Abstract
We had previously demonstrated the persistence of donor leukocytes in the peripheral blood and tissues of long-surviving kidneyl and live2-4 recipients who had stable graft function many years after transplantation.1-6 Donor cell chimerism has since been noted by other investigators in recipients of heart,7 liver,8 kidney,9 and lungl0 transplants. In an attempt to augment chimerism, and thereby facilitate graft function, we initiated a prospective trial to enhance this phenomenon by infusing 3 × l08/kg unaltered donor bone marrow cells perioperatively into an unmodified recipient of whole organ from the same donor. Additionally, 53 recipients of whole organ alone were monitored as controls. Reported herein are the first 20 of 64 study patients and 33 of 53 control patients who are more than 120 days posttransplantation.
MATERIALS AND METHODS
Patients
Since December 1992, 64 patients have been simultaneously transplanted with donor bone marrow and liver (n = 28), liver and islets (n = 1), kidney (n = 17), kidney and pancreas (n = 2), kidney and islets (n = 6), heart (n = 8), and lungs (n = 2). Immediately after whole organ placement and without any recipient conditioning, unmodified donor bone marrow cells (3 × 108/kg), harvested from the vertebral bodies, were infused through a central intravenous line. All patients were maintained on routine immunosuppression with Tacrolimus (FK 506; Prograf) and prednisone. All episodes of acute graft-versus-host (GVH) and host-versus-graft (HVG)reactions were treated with transient alterations in immunosuppression that in some patients included a methylprednisolone recycle (1 g tapered to 20 mg/d over a 6-day period). Rejection episodes that were not amenable to conventional therapy were treated additionally with azathioprine and OKT3. In seven type I diabetic patients, donor pancreatic islets (0.57−1.5 × 106/recipient), isolated by a modification of the automated method,11 were infused through the portal vein before bone marrow infusion but after organ placement. Additionally, 53 recipients of whole-organ allografts in whom consent to retrieve donor vertebral column was not available were monitored as contemporaneous controls.
Determination of Chimerism
Recipients’ peripheral blood mononuclear cells (PBMC) were prepared weekly in the first month and monthly thereafter for the detection of donor cells by the following techniques.
Flow Cytometry
In all immunostaining procedures, fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (MAbs; mouse-α-human) directed against the polymorphic epitopes of HLA class I or II were used to identify donor and recipient cells. The specificity and optimal dilution of each antibody was determined by staining donor spleen cells and recipient’s pretransplant peripheral blood mononuclear cells (PBMCs). To delineate lineages, phycoerythrin (PE)-conjugated mouse-α-human MAbs directed against various leukocyte cell surface receptors were used. Single-color fluorescence-activated cell sorting (FACS) analysis was performed to identify and or sort donor cells. Fifty thousand events were acquired, and values of circulating donor cells less than 0.5% were considered not quantifiable.
Fluorescent In Situ Hybridization
PBMCs isolated from female recipients of male organs were used to prepare cytospins for subsequent staining with a biotinylated Y-chromosome–specific probe. Fluorescein isothiocyanate–conjugated avidin was used to visualize positive cells.
Polymerase Chain Reaction
Oligonucleotides specific for either the sex-determining region of the Y-chromosome (male → female transplants) or for the appropriate mismatched MHC class II alleles were used to detect donor DNA in the recipient’s PBMCs by either polymerase chain reaction (PCR) or competitive PCR (cPCR). The analysis was conducted via a method described previously. 12
In Vitro Immune Monitoring
Pre- and posttransplant monitoring of recipients’ immune status was conducted via evaluating proliferative responses of their PBMCs to mitogens (concanavalin A and phytohemagglutinin), recall antigens (tetanus toxoid), and by mixed lymphocyte reactions (MLRs).
RESULTS AND DISCUSSION
No complication of bone marrow infusion was observed in any of the 64 patients who simultaneously received whole-organ allografts. The first 17 of 20 bone marrow–augmented patients with a follow-up of 180 to 612 days have excellent graft function. One heart and bone marrow recipient died 267 days after transplant due to causes unrelated to marrow infusion. At autopsy, no evidence of rejection was found in the allograft; however, a relatively large thromboembolus was found in the right pulmonary artery, which appeared to be infected. The exact cause of death is still unknown, but it is of interest to note that a relatively high potassium level (9.5 mEq/L) was found in the specimen of vitreous humor that was recovered approximately 10 hours after his death.
