Abstract
Light microscopic, immunohistochemical and ultrastructural analysis of protocol before transplantation and after reperfusion biopsy specimens from 87 randomly selected patients was performed to assess the contribution of preservation and immunological injury to early graft failure. Most biopsy specimens were essentially normal by light microscopy before transplantation, and no particular feature could be relied on to predict function after transplantation. Ultrastructural examination of biopsy specimens before transplantation demonstrated preferential degeneration of sinusoidal lining cells, but no strict correlation was seen between ultrastructural sinusoidal integrity before transplantation and function after transplantation. The presence of zonal or severe focal necrosis and a severe neutrophilic exudate in biopsy specimens after reperfusion presaged a poor early postoperative course in most, but not all, patients. The presence of preformed lymphocytotoxic antibodies had no effect on the early clinical course, but was associated with Kupffer cell hypertrophy in needle biopsy specimens taken after transplantation. No definite evidence was seen of hyperacute rejection as a result of preformed lymphocytotoxic antibodies as detected in conventional assays. These findings suggest that preservation injury accounts for only a subset of grafts that fail to function after transplantation. Other perioperative or “recipient” factors may be of equal or greater importance in early graft dysfunction or failure.
At the University of Pittsburgh and other institutions, as many as 10% of human orthotopic liver allografts never function properly and require urgent replacement in the first several weeks after transplantation (1–3). When no apparent technical or immunological cause of early allograft failure can be identified, the term primary nonfunction has been used, and preservation injury is often blamed. Considering all the potential insults and the chaotic metabolic environment into which the new liver is placed, the 10% rate of primary graft nonfunction is surprisingly low.
Among the many potentially noxious insults that can cause early graft damage, immunological injury has been considered one of the least important. In fact, no early deleterious effect has been seen in liver transplant recipients who harbor preformed T-warm antibodies (4–6), and these antibodies may disappear from the recipient circulation shortly after reperfusion of the allograft (7).
Only transplantation of a diseased liver (8) or violation of the major ABO blood group barriers reliably predicts poor early functioning or failure after transplantation (9). The following study is aimed at investigating the contributions of “preservation” and other forms of immunological injury to primary graft nonfunction.
PATIENTS AND METHODS
Eighty-seven patients were randomly chosen at the discretion of the operative surgeons from among 645 adults who received orthotopic liver transplants between October 1986 and October 1988 at the Presbyterian University Hospital at Pittsburgh for protocol biopsy evaluation before transplantation and after reperfusion. All procedures discussed in this study were done as a part of the standard clinical management of the transplant patients. Biopsy specimens were obtained before transplantation after organ procurement and cold preservation using standard methods (10). Biopsy specimens were obtained after reperfusion after complete revascularization of the inferior vena cava, the portal vein and the hepatic artery from the grossly normal medial or anterior segment of the allograft (11). Seventy-six of the allografts were primary grafts, nine were secondary and two were tertiary, where primary is the first graft, secondary the second graft and tertiary the third graft. Fifty-one grafts were preserved in Eurocollins’ solution, and 36 grafts were stored in University of Wisconsin (UW) solution (1, 12). Cold ischemic time varied from 3 to 21.5 hr, with a mean of 6 hr for those preserved with Eurocollins’ solution and a mean of 8 hr for organs kept in UW solution. No attempt was made to correlate the type of preservation fluid with the postoperative clinical course because those organs kept in UW solution were generally preserved for longer periods than those stored in Eurocollins’ solution.
All patients received grafts with a compatible ABO blood type. Of the 77 patients for whom crossmatches were performed, 16 had a positive or strongly positive lymphocytotoxic crossmatch using standard complement-dependent cytotoxicity assays. No further studies were performed to isotype the reactive antibodies.
The major portion of each biopsy specimen was fixed in 10% neutral buffered formalin and routinely stained with hematoxylin and eosin. A smaller portion of the biopsy specimen was fixed with 2% glutaraldehyde and was embedded in Epon-Araldite for transmission electron microscopy. All biopsy specimens from the 11 patients with a strongly positive crossmatch, 10 other crossmatch negative patients, all 11 nonprimary and the five failed allografts were selected for immunohistochemical evaluation by staining for the presence of IgG, IgM, Clq, fibrinogen, lysozyme and factor VIII–related antigen using paraffin-embedded tissue (13) and standard avidin-biotin-peroxidase methods using commercially available reagents (Dakopatts, Copenhagen, Denmark) (14).
Specific histological criteria and the results of immunoperoxidase staining were blindly and independently assessed for each biopsy specimen pair by two of the authors (S.K. and A.J.D.). The histological features examined were the severity, type and location of necrosis, inflammation and steatosis and the location and severity of hepatocellular swelling (Fig. 1) and cytoaggregation. Cytoaggregation refers to a reversible form of cell injury manifest morphologically by a “rounding-up” of the hepatocyte, so that the cell assumes a rounded appearance instead of the normal polygonal configuration. The severity of inflammation was based on the average number of inflammatory cells per high power field in the most prominently involved areas, with 0 to 1 = none; 2 to 5 = minimal; 6 to 10 = mild; 11 to 20 = moderate and more than 20 = severe. The severity of necrosis, steatosis, cytoaggregation and hepatocyte swelling was based on the estimated percentage of cells demonstrating that particular change, with 0 = none; < 10% = minimal; 10% to 40% = mild; 40% to 70% = moderate and 70% to 100% = severe. The biopsy specimens were categorized as “poor” if the inflammation or necrosis, or both, was moderate or severe, otherwise it was considered “good” (Fig. 2). The immunohistochemical stains were graded according to their intensity (negative to strong positive) and location.
Fig. 1.
