Abstract
This study determined ultrasonographic parameters of fetuses and uterine adnexa in late pregnancy in normal, cloned, and high-risk pregnancies in relation to perinatal and neonatal outcome. Ten cows with normal pregnancies (CONTROL, mean pregnancy length 273 d), 10 sick cows with potentially compromised pregnancies (HIGH-RISK, mean pregnancy length 267 d), and 10 heifers with cloned pregnancies (CLONED, mean pregnancy length 274 d) were examined at more than 260 d of gestation. There was no difference in mean fetal heart rates among the groups. The cloned calves were heavier (57 ± 8 kg) than calves from CONTROL group (36 ± 7 kg), and calves from HIGH-RISK group (37 ± 13 kg) (P = 0.003). The diameter of the thoracic aorta was positively correlated (R = 0.62) with fetal birth weight in the CONTROL group (P = 0.01). Fetal activity was not associated with survival. The results suggest that transabdominal ultrasonographic assessment of the fetal well-being may serve as a potential tool for evaluation of the fetoplacental unit.
Résumé
Évaluation par échographie du bien-être fœtal chez le bétail pour des gestations normales, à risque élevé et celles d’animaux clonés. Cette étude a déterminé les paramètres échographiques des fœtus et des annexes utérines à la fin de la gestation pour des gestations normales et à risque élevé par rapport aux résultats périnataux et néonataux. Dix vaches avec des gestations normales (TÉMOIN, durée moyenne de la gestation de 273 jours), 10 vaches malades avec des gestations potentiellement compromises (RISQUE ÉLEVÉ, durée moyenne de gestation de 267 jours) et 10 génisses avec des grossesses d’animaux clonés (CLONÉS, durée moyenne de gestation de 274 jours) ont été examinées à plus de 260 jours de gestation. Il n’y avait aucune différence au niveau des rythmes cardiaques fœtaux moyens parmi les groupes. Les veaux clonés étaient plus lourds (57 ± 8 kg) que les veaux du groupe TÉMOIN (36 ± 7 kg) et que les veaux du groupe à RISQUE ÉLEVÉ (37 ± 13 kg) (P = 0,003). Le diamètre de l’aorte thoracique présentait une corrélation positive (R = 0,62) avec le poids fœtal à la naissance du groupe TÉMOIN (P = 0,01). L’activité fœtale n’était pas associée à la survie. Les résultats suggèrent qu’une évaluation transabdominale du bien-être fœtal peut servir d’outil potentiel pour l’évaluation de l’unité fœto-placentaire.
(Traduit par Isabelle Vallières)
Introduction
The main objective of the fetal well-being assessment is to detect fetal or adnexal anomalies (1,2). Fetal well-being is recognized as the normal physical integrity of the fetus that is conditioned by its normal anatomical and physiological development (1,2). Ultrasonographic assessment of fetal well-being has been described in human (3,4), equine (5–7) and ovine (8) species. Invasive and noninvasive methods are available and can be used in normal and high-risk pregnancies (1,7). High-risk or compromised pregnancy is any pregnancy in which maternal or placental disorders can interfere with fetal health and is associated with an increased risk of pregnancy loss (7). Potential applications of fetal well-being assessment in bovine pregnancy, particularly in high-risk pregnancy such as somatic cloning pregnancy have been reviewed (2). Data on ultrasonographic findings in the near term bovine pregnancyare scant (9–12). Bovine cloned pregnancies are considered by many authors as high-risk pregnancies since uterine, placental, and fetal abnormalities may compromise the pregnancy (10,11). Various health issues of the neonatal clones have been described including respiratory distress leading to death (13). Some medical problems can be successfully treated but may require intensive care (13).
Uterine or maternal disease can also have a detrimental impact on fetal well-being and growth (4). In horses, high-risk or compromised pregnancies are associated with increased risk of premature delivery, intrauterine growth retardation (IUGR), fetal and perinatal morbidity and mortality (6,14).
Alterations of the placenta, fetal fluids, fetal heart rate (FHR), fetal heart rate variability (FHRV), and fetal movements are the most useful clinical parameters in fetal well-being assessment in humans and horses (3–6). These parameters have been assessed successfully by transabdominal ultrasonography in equine fetal evaluation (5,6,14,15). Although some studies have focused on fetal ultrasonography in the bovine late-term pregnancy (9,10,12,16,17), only a small proportion of them focused on FHR, fetal movements, and the fetal adnexa (10,17).