Another patient, a recipient of kidney and bone marrow, was admitted on postoperative day 499 with pancreatitis and acute cellular rejection. His creatinine at the time of admission was 32 mg/dL and, given his worsening renal function, he was put on dialysis. Because of ongoing domestic problems, the patient had become noncompliant in the 6 weeks before admission, which included failure to take immunosuppression regularly and resumption of excessive alcohol intake. The patient is currently on dialysis. It is unfortunate that this patient became noncompliant, because he had a rejection-free postoperative course and excellent graft function until his last admission. He also had evidence of circulating donor cells and exhibited stable donor-specific hyporeactivity in serial in vitro assays. The deliberate withdrawal of immunosuppression by this patient and its undesirable outcome have further reinforced our earlier contention that premature weaning or withdrawal of immunosuppression might have serious repercussions both for the patient and the graft.
A third patient who received cadaveric bone marrow plus a kidney was admitted on postoperative day 456 with renal dysfunction (serum creatinine 3.4 mg/dL) and evidence of rejection on fine-needle aspiration biopsy. Since she had experienced previous rejection episodes, all of which responded to manipulations in her routine immunosuppression, she was started on a steroid recycle (1 g bolus tapered to 20 mg/d). However, despite this ongoing therapy, renal function deteriorated, which prompted the initiation of treatment with antithymocyte globulin (ATGAM). Eleven days after termination of ATGAM therapy, she presented with signs and symptoms of serum sickness for which she is being treated. She is currently on dialysis.
Of the 53 control patients (recipients of whole organs alone), eight (liver n = 7, kidney n = 1) have died at varying times after transplantation.
Although none of the bone marrow-augmented or control patients are off Tacrolimus, it is noteworthy that four of nine kidney recipients in the study group are off steroids, whereas in none of the recipients of kidney transplant alone has a steroid-free existence been achieved (Table 1). Liver function, on the contrary, was similar in both groups (Table 1). After a relatively high circulating C-peptide activity, islet graft function was lost to rejection in two of three (liver plus islets, n = 1; kidney plus islets, n = 1) patients. Islet function in the third patient is high enough to warrant an insulin-free existence if and when immunosuppression, with its diabetogenic side effects, can be discontinued. Nevertheless, all three recipients of pancreatic islet cells are currently being maintained euglycemic by exogenous insulin (Table 1).
Table 1.
Organ Function of Recipients With or Without Donor Bone Marrow Augmentation
| Type of Transplantation | n | Postoperative day | T. Bilirubin (mg/dL) | Creatinine (mg/dl) | C-Peptide (pM/ml) | Insulin (U/day) | No. of Patients Off Steroids |
|---|---|---|---|---|---|---|---|
| With bone marrow | |||||||
| OLT | 7 | 355 ± 103* | 0.5 ± 0.3* | — | — | — | 4 |
| OLT + islets | 1 | 326 | 0.3 | — | 0.02 | 29 | 0 |
| CRT | 9 | 395 ± 138* | — | 1.7 ± 0.9* | — | — | 4 |
| CRT + islets | 2 | 411, 422 | — | 2.2, 1.9 | 0.44, 0.11 | 32,32 | 1 |
| Without bone marrow | |||||||
| OLT | 14 | 235 ± 35* | 0.8 ± 0.4* | — | — | — | 8 |
| CRT | 9 | 250 ± 33* | — | 3.0 ± 1.6* | — | — | 0 |
| HT | 2 | 197, 197 | Optimal cardiac function | 0 |
OLT, orthotopic liver transplantation; CRT, cadaveric renal transplantation.
Means ± SD.
HVG and GVH reactions were expected and encountered. Mild to moderate acute cellular rejection episodes were witnessed in 11 of 19 bone marrow–augmented (58%) and in 16 of 25 control patients (64%) who survived for the duration of this study. Furthermore, histopathology of allograft rejection was similar in both groups, resolving completely with transient increase in routine immunosuppression. Asymptomatic GVH reaction of the skin was encountered in only two patients, both recipients of bone marrow and liver, whose resolution in one did not require any exogenous intervention, whereas a slight increase in routine steroid therapy resulted in complete regression in the other.13
Evidence of circulating donor cells was present in 18 of 18 study patients and in nine of 21 control patients in whom detection was possible by flow cytometry (Fig 1), PCR, or fluorescence in situ hybridization. Furthermore, evidence of evolving donor-specific hyporeactivity was found in seven of 17 (41%) bone marrow–augmented patients and in four of 22 (18%) control patients, in whom in vitro testing by MLR was feasible. A more perceptible modulation of immune reactivity was seen in the liver and bone marrow recipients, in whom 62% showed donor-specific hyporeactivity as compared with 21% of the controls.14
Fig 1.