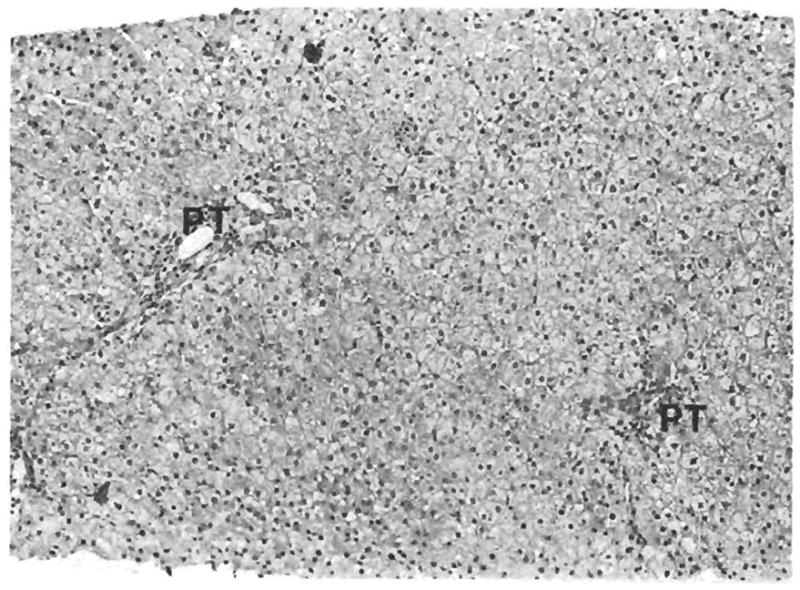
Moderate diffuse hepatocellular swelling in a biopsy specimen after reperfusion. PT = portal tract. (H & E, original magnification × 100.)
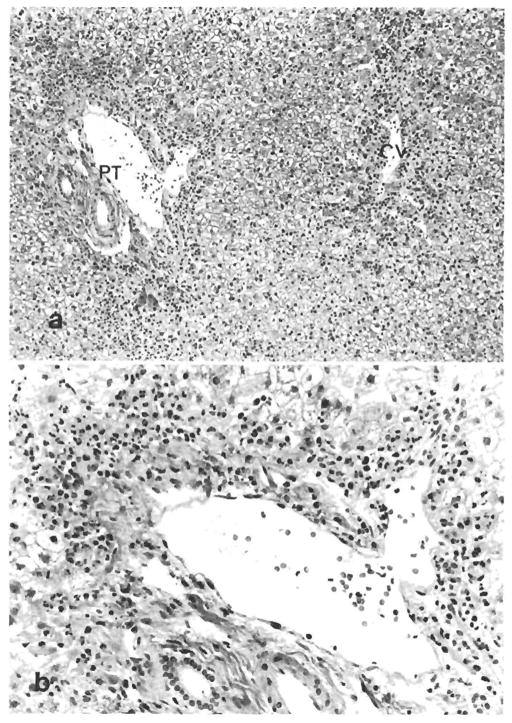
Fig. 2.
(a) Zonal necrosis associated with a severe neutrophilic infiltrate in centrizonal and periportal areas is seen in this biopsy specimen after reperfusion. CV = central vein, PT = portal tract. (H & E, original magnification × 100.) (b) Higher magnification demonstrates periportal zonal hepatocellular necrosis associated with a neutrophilic infiltrate. (H & E, original magnification × 250.)
Good organ function was defined by criteria similar to those of Makowka et al. (15) that specify peak serum values of AST always < 1,500 IU/L and ALT always < 1,000 IU/L during the first postoperative week, based on daily determinations. Poor function was characterized by peak serum values of AST > 1,500 IU/L or ALT > 1,000 IU/L on any day during the first week. The prothrombin time that was included by Makowka et al. (15) was neglected because of unavailability of complete data, and the reported values may have been influenced by the use of fresh frozen Plasmanate. Complete clinical data were available in 59 of the 87 patients and included donor age, sex, cause of death, cold ischemic time, type of preservation fluid, results of the lymphocyte crossmatch and the priority status of the recipient. All patients were followed for at least 1 mo after transplantation and all follow-up biopsy specimens were reviewed (n = 126, 58 patients) after transplantation.
RESULTS
Light Microscopy
Most biopsy specimens were essentially normal before transplantation except for focal mild spotty acidophilic necrosis, a slight increase in sinusoidal inflammatory cells and mild hepatocellular swelling. The integrity of the sinusoidal lining cells could not be reliably evaluated with immersion fixed, paraffin-embedded and hematoxylin and eosin-stained slides of biopsy specimens before transplantation.
Samples after reperfusion, on the other hand, demonstrated a range of pathological findings, some of which were similar to those seen in biopsy specimens taken during other types of abdominal surgical procedures (16).
Necrosis
Mild, spotty single-cell acidophilic necrosis was observed in six of 87 biopsy specimens; before transplantation the remainder had no hepatocellular necrosis. In biopsy specimens after reperfusion from two patients who had necrosis in the biopsy specimen before transplantation, larger areas of necrosis appeared that were classified as focal or zonal. Ten other biopsy specimens after reperfusion contained focal or zonal necrosis that was either centrilobular, periportal, or both in distribution.
Inflammation
Eighty-five of the 87 biopsy specimens contained little to no inflammation before transplantation; the remaining two showed moderate inflammation that consisted of neutrophils or neutrophils mixed with lymphocytes and cellular debris in the sinusoids.
In general, the degree of inflammation increased after revascularization and paralleled the degree of necrosis, although focal sinusoidal neutrophilia without necrosis was not uncommon. Sixty biopsy specimens showed inflammation ranging from none to mild, after reperfusion, and 27 specimens showed moderate to severe inflammation, mostly consisting of neutrophils, fewer macrophages and lymphocytes that were frequently sludged in the sinusoids associated with areas of hepatocyte necrosis and sinusoidal debris or both (Fig. 2). In summary, 47 patients had a good clinical course and histological findings, whereas 13 had a good clinical course but poor histological findings. By contrast, 15 patients had both a poor clinical course and histological findings, whereas 13 had poor histological findings but a good clinical course. Chi-squared analysis revealed a significance level < 0.05.
Steatosis, Cytoaggregation and Hepatocyte Swelling
Microvesicular steatosis was the predominant type of fatty metamorphosis, although mild focal macrovesicular change was detected in an occasional biopsy specimen. Generally, the severity of microvesicular steatosis increased in biopsy samples after reperfusion when compared with specimens before transplantation. Focal hepatocellular cytoaggregation was seen in 10 biopsy specimens before transplantation and 35 biopsy specimens after reperfusion. It generally increased after transplantation, especially in the periportal areas. Mild hydropic cell swelling was detected in both biopsy samples before transplantation and after reperfusion. However, a periportal location was slightly more prevalent in samples after reperfusion.
