only peripheral white matter abnormality.
Figure 1. Central cord inflammation.
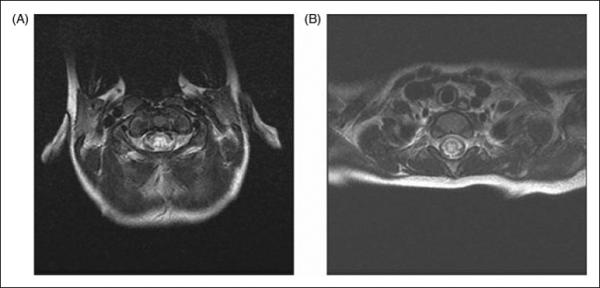
Axial fast spin echo T2W sequences reveal increased intensity signal in the center of the cord with variable intensity (A and B).
Seven out of 21 patients revealed lesions less than 3 segments, while 14/21 patients had lesions with a length equal to or more than 3 segments. The mean segment length was 6.4 ±6.1 (0.5-21.0), and the median segment length was 5.0 (0.5-21.0). Figure 2 shows the distribution of segment length. In patients with multiple lesions, the longest lesion was analyzed. To be consistent with the current literature and relevant to discussions of “longitudinally extending transverse myelitis” (LETM) in MS and NMO, long segment is defined as ≥ 3 vertebral segments [8]. A majority (67%) of patients demonstrated long segment lesions (Figure 3).
Figure 2. Segment length distribution.
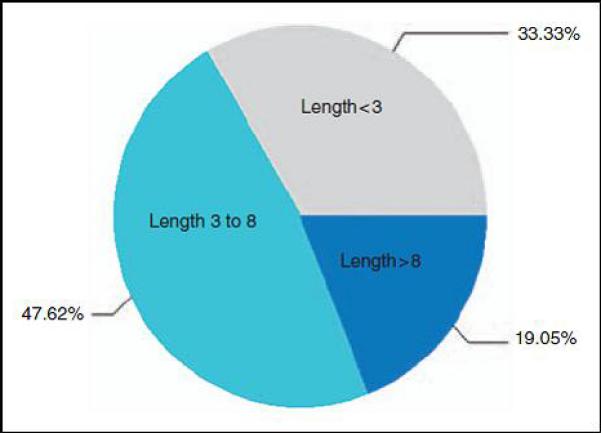
Distribution of segment length in children with idiopathic ATM
Figure 3. LETM.
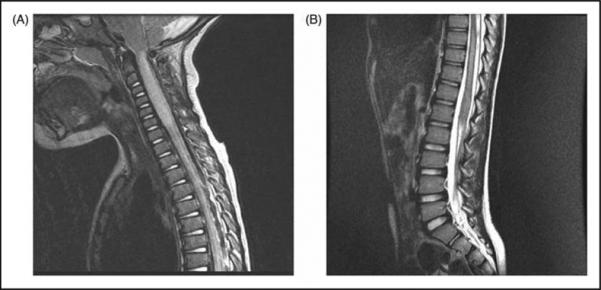
Sagittal fast spin echo T2W images demonstrate long segment lesions, A) along with cord expansion, B) not associated with cord expansion.
None of the patients had hemorrhage or syrinx on the initial MRIs.
Brain MRIs
Brain MRIs were performed in 25/27 patients at onset. In one patient, MRI showed asymptomatic patchy T2 hyperintensities at bilateral periatrial regions, which resolved one-month later and remained normal at one-year follow-up. NMO-IgG was negative. This patient has been followed for two years, and remains monophasic. Two patients had lower brainstem lesions contiguous to cervical cord lesion; these were not considered as brain lesion. In two children, brain MRIs were not available at clinical onset. These patients had symptoms and findings only attributable to spinal cord with clearly defined sensory levels. They were followed for 11.5 and 12.8 years and remained monophasic.
DISCUSSION
The diagnosis of ATM is usually made from a combination of history, laboratory tests and imaging. MRI has been the most useful diagnostic tool; despite its non-specificity, the constellation of changes in the appropriate clinical setting can offer a strong presumptive diagnosis of ATM.
There is lack of reported MRI studies of ATM in children [9]. The radiological features of ATM have been described mainly in adult patients. As ATM is a diagnosis of heterogenic etiology, results of the some studies provide information on a mixed population, including both idiopathic and disease-associated myelitis. Involvement of longer segments extending over 3 to 4 segments is a common finding of ATM in adults (reported between 53-71 %) after excluding the ATM secondary to identifiable causes [10-12]. Other important features described in adult patients include cord expansion, no enhancement, or diffuse and inhomogeneous, peripheral or slightly nodular (small focus) enhancement [10, 13-14]. Variable presence of cord expansion was seen in 47 % of patients [10] and found in 43% in the present study.
