Abstract
O-Linked attachment of β-N-acetyl-glucosamine (O-GlcNAc) on serine and threonine residues of nuclear and cytoplasmic proteins is a highly dynamic posttranslational modification that plays a key role in signal transduction pathways. Preliminary data show that O-GlcNAcylation may represent a key regulatory mechanism in the vasculature, modulating contractile and relaxant responses. Proteins with an important role in vascular function, such as endothelial nitric oxide synthase, sarcoplasmic reticulum Ca2+-ATPase, protein kinase C, mitogen-activated protein kinases, and proteins involved in cytoskeleton regulation and microtubule assembly are targets for O-GlcNAcylation, indicating that this posttranslational modification may play an important role in vascular reactivity. Here, we will focus on a few specific pathways that contribute to vascular function and cardiovascular disease–associated vascular dysfunction, and the implications of their modification by O-GlcNAc. New chemical tools have been developed to detect and study O-GlcNAcylation, including inhibitors of O-GlcNAc enzymes, chemoenzymatic tagging methods, and quantitative proteomics strategies; these will also be briefly addressed. An exciting challenge in the future will be to better understand the cellular dynamics of this posttranslational modification, as well as the signaling pathways and mechanisms by which O-GlcNAc is regulated on specific proteins in the vasculature in health and disease.
Keywords: O-Linked β-N-acetylglucosaminylation (O-GlcNAc), vascular (dys)function, protein kinases
Introduction
Signaling Pathways: Focus on O-GlcNAcylation
Various neurotransmitters (norepinephrine, dopamine), hormones (angiotensin II, vasopressin), bloodborne factors (serotonin, histamine), and endothelium-derived products (endothelin-1, prostanoids) bind to specific receptors in vascular smooth muscle cells leading to changes in their function via activation of signal transduction pathways.
Signal transduction refers to any process by which a cell converts one kind of signal or stimulus into another. The number of proteins and other molecules participating in the events involving signal transduction increases as the process emanates from the initial stimulus, which is referred to as amplification of the signal. Therefore an initial stimulus can trigger the activation of any number of complex physiological events, including changes in vascular tone.1,2
Most intracellular proteins activated by an agonist-receptor interaction possess enzymatic activity. These enzymes include heterotrimeric G proteins, small GTPases, various serine/threonine protein kinases, phosphatases, tyrosine kinases, and mitogen-activated protein kinases (MAPKs), which ultimately will modify intracellular levels of calcium (Ca2+) and smooth muscle tone.3,4 Therefore, protein phosphorylation-dephosphorylation via kinases and phosphatases, respectively, serves as a molecular switch for turning on and off cellular systems and events in the vasculature, such as contraction.5
As we will further address, O-GlcNAcylation, or the O-linked attachment of β-N-acetylglucosamine (O-GlcNAc) to serine and threonine residues in cytoplasm and nuclear proteins, can also modify the activity of enzymes that participate in signal transduction events. Both O-GlcNAc and O-phosphate, in the O-GlcNAcylation and phosphorylation processes, respectively, are dynamically added and removed from proteins in response to cellular signals, and both alter the functions and associations of the modified protein.6–8 In addition, many phosphorylation sites are also known glycosylation sites, and this reciprocal occupancy may produce different activities or alter stability in the protein.6–9 Numerous proteins, including kinases, phosphatases, transcription factors, metabolic enzymes, and cytoskeletal proteins have been identified as targets of O-GlcNAcylation.10–14 Considering that proteins with an important role in vascular function are also targets for O-GlcNAcylation, in this review we will focus on the effects of O-GlcNAcylation in vascular function. We will briefly discuss the hexosamine biosynthetic pathway, the enzymes involved in O-GlcNAcylation, and present evidence that this posttranslational modification modulates the activity of key enzymes involved in functional and structural processes in the vasculature. In addition, a potential role for O-GlcNAcylation in vascular dysfunction associated with cardiovascular diseases will be discussed, along with the available tools to study O-GlcNAcylation in the vasculature.
O-GlcNAcylation
Glycosylation, which is the site-specific enzymatic addition of saccharides to proteins and lipids, is one of the most complex posttranslational modifications. There are many types of glycosylation, but great interest has been directed to O-GlcNAcylation, or glycosylation with O-linked β-N-acetylglucosamine or beta-O-linked 2-acetamido-2-deoxy-d-glycopyranose (O-GlcNAc).6,15,16 In this unusual form of protein glycosylation, a single-sugar (β-N-acetylglucosamine) is added to serine and threonine residues of nuclear or cytoplasmic proteins.6,15,16 The cycling of O-GlcNAc on serine or threonine residues of target proteins is controlled by two highly conserved enzymes, O-GlcNAc transferase (OGT or uridine diphospho-N-acetyl glucosamine: polypeptide β-N-acetylglucosaminyl transferase; UDP-NAc transferase) and β-N-acetylglucosaminidase (O-GlcNAcase or OGA). Whereas OGT catalyses the addition of O-GlcNAc to the hydroxyl group of serine or threonine residues of a target protein, OGA catalyses the hydrolytic cleavage of O-GlcNAc from posttranslationally modified proteins.15,17,18
The OGT enzyme is a soluble protein that is found in the cytosol, nucleus, and mitochondria, rather than in the endoplasmic reticulum or Golgi.19 Three distinct isoforms of OGT have been identified, including a 110-kDa and a 78-kDa isoform, which can assemble into multimers, and smaller mitochondrial isoforms.18,20–22 Each variant contains a C-terminal catalytic domain, but differs in the number of tetratricopeptide repeats within its N-terminal domain. The tetratricopeptide repeats serve as protein-protein interaction modules that appear to target OGT to accessory proteins and potential substrates, such as the related O-GlcNAc transferase interacting protein (OIP106) and protein phosphatase-1 (PP1).23,24 The association between OGT and PP124 is particularly intriguing because it may provide a direct mechanism to couple O-GlcNAc to dephosphorylation of specific substrates.
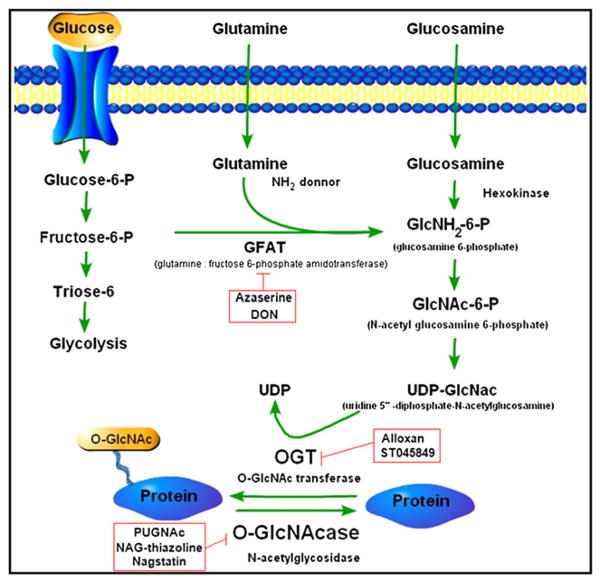
The overall catalytic activity of OGT is controlled by the concentration of its donor substrate, UDP-GlcNAc or uridine 5′-diphosphate-N-acetylglucosamine. UDP-GlcNAc is highly sensitive to flux in nutrients and energy, mainly through the hexosamine biosynthetic pathway (HBP).25,26 It is found in high concentrations, and it has been estimated that 2% to 5% of glucose is used to generate this sugar nucleotide.16,24,25,27 Increased flux through the HBP, either through increased glucose uptake or glucosamine treatment, which is distal to glutamine:fructose-6-phosphate transferase, increases the production of UDP-GlcNAc and stimulates O-GlcNAc modification of proteins (Figure 1). Glutamine:fructose-6-phosphate transferase is the rate-limiting enzyme of the pathway, converting fructose 6-phosphate to glucosamine 6-phosphate, with glutamine as the amine donor. Because OGT activity is exquisitely sensitive to UDP-GlcNAc concentrations,28 O-GlcNAcylation may act as a sensor for the general metabolic state of the cell. Consistent with this idea, O-GlcNAc has been intricately linked to cell survival29,30 induced by many forms of cell stress.31,32
Figure 1.
The hexosamine biosynthetic pathway (HBP), including the most well-known pharmacological inhibitors of this pathway.
As with OGT, OGA is also found in the nucleus and cytosol.17 OGA contains an N-terminal glycosidase domain and a putative C-terminal histone acetyltransferase domain.33 Two distinct isoforms of OGA exist, a 130-kDa and a 75-kDa variant, which share the same catalytic domain but differ in their C terminus.34 The potential histone acetyltransferase activity of OGA may provide an interesting mechanism for coupling deglycosylation of nuclear proteins to transcriptional activation. As with OGT, OGA has been shown to interact with specific proteins, including protein phosphatase-2B.17
Almost every functional class of proteins is subject to O-GlcNAcylation.15,27 However, those involved in transcription or translation, stress responses, and energy metabolism have been mainly implicated. O-GlcNAcylation can either suppress or enhance transcription, depending on the promoter involved and other associated proteins. Proteins with an important role in vascular function are also targets for O-GlcNAcylation. Examples are endothelial nitric oxide synthase (eNOS), sarcoplasmic reticulum Ca2+-AT-Pase, phospholipase C, protein kinase C (PKC), phosphoinositide-3 kinase (PI3K), and proteins involved in cytoskeleton regulation and microtubule assembly,8,15,27,35 indicating that this posttranslational modification may play an important role in vascular reactivity.