The first objective of this study was to establish ultrasonographic parameters of the placenta, fetal fluids, fetus, FHR, FHRV, and fetal movements during the last week of pregnancy in normal, compromised non-cloned, and cloned pregnancies. A second objective was to correlate these parameters with neonatal morbidity and mortality. The third objective was to study potential indicators of bovine fetal demise that may help in the management of high-risk pregnancies.
Materials and methods
The ultrasonographic protocol was approved by the institutional animal care committee of the Faculté de Médecine Vétérinaire, Université de Montréal. The animals studied were from 260 to 272 d pregnant as determined by the insemination date or age of the morula implanted for cloned pregnancies. Thirty Holstein cows carrying a single fetus were enrolled into one of 3 groups. The CONTROL group had 8 cows and 2 heifers with normal pregnancies (determined after a normal physical, vaginal, and transrectal examination of the dam). The HIGH-RISK group consisted of 10 cows that had been admitted to the Centre Hospitalier Universitaire Vétérinaire (CHUV) with severe systemic disease that could potentially affect fetal health. The diseases included right displaced abomasum with severe dehydration treated by omentopexy (n = 3), vagal indigestion (n = 2), hepatic lipidosis (n = 2), respiratory distress secondary to pharyngeal laceration (n = 1), downer cow with bleeding abomasal ulcers (n = 1), and downer cow (n = 1). Parturitions were induced with dexamethasone (Dexamethasone 5; Vétoquinol, Lavaltrie, Quebec), 25 mg, IM and followed by caesarean-section 24 h later in 4 cases and by assisted vaginal delivery in 6 cases. The CLONED group consisted of 10 heifers carrying somatic-cell cloned fetuses derived from fibroblastic cells. All animals from the CONTROL and CLONED groups received dexamethasone, 25 mg, IM 24 h prior to the elective caesarean section. The cloned calves were delivered and immediately monitored in a neonatal intensive care unit (NICU) at the CHUV. Reanimation and cardiorespiratory resuscitation were performed when necessary.
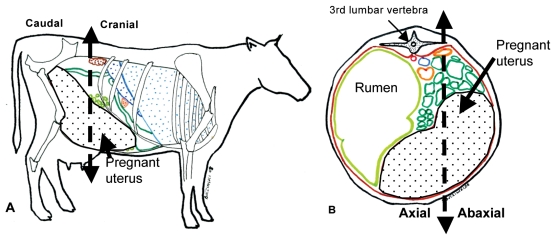
All cows were placed in a tie stall and examined without sedation by one of the authors (SB) less than 10 d from the expected delivery date. Transabdominal ultrasonography was performed in the last week of pregnancy in most cases since a caesarean-section was scheduled in the majority of cows. Cows without induction of parturition had at least 1 examination per week until calving. The last examination results were used for this study. Based on the anatomic projection of the pregnant uterus in late pregnancy (Figure 1) (18), the ventral abdomen was clipped from the udder caudally to the xiphoid process cranially, up to the mid flank on the right and to the ventral region on the left. Transabdominal ultrasonography was then performed using a 3.5 MHz probe in a portable ultrasound unit (Aloka 1700, Tokyo, Japan) after coupling gel was applied to the area of interest. The uterus, fetal fluids, and fetuses were observed for approximately 30 min. The ultrasonographic examination was performed in the afternoon. The parameters recorded were the interval between the day of the ultrasonographic examination and day of calving (Interval), mean fetal heart rate (FHRm), mean fetal aortic diameter (Ao), fetal activity (ACTIV), mean placentome area (PLAC), maximal placentome area (PLACmax), maximal length (LENGTH) and width (WIDTH) of the placentomes during each examination, maximal depth of fetal fluid (DEPTH), amniotic membrane thickness (Mthick) and echogenicity of allantoic and amniotic fluids.
Figure 1.
Anatomic topography of the gravid uterus in bovine late pregnancy (right lateral and transversal views). Adapted from Barone (18). The gravid uterus (white area with black dots) in the late pregnancy is occupying the ventral right flank (A). A transverse view at the level of the third lumbar vertebra shows that the gravid uterus extends on the left side of the linea alba and up to the mid right flank. The dotted lines show how the division of the uterine quadrants was determined for the placentome measurements. The cranial and caudal parts of the uterus are represented (A) as well as the axial and abaxial sides of the gravid uterus (B).
