Abstract
From January 1981 to December 1990, 2180 patients underwent orthotopic liver transplantation at the University of Pittsburgh. Thirty-two patients (1.5%) were identified with invasive aspergillosis (29 lung, 2 intraabdominal, 1 meningitis). Of 29 patients with invasive lung disease, only 23 (79%) had positive culture (Aspergillus fumigatus, 20; Aspergillus flavus, 3). Forty-eight variables were analyzed and compared in 23 patients with invasive disease with positive cultures and 9 patients with colonization only. The variables associated with pulmonary invasive disease, by univariate analysis, were surgical time (P = .03), presence of laparotomies (P = .02), higher creatinine level at time of Aspergillus isolation (P = .01), and use of OKT3 (P = .02). However, in a multivariate analysis, only the last two (creatinine, OKT3) were associated with invasive lung aspergillosis. Of 4 patients with positive abdominal wound culture, 2 had local invasive aspergillosis. Therefore, positive cultures of Aspergillus organisms from respiratory secretions and wound drainage may represent invasive disease and should not be ignored.
Fungal infections remain an important cause of high morbidity and mortality after orthotopic liver transplantation [1–3]. Aspergillosis is the second most important cause of fungal infection after candidiasis and is associated with a mortality approaching 100% [1–4]. The significance of isolating Aspergillus species from any clinical specimen is still unclear, and the distinction between disease and colonization is difficult. This study assessed the significance of such cultures and identified factors associated with the development of invasive lung aspergillosis after liver transplantation.
Materials and Methods
Study population
During a 10-year period from January 1981 through December 1990, 2180 adults underwent orthotopic liver transplantation at Presbyterian University Hospital, University of Pittsburgh. Surveillance for fungi was not routinely done; specimens were usually submitted for culture when clinically indicated. All cases after liver transplantation with positive culture or histology for Aspergillus organisms at any site were retrospectively identified from clinical microbiology and pathology records. Standard methods of fungal culture and identification were used. Histologic sections were stained with hematoxylin-eosin, methenamine silver, and occasionally periodic acid–Schiff stains. Septate hyphae with dichotomous branching at 45° angles were considered histologic evidence of aspergillosis. The medical records of these cases were reviewed.
Definitions of invasive aspergillosis and colonization
Definitive invasive aspergillosis included patients diagnosed by histology (biopsy or autopsy). Probable invasive aspergillosis included patients with a characteristic clinical and radiologic picture, a culture positive for Aspergillus species, and a lack of response to antibacterial therapy [5]. Patients who had positive culture with no evidence of invasive disease were classified as having colonization. Nosocomial aspergillosis was defined as a case that occurred during the patient’s hospital stay without evidence of its presence at admission.
Patient management and immunosuppression
All patients underwent orthotopic liver transplant as described by Starzl et al. [6], either with primary anastomosis of the bile duct or with Roux-en-Y choledochojejunostomy. Two immunosuppression regimens were used. The first included cyclosporine (Sandoz, East Hanover, NJ) and steroids. Cyclosporine was initially administered intravenously (3 mg/kg/day). Oral cyclosporine was begun with the return of bowel function and overlapped with the intravenous dose for a short period. Since 1983, cyclosporine has been adjusted to obtain blood levels of 800–1000 ng/mL by RIA. An intravenous intraoperative dose of 1 g of methylprednisolone was followed by a 5-day taper from 200 to 20 mg. Azathioprine was also given to some patients. The second regimen included the new immunosuppressive agent FK506 (Fujisawa Pharmaceutical, Osaka, Japan) and low-dose steroids (20 mg/day) starting immediately after transplantation. Rejection episodes occurring with either regimen were treated with increased dose of steroids, either a 1-g “pulse” dose or a 5-day recycle of methylprednisolone. Steroid-resistant rejection was treated with intravenous OKT3 monoclonal antibody (Ortho Pharmaceuticals, Raritan, NJ).
Bacterial prophylaxis included intravenous cefotaxime and ampicillin (both, 4 g/day) given for 3 days. Fungal prophylaxis included oral nystatin (2 million units daily) begun at the time of surgery and continued throughout the hospitalization.