The most striking result of this study is the presence of central cord hyperintensity involving the gray matter and nearby surrounding white matter. Gray matter involvement has not been previously reported in childhood ATM. Centrally located lesions occupying more than two-thirds of the cross-sectional area were reported in adults as well [10]. Our radiological results demonstrate that lesions do not clearly demonstrate a predilection to the white matter in isolated monophasic transverse myelitis.
Similar to adult patients, idiopathic ATM in children involves long segments; the lesion tends to be vertically extensive. In a recent study, LETM was reported in 9 out of 12 children who were seronegative for NMO –IgG antibody and had monophasic ATM [15]. Andronikou et al reported long segment involvement in three children [16]. These results support our observation that LETM, defined as lesions spanning over 2 segments, is a common radiological feature of acute isolated monophasic transverse myelitis in children and is not unique to NMO.
In adult patients with MS, spinal lesions are usually less than a 2-segment length [12-13, 17-20] and are usually located in the posterolateral aspect of the spinal cord [12]; cord swelling is rare in MS [20-21]. The detailed features of the ATM associated with polyfocal CNS demyelination (ADEM and MS) are yet to be studied in children. In adult cohorts, the reported risk of MS is higher when partial forms are considered or included only the patients with short segment lesions [22-24]. Acute partial transverse myelitis is known as the major spinal expression of MS, with complete transverse myelitis being extremely rare [23-24]. TMCWG criteria do not distinguish between the partial and complete myelitis. While some adult studies find this classification as a useful outcome parameter, we avoided the clinical definition of “partial myelitis” as there were many patients falling into gray areas. It is even less applicable to younger children in whom the extension of sensory deficits would be more difficult to determine.
Some questions remain about the low ratio of lesion enhancement in our ATM cohort. No data are available correlating the presence of enhancement and timing of the study in children. In an adult study Choi et al found greater frequency of positive enhancement in patients with subacute stage than those in acute stage [10]. Most of our patients underwent imaging studies on admission that short interval from symptom onset to MRI study (mean; 1.7 days) may be an explanation for lack of enhancement. Normal spinal MRIs were also reported in other pediatric series [2,3,6,25].
Inflammatory transverse myelitis in the absence of a specific cause is the most common cause of acute myelitis [26]. However, idiopathic nature is a diagnosis of exclusion and other causes need to be excluded. Acute infectious myelitis is usually diagnosed by prominent CSF inflammation (pleocytosis above the usual post-infectious range with neutrophilic predominance and significantly elevated protein), presence of systemic infectious symptoms (fever, rash, meningeal signs, concurrent infectious illness), and finally with the confirmation of microorganism in CSF. Connective tissue disorders (systemic lupus erythematosus, Sjogren's syndrome, mixed connective tissue disorders, Behcet's disease) and sarcoidosis may present with acute myelitis, but it is rare for myelitis to be a presenting symptom [26]. Until more autoimmune biomarkers are identified some causes of ATM can be proved only by “time”, therefore long-term follow-up information is crucial .Vascular etiologies and fibrocartilaginous embolism are very rare in children but should be considered in the presence of hyperacute presentation (time to nadir less than 4 hours), and normal CSF. History of trauma or lifting heavy weight should be carefully documented in the history. Clinical and radiological evidence of anterior cord syndrome should raise the suspicion for anterior spinal artery occlusion.
It is well known that ATM may occur as part of a multifocal CNS demyelination. LETM is reported in association with ADEM [15], however ADEM is characterized by multifocal involvement (cerebral, cerebellar, optic nerve and spinal cord) therefore isolated spinal cord syndrome is not consistent with ADEM by definition [27]. NMO as one of the relapsing CNS diseases, may present with isolated transverse myelitis and characteristically involves long segments. NMO, both clinically and radiologically, may not be distinguishable from idiopathic ATM at onset. Therefore, obtaining NMO-IgG antibodies is crucial for distinguishing two entities. On the other hand, isolated ATM is not likely to be an initial manifestation of MS in children. Only 1 of 47 children with transverse myelitis was diagnosed with MS after a mean follow-up of 8 years [2] and none (0/24) reported by Defresne et al. after a mean duration of 7 years [6]. Among patients with a final diagnosis of MS, 2 out of 29 were considered to have a focal episode of transverse myelitis at the first event [3]. The presence of oligoclonal bands in ATM has been associated with a higher risk of an MS diagnosis [28]; none of our patients had oligoclonal bands, a result that supports the observations of previous studies.