The fetal heart rate was counted manually in a 15-second period when observing the fetal thorax (3 to 5 measurements at 5 min intervals), the mean fetal heart rate (FHRm) was then calculated. If the fetal thorax could not be identified, the examination was delayed several hours and the ultrasonographic data were collected in the subsequent attempt. This occurred in 4 of 30 cases, which needed a second examination. The mean fetal thoracic aortic diameter was determined as described (Ao; 2 to 3 measurements) in horses (5,6) when the thoracic aorta was identified. The fetal activity (ACTIV) was recognized by movements of the fetuses during the ultrasonographic examination and graded from 0 to 3 as described by Reef et al (5,6). The fetal activity was graded 0 if no movement was detected, 1 if small movements were detected < 1/3 of the examination time, 2 if the fetus showed activity > 1/3 but < 2/3 of the examination time, and 3 if the fetus showed activity > 2/3 of the examination time.
The pregnant uterus was divided into 4 quadrants [axial and abaxial cranial, axial and abaxial caudal (Figure 1)]. The first placentome observed in each quadrant was used to obtain measurements with a frozen picture. The length and width of the placentome were measured by electronic callipers. Due to the elliptical form of the bovine placentome (18,19), the placentome area was calculated by the formula (width × length) × π/4 (19). The mean placentome area (PLAC) was then calculated using the 4 placentome areas. The maximal placentome area was recorded for each pregnancy (PLACmax). The maximal length (LENGTH) and width (WIDTH) were recorded for each cow. The maximal depth of fetal fluid (DEPTH) was also measured up to 20 cm (limit of the 3.5 MHz probe). The depth was classified in 2 categories (< 20 cm and ≥ 20 cm) for statistical analysis. The amniotic fluid was identified based on its relatively higher echogenicity when compared to the allantoic fluid which is almost anechoic (10). An echogenicity scale from 0 to 3 was used to assess both fetal fluid transparency based on the profile developed in the mare by Reef et al (5,6). The fetal fluids were scored 0 if they were anechoic, 1 if only a small amount of echogenic particles were detected, 2 if a moderate amount of echogenic particles were observed, and 3 if echogenic particles were present continuously throughout the examination. Two or 3 measurements of the thickness of the amniotic membrane that separates the allantoic and amniotic fluids were used to estimate a mean amniotic membrane thickness (Mthick). These measurements were taken as soon as the membrane was accurately identified.
At birth, body weight (BW) was obtained and newborns were treated by the clinicians according to their cardiovascular and respiratory status (neonatal resuscitation, endotracheal intubation and manual ventilation of intranasal oxygen delivery). Outcome in the first week of life was categorized based on the medical files as: NS (dead fetus or calf dead within 48 h after delivery, non-survivor calves), S (survivor calves). The S calves were separated in 2 categories: calves exhibiting any degree of respiratory distress (RD) with tachypnea, dyspnea of arterial blood gas indicating ventilation-perfusion mismatch and survivor calves without clinical signs of respiratory problem (NoRD).
Statistical analysis
All data were analyzed with statistical analysis software (SAS version 9.1, SAS Institute, Cary, North Carolina, USA). A t-test was used to assess association between the following quantitative data: Interval, FHRm, Ao, PLAC, PLACmax, Mthick, BW, and the outcome of calves (NS versus S). The same analysis was used to assess the same parameters according to the 2 categories of S calves (RD versus NoRD). An association between ACTIV and FHRm was investigated creating a linear model with the parameter ACTIV as a fixed factor. A chi-squared test was used to assess qualitative data (ACTIV, DEPTH, fluid score) relative to the outcome of calves (NS versus S), and the outcome of S calves (RD versus NoRD). A linear regression model was used to assess the relationship between Ao and the BW in each group and to investigate the relation between the FHRm and ACTIV. The exact chi-squared test was used to assess the effect of the type of pregnancy on ACTIV, DEPTH, fluid score, outcome, presence of respiratory distress. The quantitative data (Interval, FHRm, Ao, BW, Mthick, PLAC, PLACmax, LENGTH, and WIDTH) were compared in each group using a linear model with the pregnancy groups (CONTROL, HIGH-RISK, CLONED) as the between subject factor.
