Since its introduction into clinical trials, FK 506 has proven to be the most potent oral immunosuppressant available.1-3 Our previous studies have documented the nephrotoxicity of FK 506, and have suggested that a reduction in renal blood flow is central to its pathogenesis.4,5
The clinical management has been constantly evolving. The starting dosage of FK 506 has gradually decreased and the IV infusion was changed from a 4-hour infusion to 12-hour infusions to attenuate the early perioperative nephrotoxicity. We will present, herein, the results of our experience with nephrotoxicity during the first year of primary hepatic transplantation under FK 506. This represents the results of a high-dose period and the steepest slope of our learning curve in the use of FK 506. Current practices emphasize lower initial doses, lower FK 506 levels, and progressive dosage reductions. Maintaining the optimal drug level which minimizes nephrotoxicity while avoiding rejection, will likely be assisted by a computer-based artificial intelligence program which has only recently been developed at the University of Pittsburgh.6
PATIENTS AND METHODS
All of the 282 adult patients receiving orthotopic liver transplants at the University of Pittsburgh between September 1989 and July 1990 were included in this study. The immunosuppression schedules have been published elsewhere, and were under evolution during this study. In brief, FK 506 was administered at doses of 0.075 mg/kg or 0.15 mg/kg IV over 4 hours, then repeated at 0.075 mg/kg every 12 hours until oral conversion was possible. At that time patients received 0.15 mg/kg per day. The oral and IV doses frequently overlapped for approximately 24 hours. Prednisone, with a 5-day IV burst, was employed in many patients. Others received between 200 mg or 20 mg/kg per day as the starting dose. Rejection episodes were treated with IV Solumedrol bolus, prednisone recycle, or OKT3. All patients received Bactrim (one single-strength tablet twice per day) and acyclovir adjusted to renal function.
Plasma FK 506 levels were measured by enzyme immunoassay, and done so daily while in hospital and during each clinic visit. Standard laboratory studies included serum creatinine (SCr), BUN, electrolytes, liver enzymes, cholesterol, uric acid, magnesium, and total protein, among others. Values are expressed as mean ± standard deviation. Differences between means were determined by Student’s t test or analysis of variance when appropriate.
RESULTS
The changes in SCr over time are depicted in Fig 1. Invariably, SCr rose in the perioperative period. The change in SCr was generally mild to moderate; 76% of patients had values <2.0 mg/dL at 7 days postoperatively. Hemodialysis was required for the first time in 43 patients. Thirty-two patients were dialyzed within the first 30 days, and 11 patients after 30 days (range 38 to 568 days). Patients requiring late dialysis were typically recipients of multiple liver transplants, septic or developed acute tubular necrosis (ATN) due to hypotension, or nephrotoxic antibiotics. Seventeen patients had either ARF or chronic renal failure at the time of transplantation. At latest follow-up, one patient requiring dialysis prior to transplantation is on regular dialysis, and is listed for a cadaveric renal transplant; one patient with early renal failure has required prolonged dialysis (6 months); and two patients with late renal failure require regular dialysis. The latter two patients had renal insufficiency at the time of transplantation. Mean SCr on postoperative day 450 was 1.6 ± 0.6 mg/dL (Fig 1).
Fig 1.
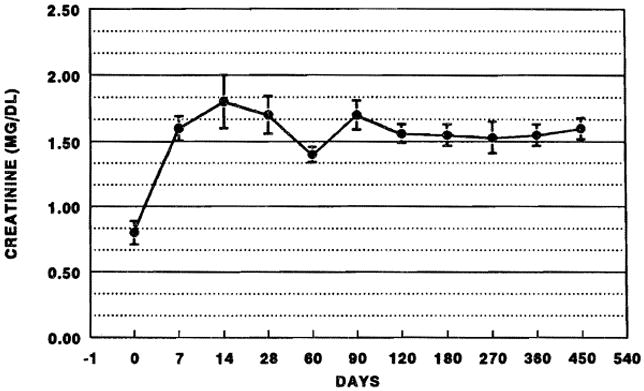
SCR after liver transplantation. Error bars are ± SEM.
In selected patients, studies fot effective renal plasma flow (ERPF) and glomerular filtration rate (GFR) were performed. Fig 2 illustrates the early changes in one patient. In essentially all patients, ERPF and GFR decreased in parallel after transplantation. A significant fall in GFR without a proportionate decrease in ERPF was seen in patients with ATN from other causes.
Fig 2.
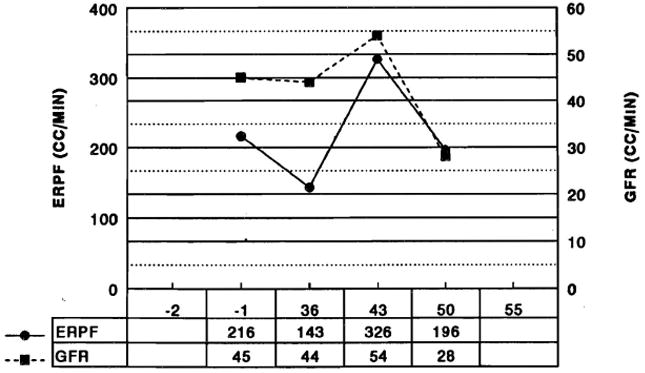
ERPF and GFR in one patient after liver transplantation.