Although a variety of conditions should be considered, our results showed that the disease presenting with non-compressive acute spinal cord syndrome in children is most likely inflammatory in origin and not associated with another CNS or systemic disease process. Long-term follow-up in this study demonstrated that this acute inflammatory disease limited to spinal cord is not likely to relapse and thus become monophasic in the majority (94%) of children. Disease-associated ATM was found in only 6% of patients in this cohort with NMO being the most likely diagnosis. The risk of MS in children who experience isolated spinal cord syndrome as a first demyelinating event appears to be low. Future studies in pediatric patients comparing initial spinal cord lesions of idiopathic ATM to those who are subsequently diagnosed with MS and NMO would be critical.
This study provides a comprehensive analysis on MRI findings of idiopathic ATM.in children.. In summary, lesion is located in the central cord involving gray and nearby surrounding white matter. Most of the lesions are longitudinally extending characterized by involvement of three or more segments. Childhood ATM typically has a monophasic course and remains a major subset of acute inflammatory CNS disorders.
Acknowledgement
The authors thank Clinical and Translational Science Institute (CTSI, University of Pittsburgh) for data analysis; and John M. Gilmore, the project coordinator (Division of Child Neurology, Department of Pediatrics) for technical assistance.
REFERENCES
- 1.Transverse Myelitis Consortium Working group Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59:499–505. doi: 10.1212/wnl.59.4.499. [DOI] [PubMed] [Google Scholar]
- 2.Pidcock FS, Krishnan C, Crawford TO, Salorio CF, Trovato M, Kerr DA. Acute transverse myelitis in childhood: Center-based analysis of 47 cases. Neurology. 2007;68:1474–1480. doi: 10.1212/01.wnl.0000260609.11357.6f. [DOI] [PubMed] [Google Scholar]
- 3.Mikaeloff Y, Suissa S, Vallee L, Lubetzki C, Ponsot G, Confavreux C, et al. KIDMUS Study Group. First episode of acute CNS inflammatory demyelination in childhood: prognostic factors for multiple sclerosis and disability. J Pediatr. 2004;144:246–252. doi: 10.1016/j.jpeds.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 4.Banwell B, Kennedy J, Sadovnick D, Arnold DL, Magalhaes S, Wambera K, et al. Incidence of acquired demyelination of the CNS in Canadian children. Neurology. 2009;72:232–239. doi: 10.1212/01.wnl.0000339482.84392.bd. [DOI] [PubMed] [Google Scholar]
- 5.Alper G, Heyman R, Wang L. Multiple sclerosis and acute disseminated encephalomyelitis diagnosed in children after long-term follow-up: comparison of presenting features. Dev Med Child Neurol. 2009;51:480–486. doi: 10.1111/j.1469-8749.2008.03136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Defresne P, Hollenberg H, Husson B, Tabarki B, Landrieu B, Tardieu M, et al. Acute transverse myelitis in children: clinical course and prognostic factors. J Child Neurol. 2003;18:401–406. doi: 10.1177/08830738030180060601. [DOI] [PubMed] [Google Scholar]
- 7.Miyazawa R, Ikeuchi Y, Tomomasa T, Ushiku H, Ogawa T, Morikawa A. Determinants of prognosis of acute transverse myelitis in children. Pediatr Int. 2003;45:512–516. doi: 10.1046/j.1442-200x.2003.01773.x. [DOI] [PubMed] [Google Scholar]
- 8.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 9.Pittock SJ, Lucchinetti CF. Inflammatory transverse myelitis: evolving concepts. Curr Opin Neurol. 2006;19:362–368. doi: 10.1097/01.wco.0000236615.59215.d3. [DOI] [PubMed] [Google Scholar]
- 10.Choi KH, Lee KS, Chung SO, Park JM, Kim YJ, Kim HS, et al. Idiopathic transverse myelitis: MR characteristics. AJNR. 1996;17:1151–1160. [PMC free article] [PubMed] [Google Scholar]
- 11.al Deeb SM, Yaqub BA, Bruyn GW, Biary NM. Acute transverse myelitis. A localized form of postinfectious encephalomyelitis. Brain. 1997;120:1115–1122. doi: 10.1093/brain/120.7.1115. [DOI] [PubMed] [Google Scholar]