The level of significance was set at P < 0.05. Due to the small number of cows in each group for 0.05 < P ≤ 0.10, the results were considered to reflect a tendency.
Results
The high-risk pregnancies were attributed to various diseases that could affect fetal well-being and pregnancy secondary to decreased placental blood flow, decreased oxygen delivery, or severe inflammatory process. There was a tendency to have less calf mortality in CONTROL (0 out of 10) than in HIGH-RISK and CLONED pregnancies (4 out of 10) (P = 0.08, Table 1). In the cloned group, 2 calves were euthanized due to severe arthrogryposis. When the survivor calves were divided into RD versus NoRD, there was no statistical differences between CONTROL (1 RD calf out of 10 live calves), HIGH-RISK (1 RD calf out of 6 live calves) and CLONED pregnancies (2 RD calves out of 6 live calves) (P = 0.78).
Table 1.
Quantitative data obtained by transabdominal ultrasonography in CONTROL, HIGH-RISK, and CLONED pregnancies
| CONTROL |
HIGH-RISK |
CLONED |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Mean +/− standard deviation | Range | n | Mean +/− standard deviation | Range | n | Mean +/− standard deviation | Range | n |
| Interval (d) | 1.9 +/− 1.1 | 0–9 | 10 | 2.2 +/− 0.9 | 0–9 | 10 | 3.4 +/− 3.0 | 0–10 | 10 |
| FHRm (bpm) | 115 +/− 10 | 96–128 | 10 | 117 +/−11 | 106–129 | 8 | 119 +/− 12 | 100–145 | 10 |
| Ao (cm) | 1.99 +/− 0.37 | 1.2–2.4 | 9 | 2.04 +/− 0.38 | 1.6–2.6 | 6 | 2.04 +/− 0.26 | 1.8–2.4 | 7 |
| PLAC (cm2) | 20.4 +/− 4.9 | 13.4–27.6 | 10 | 22.0 +/− 8.8 | 13.4–37.5 | 10 | 23.4 ±/− 7.7 | 7.4–33.5 | 10 |
| PLACmax (cm2) | 28.4 +/− 8.9 | 16.6–40.8 | 10 | 30.1 +/− 9.8 | 18.9–48.0 | 10 | 37.5 +/− 11.8 | 11.5–62.5 | 10 |
| LENGTH | 8.5 +/− 1.6 | 6.9–12.3 | 10 | 9.4 +/− 1.7 | 7.3–11.9 | 10 | 9.4 +/− 2.1 | 6.4–12.2 | 10 |
| WIDTH | 4.6 +/− 0.8 | 3.3–5.7 | 10 | 4.3 +/− 0.9 | 3.3–5.9 | 10 | 4.5 +/− 1.2 | 2.7–6.8 | 10 |
| Mthick (cm) | 0.6 +/− 0.2 | 0.3–1.0 | 10 | 0.5 +/− 0.2 | 0.2–0.9 | 10 | 0.6 +/− 0.34 | 0.2–1.0 | 5 |
| BW (kg) | 36a +/− 7 | 29–45 | 9 | 37a +/− 13 | 22–47 | 4 | 57b +/− 8 | 49–72 | 8 |
n — Number of pregnancies in which the parameters were determined.
Interval — days between the examination day and calving; FHRm: mean fetal heart rate in beats per minute; Ao — thoracic aortic diameter; BW — birth weight; PLAC — mean placentome area (mean of the results for 4 placentomes per case); PLACmax — maximal placentome area (for the 4 placentomes measured); LENGTH — maximal length of the placentome; WIDTH — maximal width of the placentome; Mthick — allantoamniotic membrane thickness.
Different letters mean there was a significant difference between the birth weight of calves in the CLONED groupb when compared with HIGH-RISK and CONTROL groups (a) with a P-value of 0.003.