Tools to Study O-GLcNAc in the Vasculature
Over the last several years, new chemical tools have been developed to detect and study O-GlcNAcylation, including inhibitors of O-GlcNAc enzymes, chemoenzymatic tagging methods, and quantitative proteomics strategies.36–38 Two excellent and recent reviews describe in detail how these new chemical approaches have been used to meet the specific challenges associated with studying O-GlcNAcylation.39,40
Pharmacological Inhibition of OGT and OGA
The discovery of potent and selective inhibitors of OGT and OGA represent powerful tools with which to evaluate O-GlcNAcylation in isolated cells/tissues and in vivo. Furthermore, pharmacological agents to inhibit OGT and OGA complement the use of conventional genetic tools to elucidate the role of O-GlcNAc and OGT/OGA in cellular processes.29,41–44 A list of the most common pharmacological inhibitors of OGT and OGA, as well as glutamine antagonists40,44–57 can be found in Table 1.
Table 1.
Pharmacological inhibitors that interfere with the O-GlcNAcylation process
| Compound Name | Chemical Formula | Target | Advantage | Disadvantage | References |
|---|---|---|---|---|---|
| [PUGNAc] | 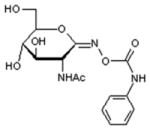 |
||||
| O-(2-acetamido-2-deoxy-D-glucopyranosylidene) amino-N-phenylcarbamate | OGA | Widely used; commercially available | Exhibits nonspecific activity; including inhibition of β-hexosaminidase | 40, 50, 51 | |
| [NAG-thiazoline] | 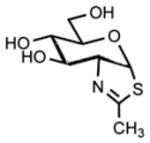 |
||||
| 1,2-dideoxy-2′-methyl-alpha-d-glucopyranoso[2,1-d]-Delta2′-thiazoline | OGA | Shows greater than 3000-fold selective inhibition of OGA over β-hexosaminidase | Exhibits nonspecific activity; including inhibition of family 20 hexosaminidases | 40, 52, 53 | |
| Nagstatin derivatives | 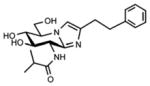 |
OGA | These inhibitors have been rationally designed using information about the enzyme active site | Compounds are not commercially available | 40, 54, 55 |
| *[STZ] | 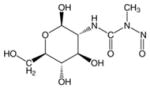 |
||||
| Streptozotocin | OGA | — | Very toxic Inhibitor due to its ability to generate reactive oxygen species | 40, 47–49 | |
| [Alloxan] | 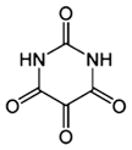 |
||||
| 2,4,5,6-tetraoxypyrimidine; 2,4,5,6-pyrimidinetetrone | OGT | Extensively used as an OGT inhibitor | Multiple nonspecific effects, including inhibition of OGA, glucokinase and formation of superoxide radicals | 45–47 | |
| [ST045849] | 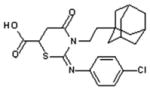 |
||||
| 3-(2-adamantanylethyl)-2-[(4-chlorophenyl)azamethylene]-4-oxo-1,3-thiazaperhyd roine-6-carboxylic acid | OGT | — | Few reports in the literature | 44 | |
| [DON] | 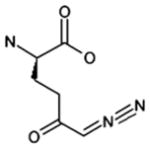 |
||||
| 6-diazo-5-oxo-L-norleucine | GFAT | Well-established glutamine antagonist | This compound inhibits various amidotransferases in a number of metabolic pathways that use glutamine as a substrate | 25, 40, 57 | |
| [Azaserine] | 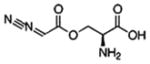 |
||||
| O-diazoacetyl-L-serine | GFAT | The effects of this glutamine antagonist appear to be specific and nontoxic based on several criteria | Like DON, it inhibits various amidotransferases in a number of metabolic pathways that use glutamine as a substrate | 25, 40, 56 |
OGT, O-GlcNAc transferase or uridine diphospho-N-acetyl glucosamine; polypeptide β-N, acetylglucosaminyl transferase; OGA, O-GlcNAcase or β-N-acetylglucosaminidase; GFAT, glutamine:fructose-6-phosphate amidotransferase.
A major challenge in the field has been the difficulty of detecting and studying O-GlcNAcylation in vivo. The lack of a well-defined consensus sequence for OGT has precluded the determination of in vivo modification sites on the basis of the primary sequence. New sensitive and selective OGT and OGA inhibitors, especially compounds that can be used in vivo, will be important tools for finely dissecting the role of each enzyme in vascular function and dysfunction.
Immunodetection of O-GlcNAc
Antibodies against O-GlcNAc (CTD110.6 and RL-2) represent helpful tool for measuring global changes in glycosylation in response to cellular stimuli.58 Accordingly, the relative ease of use represents one of the strengths of this technique, which is restricted by the lack of epitope specificity. However, a limitation of these antibodies is that they detect only a small subset of the O-GlcNAc–modified proteins.59,60 Moreover, it remains difficult to identify the specific proteins that undergo changes in glycosylation. In response to these challenges, Khidekel and coworkers have developed a method to probe the dynamics of O-GlcNAc glycosylation in vivo using quantitative isotopic and chemo-enzymatic tagging (QUIC-Tag) approaches.60 This allows identification of proteins undergoing dynamic changes in glycosylation and monitoring of glycosylation changes at specific sites within proteins. Immunoaffinity chromatography in conjunction with stable isotope labeling with amino acids in cell culture has also been used to study the dynamic interplay between O-GlcNAc and phosphorylation.61
Chemical Tagging and Identification of O-GlcNAc–modified Proteins
Methods for chemically tagging and identifying O-GlcNAc–modified proteins have vastly expanded the number of proteins targeted by O-GlcNAcylation.62 Combined with recent advances in mass spectrometry,63,64 these powerful tools have provided an unprecedented opportunity to explore the O-GlcNAc proteome, manipulate glycosylation levels and study the dynamics of this modification in vivo.
As with other posttranslational modifications, O-GlcNAc is often dynamic, substoichiometric, targeted to specific subcellular compartments, and more often observed in low-abundance regulatory proteins. The sugar (β-N-acetyl-glucosamine) is also enzymatically and chemically labile, being subject to hydrolysis by cellular glycosidases or cleavage on a mass spectrometer. Traditional methods for detecting O-GlcNAcylation include the use of wheat germ agglutinin lectin,65 pan-specific O-GlcNAc antibodies,31,66 and radiolabeling using 1,4-galactosyltransferase,67 which transfers [3H]Gal from UDP-[3H]galactose to terminal GlcNAc groups. More recently, chemical approaches have been developed to tag O-GlcNAc proteins with reporter groups such as biotin, UDP-ketogalactose substrate, N-azido-acetylglucosamine.59,68–72 This enables more rapid and sensitive detection of O-GlcNAc–modified proteins and, in specific situations, represents a powerful strategy for the detection of O-GlcNAc proteins in living cells.
Proteome Analyses
Chemical strategies to tag, enrich, and detect O-GlcNAc peptides and proteins have been instrumental to proteome analysis.37,73,74 The tagging approach in combination with high-throughput liquid chromatography tandem mass spectrometry and mass spectrometry analysis has allowed identification of a number of O-GlcNAc–modified proteins from the mammalian brain.39,59,75 Antibodies and lectins have also been used for proteomic analyses of O-GlcNAc–modified proteins.61,74,76 The weak binding affinity of antibodies and lectins necessitates gentle washing conditions and can lead to false positives, such as interacting proteins or nonspecific binding proteins. In many cases, further confirmation of the presence of the modification can be provided by evaluating individually immunoprecipitated proteins or by directly detecting O-GlcNAc–modified peptides by mass spectrometry analysis. As is the case for other cell types, it will be interesting to determine whether O-GlcNAc proteins in the vasculature participate in the regulation of gene expression and cell signaling.
Mapping of O-GlcNAc Glycosylation Sites
Mapping the sites of O-GlcNAc glycosylation within proteins is essential for elucidating the functional roles of O-GlcNAcylation.77 As previously mentioned, although OGT seems to favor sequences rich in proline, serine, and threonine residues,75 there is no apparent consensus sequence that directs the action of OGT. The development of chemical tools coupled to mass spectrometry has greatly facilitated the localization of O-GlcNAc to short peptide sequences within proteins, and this combination can be used to determine exact glycosylation sites. Because direct observation of the O-GlcNAc moiety by mass spectrometry during collision-induced dissociation is difficult, as the glycosidic linkage is labile and readily cleaved,75 alternative and newer approaches, such as β-elimination followed by Michael addition with dithiothreitol, electron transfer dissociation, and electron capture dissociation have been used.58,60,76,78 In addition, ketogalactose-biotin tagging, or azidogalactose-biotin tagging have been combined with β-elimination followed by Michael addition with dithiothreitol to identify specific glycosylation sites.61,75
As previously mentioned, an exciting challenge in the future will be to understand the cellular dynamics of O-GlcNAcylation, as well as the signaling pathways and mechanisms by which O-GlcNAc is regulated on specific proteins. Accordingly, several chemical approaches to monitor changes in O-GlcNAcylation levels in response to cellular stimuli were lately developed. A fluorescence resonance energy transfer–based sensor has been recently developed for the detection of O-GlcNAc dynamics in living cells.79 This may be used to examine changes in OGT activity in response to a variety of vasoactive compounds. Furthermore, monitoring OGT activity, identifying the intracellular signaling pathways and dynamics of O-GlcNAcylation on specific protein substrates will help to clarify the role of this posttranslational modification on vascular function.