Factors associated with invasive disease
Forty-eight clinical variables were analyzed and compared between patients with pulmonary invasive aspergillosis and patients with colonization of respiratory secretions with Aspergillus species. Laboratory variables at day of diagnosis of invasive aspergillosis were compared with the same variables at the day of the first positive Aspergillus culture in the colonization group. The variables analyzed before transplant were age, sex, liver disease, use of steroids and other immunosuppressive agents, use of intravenous antibiotics in the preceding month, and liver function tests. Intraoperatively analyzed were cumulative intraoperative time (hours), retransplantation operations, other abdominal operations (laparotomies), and total number of blood products given in the operating room. Posttransplant variables analyzed were immunosuppressive agents (cyclosporine, FK506, azathioprine, OKT3, steroids) given, the use and duration of nonprophylactic intravenous antibiotics, Aspergillus isolation (species, site, time after transplantation, and association with Candida species), clinical complaints, associated infections, presence of diabetes, requirement for hemodialysis, and laboratory values (levels or counts of total white blood cells, polymorphonuclear leukocytes, immature band forms, hematocrit, platelets, prothrombin time, partial thromboplastin time, total bilirubin, alkaline phosphatase, aspartate aminotransferase [AST], alanine aminotransferase, and serum albumin).
Statistical analysis
We did univariate logistic analyses to identify factors associated with invasive aspergillosis. The likelihood ratio χ2 test (two-tailed) was used to assess whether a factor was associated with invasive disease. On the basis of results of the univariate analyses, we did a multivariate logistic analysis. Variables were included in the multivariate analysis if they had P < .25 or were known to be biologically important. The backward elimination method was used as a variable selection technique. The likelihood ratio χ2 test (two-tailed) was used to assess the importance of each factor to the model (i.e., whether a factor was to be excluded from the model) [7].
Results
During the 10-year study period, 32 (1.5%) of 2180 liver transplant recipients developed invasive aspergillosis. The diagnosis was premortem in 19 patients (59%) and postmortem in 13 (41 %). They included 29 patients with pulmonary disease (definitive in 21, probable in 8), 2 with definitive intraabdominal disease, and 1 with Aspergillus meningitis who was diagnosed at autopsy. In 27 patients the diagnosis was done in the initial posttransplant period and in 5 patients at subsequent admissions. Of these 5 late admissions, 3 patients were readmitted for retransplantation. All cases but 1 were nosocomial infections. Of the 29 cases with invasive pulmonary aspergillosis, 23 (79%) had positive cultures (20 with Aspergillus fumigatus, 3 with Aspergillus flavus). The 6 remaining patients had definitive pulmonary aspergillosis with negative cultures in respiratory secretions (21 %). Both patients with intraabdominal invasive aspergillosis, had positive abdominal wound culture (A. fumigatus).
We subsequently identified 11 patients who had positive Aspergillus cultures without evidence of invasive aspergillosis and were never given amphotericin B. They were classified as having only colonization. In 9 patients, Aspergillus species were isolated from respiratory secretions (A. fumigatus in 5, A. flavus in 2, Aspergillus niger in 2) and in 2 patients from abdominal wound drainage (A. fumigatus in both). Nine of these patients survived and are still alive with a follow-up between 8 months and 8 years. Two patients died with abdominal sepsis at days 27 and 97; invasive aspergillosis was not found at autopsy.