The results of the ultrasonographic profiles are presented in tables 1, 2, and 3 for the 3 groups. The mean gestation lengths were 273 d, 274 d, and 267 d for CONTROL, CLONED, and HIGH-RISK pregnancies, respectively. Birth weight was different between calves from each group. Cloned calves were heavier (mean = 57 ± 8 kg) than calves from control (36 ± 7 kg) and high-risk pregnancies (37 ± 13 kg) (P = 0.003). There was a tendency for calves that died to be heavier (mean BW = 55 kg ± 11 kg) than calves that survived (mean BW = 42 kg ± 12 kg) (P = 0.09). Other parameters were not statistically different among the pregnancy groups (Table 1). Two fetuses from the HIGH-RISK group had no heart beats detectable when imaging the thorax. Both fetuses were dead at calving. The FHRm was not significantly different among the groups (P = 0.14). In the HIGH-RISK group the FHRm was 93 ± 50 bpm when all data were compiled or 117 ± 11 bpm when the 2 values from the dead fetuses were not considered. No association was found between FHRm and fetal activity (P = 0.32) (Table 2). The fetal activity score did not differ among the groups (Table 2). Most fetuses were moving < 1/3 of the total examination time or between 1/3 and 2/3 of the total examination time. The fetuses that were totally inactive throughout the entire examination period (n = 2, HIGH RISK group) also had absent heart rate and were dead at the time of calving. Two fetuses were moving > 2/3 of the total examination time. One of these fetuses was a calf from a compromised pregnancy. This fetus was an NS calf. The other fetus was from a control pregnancy and did not have any problem after birth.
Table 2.
Fetal activity scores and mean (sd) fetal heart rate (FHRm) according to fetal activity
| Fetal activity | Score 0 | Score 1 | Score 2 | Score 3 |
|---|---|---|---|---|
| CONTROL* | 0 | 7 | 2 | 1 |
| HIGH-RISK* | 2 | 4 | 3 | 1 |
| CLONED* | 1 | 7 | 2 | 0 |
| Total | 3 | 18 | 7 | 2 |
| FHRm (bpm) | 39** +/− 67 | 115 +/− 12 | 120 +/− 8 | 123 +/− 2 |
The score of 0 was assigned to fetuses that did not move during the entire ultrasonographic examination, the score of 1 was given to fetuses that moved < 1/3 of the total examination time, the score of 2 was assigned to fetuses that were active < 2/3 but > 1/3 of the total examination time, the score of 3 was given to fetuses that moved for > 2/3 of the examination time.
There were no significant differences between the type of pregnancy and the activity score of the fetus (chi-squared test, P = 0.66).
Two of the 3 fetuses had no heart beat when their thorax was imaged. There was no association between the FHRm and the level of activity (P = 0.32) (fetuses with no activity were removed for the analysis).
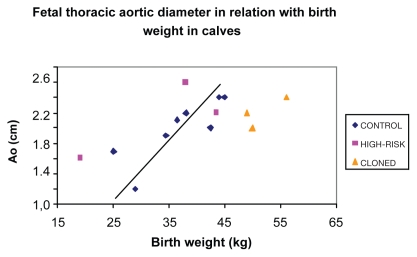
Diameter of the thoracic aorta (n = 19) was obtained and compared with fetal birth weight (n = 22). It was possible to obtain both data in only 14 cases. A significant positive linear correlation was found with the linear regression model between Ao and BW in CONTROL group (n = 8) (R = 0.62; P = 0.01) (Figure 2). This correlation was better estimated by the equation:
Figure 2.
Association between thoracic aortic diameters determined by transabdominal ultrasonography and newborn body weight in CONTROL (n = 8), HIGH-RISK (n = 3), and CLONED pregnancies (n = 3). This figure represents the 14 cases in which both the fetal aortic diameter (Ao) and the birth weight (BW) were available. The regression curve has been drawn on the graphs for the fetuses of CONTROL (♦) pregnancies in which a positive correlation (R = 0.62; P = 0.01) between Ao and BW was observed. Fetuses from the CONTROL (♦), HIGH-RISK (▪), and CLONED groups (▴) are represented. No association was found between Ao and BW in fetuses from HIGH-RISK or CLONED group.
No significant correlation was found for HIGH-RISK (n = 3; P = 0.40) or CLONED (n = 3; P = 0.42) group.
The uterine fluid depth was frequently above 20 cm in 20 of 30 cases. There was a tendency for pregnancies with DEPTH > 20 cm to have NS calves (P = 0.07). The echogenicity score of the uterine fluid was not correlated with the calf’s outcome (P = 1.0).