Immunohistochemistry
No endothelial staining for immunoglobulin or complement components was seen in any of the biopsy specimens before transplantation, but mild focal positivity for IgG and IgM in the cytoplasm of an occasional Kupffer cell, plasma cells and spindle-shaped cells in the portal tract connective tissue was detected in a few cases. The results were not influenced by whether the donor had received a blood transfusion. Lysozyme staining of biopsy specimens to detect Kupffer cells before transplantation showed considerable variability in the number of positive cells and the amount of positive staining cytoplasm. Staining for factor VIII–related antigen accentuated the integrity of the endothelium of the larger vessels, but the sinusoidal endothelial cells did not reliably stain. No intravascular fibrinogen deposits were detected in the biopsy specimens before transplantation.
Many of the biopsy specimens after reperfusion revealed a faint interrupted linear sinusoidal positivity for IgG and IgM, regardless of the presence or absence of preformed lymphocytotoxic antibodies (data not shown). It was, however, difficult to separate nonspecific serum coating of the sinusoidal cells from specific binding, and necrotic hepatocytes stained nonspecifically for immunoglobulins. Occasional crossmatch-positive patients demonstrated a more intense uninterrupted linear sinusoidal staining for immunoglobulins, but the pattern of immune staining could not be used to blindly identify patients with positive crossmatch. Regardless of the crossmatch results, intrasinusoidal and perisinusoidal fibrinogen deposition was often detected in the areas of hepatocyte necrosis and inflammation, particularly in the periportal regions and near the hepatic veins. The only observed distinction on immunohistochemical staining between patients with a positive crossmatch vs. those without a positive crossmatch was a tendency for increased nuclear and cytoplasmic size, positive staining for lysozyme of the Kupffer cells and increased numbers of macrophages in biopsy specimens from patients with a positive crossmatch after reperfusion. Staining patterns in the nonprimary grafts were similar to those described in the grafts with positive crossmatch.
Ultrastructural Findings
Most biopsy specimens from patients before transplantation whose biopsy specimen after reperfusion significantly deteriorated when compared with the specimen before transplantation demonstrated focal abnormalities of sinusoidal lining cells. These changes included endothelial cell vacuolization and a partial or complete detachment of individual cells, resulting in denudation with loss of the space of Disse (Fig. 3). The sinusoids contained cellular debris, presumably fragments of hepatocytes, detached endothelial cells and occasional inflammatory cells. The hepatocellular changes detected in the samples before transplantation were relatively mild and included cytoplasmic fat vacuolization, a decrease in the mitochondrial matrix, formation of hepatocellular cytoplasmic blebs protruding into the sinusoids and occasional loss of hepatocyte microvilli on the sinusoidal surface. Bile canalicular microvilli were generally intact. Glycogen was usually detectable and the rough endoplasmic reticulum was generally intact with only mild swelling (Fig. 4). On the other hand, sinusoidal lining cells were intact in four of seven biopsy specimens before transplantation (Fig. 4) in which there was no significant histological deterioration between the biopsy specimens when inspected using light microscopy before transplantation and after reperfusion.
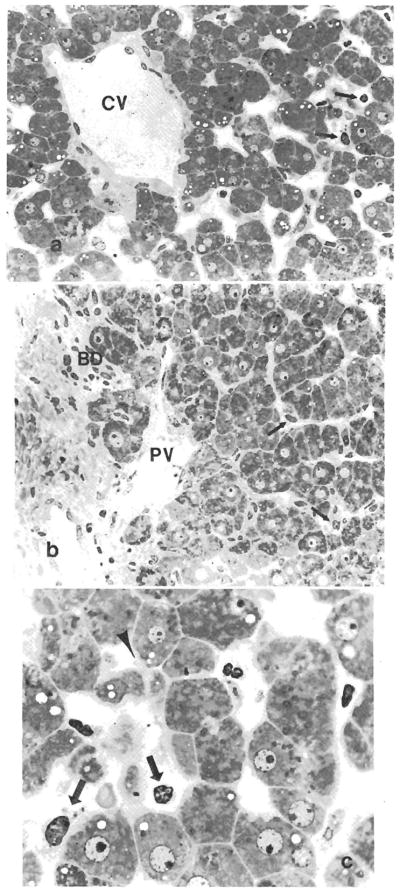
Fig. 3.
Plastic-embedded section of a biopsy specimen before transplantation obtained from a graft that exhibited histological evidence of damage after reperfusion. (a) The centrizonal sinusoidal lining cells demonstrate a rounded configuration instead of the slender elongated appearance (arrows) and focal denudation. The central vein endothelium is intact. CV = central vein. (Toluidine blue 0, original magnification × 250.) (b) Periportal sinusoidal lining cells (arrows) are less severely damaged in the same patient and the portal vein endothelium is intact. PV = portal vein; BD = bile duct. (Toluidine blue 0, original magnification × 250.) (c) Higher magnification of the centrizonal sinusoids demonstrates the endothelial cell damage (arrows) and hepatocellular blebs (arrow head) (Toluidine blue 0, original magnification × 1,000.)
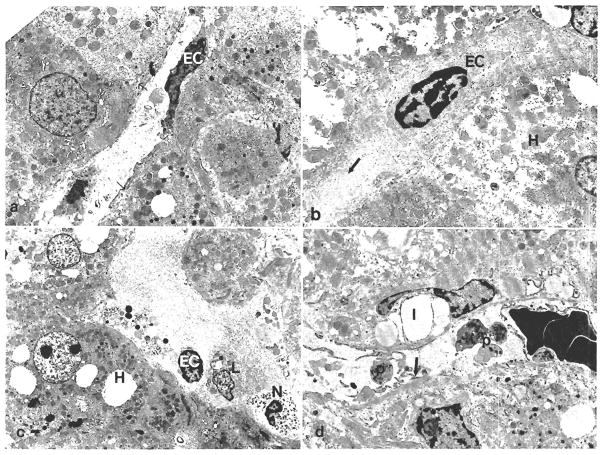
Fig. 4.