The number of samples submitted for fungal culture in patients with invasive aspergillosis was four times higher than in patients with colonization (mean, 6 vs. 1.5 samples/patient, respectively).
Patients with Positive Culture in Respiratory Secretions
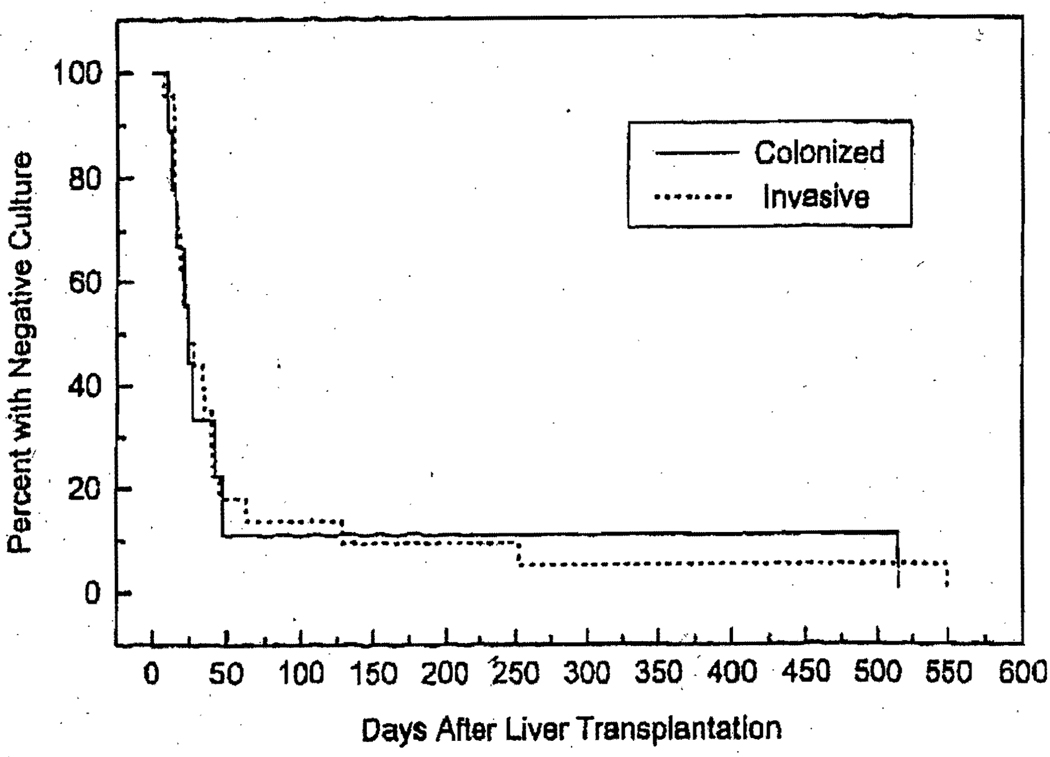
Aspergillus organisms were isolated from the respiratory secretions of 32 patients (1.5%). To assess the significance of this, we compared 23 patients with invasive lung disease (who also had positive culture of respiratory secretions) to 9 patients with colonization. The two groups were comparable with respect to liver disease, sex ratio, and age. There was no statistical difference between the time of first positive culture and the time of diagnosis of invasive disease (invasive group). The median time of diagnosis of invasive disease, expressed in days after the first liver transplant operation, was 38 days (range, 7–550), and the median time of the first positive culture in these patients was 24 days (range, 7–550). Figure 1 shows the time of occurrence of the first positive Aspergillus culture in relation to the first transplant operation in the two groups. Fifty percent of the patients were diagnosed with the disease <1 month after transplantation. The median time of diagnosis was 1.2 months. Three patients were diagnosed >4 months after transplantation. The maximum follow-up was 80 months.
Figure 1.
Time of occurrence of positive culture for Aspergillus organisms in respiratory secretions in 23 patients with invasive aspergillosis and 9 with colonization after liver transplantation. In invasive group, this time is also time of diagnosis of invasive disease.
Of the 32 patients, 31 had temperature elevations >38°C at time of Aspergillus isolation; the exception was a patient in the colonization group.
Univariate analysis
Table 1 shows the variables that were found by univariate analysis to be associated with invasive disease in the lung. All variables in the table have a positive association with invasive disease (i.e., by increasing the level of each factor, we increase the risk of invasive disease). Four factors were statistically associated (P < .05) with invasive disease: cumulative surgical time (hours) before diagnosis (P = .028), laparotomies, excluding those done for transplantation (P = .024), creatinine level at time of diagnosis (P = .015), and OKT3 administration (P = .015). The remaining seven variables were not statistically associated with invasive disease but either were known to be biologically important or met the criteria for inclusion in the multivariate analysis (i.e., P < .25). These variables included use of additional immunosuppressive agents (steroids, particularly repeat cycles, and azathioprine), multiple transplant operations, number of units of red blood cells transfused in transplant operations, the increase in AST at time of diagnosis relative to the pretransplant value, and the number of days intravenous antibiotics were given for treatment of infections (we did not count the number of days intravenous antibiotics were given for prophylaxis).
Table 1.
Factors associated with invasive disease, univariate analysis, in liver transplant recipients, with aspergillosis.
| Variable | Colonization | Invasive disease |
Odds ratio |
P* |
|---|---|---|---|---|
| Surgical time, h | 13.7(7.1) | 21.1 (9.6) | 1.12 | .03 |
| Additional steroids | 33.3 | 65.2 | 3.75 | .10 |
| Azathioprine | 22.2 | 52.2 | 3.82 | .12 |
| Red blood cells, units | 19.8 (17.6) | 33.7 (25.7) | 1.04 | .10 |
| Laparotomies | 11.1 | 52.2 | 8.73 | .02 |
| Creatinine, mg/dL | 2.1 (1.2) | 3.7(1.8) | 2.10 | .01 |
| Change in aspartate aminotransferase, IU/L | 0.1 (1.5) | 3.7(1.8) | 1.21 | .20 |
| Repeat cycle | 11.1 | 43.5 | 6.15 | .07 |
| OKT3 monoclonal antibody | 22.2 | 69.6 | 8.00 | .02 |
| Retransplantation | 33.3 | 60.9 | 3.11 | .16 |
| Days of antibiotics | 10.3 (7.5) | 16.7 (14.5) | 1.05 | .18 |
NOTE. Data are mean (SD) or % of patients.