Discussion
This is the first study that attempted to use ultrasonographic assessment of fetal well-being in late pregnancy to predict perinatal and neonatal outcome in cows. Sporadic ultrasonographic assessment, however, may not be accurate enough to provide a complete evaluation of the fetoplacental unit and to predict perinatal and neonatal outcome. The HIGH-RISK pregnancy convenience group with severe maternal disease did not seem to suffer a reduced fetal survival rate. Prediction of an outcome was only possible in cases where dramatic reduction of vital signs (such as, complete absence of fetal movements) was observed.
The serial FHR measurement was not a good predictor of neonatal outcome. The FHR is an integral part of the fetal assessment since any fetal injury may lead to variations of FHR (3–6,20). However, continuous FHR monitoring may be more informative than serial FHR measurement (20). In this study, the serial assessment of the FHR could only detect fetal mortality (9).
Ultrasonographic assessment of fetal activity brings limited information about fetal well-being. Fetal inactivity (fetuses with no observable movements during the examination period) was associated with fetal death in 2 of 3 cases. A poor prognosis was observed in 1 of 2 fetuses moving more than 66% of the examination period (hyperactive fetuses). The limited number of the fetuses precludes further extrapolations; however, fetal hyperactivity or inactivity has been frequently associated with abnormal outcome in equine pregnancies (21). In horses, fetal inactivity is an indicator of fetal demise since equine fetuses have fewer sleeping periods than human fetuses (6). Fetal inactivity occurred in 3 of 10 heifers in the last 24 h of pregnancy when the fetus was observed for a 10-minute period without any detrimental effect on its health (22). Further characterization of the clinical significance of fetal movements on perinatal health should be determined. The main movements that were recognized by the authors were movements of the limbs and rotational movements about the fetal long-axis. It was difficult to precisely identify the portion of bone or joint that was visualized. The identification of the specific parts of the fetus that are moving is of potential interest, especially since musculoskeletal anomalies such as arthrogryposis are frequently encountered in cloned calves (13). This was the reason for euthanizing 2 cloned calves in this study. These malformations were not anticipated after the ultrasonographic examination. The precise identification of the fetal metacarpo-phalangeal or metatarso-phalangeal joints during the ultrasonographic examination as well as the ability to observe movements of these joints could potentially help to detect these anomalies in utero.
Estimation of fetal birth weight is a useful parameter to detect IUGR fetuses (1) and heavy fetuses which have a higher risk of periparturient complications (1,23). The diameter of the thoracic aorta is a useful tool to estimate equine fetal birth weight in the last week of pregnancy with a correlation coefficient (R) from 0.71 to 0.94 (5,24). This correlation also exists in control bovine pregnancies although it was slightly lower in the present study. The thoracic aorta may be difficult to observe systematically, because of the fetal position or movements. More than 1 examination may be required to obtain the aortic measurements. Further studies with a larger number of pregnancies should be performed to confirm the usefulness of the parameter especially as an early indicator of fetal-maternal disproportion to decide whether a caesarean section is needed or not.
Various placental anomalies have been described in late term cloned pregnancies (10,25,26–28). They include large placentomes (11) and accessory placentomes (small placentomes between 1 and 3 cm long) (26–28). The number of placentomes may also be variable in cloned pregnancy (normal from 70 to 120) (26,29) or decreased (< 50 placentomes) (26,27). The ultrasonographic assessment of the placentomes in late pregnancy was performed in several studies (11,28). Heyman (11) found that the mean placentome area was significantly greater in cloned pregnancies when compared with control pregnancies at day 232 but not at day 247 nor 262 (11). In another study, Kohan-Ghadr et al (28) reported that the maximal length of the placentome when measured by ultrasonography was significantly higher in cloned pregnancies than in control pregnancies before day 240 of gestation, but no information was available after this date (28). Our results did not show that the mean placentome area or the maximal length of the placentome was significantly different when comparing cloned pregnancies to normal and high-risk pregnancies after day 260. The maximal placentome area tended to be greater in the cloned pregnancies, although this was not significant. The first placentome to be observed in each uterine quadrant was selected for measurement which is not a true random selection; the measurement of an accessory placentome could have artificially decreased the mean placentome area in cloned pregnancies in our study. However, the elaboration of a more objective protocol for assessing the uterus may be difficult since the placentome observation is highly dependent on the position of the fetus. It is impossible to observe all the placentomes during ultrasonographic examination in late pregnancy. The echogenicity of the placentomes may also be altered in cloned pregnancies (27), but this information was not systematically recorded during the study.
