Liver allografts are resistant to hyperacute or antibody mediated rejection from preformed antibodies. No consistent syndrome develops following transplantation when a recipient harbors preformed lymphocytotoxic antibodies.1,2 The only apparent deleterious effect of preformed lymphocytotoxic antibodies is a higher incidence of the “vanishing bile duct syndrome.”3 Despite these clinical observations, a phenomenon akin to “hyperacute” rejection can be seen following liver transplantation in experimental animals who were previously sensitized to donor antigens.4,5 Additionally, isolated case reports thought to represent hyperacute rejection of the liver in humans, have appeared over the years.6,7 However, no pathophysiologic mechanisms or means of predicting its occurrence in humans have been proposed. The following study was designed to address this problem.
The syndrome of hyperacute rejection was first recognized in renal transplantation when crossing ABO blood group barriers.8 A similar process developed when a recipient harbored preformed lymphocytotoxic antibodies.9 We therefore took advantage of this historical perspective and embarked on a detailed study of liver allografts across ABO blood group barriers.
Analysis of the patient’s course and pathology specimens revealed a significantly (p < 0.05) higher rate of early graft failure (<3 weeks) in ABO incompatible (ABO-I) grafts when compared to ABO compatible (ABO-C), age, sex and priority matched controls. Study of these cases allowed for the recognition of antibody mediated rejection of human liver allografts and for the development of criteria for establishing the diagnosis.
MATERIALS AND METHODS
Case Selection
All adult and pediatric patients who received a primary ABO incompatible liver graft (non O → O, AB → non AB, B → A, or A → B) at the University of Pittsburgh between 1981 and 1987 were studied. The age, sex and priority status of each of the ABO-I cases was recorded. Priority status refers to the condition of the patient prior to transplantation. Priority 1 patients are the least sick and those with a priority of 6, are in the intensive care unit on ventilatory support. These primary ABO-I cases were then compared to two groups of control patients.
All patients at the, University of Pittsburgh received a sequential OLT number to designate their position in the series and the time of their initial transplant operation. The first group of controls was selected on the basis of the time of initial liver transplantation by the OLT number. These are referred to subsequently as “clinical” controls. For example, if a patient receiving an ABO-I liver graft was given OLT 1003, the immediately prior and consecutive age matched, ABO-C patient was selected as a control. Therefore, each ABO-I patient had two controls. However, in some cases the ABO-I cases were close or consecutive in OLT number. When this occurred, a single case was used as a control for two different ABO-I cases. The age, sex and priority status of each of these controls were recorded. The results of the T-warm lymphocytotoxic crossmatch in this group were not considered. The numerical method of selecting control cases was chosen to compensate for the variable handling of patients over the years.
Initial analysis of the data from the two groups revealed a much higher incidence of early graft failure because of hemorrhagic necrosis in the ABO-I compared to the ABO-C clinical control group. Therefore, we chose a second control group, referred to as “pathologic” controls. This group consisted of 10 ABO-C, cross-match negative patients, transplanted over the same period of time, who also lost their grafts within the first several weeks. These cases were chosen as histo- and immunopathologic controls since we hypothesized that all early graft failures may have a similar appearance, unrelated to prior sensitization. However, we did exclude patients with a positive T-warm lymphocytotoxic crossmatch from this group since it was possible, but unlikely, that the sensitization led to early graft failure.
Patient Demographic Data
There were 24 patients (5 adults, 19 children) who received primary ABO-I hepatic grafts. The clinical control group contained 38 patients (10 adults, 28 children). These two groups were compared for graft survival, and will be referred to as the “study group.” The study group was primarily pediatric; average for pediatric patients was 5.8 years and for adults 45.3 years, with 48 (77%) under the age of 18 at the time of transplantation. Among the ABO-I patients, 83% were under the age of 18 compared with 74% in the control group (p > 0.05). The overall distribution of the priority scores did not differ significantly between these two groups (p > 0.05).
Pathologic Studies
All pathologic specimens from the three groups were reviewed. Particular attention was given to histopathologic findings of platelet-fibrin thrombi, vasculitis, congestion, hemorrhage, neutrophilic exudation and ischemic necrosis. Evidence for the deposition of IgM, IgG, IgA, Clq, C3, C4, and fibrinogen was sought for in the ABO-I cases and pathologic controls using a direct immunofluorescent and an indirect immunoperoxidase technique.