O-GLcNAc and Vascular Function
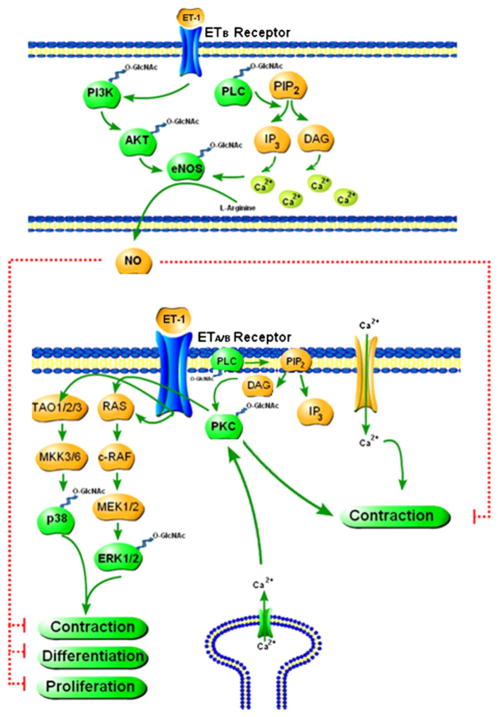
The dynamic nature of O-GlcNAc is a unique characteristic that distinguishes it from other forms of glycosylation. This feature has important implications for the regulation of protein structure and function and the interplay with other post-translational modifications. Several reviews have described the roles of O-GlcNAc in cellular processes, such as transcription,19,80 stress response,10,32,81,82 apoptosis,82–84 signal transduction,19,82,85 glucose sensing,81,86 and proteasomal degradation.81 With respect to vascular function, O-GlcNAc is a relatively unexplored area. Here, we will focus on a few specific pathways (PKC, MAPK, and PI3K/Akt) that contribute to vascular function and the implications of their interaction with O-GlcNAc. These signaling pathways are also closely implicated in vascular dysfunction associated with arterial hypertension.87,88 In addition, one potential mechanism by which O-GlcNAcylation may change vascular function includes the complex interplay between O-GlcNAcylation and phosphorylation.89 An exciting challenge in the future will be to better understand the cellular dynamics of the modification, as well as the signaling pathways and mechanisms by which O-GlcNAc is regulated on specific proteins in the vasculature (Figure 2).
Figure 2.
Proteins with an important role in vascular function are targets for O-GlcNAcylation indicating that this posttranslational modification may play an important role in vascular reactivity (please refer to the text for more details).
PKC and O-GlcNAc
It will be important to determine whether signaling pathways, such as PKC, normally activated by vasoactive agents, such as angiotensin II and endothelin-1, can interfere with O-GlcNAcylation and vice versa. Accordingly, early studies indicated that activation of PKC or cAMP-dependent protein kinase significantly decreased overall O-GlcNAcylation in neuronal cytoskeletal proteins.90 Conversely, inhibition of PKC, cAMP-dependent protein kinas, cyclin-dependent protein kinases, or S6 kinase increased overall O-GlcNAc levels in fractions from these cells. Stimulation of the transactivation of Sp1, which is O-GlcNAcylation–dependent, can be blocked by molecular and pharmacological inhibition of PKC.91 In cerebellar neurons from early postnatal mice, activation of cAMP-dependent protein kinase or PKC results in reduced levels of O-GlcNAc specifically in the fraction of cytoskeletal and cytoskeleton-associated proteins, whereas inhibition of the same kinases results in increased levels of O-GlcNAc.92
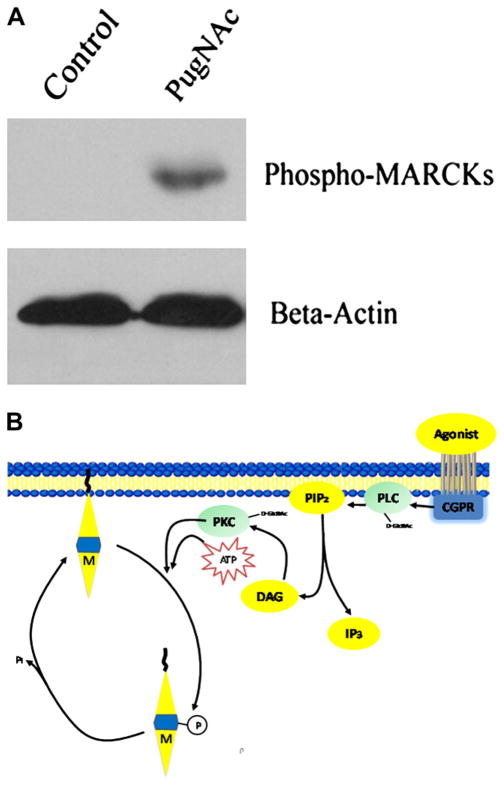
In the opposite direction, all PKC isozymes expressed in rat hepatocytes are dynamically modified by O-GlcNAc. O-GlcNAcylation of PKC-alpha correlated negatively with enzyme activity.93 Increased O-GlcNAc modification in a human astroglial cell line, in response to glucosamine (which increases the production of glucosamine 6-phosphate and stimulates O-GlcNAc modification of proteins) or PUGNAc (which blocks O-GlcNAcase activity, mimicking the enzyme-stabilized transition state), resulted in a decrease in membrane-associated PKC-epsilon and PKC-alpha, but not PKC-iota, indicating that increased levels of the O-GlcNAc modification regulates specific PKC isoforms.94 Preliminary data from our laboratory show that PUGNAc incubation for 3 hours, which increases vascular content of O-GlcNAc proteins, increases phosphorylation of myristoylated alanine rich C-kinases, a specific PKC substrate (Figure 3). Therefore, it is likely that O-GlcNAc modification of PKC isoforms found in the vasculature, such as PKC-alpha, PKC-beta, PKC-delta, PKC-gamma, PKC-epsilon, and PKC-zeta,87 can interfere with vascular processes regulated by these enzymes.
Figure 3.
Myristolated alanine rich C kinases (MARCKs) are phosphorylated demonstrating PKC activity. (A) Sprague-Dawley rat aortic rings were exposed to PUGNAc [O-(2-acetamido-2-deoxy-D-glucopyranosylidene) amino-N-phenylcarbamate; 100 μM], and inhibitor of β-N-acetylglucosaminidase (OGA), or control (methanol) for 3 hours. PUGNAc stimulates MARCKs phosphorylation. B-actin is shown for normalization of protein content. (B) Model demonstrating MARCKs as a specific substrate of protein kinase C (PKC). Nonphosphorylated MARCKs (M) is associated with the cytoplasmic side of the membrane. After PKC phosphorylates MARCKs, it is released from the membrane into the cytosol, where it remains until dephosphorylated.
MAPKs and O-GlcNAc
Phosphorylation-induced activation of the MAPKs leads to functional changes in the vasculature, and also modulates growth, differentiation, oxidative, and inflammatory responses.95 The MAPKs p38 and extracellular signal-related kinase (ERK1/2) have been reported to be phosphorylated in response to increased O-GlcNAc levels (95). A positive correlation between phosphorylation of the MAPK cascade (ERK1/2 and p38) and nuclear O-GlcNAcylation was observed in fetal human cardiac myocytes exposed to high glucose.44 In isolated rat hearts, perfusion with 5 mM glucosamine increased O-GlcNAc levels and conferred cardioprotection after ischemia-reperfusion.96 Interestingly, although glucosamine did not alter the response of either ERK1/2 or Akt (protein kinase B) to ischemia-reperfusion, it significantly attenuated the ischemia-induced increase in p38 phosphorylation, as well as the increased p38 phosphorylation at the end of reperfusion, suggesting that glucosamine-induced cardio-protection may be mediated via the p38 MAPK pathway.97
Augmented O-GlcNAc levels in mouse hippocampal synapses increased phosphorylation of synapsin I/II at serine 9 (cAMP-dependent protein kinase substrate site), serine 62/67 (ERK1/2 [MAPK 1/2] substrate site), and serine 603 (calmodulin kinase II site). Activation-specific phosphorylation events on ERK1/2 and calmodulin kinase II were also increased in response to elevation of O-GlcNAc levels.39 Advanced glycation end-products induce reactive oxygen species accumulation, apoptosis, MAPK activation, and nuclear O-GlcNAcylation in human cardiac myocytes.98 In addition, exposure of neutrophils to PUGNAc or glucosamine also stimulates the small GTPase Rac, which is an important upstream regulatory element in p38 and p44/42 MAPK signaling in neutrophils, and these MAPKs are implicated in chemotactic signal transduction.
Conversely, alterations in MAPK pathways can also have effects on the enzymes responsible for the regulation of O-GlcNAc.95 In neuro-2a neuroblastoma cells, increased OGT expression on glucose deprivation occurs in an AMP-activated protein kinase–dependent manner, whereas OGT enzymatic activity is regulated in a p38 MAPK-dependent manner. OGT is not phosphorylated by p38, but rather it interacts directly with p38 through its C terminus. The interaction with p38 does not change the catalytic activity of OGT, but p38 regulates OGT activity within the cell by recruiting it to specific targets.99 Together, these data indicate that O-GlcNAcylation is an important signaling element and it modulates the activities of several critical signaling kinases.100 Thus, we speculate that signaling kinases, such as proteins from MAPK and RhoA/Rho kinase pathways, are also regulated by O-GlcNAc modifications in the vasculature and that this posttranslational modification not only modulates vascular responses to constrictor stimuli, but also may play a role in the abnormal function of kinases involved in hypertension-associated vascular dysfunction.