This preliminary study showed that transabdominal ultrasonography is a feasible technique for the evaluation of fetuses and the fetal adnexa in the late pregnancy in cattle. However, a more detailed ultrasonographic assessment of the fetomaternal unit is necessary to diagnose more subtle anomalies and improve management of high-risk pregnancies.
Acknowledgments
The authors thank L’Alliance Boviteq for providing the cloned pregnancies that were used in this study; they also thank Guy Beauchamp for his valuable help in the statistical analysis of the data. CVJ
Footnotes
The study was performed at the Centre Hospitalier Universitaire Vétérinaire (CHUV) of the Faculté de Médecine Vétérinaire.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Lerner JP. Fetal growth and well-being. Obstet Gynecol Clin North Am. 2004;31:159–176. doi: 10.1016/S0889-8545(03)00121-9. [DOI] [PubMed] [Google Scholar]
- 2.Buczinski S, Fecteau G, Lefebvre RC, Smith LC. Fetal wellbeing assessment in bovine near-term gestations: Current knowledge and future perspectives arising from comparative medicine. Can Vet J. 2007;48:178–183. [PMC free article] [PubMed] [Google Scholar]
- 3.Manning FA. The fetal biophysical profile score. Obstet Gynecol Clin North Am. 1999;26:557–577. doi: 10.1016/s0889-8545(05)70099-1. [DOI] [PubMed] [Google Scholar]
- 4.Manning FA. Ultrasonography in perinatal medicine. In: Avery GB, editor. Neonatalogy: Pathophysiology and Management of the Newborn. 3rd ed. Philadelphia: Lippincott; 1987. pp. 110–129. [Google Scholar]
- 5.Reef VB, Vaala WE, Worth LT, et al. Ultrasonographic evaluation of the fetus and intrauterine environment in healthy mares during late gestation. Vet Radiol Ultrasound. 1995;36:533–541. [Google Scholar]
- 6.Reef VB, Vaala WE, Worth LT, et al. Ultrasonographic assessment of fetal well-being during late gestation: Development of an equine biophysical profile. Equine Vet J. 1996;28:200–208. doi: 10.1111/j.2042-3306.1996.tb03773.x. [DOI] [PubMed] [Google Scholar]
- 7.Bucca S. Diagnosis of the compromised equine pregnancy. Vet Clin North Am Eq Pract. 2006;22:749–761. doi: 10.1016/j.cveq.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Galan HL, Hussey MJ, Barbera A, et al. Relationship of fetal growth to duration of heat stress in an ovine model of placental insufficiency. Am J Obstet Gynecol. 1999;180:1278–1282. doi: 10.1016/s0002-9378(99)70629-0. [DOI] [PubMed] [Google Scholar]
- 9.Jonker FH. Fetal death: Comparative aspects in large domestic animals. Anim Reprod Sci. 2004;82:415–430. doi: 10.1016/j.anireprosci.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Buczinski S, Fecteau G, Comeau G, et al. Fetal well-being assessment, neonatal and post partum findings of cloned pregnancy in the bovine species: 10 fetuses and calves. Can Vet J. 2009;50:261–269. [PMC free article] [PubMed] [Google Scholar]
- 11.Heyman Y, Chavatte-Palmer P, LeBourhis D, et al. Frequency and occurrence of late-gestation losses from cattle cloned embryos. Biol Reprod. 2002;66:6–13. doi: 10.1095/biolreprod66.1.6. [DOI] [PubMed] [Google Scholar]
- 12.Taverne MAM, Breukelman SP, Perenyi Z, et al. The monitoring of bovine pregnancies derived from transfer of in vitro produced embryos. Reprod Nutr Dev. 2002;42:613–624. doi: 10.1051/rnd:2002047. [DOI] [PubMed] [Google Scholar]
- 13.Fecteau ME, Palmer JE, Wilkins PA. Neonatal care of high-risk cloned and transgenic calves. Vet Clin North Am (Food Anim Pract) 2005;21:637–653. doi: 10.1016/j.cvfa.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Pantaleon LG, Bain FT, Zent W, et al. Equine fetal growth and development. Compend Contin Educ Pract Vet. 2003;25:470–477. [Google Scholar]