Hypomagnesemia was common after transplantation. Fig 3 illustrates the percentage of patients with hypomagnesemia at selected times after transplantation. Ninety days after transplantation, 11.8% of patients had values <1.0 mg/dL. The prevalence of hypomagnesemia tended to decrease after 60 to 90 days. The hypomagnesemia was surprisingly well tolerated, even at levels less than 1.0 mg/dL. No seizures, hypocalcemia, or hypokalemia could be attributed to hypomagnesemia, although it may have lowered the seizure threshold in several patients with seizures from other causes.
Fig 3.
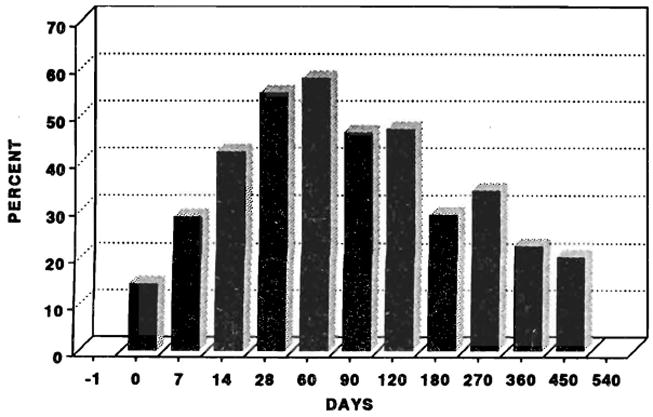
Percentage of patients with hypomagnesemia (magnesium <1.3 mg/dL).
Hyperkalemia was also a common complication of FK 506 therapy. At latest follow-up, 55% of the patients require fludrocortisone acetate (Florinef) to control hyperkalemia. These patients have been exquisitely sensitive to this agent. Common complications of this therapy, however, were peripheral edema and worsening hypertension due to sodium and water retention. Two patterns of hyperkalemia were seen: (1) in association with hyperchloremic metabolic acidosis (type IV RTA); and (2) in association with renal insufficiency without acidosis.
A very low incidence of hypertension, despite the hypertensive stimulus of fludrocortisone, was again apparent. At latest follow-up, 37 patients (16.4%) required antihypertensive medication. Of these, 30 (81.1%) require only one medication, 6 (16.2%) require two, and 1 (2.7%) requires three medications to control hypertension. Patients with a previous history of hypertension, although normotensive immediately prior to transplantation, developed elevated pressures once hepatic function improved. Serum creatinine was also greater in patients with a hypertensive history (1.8 ± 1.3 mg/dL) than their normotensive counterparts (1.4 ± 0.6 mg/dL), P < .05.
DISCUSSION
The nephrotoxicity of FK 506 has been described previously. The patients reported in this series represent the results of empiric trials of FK 506 drug dosing and an earlier practice of maintaining relatively high oral doses months after transplantation. During this early learning phase, the standard dose and schedule of IV administration has changed, and the target FK 506 level has fallen dramatically.
Although the patients in this series have received what will likely come to be regarded as very high doses of FK 506, renal function has been relatively well preserved compared to published trials with cyclosporine and in the randomized control trial of liver transplants at the University of Pittsburgh.7
The mechanism of FK 506 nephrotoxicity is currently under exploration. Our studies demonstrate that ERPF and GFR both decrease after initiation of FK 506, and often parallel changes in FK 506 level—decreasing with high levels and increasing with lower levels. No precise linear relationship exists for SCr and FK 506 levels, but elevations in FK 506 are associated with deterioration of renal function. Many of the findings of in vitro studies reported are similar to those for CyA, but are usually milder.8,9 It is now certain that the nephrotoxicity of FK 506 is dose-dependent and measures to decrease the quantity of drug required will improve renal function.
In the early phases of clinical use, the nephrotoxicity of FK 506 has been clearly demonstrated. Although our clinical experience, to date, suggests that FK 506 is approximately as nephrotoxic as CyA when used at maximal doses, it is likely that the measures now being planned will further reduce its severity.7 Among these include, the addition of a third drug such as azathioprine or the newer agents in this class. This will likely allow further reduction in FK 506 doses. A novel computer-assisted drug prescription program is now being developed at the University of Pittsburgh.6 With this system, a user-defined target FK 506 level will be maintained by dose adjustments which correct for drug toxicity. Additional potential areas of study include direct measures to reduce nephrotoxicity by preoperative calcium channel blockade, or prostaglandin administration, among others.
Acknowledgments
Supported by research grants from the Veterans Administration and Project Grant No. 29961 from the National Institutes of Health, Bethesda, Maryland.
References
- 1.Starzl TE, Todo S, Fung JJ, et al. Lancet. 1989;ii:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Abu-Elmagd, Tzakis A, et al. Transplant Proc. 1991;23:914. [PubMed] [Google Scholar]
- 3.Fung JJ, Todo S, Jain A, et al. Transplant Proc. 1990;22:6. [PMC free article] [PubMed] [Google Scholar]
- 4.McCauley J, Fung JJ, Jain A, et al. Transplant Proc. 1990;22:17. [PMC free article] [PubMed] [Google Scholar]
- 5.McCauley J, Fung JJ, Todo S, et al. Transplant Proc. 1991;23:1444. [PMC free article] [PubMed] [Google Scholar]
- 6.McMichaels J, et al. Transplant Proc. (this issue) [Google Scholar]
- 7.Fung J, et al. Transplant Proc. (this issue) [Google Scholar]
- 8.McCauley J, Farkus Z, Prasad S, et al. Transplant Proc. (this issue) [PubMed] [Google Scholar]
- 9.McCauley J, Studer R, Craven P, et al. Transplant Proc. (this issue) [PubMed] [Google Scholar]