PI3K/Akt/eNOS Pathway and O-GlcNAc
On activation, Akt phosphorylates eNOS leading to increased nitric oxide production. However, O-GlcNAc–modified Akt is not able to activate eNOS, thereby leading to decreased nitric oxide production.101 In addition, it has been shown in both vascular and penile tissues from diabetic animals that increased O-GlcNAc modification of eNOS, which is associated with decreased phosphorylation of eNOS at Ser1177 (eNOS- Ser1177), inhibits eNOS and impairs endothelial function.102–104 Furthermore, increased platelet aggregation induced by advanced glycation end-products is associated with decreased eNOS-Ser1177 and increased O-glycosylation of eNOS.105
Preliminary studies conducted by our laboratory showed that PUGNAc, which blocks O-GlcNAcase activity and increases O-GlcNAcylation, decreases phosphorylation of eNOS-Ser1177 as well as phosphorylation of Akt-Ser.473 Because PUGNAc also enhances vascular reactivity to constrictor stimuli, these results suggest that a reduction of Akt-mediated eNOS phosphorylation at Ser1177 plays a key role to modulate vascular reactivity.106 O-GlcNAcylation–induced changes in the Akt/eNOS signaling may have other effects beyond changing vascular reactivity. Accordingly, elevated O-GlcNAc levels and decreased Akt activity in endothelial cells is associated with impaired angiogenesis in mouse aortic rings (determined by vascular sprouting from mouse aortic rings, migration, and capillary-like tube formation of endothelial cells), whereas decreased O-GlcNAc, through overexpression of OGA, enhances these angiogenic responses.107
We hypothesize that increased O-GlcNAcylation disrupts the balance in cellular signaling mechanisms that promote contraction and relaxation of vascular smooth muscle cells, making the vasculature more sensitive to normal stimuli. O-GlcNAcylation-induced change in Akt/eNOS signaling is one of the contributing mechanisms, but as mentioned previously, O-GlcNAc modification of PKC isoforms as well as MAPKs may also lead to functional vascular changes, both in physiological and pathological conditions.
O-GLcNAc and Vascular Dysfunction Associated with Hypertension
Abnormal O-GlcNAcylation seems to contribute to the etiology of important human diseases, particularly diabetes.108,109 Vascular complications are the leading cause for morbidity and mortality in diabetic patients, and hyperglycemia is the primary factor in their pathogenesis.110,111 Glucose metabolism through the HBP has been implicated in many of the adverse effects of hyperglycemia, such as insulin resistance in peripheral tissues and diabetic vascular complications.19,25,81 Accordingly, elevated O-GlcNAcylation on insulin receptor substrate reduces its interactions with PI3K, thus blocking insulin signaling at an early stage.112,113 Transgenic mice overexpressing glutamine: fructose-6-phosphate transferase in skeletal muscle and adipose tissue develop insulin resistance and hyperleptinemia.114,115 Glucosamine infusion in vivo or prolonged incubation with PUGNAc also results in skeletal muscle insulin resistance.116–118 In addition, hyperglycemia, hyperlipidemia, or hyperinsulinemia were all shown to produce increased O-GlcNAcylation, disturbing signaling, transcription, and other cellular functions.25,73,119,120
Hypertension is also a disease marked by vascular dysfunction, including enhanced reactivity to constrictors, endothelial dysfunction, impaired relaxation, and remodeling.88,121 Few studies have investigated the role of O-GlcNAcylation in the vasculature of normotensive and hypertensive models. Studies from our laboratory have indicated a link between hypertension-related vascular dysfunction and O-GlcNAc. In DOCA-salt hypertensive rats, O-GlcNAc levels as well as vascular reactivity are significantly increased in both resistance (mesenteric) and conduit (aorta) arteries.122 Treatment with PUGNAc, which increases vascular O-GlcNAc levels, augments reactivity to contractile stimuli in arteries from normotensive, but not hypertensive animals, indicating that O-GlcNAc may contribute to hypertension-associated vascular hyperreactivity.122 Further support to the hypothesis that increased O-GlcNAc levels may lead to vascular dysfunction emerged from studies with eNOS.16,19 eNOS activity in cultured human coronary and bovine endothelial cells is inhibited by hyperglycemia through O-GlcNAc modification and by reciprocal reduction in phosphorylation of Ser.1177 Also, aortas from diabetic animals exhibit increased O-GlcNAc modification of eNOS and decreased eNOS (Ser1177) phosphorylation, which could contribute to reduced endothelium-dependent vasodilatation in diabetic patients. In addition, Musicki et al103 demonstrated that diabetes-related erectile dysfunction is associated with hyperglycemia-induced O-GlcNAc modification of eNOS. We have demonstrated that vessels from mineralocorticoid hypertensive rats also exhibit increased levels of O-GlcNAc–modified eNOS.122
Whereas inhibition of eNOS activity by O-GlcNAc may represent one of the mechanisms leading to impaired vascular reactivity in hypertension,122 the enzymatic pathways that modulate protein O-glycosylation remain incompletely understood. In a recent report, Li and colleagues showed that accumulation of advanced glycation end-products, induced either by high glucose or the advanced glycation end-product carbon precursor methylglyoxal, induces generation of reactive oxygen species and increased O-GlcNAcylation in fetal human cardiac myocytes.98 In addition, exposure of COS-731 or HeLa and HepG-2123 cells to a wide variety of stresses, including the pro-oxidant agent hydrogen peroxide (H2O2), increases O-GlcNAc levels. In COS-7 cells, H2O2 also increased OGT expression.31 Therefore, it is possible that increased reactive oxygen species generation provides a mechanism leading to increased vascular O-GlcNAcylation in hypertension.
Recent evidence shows that increased O-GlcNAcylation also interferes with many aspects of the inflammatory process, which represents another important feature of the hypertensive disease.124 For example, in rat mesangial cells increased flux through the HBP leads to nuclear factor-κB promoter activation and increased expression of inflammatory cytokines, which may contribute to glomerulosclerosis.125 Augmentation of O-GlcNAc levels in human neutrophil leukocytes, by glucosamine or PUGNAc administration, enhances agonist-induced chemotaxis and also stimulates chemotaxis in the absence of stimuli.126 In addition, increased flux through the HBP stimulates the expression of prosclerotic genes in mesangial cells in vitro, such as transforming growth factor-beta1, plasminogen activator inhibitor-1, fibronectin, and laminin,113,127–129 suggesting that these changes contribute to glomerular extracellular matrix accumulation and mesangial expansion in vivo, which are characteristic of diabetic and hypertensive nephropathy. In contrast, anti-inflammatory actions have also been associated with increased O-GlcNAc levels.130 Glucosamine suppresses expression of the proinflammatory mediators interleukin-6 and COX-2; inhibits nuclear factor-κB activation and interleukin-1β bioactivity in chondrocytes; downregulates tumor necrosis factor-α–induced expression of intercellular adhesion molecule in epithelial cells; and suppresses neutrophil functions such as superoxide generation, phagocytosis, granule enzyme release, and chemotaxis.131–136 The mechanism by which glucosamine acts as an anti-inflammatory agent is likely linked to augmentation of O-GlcNAc–modified protein levels, but glucosamine effects may be mediated via other pathways that should be further addressed.96,130
One possible explanation for the potential contradiction between the beneficial and adverse effects of increased O-GlcNAcylation would be that target proteins may differentially respond to short-term increases in O-GlcNAc relative to a sustained increase in flux through OGT. Another alternative explanation, suggested by Fulop and colleagues, is the concept of allostasis.10 Accordingly, the initial biological response to an acute stress is the activation of processes that are protective and improve survival. As the allostatic load increases and the stress becomes more frequent or continuous, activation of the same pathways results in the development of pathophysiology.137 Therefore, if on one hand increased O-GlcNAcylation is an acute survival response, the pathophysiological effects may be a consequence of chronic activation, as seen in metabolic diseases.
In our studies, a temporal relationship was observed not only between increased blood pressure and the higher vascular O-GlcNAcylation content, but also between augmented O-GlcNAc levels and decreased expression of OGA and OGT. Whether the same vascular proteins are modified by O-GlcNAc at the beginning and at later stages of the hypertension process is unclear. According to the concept of allostasis, it is also possible that vascular O-GlcNAcylation may be protective or may represent the initial biological response to the augmented blood pressure levels, but as the hypertensive process continues, activation of the same pathways may result in vascular damage.
Conclusion
Over the past decade, a surge of discoveries in O-GlcNAc glycosylation has revealed new roles for this modification in the cardiovascular system, as was highlighted in an excellent review by Laczy and colleagues.95 However, one of the central challenges, specifically to vascular function, will be to understand the unique molecular and cellular O-GlcNAc signaling as it relates to contraction and dilation. Sensitive methods to detect the modification on small subpopulations of vascular cells or proteins will be required to dissect the role of O-GlcNAc in contraction, dilation, and structural remodeling. Despite significant progress, faster, higher throughput methods are still needed to identify O-GlcNAc proteins and study O-GlcNAc dynamics in vivo.