Elution studies were performed as follows. Frozen graft tissue (2-5 grams) were minced with the use of a tissue homogenizer. The tissue was washed (4×) in 6% bovine albumin in the 4°C. A heat elution was performed at 56°C for fifteen minutes into 2-3 ml of albumin, then the tissue was centrifuged at 4,500 ×G for five minutes. The supernatant was tested by hemaglutination against A1, B and O red cells with readings at immediate spin, 30 minutes and 37°C in polyspecific antiglobulin. The immunoglobulin class of eluted antibodies was determined by a) the use of anti-IgG anti-globulin re-agent and treatment with dithiothreitol to inactivate IgM. The last wash solution was tested negative for ABO antibody served as control. Testing was performed without knowledge of the donor and/or recipient ABO types.
RESULTS
Graft Survival Analysis
All patients in the study group were followed for a minimum of 30 days (up to 2,141 days). The results are shown graphically in Fig 1. Among the clinical controls 4 (11%) of grafts failed within 30 days, compared to 11 (46%) of the primary ABO-I cases (p < 0.05). A ratio of graft failure within 30 days of transplantation defined by each of the independent variables (gender, age group and priority status) at the time of transplant was calculated. A statistically significant (p < 0.05) odds ratio greater than 1, implying a greater risk of early graft failure among those receiving incompatible grafts than among those receiving compatible grafts, was found for females, pediatric patients and for patients in the hospital (not in the ICU ) at the time of transplantation. A multi-variant model (logistic regression) was used to determine whether there was an effect of ABO compatibility on graft survival, simultaneously adjusting for the effect of gender, age and priority status. When the full model was fit, a statistically significant effect (p < 0.05) was found for ABO compatibility but not for any of the other independent variables. The adjusted odds ratio was 11.8 with a 95% confidence interval of (2.5, 56.6). This implies that adjusting for age, gender and priority status, the odds of a recipient suffering graft failure within the first 30 days of liver transplantation is about 12 times greater among those receiving incompatible than among those receiving compatible grafts. None of the other independent variables (age, sex, or priority status) examined were found to be statistically significant (p > 0.05), adjusting for a case or control status.
Fig 1.
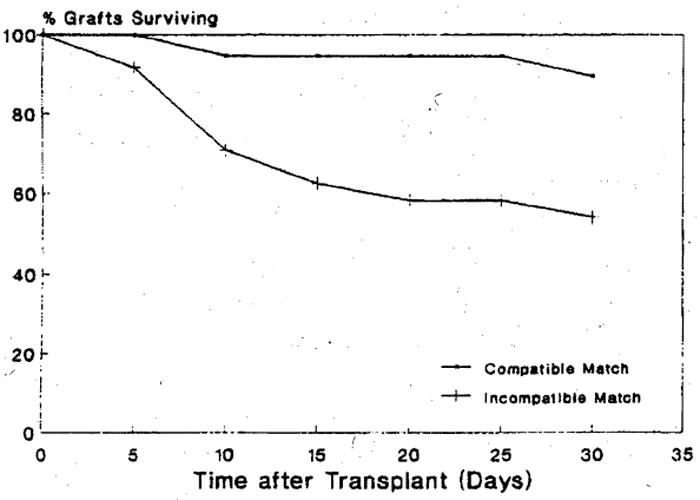
Comparison of graft survival in first 30 days post-transplantation of ABO-I and ABO-C liver allografts.
Individual Case Analysis
A detailed review of each patient’s clinical course and subsequent classification was performed and reported elsewhere.10 In summary, among the ABO-I patients 10 (42%) still have functional grafts. Fourteen of these patients (58%) have experienced graft failure and were retransplanted or expired. Four of these graft failures were directly attributable to humoral rejection due to preformed isoagglutinins. In eight others, humoral rejection was thought to play a signifinicant, if not the primary role in graft failure. Recurrent hepatitis B was responsible for graft failure in one case. In the remaining patient, there was evidence of antibody mediated graft damage, but graft failure was temporally too remote (147 days) from the operation to implicate antibodies as the primary reason for graft failure. The average graft survival for failed graft was 32 days. However, most of the grafts that failed, did so within the first 2-3 weeks. The few late graft failures skewed the mean.