Electron microscopic findings in biopsy specimens before transplantation. (a) Demonstrates an area of well-maintained sinusoidal endothelial cells. EC = endothelial cell, arrows = space of Disse. (Original magnification × 4,700.) (b) Partial retraction of endothelial cells (EC) from the underlying tissue. H = hepatocytes, arrow = bleb. (Original magnification × 4,700.) (c) Inflammatory cells can be seen directly adherent to the hepatocytes (H) where the sinusoidal lining cells are denuded. Hepatocytes show cytoplasmic fat droplets, the other organelles are fairly well maintained. L = lymphocytes, N = neutrophil, EC = endothelial cell. (d) Sinusoidal platelets (P), cellular debris and fibrin deposition (arrow) were more easily detected in postreperfusion biopsy specimens from patients with a positive cross match. I = Ito cell. (Original magnification × 4,700.)
In specimens taken after reperfusion, both groups showed increased sinusoidal cellular debris, focal sinusoidal endothelial cell denudation and occasional active appearing Kupffer cells that contained cytoplasmic vacuoles and electron-dense material. Inflammatory cells were often clustered in areas of microarchitectural distortion and sinusoidal lining cell denudation. They were also seen near Kupffer cells and directly adherent to hepatocytes or amidst cellular debris.
Hepatocyte alterations were similar to those seen in the specimens before transplantation and in most cases were relatively mild. The changes included an increase in lipid vacuolization, detachment of cytoplasmic blebs and, in some areas, formation of electron-dense material in the cytoplasm. The mitochondria in some cases showed mild swelling, and the rough endoplasmic reticulum showed focal mild fusiform dilatation when compared with samples taken before transplantation. Sinusoidal platelets and fibrin (Fig. 4d) were more easily seen in two biopsy specimens taken after reperfusion from patients with a strongly positive T-warm crossmatch than in the cases with a negative crossmatch, but no definitive electron-dense material suggestive of immune deposits was seen.
Correlation of Donor Variables and Histological Findings with the Clinical Course after Transplantation
Neither the donor variables examined nor the recipient status code demonstrated a significant correlation with either the histological findings before or after reperfusion biopsy specimens or the clinical course after transplantation, other than a much higher incidence of graft failure or death in patients who had received a nonprimary graft (Tables 1 and 2). During the 1 mo follow-up period after transplantation, six of 59 patients who experienced a good early clinical course died or experienced graft failure and required retransplantation (four deaths, two retransplants), and six of 28 patients who experienced a poor early clinical course died or required retransplantation (three deaths, three retransplants). However, all the patients whose graft failed or who died because of graft dysfunction experienced a poor early clinical course.
Table 1.
Data of patients experiencing a good early clinical course
| Patient no. | Graft no. | Type of solution | Ischemic time (hr:min) | Warm T-cell crossmatch | Patient/graft statusa | Cause of death/fail | Pre-OLT endothelial cell damage |
|---|---|---|---|---|---|---|---|
| Post-OLT histological diagnosis: good | |||||||
| 1b | 2 | COL | NA | NA | Died (8) | Sep Before OLT | None |
| 2 | 1 | COL | 08:58 | Wk Pos | Func | NA | |
| 3 | 1 | COL | NA | Negative | Func | NA | |
| 4 | 1 | COL | 05:04 | Wk Pos | Func | NA | |
| 5 | 1 | COL | 05:22 | Negative | Func | NA | |
| 6b | 1 | COL | 04:48 | Negative | Func | None | |
| 7 | 1 | COL | NA | NA | Func | NA | |
| 8 | 1 | COL | NA | NA | Func | NA | |
| 9 | 1 | COL | 06:10 | NA | Func | NA | |
| 10 | 1 | COL | 06:33 | St Pos | Func | NA | |
| 11b | 1 | COL | 08:24 | Negative | Func | Mild | |
| 12 | 1 | COL | 05:56 | St Pos | Func | NA | |
| 13b | 1 | COL | 04:35 | Db Pos | Func | None | |
| 14 | 1 | COL | 07:53 | Db Pos | Func | NA | |
| 15 | 1 | COL | 05:26 | Db Pos | Func | NA | |
| 16 | 1 | COL | 07:20 | Negative | Func | NA | |
| 17 | 1 | COL | 07:43 | Wk Pos | Func | NA | |
| 18 | 1 | COL | 08:21 | Negative | Func | NA | |
| 19 | 1 | COL | 04:10 | Negative | Func | NA | |
| 20 | 1 | COL | 07:34 | Db Pos | Func | NA | |
| 21 | 1 | COL | 06:07 | Wk Pos | Func | NA | |
| 22 | 1 | COL | 04:30 | Negative | Func | NA | |
| 23 | 2 | COL | NA | Positive | Func | NA | |
| 24 | 1 | COL | 05:26 | Wk Pos | Func | NA | |
| 25 | 1 | COL | 05:16 | Db Pos | Func | NA | |
| 26 | 3 | COL | 06:46 | Negative | Died (16) | Sep and MI | NA |
| 27 | 2 | UW | 06:58 | Db Pos | Func | NA | |
| 28b | 1 | UW | 05:44 | Negative | Func | None | |
| 29 | 1 | UW | 05:12 | Db Pos | Func | NA | |
| 30 | 1 | UW | 06:22 | Positive | Func | NA | |
| 31 | 1 | UW | 09:10 | St Pos | Func | NA | |
| 32 | 1 | UW | 05:00 | Db Pos | Func | NA | |