O-GlcNAc is abundant in the heart and arteries and is present on many diverse proteins involved in transcription, signaling, and cardiovascular function. Indeed, recent studies have begun to uncover the functional roles of O-GlcNAc, its complex dynamics in the heart, cardiac myocytes, and the vasculature and the interplay between O-GlcNAc and phosphorylation. Many of these discoveries have been accelerated by the development of new chemical tools, such as those for detecting the O-GlcNAc modification in cells and for inhibiting OGT and OGA.
Although the pace and scope of understanding O-GlcNAc has expanded considerably, much remains to be discovered. Owing to the challenge of studying the modification, evidence linking O-GlcNAc to specific biological functions has often been indirect or correlative. This is particularly true in the cardiovascular system, where the complexity of the function of the vasculature renders O-GlcNAc more difficult to investigate. Nonetheless, in-depth functional studies on proteins will be essential in the future to determine the roles of O-GlcNAc in vascular-specific contexts. The development of new chemical tools to produce homogeneously glycosylated proteins will represent an important step toward this goal.
In this sense, the discovery of more selective and potent inhibitors of OGT and OGA, especially compounds that can be used in vivo, will allow very rapid advances in the area. Accordingly it will be very interesting to test whether inhibition of OGT during the development of cardiovascular disease, particularly hypertension will ameliorate vascular dysfunction associated with this pathological condition.
Given the diversity of OGT and OGA substrates, and the lethality of deleting the OGT gene in mice, creative new genetic or chemical approaches are needed to more selectively target functional subsets of OGT and OGA by interfering, for instance, with the enzymes in certain subcellular compartments. From the time of its discovery, the appeal of O-GlcNAc has been both the intrigue of understanding its unique biology and the great technical challenges associated with its study. Over the past 5 years, we have seen a surge of new chemistry designed to meet these obstacles. Strengthened by an arsenal of chemical tools, the future of O-GlcNAc is primed for new and exciting discoveries.
Acknowledgments
Supported by the National Institutes of Health and Cardiovascular Discovery Institute, EUA and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Brazil.
References
- 1.Hancock JT. Cell signalling. England: Addison-Wesley; 1997. [Google Scholar]
- 2.Gomperts BD, Kramer IM, Tatham PER. Signal transduction. San Diego: Academic Press; 2002. [Google Scholar]
- 3.Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ. 2003;27:201–6. doi: 10.1152/advan.00025.2003. [DOI] [PubMed] [Google Scholar]
- 4.Tostes RC, Leite R, Webb RC. Vascular smooth muscle contraction and relaxation. In: Izzo JL, Sica D, Black HR, editors. Hypertension primer. The essentials of high blood pressure. 4. Chapter A10. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 34–7. [Google Scholar]
- 5.Booz GW, Baker KM. Protein phosphorylation. In: Izzo JL, Sica D, Black HR, editors. Hypertension primer: The essentials of high blood pressure. 4. Chapter A5. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 16–21. [Google Scholar]
- 6.Hart GW, Haltiwanger RS, Holt GD, Kelly WG. Glycosylation in the nucleus and cytoplasm. Annu Rev Biochem. 1989;58:841–74. doi: 10.1146/annurev.bi.58.070189.004205. [DOI] [PubMed] [Google Scholar]
- 7.Kamemura K, Hayes BK, Comer FI, Hart GW. Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: alternative glycosylation/phosphorylation of THR-58, a known mutational hot spot of c-Myc in lymphomas, is regulated by mitogens. J Biol Chem. 2002;277:19229–35. doi: 10.1074/jbc.M201729200. [DOI] [PubMed] [Google Scholar]
- 8.Comer FI, Hart GW. Reciprocity between O-GlcNAc and O-phosphate on the carboxyl terminal domain of RNA polymerase II. Biochemistry. 2001;40:7845–52. doi: 10.1021/bi0027480. [DOI] [PubMed] [Google Scholar]
- 9.Hart GW, Greis KD, Dong LY, Blomberg MA, Chou TY, Jiang MS, et al. O-linked N-acetylglucosamine: the “yin-yang” of Ser/Thr phosphorylation? Nuclear and cytoplasmic glycosylation. Adv Exp Med Biol. 1995;376:115–23. [PubMed] [Google Scholar]
- 10.Fulop N, Marchase RB, Chatham JC. Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res. 2007;73:288–97. doi: 10.1016/j.cardiores.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart GW, Housley MP, Slawson C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–22. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 12.Issad T, Kuo M. O-GlcNAc modification of transcription factors, glucose sensing and glucotoxicity. Trends Endocrinol Metab. 2008;19:380–9. doi: 10.1016/j.tem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Slawson C, Lakshmanan T, Knapp S, Hart GW. A mitotic GlcNAcylation/phosphorylation signaling complex alters the posttranslational state of the cytoskeletal protein vimentin. Mol Biol Cell. 2008;19:4130–40. doi: 10.1091/mbc.E07-11-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reason AJ, Morris HR, Panico M, Marais R, Treisman RH, Haltiwanger RS, et al. Localization of O-GlcNAc modification on the serum response transcription factor. J Biol Chem. 1992;267:16911–21. [PubMed] [Google Scholar]
- 15.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–22. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 16.Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001;291:2376–8. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- 17.Wells L, Gao Y, Mahoney JA, Vosseller K, Chen C, Rosen A, et al. Dynamic O-glycosylation of nuclear and cytosolic proteins: further characterization of the nucleocytoplasmic beta-N-acetylglucosaminidase, O-GlcNAcase. J Biol Chem. 2002;277:1755–61. doi: 10.1074/jbc.m109656200. [DOI] [PubMed] [Google Scholar]
- 18.Lubas WA, Hanover JA. Functional expression of O-linked GlcNAc transferase. Domain structure and substrate specificity. J Biol Chem. 2000;275:10983–8. doi: 10.1074/jbc.275.15.10983. [DOI] [PubMed] [Google Scholar]
- 19.Love DC, Hanover JA. The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci STKE. 2005;2005:re13. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- 20.Lazarus BD, Love DC, Hanover JA. Recombinant O-GlcNAc transferase isoforms: identification of O-GlcNAcase, yes tyrosine kinase, and tau as isoform-specific substrates. Glycobiology. 2006;16:415–21. doi: 10.1093/glycob/cwj078. [DOI] [PubMed] [Google Scholar]
- 21.Kreppel LK, Hart GW. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem. 1999;274:32015–22. doi: 10.1074/jbc.274.45.32015. [DOI] [PubMed] [Google Scholar]
- 22.Jinek M, Rehwinkel J, Lazarus BD, Izaurralde E, Hanover JA, Conti E. The superhelical TPR-repeat domain of O-linked GlcNAc transferase exhibits structural similarities to importin alpha. Nat Struct Mol Biol. 2004;11:1001–7. doi: 10.1038/nsmb833. [DOI] [PubMed] [Google Scholar]
- 23.Iyer SP, Akimoto Y, Hart GW. Identification and cloning of a novel family of coiled-coil domain proteins that interact with O-GlcNAc transferase. J Biol Chem. 2003;278:5399–409. doi: 10.1074/jbc.M209384200. [DOI] [PubMed] [Google Scholar]
- 24.Wells L, Kreppel LK, Comer FI, Wadzinski BE, Hart GW. O-GlcNAc transferase is in a functional complex with protein phosphatase 1 catalytic subunits. J Biol Chem. 2004;279:38466–70. doi: 10.1074/jbc.M406481200. [DOI] [PubMed] [Google Scholar]
- 25.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266:4706–12. [PubMed] [Google Scholar]
- 26.Buse MG. Hexosamines, insulin resistance, and the complications of diabetes: current status. Am J Physiol Endocrinol Metab. 2006;290:E1–8. doi: 10.1152/ajpendo.00329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zachara NE, Hart GW. Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta. 2006;1761:599–617. doi: 10.1016/j.bbalip.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Haltiwanger RS, Blomberg MA, Hart GW. Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-N-acetyl-glucosamine:polypeptide beta-N-acetylglucosaminyl-transferase. J Biol Chem. 1992;267:9005–13. [PubMed] [Google Scholar]