In the clinical control group, 24 (63%) of the grafts are still functioning. However, the causes of graft failure differed significantly from that seen in the primary ABO-I group. Additionally, the average graft survival for failed grafts in this group was 197 days; significantly (p < 0.05) longer than the failed primary ABO-I grafts.
Among the pathology controls, all experienced graft failure within the first two weeks. Eight grafts failed from hepatic artery or iliac graft thrombosis and/or arterial mural dissection, which according to the operating surgeon, was explainable on technical grounds. One patient had no portal vein for anastomosis requiring an alternative, but unsuccessful, venous anastomosis. The cause of graft failure in the remaining patient was determined to be the result of preservation injury.
Pathologic Findings
A majority of the pathologic samples were taken as a result of the onset of early graft dysfunction. However, protocol pre- and post-implantation biopsies were available in five patients. The following description is a summary of the pathologic findings from several ABO-I cases.
Pre-implantation biopsies generally contained minimal to no pathologic alterations. Samples taken 2-6 hours after implantation showed a rather impressive clustering of neutrophils, fibrin deposition and red blood cell sludging in the sinusoids. This was seen in association with focal hemorrhage in the space of Disse, focal hepatocellular aggregation or single cell acidophilic necrosis. Biopsies one to two days later continued to show the above changes but in addition, small clusters of hepatocytes demonstrating coagulative necrosis were seen. Small portal arteries may or may not show fibrinoid degeneration or inflammation at this time. A mild neutrophilic portal exudate may begin to appear with focal duct proliferation as a sign of regeneration. At this stage, the histologic changes can be quite difficult if not impossible to separate from those of prolonged preservation injury. Thereafter, a progressive patchy geographic hemorrhagic infarction of the organ ensues. The early progression of the changes may or may not be detectable in biopsies because of the patchy nature of the process and subsequent sampling problems. Once the process is widespread, coagulopathy, submassive or massive necrosis and hepatic failure become evident necessitating retransplantation.
Gross examination of failed ABO-I grafts revealed enlarged, hemorrhagic organs mottled with random areas of necrosis with or without large vessel thrombosis. The capsule had actually ruptured in one liver.
Microscopically, focal fibrinoid necrosis or inflammatory vasculitis of small or medium size arteries was detectable in only four cases. More prevalant findings included arterial and venous endothelial cell reactivity, with mild lumenal platelet and neutrophil sludging. The most evident finding was the presence of focal fibrin masses attached to a partially disrupted vascular wall, extending in a flame-like fashion into the vessel lumen. These were particularly common in venous channels. However, similar arterial deposits could also be seen. Throughout the hepatic parenchyma most all ABO-I cases showed severe and widespread geographic areas of hemorrhagic necrosis. The necrotic tissue was intermixed with neutrophils and there was often no particular zonal distribution.
Clinical and Pathologic Control Cases
Pathologic specimens from the clinical control group showed little resemblance to the findings described in the ABO-I cases. The exception to this statement is that biopsies obtained in the first several days post-transplant from controls often contained focal sinusoidal neutrophil clusters, congestion and mild acidophilic necrosis of hepatocytes similar to that described in the early course of the ABO-I patients. However, the magnitude of these changes in the control groups was less than those seen in the ABO-I group.
Failed grafts from the pathologic control group with arterial thrombosis and hilar abscesses, demonstrated areas of necrosis which appeared reminiscent of necrosis seen in the ABO-I livers. However, necrotizing and/or inflammatory vasculitis was not found.
Immunofluorescent Studies
All cases categorized as primary antibody mediated rejection demonstrated the presence of focal but intense deposition of IgM and Clq, with lesser amounts of C3, usually in the arterial walls. Focal IgG deposits were found in only one graft. Patchy sinusoidal and venous staining could also be seen but was much less impressive than deposits in the arteries. In general, the arterial deposits were not uniformly distributed throughout the liver. In fact some subcapsular sections were negative while those taken closer to the hilum demonstrated impressive arterial deposition. Sequential staining of biopsy specimens from several ABO-I cases revealed intense IgM, IgG and C3 in the sinusoidal, arterial and venous systems, prior to graft failure. However, examination of the immunofluorescent staining days or weeks later contained only focal IgM and Clq in the arteries.