| 33 | 1 | UW | 05:56 | Db Pos | Func | NA | |
| 34 | 1 | UW | 05:06 | Negative | Func | NA | |
| 35 | 1 | UW | 21:30 | NA | Func | NA | |
| 36 | 1 | UW | 07:35 | NA | Died (30) | Sep | NA |
| 37 | 1 | UW | 18:30 | Negative | Func | NA | |
| 38 | 1 | UW | 15:21 | Db Pos | Func | NA | |
| 39 | 1 | UW | 07:14 | Negative | Func | NA | |
| 40 | 3 | UW | 15:25 | Wk Pos | Died (5) | ARDS and Sep | NA |
| 41 | 1 | UW | 05:19 | Negative | Func | NA | |
| 42 | 1 | UW | 10:53 | St Pos | Func | NA | |
| 43 | 2 | UW | 09:23 | Negative | Func | NA | |
| 44 | 1 | UW | 08:30 | NA | Func | NA | |
| 45 | 1 | UW | 12:22 | Negative | Func | NA | |
| 46 | 1 | UW | 18:29 | Negative | R-OLT (4) | Hilar necrosis | NA |
| 47 | 1 | UW | 08:42 | NA | Func | NA | |
| Post-OLT histological diagnosis: poor | |||||||
| 48 | 1 | COL | NA | Positive | Func | NA | |
| 49 | 1 | COL | 05:05 | Negative | Func | NA | |
| 50 | 1 | COL | 04:43 | Db Pos | Func | NA | |
| 51 | 1 | COL | 06:02 | Negative | Func | NA | |
| 52 | 1 | COL | NA | Negative | Func | NA | |
| 53 | 1 | COL | 08:23 | Negative | Func | NA | |
| 54 | 1 | UW | 08:40 | Positive | Func | NA | |
| 55 | 1 | UW | 16:45 | Negative | Func | NA | |
| 56b | 1 | UW | 10:00 | Negative | Func | Mild | |
| 57 | 1 | UW | 03:16 | Negative | R-OLT (16) | ACR | NA |
| 58 | 1 | UW | 04:07 | Negative | Func | NA | |
| 59 | 1 | UW | 10:20 | St Pos | Func | NA | |
OLT = transplant; COL = Eurocollins’ solution; UW = University of Wisconsin solution; Pos = positive; Wk = Weak; Db = doubtful; St = strong; Func = functioning; R-OLT = retransplant; Sep = sepsis; ARDS = adult respiratory distress syndrome; MI = myocardial infarction; ACR = acute cellular rejection; NA = not available.
Patient/graft status at 1 mo (with survival in days).
Ultrastructural examination performed in these grafts.
Table 2.
Data of patients experiencing a poor early clinical course
| Patient no. | Graft no. | Type of solution | Ischemic time (hr:min) | Warm T-cell crossmatch | Patient/graft statusa | Cause of death/fail | Pre-OLT endothelial cell damage |
|---|---|---|---|---|---|---|---|
| Post-OLT histological diagnosis: good | |||||||
| 60 | 1 | COL | 05:25 | Negative | Func | NA | |
| 61 | 1 | COL | 05:34 | Db Pos | Func | NA | |
| 62b | 1 | COL | 05:07 | Db Pos | Func | Moderate | |
| 63 | 1 | COL | 08:16 | Negative | Func | NA | |
| 64b | 1 | COL | 04:35 | Negative | Func | Mild | |
| 65 | 1 | COL | 05:36 | St Pos | Func | NA | |
| 66 | 1 | COL | 05:55 | Negative | Func | NA | |
| 67 | 1 | COL | 05:54 | Negative | Func | NA | |
| 68 | 1 | COL | 07:28 | Db Pos | Func | NA | |
| 69 | 1 | UW | 18:36 | Db Pos | Func | NA | |
| 70 | 2 | UW | 07:00 | Negative | Func | NA | |
| 71 | 1 | UW | 16:21 | Pos | Func | NA | |
| 72 | 2 | UW | 06:20 | Negative | Died (24) | Sep | NA |
| Post-OLT histological diagnosis: poor | |||||||
| 73 | 1 | COL | 05:58 | St Pos | Func | NA | |
| 74 | 1 | COL | NA | Negative | R-OLT (13) | Dys:Hypo | NA |
| 75b | 1 | COL | 06:20 | Negative | Func | Mild | |
| 76b | 1 | COL | 05:59 | Negative | Func | Mild | |
| 77b | 1 | COL | NA | Db Pos | Func | Mild | |
| 78 | 1 | COL | NA | St Pos | Func | NA | |
| 79 | 2 | COL | NA | NA | Died (8) | Dys:HAT | NA |
| 80b | 1 | COL | 05:58 | Wk Pos | Func | Mild | |
| 81b | 1 | COL | NA | St Pos | Func | Moderate | |
| 82 | 2 | COL | 07:47 | NA | Died (2) | Dys:Hypo | NA |
| 83 | 1 | UW | 06:48 | Negative | Func | NA | |
| 84 | 1 | UW | 04:26 | Negative | Func | NA | |
| 85b | 1 | UW | 08:29 | St Pos | R-OLT (3) | PNF | Moderate |
| 86b | 1 | UW | 11:19 | Negative | R-OLT (3) | Dys:Hypo | Mild |
| 87b | 1 | UW | 14:56 | St Pos | Func | Moderate | |
OLT = transplant; COL = Eurocollins’ solution; UW = University of Wisconsin solution; Pos = positive; Wk = Weak; Db = doubtful; St = strong; R-OLT = retransplant; Func = functioning; Sep = sepsis; Dys = graft dysfunction; Hypo = hypotension; HAT = hepatic artery thrombosis; PNF = primary graft nonfunction; NA = not available.
Patient/graft status at one month (with survival in days).
Ultrastructural examination performed in these grafts.
No finding in the biopsy specimen before transplantation was able to predict organ function after transplantation. Fifteen of the 27 patients with “poor” histological findings (i.e., moderate or severe inflammation and necrosis or both) experienced a poor clinical course, whereas the other 12 had a good course. By contrast, only 13 of the 60 patients who had “good” histological findings after transplantation experienced a poor clinical course. The combination of zonal necrosis and severe inflammation in the biopsy specimen after reperfusion, however, presaged a poor clinical course in five of seven patients who demonstrated these findings; two died within a week after transplantation. Neither the severity nor location of microvesicular steatosis, cytoaggregation or hepatocyte swelling was associated with the clinical course.