- 12.Sheerin F, Collison K, Quaghebeur G. Magnetic resonance imaging of acute intramedullary myelopathy: radiological differential diagnosis for the on-call radiologist. Clin Radiol. 2009;64:84–94. doi: 10.1016/j.crad.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Tartaglino LM, Croul SE, Flanders AE, Sweeney JD, Schwartzman RJ, Liem M, et al. Idiopathic acute transverse myelitis: MR imaging findings. Radiology. 1996;201:661–669. doi: 10.1148/radiology.201.3.8939212. [DOI] [PubMed] [Google Scholar]
- 14.Sanders KA, Khandji AG, Mohr JP. Gadolinium-MRI in acute transverse myelopathy. Neurology. 1990;40:1614–1616. doi: 10.1212/wnl.40.10.1614. [DOI] [PubMed] [Google Scholar]
- 15.Banwell B, Tenembaum S, Lennon VA, Ursell E, Kennedy J, Bar-Or A, et al. Neuromyelitis optica-IgG in childhood inflammatory demyelinating CNS disorders. Neurology. 2008;70:344–352. doi: 10.1212/01.wnl.0000284600.80782.d5. [DOI] [PubMed] [Google Scholar]
- 16.Andronikou S, Albuquerque-Jonathan G, Wilmshurst J, Hewlett R. MRI findings in acute idiopathic transverse myelopathy in children. Pediatr Radiol. 2003;33:624–629. doi: 10.1007/s00247-003-1004-8. [DOI] [PubMed] [Google Scholar]
- 17.de Seze J, Stojkovic T, Breteau G, Lucas C, Michon-Pasturel U, Gauvrit JY, et al. Acute myelopathies: Clinical, laboratory and outcome profiles in 79 cases. Brain. 2001;124:1509–1521. doi: 10.1093/brain/124.8.1509. [DOI] [PubMed] [Google Scholar]
- 18.Simon JH. Update on multiple sclerosis. Radiol ClinNorth Am. 2006;44:79–100. doi: 10.1016/j.rcl.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Harzheim M, Schlegel U, Urbach H, Klockgether T, Schmidt S. Discriminatory features of acute transverse myelitis: a retrospective analysis of 45 patients. J Neurol Sci. 2004;217:217–223. doi: 10.1016/j.jns.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Campi A, Filippi M, Comi G, Martinelli V, Baratti C, Rovaris M, et al. Acute transverse myelopathy: spinal and cranial MR study with clinical follow-up. AJNR. 1995;16:115–123. [PMC free article] [PubMed] [Google Scholar]
- 21.Jeffery DR, Mandler RN, Davis LE. Transverse myelitis. Retrospective analysis of 33 cases, with differentiation of cases associated with multiple sclerosis and parainfectious events. Arch Neurol. 1993;50:532–535. doi: 10.1001/archneur.1993.00540050074019. [DOI] [PubMed] [Google Scholar]
- 22.Perumal J, Zabad R, Caon C, Mackenzie M, Tselis A, Bao F, et al. Acute transverse myelitis with normal brain MRI : long-term risk of MS. J Neurol. 2008;255:89–93. doi: 10.1007/s00415-007-0686-5. [DOI] [PubMed] [Google Scholar]
- 23.Scott TF, Kassab SL, Singh S. Acute partial transverse myelitis with normal cerebral magnetic resonance imaging: transition rate to clinically definite multiple sclerosis. Mult Scler. 2005;11:373–377. doi: 10.1191/1352458505ms1194oa. [DOI] [PubMed] [Google Scholar]
- 24.Cordonnier C, de Seze J, Breteau G, Ferriby D, Michelin E, Stojkovic T, et al. Prospective study of patients presenting with acute partial transverse myelopathy. J Neurol. 2003;250:1447–1452. doi: 10.1007/s00415-003-0242-x. [DOI] [PubMed] [Google Scholar]
- 25.Knebusch M, Strassburg HM, Reiners K. Acute transverse myelitis in childhood: nine cases and review of the literature. Dev Med Child Neurol. 1998;40:631–639. doi: 10.1111/j.1469-8749.1998.tb15430.x. [DOI] [PubMed] [Google Scholar]
- 26.Anu J, Weinshenker BG. An approach to the diagnosis of acute transverse myelitis. Semin Neurol. 2008;28:105–120. doi: 10.1055/s-2007-1019132. [DOI] [PubMed] [Google Scholar]
- 27.Krupp LB, Banwell B, Tenembaum S. International Pediatric MS Study Group. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68(Suppl. 2):S7–S12. doi: 10.1212/01.wnl.0000259422.44235.a8. [DOI] [PubMed] [Google Scholar]
- 28.Bashir K, Whitaker JN. Importance of paraclinical and CSF studies in the diagnosis of MS in patients presenting with partial cervical transverse myelopathy and negative cranial MRI. Mult Scler. 2000;6:312–316. doi: 10.1177/135245850000600503. [DOI] [PubMed] [Google Scholar]