In the pathology controls, focal deposition of IgM and IgG was seen in only one liver, from a patient who had a weakly positive crossmatch. All other cases were negative. Sinusoidal staining for C1q and fibrinogen was frequently positive in both the ABO-I and the ABO-C livers, particularly in and around the central vein region. This was considered to be a non-specific finding; the result of leakage through a damaged sinusoidal wall or through Kupffer cell phagocytosis. Although not included in this study, we have seen several examples of non-specific leakage of IgM into the wall of medium-sized arteries of grafts with arterial thrombosis at the anastomosis. In general, these deposits were less intense and accompanied by the leakage of other serum components such as fibrinogen and albumin.
Elution Studies
Tissue eluates from failed primary ABO-I allografts, which were thought to demonstrate primary antibody mediated rejection all showed reactivity against the donor ABO antigens (N = 4). Analysis of the reactivity using dithiothreitol and anti-IgG antiglobulin reagent gave results characteristic of the IgM antibody class, identical to that found within the tissues. Eluates of tissues from three of the liver pathology controls were negative for isoagglutinin activity.
Clinical Observations
A clinical diagnosis of hyperacute rejection, that is a graft after initial adequate reperfusion which becomes cyanotic, mottled, flaccid and fails to produce bile was not made in this group of patients. However, retrospectively several surgeons recall a swelling and “hardening” of the ABO-I livers, three to fours hours after reperfusion. The only other possible clue to future complications in the immediate reperfusion period was the detection fibrinolysis and difficulty in achieving hemostasis.
The first several days post-transplant most patients with ABO-I grafts experienced a relentless rise in serum transaminase values, signaling a catastrophic event. Angiograms and percutaneous biopsies were often performed to rule out arterial thrombosis or other definable causes of graft malfunction. Arterial angiography in one case was particularly striking (Fig 2); it demonstrated a markedly narrowed arterial tree. This diffuse narrowing was thought to be the result of diffuse arterial spasm. The rise in transaminase values was followed by bleeding and hepatic failure.
Fig 2.
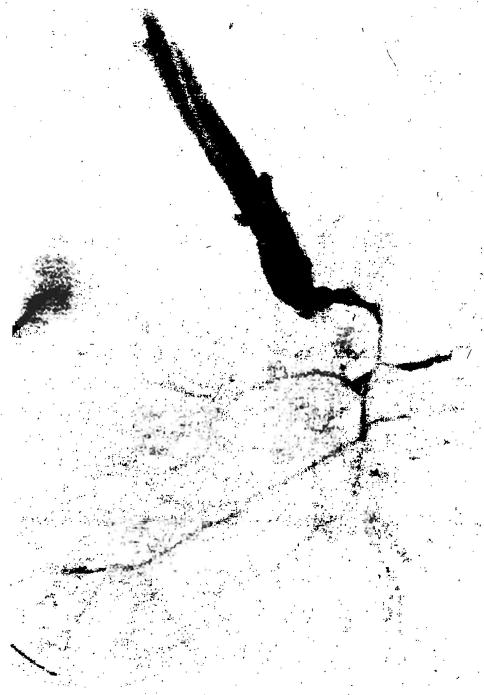
Severe and diffuse narrowing of the entire allograft intrahepatic arterial tree demonstrated on selective aortic conduit arteriogram two weeks following liver transplantation.
The appearance of the organs at re-transplantation was similar to kidneys undergoing “hyperacute” rejection. They were enlarged, mottled and cyanotic. The gross intraoperative appearance of one graft was particularly impressive. The organ was massively enlarged, cyanotic, had a capsular rupture and was bleeding from the surface.
DISCUSSION
The results of this study demonstrate that liver allografts are susceptible to antibody mediated (hyperacute) rejection from preformed (isoagglutinins) antibodies. The diagnosis, however, should be limited to cases which meet all of the following criteria: 1) early graft failure with no alternative clinical or pathological explanation, 2) consistent light immunofluorescent microscopy findings, 3) demonstration of a pre-sensitization stated in the recipient and 4) presence of donor specific antibodies in a tissue eluate from the failed graft. Strict adherence to these criteria will prevent over-diagnosis, since a similar clinical course and hemorrhagic necrosis of the graft may occur in a variety of settings.10
Hyperacute rejection of a human liver allograft has not been previously documented. It has been convincingly demonstrated that lymphocytotoxic antibodies have relatively little effect on the immediate post-transplant course.1-3 However, no detailed observations of a series of ABO-I human liver grafts has been reported. The indepth study of these patients has allowed us to recognize both the clinical and pathologic syndrome that develops following transplantation in the face of a specific type of preformed antibody. Furthermore, recognition of the syndrome then paved the way for the development of the above criteria to substantiate the diagnosis.