Clinicopathological Analysis of Graft Failure or Patient Death
Sepsis was the cause of death in all four patients (patients 1, 26, 36 and 40) who demonstrated a good early clinical course and good histological findings after reperfusion. Graft function was relatively intact near or at the time of death. Two patients in this same group had to be given another liver allograft within the 30-day follow-up period (patients 46 and 57). Patient 46 required a new liver because of necrosis of the hilum. The cause was uncertain, but it was likely related to technical problems with the operation. No vascular thrombosis was found. Graft failure in patient 57 was due to uncontrollable acute cellular rejection. Patient 72 experienced a poor early clinical course and died of sepsis 24 days later.
The cause of graft failure or death or both was more difficult to determine in those patients who experienced a poor early clinical course and had severe necrosis or inflammation or both in the biopsy specimen after reperfusion. Patient 79 received a secondary graft and the biopsy specimen after reperfusion showed midzonal hepatocellular necrosis associated with severe neutrophilic inflammation. The liver did not produce bile after transplantation, and the patient died 8 days after transplantation. On postmortem examination, the liver demonstrated massive coagulative necrosis and thrombosis of the right branch of the hepatic artery. No immunoglobulin or complement deposition was seen in either the biopsy specimen after reperfusion or the autopsy liver specimen. Three other patients (patients 74, 82, 86) were hypotensive in the operating room and this may have inflicted ischemic injury on the graft. Two of these grafts (patients 74, 86) were available for pathological evaluation; the first (patient 74) demonstrated widespread centrilobular necrosis, the other (patient 86) revealed massive coagulative necrosis. No significant immunoglobulin, complement or fibrinogen deposits were detected in the specimens from either patient. The other patient (patient 82) suffered a myocardial infarction in the operating room and died several days later; autopsy permission was not obtained. Ultrastructural examination of the biopsy sample from patient 86 before transplantation revealed focal mild sinusoidal endothelial cell damage. Hypotension or other probably nonimmunological insults or both occurring during or shortly after the operation were thought to be the underlying cause of graft failure in these four patients.
Only one patient (patient 85) demonstrated no apparent clinical reason for graft failure, although the transplant operation was described as extremely difficult because of the extensive peritoneal adhesions caused by a previous Leveen shunt operation. Ultrastructural examination of the biopsy specimen before transplantation demonstrated a more severe form of sinusoidal endothelial cell injury. The lymphocytotoxic crossmatch was strongly positive. The biopsy specimen exhibited sinusoidal immune deposits and severe inflammation with necrosis after reperfusion. The failed graft revealed widespread periportal zonal hepatocellular necrosis. Immunohistochemical stains of the failed allograft demonstrated no significant deposition of immunoglobulins or complement.
Follow-up Biopsies
Follow-up biopsies were performed in 58 of the patients included in this study but were not done according to protocol. Seven of 15 patients who had moderate or severe focal or zonal necrosis in their biopsy specimens after reperfusion demonstrated histological findings in follow-up biopsies that have been attributed to ischemic preservation injury (17). By contrast, only eight of 43 patients with little or no necrosis or inflammation in the biopsy specimen after reperfusion demonstrated similar changes in follow-up biopsy samples. Portal fibrosis was detected in late follow-up biopsy specimens taken more than 2 mo after transplantation in patients from both groups (i.e., good and poor histological findings) and no strict correlation was seen with the samples after reperfusion.
DISCUSSION
We were unable to predict organ function after transplantation by light microscopic examination of immersion-fixed, paraffin-embedded and hematoxylin and eosin stained biopsy specimens before transplantation. However, ultrastructural analysis revealed that the sinusoidal microvasculature was more sensitive to organ procurement and cold preservation than the endothelium of larger vessels or hepatocytes, which demonstrated ultrastructural changes associated with reversible injury (18–21). Obvious differences between sinusoidal and other endothelial cells are the lack of a conventional basement membrane, proximity of the Kupffer cells and the functional specialization of the sinusoidal endothelium (22, 23).
The sinusoidal lining cell damage incurred during cold preservation probably contributed to fibrinogen deposition and neutrophil accumulation in the areas of damage in biopsy specimens after reperfusion. Subsequent microvascular thrombosis and enzyme release may therefore be partially responsible for the mechancial disruption of the microcirculation after reperfusion and prevent adequate restitution of the blood supply. Although the histological appearance of the biopsy specimen after reperfusion had some prognostic significance, many patients did well even when there was evidence of severe histological damage. This was not surprising, considering the focality of necrosis in many liver allografts (24), a factor that introduces sampling problems. By contrast, the histological findings showed minimal alterations in some patients who experienced a poor early clinical course. In this circumstance, biopsies after reperfusion may be performed too soon after revascularization to detect morphological changes of irreversible ischemic injury. Despite the evidence in this study that sinusoidal cell injury was associated with cold preservation and has been used to predict function in animal studies after transplantation (19), events occurring during or shortly after implantation of the liver appeared to cause an equal or greater degree of morbidity and mortality in this group of patients.
Most animal models evaluating preservation injury allow a precisely controlled analysis (19, 20, 25–27) but ignore the contribution of the arterial blood flow and the metabolic derangements caused by a poorly functioning native liver. In humans, arterial flow plays a more vital role. The time sequence between reperfusion of the venous and arterial systems may vary considerably, particularly when difficulties are encountered with the arterial anastomosis or when an artery graft must be placed. During this time, the liver is reperfused and warmed by relatively hypoxic portal blood or the arterial supply is disrupted after having been initially intact. Either sequence of events has the potential to cause damage because the newly placed organ is devoid of arterial collaterals.
Consistent with previous studies (4–6), preformed lymphocytotoxic antibodies did not appear to directly damage the liver allografts after reperfusion and no correlation with the early clinical course was apparent. Taken as a group, the only apparent distinction was a tendency toward an increase in the number of macrophages and the amount and intensity of lysozyme-positive cytoplasm of the Kupffer cells in biopsy specimens from crossmatch-positive patients after reper-fusion compared with those without preformed antibodies. However, from our preliminary studies using an antibody described by Pulford et al. (28) (KP1 obtained from Dr. D.Y. Mason, Oxford) that is used to detect Kupffer cells, it is evident that lysozyme stains only a fraction of the Kupffer cells present, and those may be in a state of relative activation.