- 15.Bucca S, Fogarty U, Collins A, et al. Assessment of feto-placental well-being in the mare from mid-gestation to term: Transrectal and transabdominal ultrasonographic features. Therio. 2005;64:542–557. doi: 10.1016/j.theriogenology.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Breukelman S, Mulder EJH, Van Oord R, et al. Continuous fetal heart rate monitoring during late gestation in cattle by means of Doppler ultrasonography: Reference values obtained by computer-assisted analysis. Therio. 2006;65:486–498. doi: 10.1016/j.theriogenology.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 17.Buczinski S, Bélanger AM, Fecteau G, Roy JP. Prolonged gestation in two Holstein cows: Transabdominal ultrasonographic findings in late pregnancy and pathologic findings in the fetuses. J Vet Med A Physiol Pathol Clin Med. 2007;54:624–626. doi: 10.1111/j.1439-0442.2007.00985.x. [DOI] [PubMed] [Google Scholar]
- 18.Barone R. Topographie des organes abdominaux d’une vache gravide. In: Barone R, editor. Anatomie comparée des mammifères domestiques. Tome 4 : Splanchnologie II. 3rd ed. Vigot, Paris: 2001. p. 768. [Google Scholar]
- 19.Batchelder CA, Bertolini M, Mason JB, et al. Perinatal physiology in cloned and normal calves: Physical and clinical characteristics. Cloning Stem Cells. 2007;9:63–82. doi: 10.1089/clo.2006.0037. [DOI] [PubMed] [Google Scholar]
- 20.Harman CR. Assessment of fetal health. In: Creasy RK, Resnik R, Iams JD, Lockwood CJ, Moore TR, editors. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. 6th ed. Elsevier-Saunders; Philadelphia: 2009. pp. 361–395. [Google Scholar]
- 21.Reimer JM. Use of transcutaneous ultrasonography in complicated latter-middle to late gestation pregnancies in the mare: 122 cases. Proc Am Assoc Equine Pract. 1997;43:259–261. [Google Scholar]
- 22.Fraser AF. A monitored study of major physical activities in the perinatal calf. Vet Rec. 1989;125:38–40. doi: 10.1136/vr.125.2.38. [DOI] [PubMed] [Google Scholar]
- 23.Johanson JM, Berger PJ. Birth weight as a predictor of calving ease and perinatal mortality in Holstein cattle. J Dairy Sci. 2003;96:3745–3755. doi: 10.3168/jds.S0022-0302(03)73981-2. [DOI] [PubMed] [Google Scholar]
- 24.Adams-Brandemuehl CS, Pipers FS. Antepartum evaluation of the equine fetus. J Reprod Fertil. 1987;35:565–573. [PubMed] [Google Scholar]
- 25.Constant F, Guillomot M, Heyman Y, et al. Large offspring or large placenta syndrome? Morphometric analysis of late geatation bovine placentomes from somatic nuclear transfer pregnancies complicated by hydrallantois. Biol Reprod. 2000;75:122–130. doi: 10.1095/biolreprod.106.051581. [DOI] [PubMed] [Google Scholar]
- 26.Miglino MA, Pereira FTV, Visintin JA, et al. Placentation in cloned cattle: Structure and microvascular architecture. Therio. 2007;68:604–617. doi: 10.1016/j.theriogenology.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 27.Hill JR, Edwards JF, Sawyer N, et al. Placental anomalies in a viable cloned calf. Cloning. 2001;3:83–88. doi: 10.1089/15204550152475581. [DOI] [PubMed] [Google Scholar]
- 28.Kohan-Ghadr HR, Lefebvre RC, Fecteau G, et al. Ultrasonographic and histological characterization of the placenta of somatic nuclear transfer-derived pregnancies in dairy cattle. Therio. 2008;69:218–230. doi: 10.1016/j.theriogenology.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 29.Jainudeen MR, Hafez E. Gestation, prenatal physiology and parturition. In: Hafez E, editor. Reproduction in farm animals. Philadelphia: Lea and Febiger; 1993. pp. 217–219. [Google Scholar]