The pathophysiologic mechanisms underlying the cause of graft failure in hyperacute liver allograft rejection are probably similar to those in kidney or heart grafts. However, some noticeable differences may help us understand the phenomenon of hyperacute rejection in general. Undoubtedly, endothelial damage from preformed antibodies is the initial event. Conventionally, this was thought to be followed by diffuse intra-organ coagulation, which was responsible for the ischemic necrosis. However, in liver allografts although platelet and neutrophil sludging can be identified, more common is the finding of venous and arterial fibrin deposition. Also, the dynamic observations of diffuse arterial spasm may be of importance, since it is thought to play a prominent role in hyperacute rejection in animal models.11,12
Finally, we have recognized that antibody mediated (hyperacute) rejection of the liver exists, and crudely identified its characteristic features. However, much work needs to be done in this area. A search must be made for other antibodies which may be implicated in early graft failures. Presently, it appears that T-warm lymphocytotoxic antibodies are unlikely candidates in most situations. However, as yet unidentified antigen-antibody systems may be operative, since a significant percentage of early graft failures remains unexplained (unpublished observation).
References
- 1.Iwatsuki S, Iwasaki Y, Kano T, Klintmalm G, Koep LJ, Weil R, Starzl TE. Transplant Proc. 1981;13:286–288. [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon RD, Fung JJ, Markus B, Fox I, Iwatsuki S, Esquivel CO, Tzakis A, Todo S, Starzl TE. Surgery. 1986;100:705–715. [PMC free article] [PubMed] [Google Scholar]
- 3.Batts KP, Moore SB, Perkins JD, Weisner RH, Grambesch PM, Krom RA. Transplantation. 1988;45:376. doi: 10.1097/00007890-198802000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Gubernatis G, Lauchart W, Jonber M, Steinhoff G, Bornscheuer A, Newhaus P, van Es AA, Kemnitz J, Wonigeit K, Pichlmayr R. Transplant Proc. 1987;19:1082–1083. [PubMed] [Google Scholar]
- 5.Knechtle SJ, Kolbeck PC, Tsuchimoto S, Coundouriotis A, Sanfilippo F, Bollinger RR. Transplant Proc. 1987;19:1070–1076. [PubMed] [Google Scholar]
- 6.Hume DM, Williams GM. Personal communication to T.E. Starl. In: Starzl TE, editor. Experience in Hepatic Transplantation. Philadelphia: WB Saunders Co; Feb 8, 1969. p. 269. [Google Scholar]
- 7.Snover DC. Transplant Proc. 1986;18:123–127. [Google Scholar]
- 8.Starzl TE, Marchioro TL, Holmes JH, Hermann G, Brittain RS, Stonington OH, Talmage DW, Waddell WR. Surgery. 1964;55:195–200. [PMC free article] [PubMed] [Google Scholar]
- 9.Kissmeyer-Neilsen F, Olsen S, Peterson VP, Fjeldbory O. Lancet. 1966;2:662–665. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 10.Demetris AJ, Jaffe R, Tzakis A, Ramsey G, Todo S, Belle S, Esquivel C, Shapiro R, Zjako A, Markus B, Morozec E, Van Thiel DH, Sysyn G, Gordon R, Makowka L, Starzl TE. Antibody mediated rejection in human orthotopic liver allografts: A study of liver transplantation across ABO blood group barriers. Am J Pathol. 1988 in press. [PMC free article] [PubMed] [Google Scholar]
- 11.Dempster WJ. Br J Exp Pathol. 1971;52:172–188. [PMC free article] [PubMed] [Google Scholar]
- 12.Terada Y, Veno A. Transplantation. 1983;35:205–208. doi: 10.1097/00007890-198303000-00003. [DOI] [PubMed] [Google Scholar]