The donor liver secretes soluble class I major histo-compatibility complex antigens into the recipient circulation (29) that could bind the lymphocytotoxic antibodies and explain their disappearance from the serum (7, 30). The immune complexes thus formed could theoretically be removed by the Kupffer cells. Whether such apparent “protective” mechanisms do occur and whether they could be overridden in humans as is seen in highly sensitized animals (31, 32) is presently unknown. Gugenheim et al. (33, 34) found evidence for the above hypothesis by demonstrating “nontoxic” binding of immune components to the sinusoids of livers in an ex vivo perfusion model of sensitized rats. Although the immune deposits were most intense in livers from the sensitizing strain, they noted nonspecific deposition, as we did, in third-party livers as well.
The role of Kupffer cells in preservation injury has also been largely overlooked. If morphology can be equated with the functional status, the activity of the Kupffer cells varies considerably among donors. Because they are capable of clearing immune complexes, platelet aggregates, fibrin, endotoxin and metabolic products from the circulation (35, 36), Kupffer cells could help the donor liver adjust to its new environment. After allograft implantation, various host factors, including the immune system and endotoxin (37, 38), can stimulate the Kupffer cells. Once activated, these cells can secrete potent biological mediators such as tumor necrosis factor, interleukin-1, procoagulant activity, oxidative enzymes and monokines that suppress hepatocyte protein synthesis (35, 39). Therefore the Kupffer cells apparently have the ability to “protect” the transplanted liver in some instances and inhibit recovery of hepatic function or even contribute to the damage in others.
Consistent with previous studies (15, 40), the variable examined, other than nonprimary grafts, demonstrated little or no correlation to either the histological changes in biopsy specimens or the early clinical course. We believe that attention should be focused on donor factors and particularly on recipient factors that were not easily addressed in this study and not commonly implicated in early graft failure. These include the interval between reconstitution of the portal and arterial flows and as yet undefined “recipient” factors such as an endotoxemia (37, 38).
Acknowledgments
We thank Ms. Burnham, Ms. Coles and Ms. Bisceglia for performing the immunohistochemical stains; Mr. Graner and Ms. Ziesmer for preparing electron microscopy and Ms. Mient for typing this manuscript.
References
- 1.Todo S, Nery J, Yanaga K, Podesta L, Gordon RD, Starzl TE. Extended preservation of human liver grafts with UW solution. JAMA. 1989;261:711–714. [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw BW, Wood RP. Improved results with retransplantation of the liver. Transplant Proc. 1989;21:2407–2488. [PubMed] [Google Scholar]
- 3.Paulsen AW, Brajtbord D, Klintmalm GB, Ramsay MA, Swygert TH, Valek TR. Intraoperative measurements related to subsequent hepatic graft failure. Transplant Proc. 1989;21:2337–2338. [PubMed] [Google Scholar]
- 4.Iwatsuki S, Rabin BS, Shaw BW, Starzl TE. Liver transplantation against T cell positive warm crossmatches. Transplant Proc. 1984;16:1427–1429. [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon RD, Fung JJ, Markus B, Fox I, Iwatsuki S, Esquivel CO, Tzakis A, et al. The antibody crossmatch in liver transplantation. Surgery. 1986;100:705–715. [PMC free article] [PubMed] [Google Scholar]
- 6.Moore SB, Wiesner RH, Perkind JD, Nagorney DM, Sterioff S, Krom RAF. A positive lymphocyte crossmatch and major histocompatibility complex matching do not predict early rejection of liver transplants in patients treated with cyclosporine. Transplant Proc. 1987;19:2390–91. [PubMed] [Google Scholar]
- 7.Fung J, Griffin M, Duquesnoy R, Shaw BW, Starzl TE. Successful sequential liver-kidney transplantation in a patient with preformed lymphocytotoxic antibodies. Transplant Proc. 1987;19:767–768. [PMC free article] [PubMed] [Google Scholar]
- 8.Todo S, Demetris AJ, Makowka L, Teperman L, Podesta L, Shaver T, Tzakis AG, et al. Primary non-function of hepatic homografts with pre-existing fatty infiltration. Transplantation. 1989;47:903–905. doi: 10.1097/00007890-198905000-00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demetris AJ, Jaffe R, Tzakis AG, Ramsey G, Todo S, Belle S, Esquivel CO, et al. Antibody mediated rejection of human orthotopic liver allografts: a study of liver transplantation across ABO blood group barriers. Am J Pathol. 1988;132:489–502. [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Miller C, Broznick B, Makowka L. An improved technique for multiple organ harvesting. Surg Gynecol Obstet. 1987;165:343–348. [PMC free article] [PubMed] [Google Scholar]
- 11.Yanaga K, Makowka L. A simple and safe technique for pretransplant biopsy of the liver allograft. J Clin Transplant. (In press) [Google Scholar]
- 12.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673–676. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Sinclair RA, Burns J, Dunnill MS. Immunoperoxidase staining of formalin-fixed, paraffin-embedded human renal biopsies with a comparison of the peroxidase anti-peroxidase (PAP) and indirect methods. J Clin Pathol. 1981;34:859–865. doi: 10.1136/jcp.34.8.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase (ABC) complex in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedure. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 15.Makowka L, Gordon RD, Todo S, Ohkohchi N, Marsh JW, Tzakis AG, Yokoi H, et al. Analysis of donor criteria for the prediction of outcome in clinical liver transplantation. Transplant Proc. 1987;19:2378–2382. [PMC free article] [PubMed] [Google Scholar]