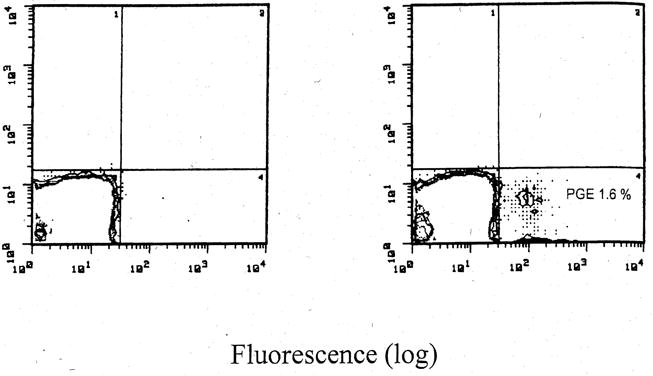
FACS profile of PBMC obtained from a recipient 314 days after bone marrow and kidney transplantation. (A) Isotype-matched irrelevant antibody used as control. (B) Monoclonal antibody specific for HLA class I (anti-B7, IgG1; HB59, ATCC, Rockville, MD) was used to identify circulating donor cells. PGE, percentage gated events.
Taken together, these data suggest that simultaneous infusion of donor bone marrow at the time of whole-organ transplantation leads to augmentation of chimerism without undue risk to the patients. Given the augmentation of donor leukocyte chimerism and evidence in some recipients of evolving donor-specific hyporeactivity, it is tempting to speculate that these patients can realistically aspire to an eventual drug-free state. However, it must be emphasized that we do not anticipate weaning these patients off immunosuppression in the near future. This cautious approach has emerged from our experience in withdrawing immunosuppression from long-surviving kidney and liver recipients, which has taught us that long-term follow-up is required to ascertain the feasibility of safely weaning patients off drugs.
Acknowledgments
This work was supported by National Institutes of Health Grant DK29961.
References
- 1.Starzl TE, Demetris AJ, Trucco M, et al. Transplantation. 1993;55:1272. doi: 10.1097/00007890-199306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Demetris AJ, Murase N, et al. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Demetris AJ, Trucco M, et al. Lancet. 1992;340:876. doi: 10.1016/0140-6736(92)93286-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Demetris AJ, Trucco M, et al. N Engl J Med. 1993;328:745. doi: 10.1056/NEJM199303183281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Demetris AJ, Murase N, et al. Immunol Today. 1993;14:326. doi: 10.1016/0167-5699(93)90054-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Demetris AJ, Trucco M, et al. Hepatology. 1993;17:1127. [PMC free article] [PubMed] [Google Scholar]
- 7.Schlitt HJ, Hundrieser J, Hisanaga M, et al. Lancet. 1994;343:1469. doi: 10.1016/s0140-6736(94)92584-4. [DOI] [PubMed] [Google Scholar]
- 8.Schlitt HJ, Raddatz G, Steinhoff G. Transplantation. 1993;56:951. doi: 10.1097/00007890-199310000-00033. [DOI] [PubMed] [Google Scholar]
- 9.McDaniel D, Naftilan J, Hulvey K, et al. Transplantation. 1994;57:852. doi: 10.1097/00007890-199403270-00014. [DOI] [PubMed] [Google Scholar]
- 10.Keenan RJ, Zeevi A, Banas R, et al. J Heart Lung Transplant. 1994;13:A9. [Google Scholar]
- 11.Ricordi C, Lacy PE, Finke EH, et al. Diabetes. 1988;37:413. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 12.Rudert WA, Kocova M, Rao AS, et al. Transplantation. doi: 10.1097/00007890-199410270-00022. in press. [DOI] [PubMed] [Google Scholar]
- 13.Fontes P, Rao AS, Demetris AJ, et al. Lancet. 1994;344:151. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeevi A, Lombardozzi S, Banas R, et al. Transplant Proc. in press. [PMC free article] [PubMed] [Google Scholar]