- 29.O’Donnell N, Zachara NE, Hart GW, Marth JD. Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol. 2004;24:1680–90. doi: 10.1128/MCB.24.4.1680-1690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ngoh GA, Jones SP. New insights into metabolic signaling and cell survival: the role of beta-O-linkage of N-acetylglucosamine. J Pharmacol Exp Ther. 2008;327:602–9. doi: 10.1124/jpet.108.143263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 2004;279:30133–42. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- 32.Chatham JC, Not LG, Fulop N, Marchase RB. Hexosamine biosynthesis and protein O-glycosylation: the first line of defense against stress, ischemia, and trauma. Shock. 2008;29:431–40. doi: 10.1097/shk.0b013e3181598bad. [DOI] [PubMed] [Google Scholar]
- 33.Toleman C, Paterson AJ, Whisenhunt TR, Kudlow JE. Characterization of the histone acetyltransferase (HAT) domain of a bifunctional protein with activable O-GlcNAcase and HAT activities. J Biol Chem. 2004;279:53665–73. doi: 10.1074/jbc.M410406200. [DOI] [PubMed] [Google Scholar]
- 34.Comtesse N, Maldener E, Meese E. Identification of a nuclear variant of MGEA5, a cytoplasmic hyaluronidase and a beta-N-acetylglucosaminidase. Biochem Biophys Res Commun. 2001;283:634–40. doi: 10.1006/bbrc.2001.4815. [DOI] [PubMed] [Google Scholar]
- 35.Cheung WD, Sakabe K, Housley MP, Dias WB, Hart GW. O-linked beta-N-acetylglucosaminyltransferase substrate specificity is regulated by myosin phosphatase targeting and other interacting proteins. J Biol Chem. 2008;283:33935–41. doi: 10.1074/jbc.M806199200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peter-Katalinic J. Methods in enzymology: O-glycosylation of proteins. Methods Enzymol. 2005;405:139–71. doi: 10.1016/S0076-6879(05)05007-X. [DOI] [PubMed] [Google Scholar]
- 37.Zachara NE. Detecting the “O-GlcNAc-ome”; detection, purification, and analysis of O-GlcNAc modified proteins. Methods Mol Biol. 2009;534:251–79. doi: 10.1007/978-1-59745-022-5_19. [DOI] [PubMed] [Google Scholar]
- 38.Zachara NE. Detection and Analysis of (O-linked beta-N-Acetylglucosamine)-modified proteins. Methods Mol Biol. 2009;464:227–54. doi: 10.1007/978-1-60327-461-6_13. [DOI] [PubMed] [Google Scholar]
- 39.Rexach JE, Clark PM, Hsieh-Wilson LC. Chemical approaches to understanding O-GlcNAc glycosylation in the brain. Nat Chem Biol. 2008;4:97–106. doi: 10.1038/nchembio.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macauley MS, Vocadlo DJ. Increasing O-GlcNAc levels: an overview of small-molecule inhibitors of O-GlcNAcase. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbagen.2009.07.028. In press. [DOI] [PubMed] [Google Scholar]
- 41.Shafi R, Iyer SP, Ellies LG, O’Donnell N, Marek KW, Chui D, et al. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci U S A. 2000;97:5735–9. doi: 10.1073/pnas.100471497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dauphinee SM, Ma M, Too CK. Role of O-linked beta-N-acetylglucosamine modification in the subcellular distribution of alpha4 phosphoprotein and Sp1 in rat lymphoma cells. J Cell Biochem. 2005;96:579–88. doi: 10.1002/jcb.20508. [DOI] [PubMed] [Google Scholar]
- 43.Forsythe ME, Love DC, Lazarus BD, Kim EJ, Prinz WA, Ashwell G, et al. Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 (O-GlcNAcase) knockout impacts O-GlcNAc cycling, metabolism, and dauer. Proc Natl Acad Sci U S A. 2006;103:11952–7. doi: 10.1073/pnas.0601931103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gross BJ, Kraybill BC, Walker S. Discovery of O-GlcNAc transferase inhibitors. J Am Chem Soc. 2005;127:14588–9. doi: 10.1021/ja0555217. [DOI] [PubMed] [Google Scholar]
- 45.Lee TN, Alborn WE, Knierman MD, Konrad RJ. Alloxan is an inhibitor of O-GlcNAc-selective N-acetyl-beta-D-glucosaminidase. Biochem Biophys Res Commun. 2006;350:1038–43. doi: 10.1016/j.bbrc.2006.09.155. [DOI] [PubMed] [Google Scholar]
- 46.Meglasson MD, Burch PT, Berner DK, Najafi H, Matschinsky FM. Identification of glucokinase as an alloxan-sensitive glucose sensor of the pancreatic beta-cell. Diabetes. 1986;35:1163–73. doi: 10.2337/diab.35.10.1163. [DOI] [PubMed] [Google Scholar]
- 47.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–46. [PubMed] [Google Scholar]
- 48.Toleman C, Paterson AJ, Shin R, Kudlow JE. Streptozotocin inhibits O-GlcNAcase via the production of a transition state analog. Biochem Biophys Res Commun. 2006;340:526–34. doi: 10.1016/j.bbrc.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 49.Pathak S, Dorfmueller HC, Borodkin VS, van Aalten DM. Chemical dissection of the link between streptozotocin, O-GlcNAc, and pancreatic cell death. Chem Biol. 2008;15:799–807. doi: 10.1016/j.chembiol.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim EJ, Amorelli B, Abdo M, Thomas CJ, Love DC, Knapp S, et al. Distinctive inhibition of O-GlcNAcase isoforms by an alpha-GlcNAc thiolsulfonate. J Am Chem Soc. 2007;129:14854–5. doi: 10.1021/ja076038u. [DOI] [PubMed] [Google Scholar]
- 51.Kim EJ, Perreira M, Thomas CJ, Hanover JA. An O-GlcNAcase-specific inhibitor and substrate engineered by the extension of the N-acetyl moiety. J Am Chem Soc. 2006;128:4234–5. doi: 10.1021/ja0582915. [DOI] [PubMed] [Google Scholar]
- 52.Knapp S, Abdo M, Ajayi K, Huhn RA, Emge TJ, Kim EJ, et al. Tautomeric modification of GlcNAc-thiazoline. Org Lett. 2007;9:2321–4. doi: 10.1021/ol0706814. [DOI] [PubMed] [Google Scholar]
- 53.Macauley MS, Whitworth GE, Debowski AW, Chin D, Vocadlo DJ. O-GlcNAcase uses substrate-assisted catalysis: kinetic analysis and development of highly selective mechanism-inspired inhibitors. J Biol Chem. 2005;280:25313–22. doi: 10.1074/jbc.M413819200. [DOI] [PubMed] [Google Scholar]
- 54.Dorfmueller HC, Borodkin VS, Schimpl M, Shepherd SM, Shpiro NA, van Aalten DM. GlcNAc-statin: a picomolar, selective O-GlcNAcase inhibitor that modulates intracellular O-glcNAcylation levels. J Am Chem Soc. 2006;128:16484–5. doi: 10.1021/ja066743n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shanmugasundaram B, Debowski AW, Dennis RJ, Davies GJ, Vocadlo DJ, Vasella A. Inhibition of O-GlcNAcase by a gluco-configured nagstatin and a PUGNAc-imidazole hybrid inhibitor. Chem Commun (Camb) 2006:4372–4. doi: 10.1039/b612154c. [DOI] [PubMed] [Google Scholar]
- 56.Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J Mol Cell Cardiol. 2007;42:177–85. doi: 10.1016/j.yjmcc.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Majumdar G, Harmon A, Candelaria R, Martinez-Hernandez A, Raghow R, Solomon SS. O-glycosylation of Sp1 and transcriptional regulation of the calmodulin gene by insulin and glucagon. Am J Physiol Endocrinol Metab. 2003;285:E584–91. doi: 10.1152/ajpendo.00140.2003. [DOI] [PubMed] [Google Scholar]
- 58.Syka JE, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc Natl Acad Sci U S A. 2004;101:9528–33. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khidekel N, Arndt S, Lamarre-Vincent N, Lippert A, Poulin-Kerstien KG, Ramakrishnan B, et al. A chemo-enzymatic approach toward the rapid and sensitive detection of O-GlcNAc posttranslational modifications. J Am Chem Soc. 2003;125:16162–3. doi: 10.1021/ja038545r. [DOI] [PubMed] [Google Scholar]
- 60.Khidekel N, Ficarro SB, Clark PM, Bryan MC, Swaney DL, Rexach JE, et al. Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics. Nat Chem Biol. 2007;3:339–48. doi: 10.1038/nchembio881. [DOI] [PubMed] [Google Scholar]
- 61.Wang Z, Pandey A, Hart GW. Dynamic interplay between O-linked N-acetylglucosaminylation and glycogen synthase kinase-3-dependent phosphorylation. Mol Cell Proteomics. 2007;6:1365–79. doi: 10.1074/mcp.M600453-MCP200. [DOI] [PubMed] [Google Scholar]
- 62.Sprung R, Nandi A, Chen Y, Kim SC, Barma D, Falck JR, et al. Tagging-via-substrate strategy for probing O-GlcNAc modified proteins. J Proteome Res. 2005;4:950–7. doi: 10.1021/pr050033j. [DOI] [PubMed] [Google Scholar]
- 63.Chalkley RJ, Thalhammer A, Schoepfer R, Burlingame AL. Identification of protein O-GlcNAcylation sites using electron transfer dissociation mass spectrometry on native peptides. Proc Natl Acad Sci U S A. 2009;106:8894–9. doi: 10.1073/pnas.0900288106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carapito C, Klemm C, Aebersold R, Domon B. Systematic LC-MS analysis of labile post-translational modifications in complex mixtures. J Proteome Res. 2009;8:2608–14. doi: 10.1021/pr800871n. [DOI] [PubMed] [Google Scholar]
- 65.Cieniewski-Bernard C, Bastide B, Lefebvre T, Lemoine J, Mounier Y, Michalski JC. Identification of O-linked N-acetylglucosamine proteins in rat skeletal muscle using two-dimensional gel electrophoresis and mass spectrometry. Mol Cell Proteomics. 2004;3:577–85. doi: 10.1074/mcp.M400024-MCP200. [DOI] [PubMed] [Google Scholar]