- 16.Christoffersen P, Poulsen H, Skeie E. Focal liver cell necrosis accompanied by infiltration of granulocytes arising during operation. Acta Hepato Splenologica. 1968;17:240–245. [PubMed] [Google Scholar]
- 17.Demetris AJ, Jaffe R, Starzl TE. A review of adult and pediatric post-transplant liver pathology. In: Rosen PP, Fechner RE, editors. Pathology Annual. Part 2. Norwalk: Appleton & Lange; 1987. pp. 347–386. [PubMed] [Google Scholar]
- 18.Bassi M, Bernelli-Zazzera A. Ultrastructural cytoplasmic changes of liver cells after reversible and irreversible ischemia. Exp Mol Pathol. 1964;3:332–350. doi: 10.1016/0014-4800(64)90006-1. [DOI] [PubMed] [Google Scholar]
- 19.McKeown CMB, Edwards V, Phillips MJ, Harvey PRC, Petrunka CN, Strasberg SM. Sinusoidal lining cell damage: the critical injury in cold preservation of liver allografts in the rat. Transplantation. 1988;46:178–191. [PubMed] [Google Scholar]
- 20.Otto G, Wolff H, David H. Preservation damage in liver transplantation: electron-microscopic findings. Transplant Proc. 1984;14:1247–1248. [PubMed] [Google Scholar]
- 21.Bassi M, Bernelli-Zazzera A, Cassi E. Electron microscopy of rat liver cells in hypoxia. J Pathol Bacteriol. 1960;79:179–183. doi: 10.1002/path.1700790124. [DOI] [PubMed] [Google Scholar]
- 22.Rappaport AM. Physioanatomic considerations. In: Schiff L, Schiff ER, editors. Diseases of the liver. 6. Philadelphia: J.B. Lippincott Co; 1987. pp. 1–46. [Google Scholar]
- 23.Fraser R, Day WA, Fernando NS. The liver sinusoidal cells: their role in disorders of the liver, lipoprotein metabolism and atherogenesis. Pathology. 1986;18:5–11. doi: 10.3109/00313028609090821. [DOI] [PubMed] [Google Scholar]
- 24.Russo RA, Yunis EJ. Subcapsular necrosis in orthotopic liver allografts. Hepatology. 1986;6:708–713. doi: 10.1002/hep.1840060428. [DOI] [PubMed] [Google Scholar]
- 25.Marotto ME, Thurman RG, Lemasters JJ. Early midzonal cell death during low-flow hypoxia in the isolated perfused rat liver: protection by allopurinol. Hepatology. 1988;8:585–590. doi: 10.1002/hep.1840080325. [DOI] [PubMed] [Google Scholar]
- 26.Matzger J, Dore SP, Lauterburg BH. Oxidant stress during reperfusion of ischemic liver: no evidence for a role of xanthine oxidase. Hepatology. 1988;8:580–584. doi: 10.1002/hep.1840080324. [DOI] [PubMed] [Google Scholar]
- 27.Lemasters JJ, Stemkowski CJ, Ji S, Thurman RG. Cell surface changes and enzyme release during hypoxia and reoxygenation in the isolated perfused rat liver. J Cell Biol. 1983;97:778–786. doi: 10.1083/jcb.97.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pulford KAF, Rigney EM, Micklem KJ, Jones M, Stross WP, Gatter KC, Mason DY. KP1: a new monoclonal antibody that detects a monocyte/macrophage associated antigen in routinely processed tissue sections. J Clin Pathol. 1989;42:414–421. doi: 10.1136/jcp.42.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies HFFS, Pollard SG, Calne RY. Soluble HLA antigens in the circulation of liver graft recipients. Transplantation. 1989;47:524–527. doi: 10.1097/00007890-198903000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Houssin D, Bellon B, Brunaud MD, Gugenheim J, Settaf A, Meriggi F, Emond J. Interactions between liver allografts and lymphocytotoxic alloantibodies in inbred rats. Hepatology. 1986;6:994–998. doi: 10.1002/hep.1840060531. [DOI] [PubMed] [Google Scholar]
- 31.Gubernatis G, Lauchart W, Jonker M, Steinhoff G, Bornscheuer A, Neuhaus P, Van Es AA, et al. Signs of hyperacute rejection of liver grafts in rhesus monkeys after donor-specific presensitization. Transplant Proc. 1987;19:1082–1083. [PubMed] [Google Scholar]
- 32.Knechtle SJ, Kolbeck PC, Tsuchimoto S, Coundouriotis A, Sanfilippo F, Bollinger RR. Humoral rejection of rat hepatic transplants by passive transfer of serum. Transplant Proc. 1987;19:1072–1076. [PubMed] [Google Scholar]
- 33.Gugenheim J, Houssin D, Emond J, Gigou M, Crougneau ST, Bismuth H. Delayed rejection of heart allografts in hypersensitized rat by extracorporeal donor-specific liver hemoperfusion. Transplantation. 1986;41:398–404. doi: 10.1097/00007890-198603000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Gugenheim J, Le Thai B, Rouger P, Gigou M, Gane P, Vial MC, Charpentier B, et al. Relationship between the liver and lymphocytotoxic alloantibodies in inbred rats: specific absorption by nonparenchymal liver cells. Transplantation. 1988;45:474–478. doi: 10.1097/00007890-198802000-00046. [DOI] [PubMed] [Google Scholar]
- 35.Wardle EN. Kupffer cells and their function. Liver. 1987;7:63–75. doi: 10.1111/j.1600-0676.1987.tb00319.x. [DOI] [PubMed] [Google Scholar]
- 36.Richards PS, Saba TM. Effect of endotoxin on fibronectin and Kupffer cell activity. Hepatology. 1985;5:32–37. doi: 10.1002/hep.1840050108. [DOI] [PubMed] [Google Scholar]
- 37.Miyata T, Imventarza O, Ueda Y, Furukawa H, Todo S, Starzl TE. Endogenous endotoxemia during orthotopic liver transplantation in dogs. Transplant Proc. 1989;21:3861–3862. [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoyama I, Todo S, Miyata T, Selby R, Tzakis AJ, Starzl TE. Endotoxemia and human liver transplantation. Transplant Proc. 1989;21:3833–3841. [PMC free article] [PubMed] [Google Scholar]
- 39.Keller GA, West MA, Cerra FB, Simmons RL. Modulation of hepatocyte protein synthesis by endotoxin activated Kupffer cells. Ann Surg. 1985;201:87–95. [PMC free article] [PubMed] [Google Scholar]
- 40.vanWoerden WF, Prium J, Knol E, Klompmaker IJ, deBruijn KM, Persijn GG, Sloof MJH. Donor data of liver grafts with primary non-function (PNF): a preliminary analysis on behalf of the European Liver Registry (ELR) Transplant Proc. 1989;21:2383–2384. [PubMed] [Google Scholar]