- 66.Liu K, Paterson AJ, Chin E, Kudlow JE. Glucose stimulates protein modification by O-linked GlcNAc in pancreatic beta cells: linkage of O-linked GlcNAc to beta cell death. Proc Natl Acad Sci U S A. 2000;97:2820–5. doi: 10.1073/pnas.97.6.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roquemore EP, Chou TY, Hart GW. Detection of O-linked N-acetylglucosamine (O-GlcNAc) on cytoplasmic and nuclear proteins. Methods Enzymol. 1994;230:443–60. doi: 10.1016/0076-6879(94)30028-3. [DOI] [PubMed] [Google Scholar]
- 68.Ramakrishnan B, Qasba PK. Structure-based design of beta 1,4-galactosyltransferase I (beta 4Gal-T1) with equally efficient N-acetylgalactosaminyltransferase activity: point mutation broadens beta 4Gal-T1 donor specificity. J Biol Chem. 2002;277:20833–9. doi: 10.1074/jbc.M111183200. [DOI] [PubMed] [Google Scholar]
- 69.Tai HC, Khidekel N, Ficarro SB, Peters EC, Hsieh-Wilson LC. Parallel identification of O-GlcNAc-modified proteins from cell lysates. J Am Chem Soc. 2004;126:10500–1. doi: 10.1021/ja047872b. [DOI] [PubMed] [Google Scholar]
- 70.Gurcel C, Vercoutter-Edouart AS, Fonbonne C, Mortuaire M, Salvador A, Michalski JC, et al. Identification of new O-GlcNAc modified proteins using a click-chemistry-based tagging. Anal Bioanal Chem. 2008;390:2089–97. doi: 10.1007/s00216-008-1950-y. [DOI] [PubMed] [Google Scholar]
- 71.Hsieh-Wilson L, Khidekel N, Tai H-C, Arndt S. Method and compositions for the detection of protein glycosylation. 20050130235. US patent. 2003 Nov 18;
- 72.Vocadlo DJ, Hang HC, Kim EJ, Hanover JA, Bertozzi CR. A chemical approach for identifying O-GlcNAc-modified proteins in cells. Proc Natl Acad Sci U S A. 2003;100:9116–21. doi: 10.1073/pnas.1632821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whelan SA, Lane MD, Hart GW. Regulation of the O-linked beta-N-acetylglucosamine transferase by insulin signaling. J Biol Chem. 2008;283:21411–7. doi: 10.1074/jbc.M800677200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whelan SA, Hart GW. Proteomic approaches to analyze the dynamic relationships between nucleocytoplasmic protein glycosylation and phosphorylation. Circ Res. 2003;93:1047–58. doi: 10.1161/01.RES.0000103190.20260.37. [DOI] [PubMed] [Google Scholar]
- 75.Khidekel N, Ficarro SB, Peters EC, Hsieh-Wilson LC. Exploring the O-GlcNAc proteome: direct identification of O-GlcNAc-modified proteins from the brain. Proc Natl Acad Sci U S A. 2004;101:13132–7. doi: 10.1073/pnas.0403471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vosseller K, Trinidad JC, Chalkley RJ, Specht CG, Thalhammer A, Lynn AJ, et al. O-linked N-acetylglucosamine proteomics of postsynaptic density preparations using lectin weak affinity chromatography and mass spectrometry. Mol Cell Proteomics. 2006;5:923–34. doi: 10.1074/mcp.T500040-MCP200. [DOI] [PubMed] [Google Scholar]
- 77.Zachara NE, Cheung WD, Hart GW. Nucleocytoplasmic glycosylation, O-GlcNAc: identification and site mapping. Methods Mol Biol. 2004;284:175–94. doi: 10.1385/1-59259-816-1:175. [DOI] [PubMed] [Google Scholar]
- 78.Wells L, Vosseller K, Cole RN, Cronshaw JM, Matunis MJ, Hart GW. Mapping sites of O-GlcNAc modification using affinity tags for serine and threonine post-translational modifications. Mol Cell Proteomics. 2002;1:791–804. doi: 10.1074/mcp.m200048-mcp200. [DOI] [PubMed] [Google Scholar]
- 79.Carrillo LD, Krishnamoorthy L, Mahal LK. A cellular FRET-based sensor for beta-O-GlcNAc, a dynamic carbohydrate modification involved in signaling. J Am Chem Soc. 2006;128:14768–9. doi: 10.1021/ja065835+. [DOI] [PubMed] [Google Scholar]
- 80.Comer FI, Hart GW. O-GlcNAc and the control of gene expression. Biochim Biophys Acta. 1999;1473:161–71. doi: 10.1016/s0304-4165(99)00176-2. [DOI] [PubMed] [Google Scholar]
- 81.Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta. 2004;1673:13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 82.Wells L, Hart GW. O-GlcNAc turns twenty: functional implications for post-translational modification of nuclear and cytosolic proteins with a sugar. FEBS Lett. 2003;546:154–8. doi: 10.1016/s0014-5793(03)00641-0. [DOI] [PubMed] [Google Scholar]
- 83.Wells L, Whelan SA, Hart GW. O-GlcNAc: a regulatory post-translational modification. Biochem Biophys Res Commun. 2003;302:435–41. doi: 10.1016/s0006-291x(03)00175-x. [DOI] [PubMed] [Google Scholar]
- 84.Park J, Kwon H, Kang Y, Kim Y. Proteomic analysis of O-GlcNAc modifications derived from streptozotocin and glucosamine induced beta-cell apoptosis. J Biochem Mol Biol. 2007;40:1058–68. doi: 10.5483/bmbrep.2007.40.6.1058. [DOI] [PubMed] [Google Scholar]
- 85.Slawson C, Hart GW. Dynamic interplay between O-GlcNAc and O-phosphate: the sweet side of protein regulation. Curr Opin Struct Biol. 2003;13:631–6. doi: 10.1016/j.sbi.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 86.Wells L, Vosseller K, Hart GW. A role for N-acetyl-glucosamine as a nutrient sensor and mediator of insulin resistance. Cell Mol Life Sci. 2003;60:222–8. doi: 10.1007/s000180300017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salamanca DA, Khalil RA. Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol. 2005;70:1537–47. doi: 10.1016/j.bcp.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Touyz RM. Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: role of angiotensin II. Exp Physiol. 2005;90:449–55. doi: 10.1113/expphysiol.2005.030080. [DOI] [PubMed] [Google Scholar]
- 89.Kamemura K, Hart GW. Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: a new paradigm for metabolic control of signal transduction and transcription. Prog Nucleic Acid Res Mol Biol. 2003;73:107–36. doi: 10.1016/s0079-6603(03)01004-3. [DOI] [PubMed] [Google Scholar]
- 90.Griffith LS, Mathes M, Schmitz B. Beta-amyloid precursor protein is modified with O-linked N-acetyl-glucosamine. J Neurosci Res. 1995;41:270–8. doi: 10.1002/jnr.490410214. [DOI] [PubMed] [Google Scholar]
- 91.Fantus GI, Goldberg HJ, Whiteside CI, Topic D. The hexosamine biosynthesis pathway: contribution to the pathogenesis of diabetic nephropathy. In: Cortes P, Mogensen CE, editors. The diabetic kidney. Humana Press; 2006. pp. 120–33. [Google Scholar]
- 92.Griffith LS, Schmitz B. O-linked N-acetylglucosamine levels in cerebellar neurons respond reciprocally to perturbations of phosphorylation. Eur J Biochem. 1999;262:824–31. doi: 10.1046/j.1432-1327.1999.00439.x. [DOI] [PubMed] [Google Scholar]
- 93.Robles-Flores M, Melendez L, Garcia W, Mendoza-Hernandez G, Lam TT, Castaneda-Patlan C, et al. Posttranslational modifications on protein kinase c isozymes. Effects of epinephrine and phorbol esters. Biochim Biophys Acta. 2008;1783:695–712. doi: 10.1016/j.bbamcr.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 94.Matthews JA, Acevedo-Duncan M, Potter RL. Selective decrease of membrane-associated PKC-alpha and PKC-epsilon in response to elevated intracellular O-GlcNAc levels in transformed human glial cells. Biochim Biophys Acta. 2005;1743:305–15. doi: 10.1016/j.bbamcr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 95.Laczy B, Hill BG, Wang K, Paterson AJ, White CR, Xing D, et al. Protein O-GlcNAcylation: a new signaling paradigm for the cardiovascular system. Am J Physiol Heart Circ Physiol. 2009;296:H13–28. doi: 10.1152/ajpheart.01056.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zou L, Yang S, Champattanachai V, Hu S, Chaudry IH, Marchase RB, et al. Glucosamine improves cardiac function following trauma-hemorrhage by increased protein O-GlcNAcylation and attenuation of NF-{kappa}B signaling. Am J Physiol Heart Circ Physiol. 2009;296:H515–23. doi: 10.1152/ajpheart.01025.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, et al. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117:1172–82. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- 98.Li SY, Sigmon VK, Babcock SA, Ren J. Advanced glycation endproduct induces ROS accumulation, apoptosis, MAP kinase activation and nuclear O-GlcNAcylation in human cardiac myocytes. Life Sci. 2007;80:1051–6. doi: 10.1016/j.lfs.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 99.Cheung WD, Hart GW. AMP-activated protein kinase and p38 MAPK activate O-GlcNAcylation of neuronal proteins during glucose deprivation. J Biol Chem. 2008;283:13009–20. doi: 10.1074/jbc.M801222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kneass ZT, Marchase RB. Protein O-GlcNAc modulates motility-associated signaling intermediates in neutrophils. J Biol Chem. 2005;280:14579–85. doi: 10.1074/jbc.M414066200. [DOI] [PubMed] [Google Scholar]
- 101.Soesanto YA, Luo B, Jones D, Taylor R, Gabrielsen JS, Parker G, et al. Regulation of Akt signaling by O-GlcNAc in euglycemia. Am J Physiol Endocrinol Metab. 2008;295:E974–80. doi: 10.1152/ajpendo.90366.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–8. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Musicki B, Kramer MF, Becker RE, Burnett AL. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2005;102:11870–5. doi: 10.1073/pnas.0502488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rammos G, Peppes V, Zakopoulos N. Transient insulin resistance in normal subjects: acute hyperglycemia inhibits endothelial-dependent vasodilatation in normal subjects. Metab Syndr Relat Disord. 2008 Fall;6:159–70. doi: 10.1089/met.2007.0036. [DOI] [PubMed] [Google Scholar]
- 105.Chen L, Liu Y, Cui B, Mi Q, Huang Y, Fan L, et al. 17Beta-oestradiol partially attenuates the inhibition of nitric oxide synthase-3 by advanced glycation end-products in human platelets. Clin Exp Pharmacol Physiol. 2007;34:972–8. doi: 10.1111/j.1440-1681.2007.04680.x. [DOI] [PubMed] [Google Scholar]
- 106.Lima VV, Giachini FR, Carneiro FS, Carneiro ZN, Fortes ZB, Carvalho MHC, et al. Increased vascular O-GlcNAcylation augments reactivity to constrictor stimuli. J Am Soc Hypertens. 2008;2:410–7. doi: 10.1016/j.jash.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luo B, Soesanto Y, McClain DA. Protein modification by O-linked GlcNAc reduces angiogenesis by inhibiting Akt activity in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:651–7. doi: 10.1161/ATVBAHA.107.159533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Akimoto Y, Hart GW, Hirano H, Kawakami H. O-GlcNAc modification of nucleocytoplasmic proteins and diabetes. Med Mol Morphol. 2005;38:84–91. doi: 10.1007/s00795-004-0264-1. [DOI] [PubMed] [Google Scholar]
- 109.Dias WB, Hart GW. O-GlcNAc modification in diabetes and Alzheimer’s disease. Mol Biosyst. 2007;3:766–72. doi: 10.1039/b704905f. [DOI] [PubMed] [Google Scholar]
- 110.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 111.Di Mario U, Pugliese G. 15th Golgi lecture: from hyperglycaemia to the dysregulation of vascular remodelling in diabetes. Diabetologia. 2001;44:674–92. doi: 10.1007/s001250051676. [DOI] [PubMed] [Google Scholar]
- 112.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, et al. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–72. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 113.Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 2002;99:5313–8. doi: 10.1073/pnas.072072399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cooksey RC, McClain DA. Transgenic mice overexpressing the rate-limiting enzyme for hexosamine synthesis in skeletal muscle or adipose tissue exhibit total body insulin resistance. Ann N Y Acad Sci. 2002;967:102–11. doi: 10.1111/j.1749-6632.2002.tb04268.x. [DOI] [PubMed] [Google Scholar]
- 115.Hebert LF, Jr, Daniels MC, Zhou J, Crook ED, Turner RL, Simmons ST, et al. Overexpression of glutamine:fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J Clin Invest. 1996;98:930–6. doi: 10.1172/JCI118876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patti ME, Virkamaki A, Landaker EJ, Kahn CR, Yki-Jarvinen H. Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance of early postreceptor insulin signaling events in skeletal muscle. Diabetes. 1999;48:1562–71. doi: 10.2337/diabetes.48.8.1562. [DOI] [PubMed] [Google Scholar]
- 117.Virkamaki A, Daniels MC, Hamalainen S, Utriainen T, McClain D, Yki-Jarvinen H. Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance in multiple insulin sensitive tissues. Endocrinology. 1997;138:2501–7. doi: 10.1210/endo.138.6.5172. [DOI] [PubMed] [Google Scholar]
- 118.Virkamaki A, Yki-Jarvinen H. Allosteric regulation of glycogen synthase and hexokinase by glucosamine-6-phosphate during glucosamine-induced insulin resistance in skeletal muscle and heart. Diabetes. 1999;48:1101–7. doi: 10.2337/diabetes.48.5.1101. [DOI] [PubMed] [Google Scholar]
- 119.McClain DA, Lubas WA, Cooksey RC, Hazel M, Parker GJ, Love DC, et al. Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc Natl Acad Sci U S A. 2002;99:10695–9. doi: 10.1073/pnas.152346899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Parker G, Taylor R, Jones D, McClain D. Hyperglycemia and inhibition of glycogen synthase in streptozotocin-treated mice: role of O-linked N-acetylglucosamine. J Biol Chem. 2004;279:20636–42. doi: 10.1074/jbc.M312139200. [DOI] [PubMed] [Google Scholar]
- 121.Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol. 2005;43:19–29. doi: 10.1016/j.vph.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 122.Lima VV, Giachini FR, Choi H, Carneiro FS, Carneiro ZN, Fortes ZB, et al. Impaired vasodilator activity in deoxycorticosterone acetate-salt hypertension is associated with increased protein O-GlcNAcylation. Hypertension. 2009;53:166–74. doi: 10.1161/HYPERTENSIONAHA.108.116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guinez C, Mir AM, Leroy Y, Cacan R, Michalski JC, Lefebvre T. Hsp70-GlcNAc-binding activity is released by stress, proteasome inhibition, and protein misfolding. Biochem Biophys Res Commun. 2007;361:414–20. doi: 10.1016/j.bbrc.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 124.Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci (Lond) 2007;112:375–84. doi: 10.1042/CS20060247. [DOI] [PubMed] [Google Scholar]
- 125.James LR, Tang D, Ingram A, Ly H, Thai K, Cai L, et al. Flux through the hexosamine pathway is a determinant of nuclear factor kappaB- dependent promoter activation. Diabetes. 2002;51:1146–56. doi: 10.2337/diabetes.51.4.1146. [DOI] [PubMed] [Google Scholar]
- 126.Kneass ZT, Marchase RB. Neutrophils exhibit rapid agonist-induced increases in protein-associated O-GlcNAc. J Biol Chem. 2004;279:45759–65. doi: 10.1074/jbc.M407911200. [DOI] [PubMed] [Google Scholar]
- 127.Daniels MC, McClain DA, Crook ED. Transcriptional regulation of transforming growth factor beta1 by glucose: investigation into the role of the hexosamine biosynthesis pathway. Am J Med Sci. 2000;319:138–42. doi: 10.1097/00000441-200003000-00002. [DOI] [PubMed] [Google Scholar]
- 128.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97:12222–6. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goldberg HJ, Whiteside CI, Hart GW, Fantus IG. Posttranslational, reversible O-glycosylation is stimulated by high glucose and mediates plasminogen activator inhibitor-1 gene expression and Sp1 transcriptional activity in glomerular mesangial cells. Endocrinology. 2006;147:222–31. doi: 10.1210/en.2005-0523. [DOI] [PubMed] [Google Scholar]
- 130.Xing D, Feng W, Not LG, Miller AP, Zhang Y, Chen YF, et al. Increased protein O-GlcNAc modification inhibits inflammatory and neointimal responses to acute endoluminal arterial injury. Am J Physiol Heart Circ Physiol. 2008;295:H335–42. doi: 10.1152/ajpheart.01259.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen JT, Liang JB, Chou CL, Chien MW, Shyu RC, Chou PI, et al. Glucosamine sulfate inhibits TNF-alpha and IFN-gamma-induced production of ICAM-1 in human retinal pigment epithelial cells in vitro. Invest Ophthalmol Vis Sci. 2006;47:664–72. doi: 10.1167/iovs.05-1008. [DOI] [PubMed] [Google Scholar]
- 132.Gouze JN, Bianchi A, Becuwe P, Dauca M, Netter P, Magdalou J, et al. Glucosamine modulates IL-1-induced activation of rat chondrocytes at a receptor level, and by inhibiting the NF-kappa B pathway. FEBS Lett. 2002;510:166–70. doi: 10.1016/s0014-5793(01)03255-0. [DOI] [PubMed] [Google Scholar]
- 133.Largo R, Alvarez-Soria MA, Diez-Ortego I, Calvo E, Sanchez-Pernaute O, Egido J, et al. Glucosamine inhibits IL-1beta-induced NFkappaB activation in human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2003;11:290–8. doi: 10.1016/s1063-4584(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 134.Shikhman AR, Kuhn K, Alaaeddine N, Lotz M. N-acetylglucosamine prevents IL-1 beta-mediated activation of human chondrocytes. J Immunol. 2001;166:5155–60. doi: 10.4049/jimmunol.166.8.5155. [DOI] [PubMed] [Google Scholar]
- 135.Yang S, Zou LY, Bounelis P, Chaudry I, Chatham JC, Marchase RB. Glucosamine administration during resuscitation improves organ function after trauma hemorrhage. Shock. 2006;25:600–7. doi: 10.1097/01.shk.0000209563.07693.db. [DOI] [PubMed] [Google Scholar]
- 136.Hua J, Sakamoto K, Nagaoka I. Inhibitory actions of glucosamine, a therapeutic agent for osteoarthritis, on the functions of neutrophils. J Leukoc Biol. 2002;71:632–40. [PubMed] [Google Scholar]
- 137.Fulop N, Zhang Z, Marchase RB, Chatham JC. Glucosamine cardioprotection in perfused rat hearts associated with increased O-linked N-acetylglucosamine protein modification and altered p38 activation. Am J Physiol Heart Circ Physiol. 2007;292:H2227–36. doi: 10.1152/ajpheart.01091.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]