P < .05 is significant, based on likelihood ratio χ2 test.
Multivariate analysis
Of the variables that met the criteria for inclusion in the multivariate analysis, one, surgical time, is a function of two other factors: the number of laparotomies and the number of transplant operations. Because of the interrelationships among surgical time, laparotomies, and transplant operations, we run the risk of colinearity in the multivariate analysis. To avoid this problem, cumulative surgical time was analyzed with the exclusion of the number of laparotomies and the number of transplant operations (model A). These two factors were subsequently analyzed with the exclusion of cumulative surgical time (model B).
The results of the multivariate analyses are presented in table 2. Both models show that the use of OKT3 monoclonal antibody is associated with invasive disease. However model B’s ability to predict invasive disease was inadequate (goodness of fit, P = .07). This model was produced when cumulative surgical time was excluded from the multivariate analysis. When the number of laparotomies and the number of transplant operations were excluded from the analysis (model A), only two variables were associated with invasive disease: the use of OKT3 monoclonal antibody and the creatinine level at diagnosis. Neither model identified cumulative surgical time or laparotomies and transplant operations as being associated with invasive disease. The fact that cumulative surgical time and laparotomies were statistically associated with invasive disease by univariate analysis but not by multivariate analysis suggests that both factors are only surrogates of some other factors. A comparison of models A and B via the likelihood ratio χ2 test indicated that the number of units of red blood cells given in the operating room was not an important predictor of invasive disease. Lung infiltrates were present in all 23 patients with invasive aspergillosis by definition but in only 33% of patients colonized. Because one of our selection criteria for invasive aspergillosis included changes on chest radiography, we excluded this factor from our multivariate analysis model.
Table 2.
Results of multivariate analysis of liver transplant recipients with aspergillosis.
| Variables | Adjusted odds ratio | P |
|---|---|---|
| Model A | ||
| Creatinine | 1.89 | .10 |
| OKT3 | 6.29 | .05 |
| Goodness of fit | .37 | |
| Model B | ||
| Units of red blood cells | 1.04 | .14 |
| Creatinine | 1.96 | .08 |
| OKT3 | 8.53 | .05 |
| Goodness of fit | .07 |
NOTE. Model A excludes number of laparotomies and number of transplant operations; model B excludes cumulative surgical time.
Patients with Positive Culture of Wound Drainage
Four patients had positive Aspergillus culture (A. fumigatus) in abdominal wound drainage. They were 3 women and 1 man with a mean age of 38 years. Two of them were only colonized with the fungus and survived. Both had bacterial peritonitis after a second transplant operation for which they received courses of antibiotics. The other 2 developed abdominal invasive disease with Aspergillus species and died. Their autopsies did not show lung involvement. In 1 case there was invasion of the diaphragm and suprahepatic abscess, and in the other there was Aspergillus invasion and thrombosis of the portal vein.
Discussion
In a survey of fungal infections after 101 consecutive liver transplants done at our institution, 4 patients were diagnosed with invasive aspergillosis and all died secondary to their infection [4]. A major obstacle for successful management of invasive aspergillosis is the difficulty in establishing early diagnosis [8]. Isolation of Aspergillus species from clinical specimens ranges between 2% and 8% in the general population [9, 10]. Weiland et at. [11] reported a rate of 3% positive respiratory cultures in kidney transplantation, and in the current study we found a rate of 1.5% after liver transplantation.
Isolation of Aspergillus species from respiratory and wound specimens does not imply disease, as this fungus may be a colonizer or a laboratory contaminant. Although its isolation has often been ignored, recent studies have shown its usefulness as a diagnostic tool in the immunocompromised host. Yu et al. [12] observed that positive cultures rarely indicate disease in noncompromised patients but are virtually diagnostic of invasive aspergillosis in leukemic patients with neutropenia.
Neutropenic patients are predisposed to Aspergillus pneumonia [13]. In contrast, solid organ transplant patients may develop Aspergillus pneumonia without neutropenia. This was first shown in kidney transplant recipients, where only a high daily dose of steroids but not neutropenia was a predictor of invasive aspergillosis [14]. In our study, no patient had neutropenia at diagnosis of invasive aspergillosis (median number of neutrophils, 7171/mm; range, 1190–21,716). This suggests that defects in neutrophil function, with normal counts, may also predispose to invasive aspergillosis.
The value of respiratory isolation of Aspergillus organisms after kidney transplantation has been emphasized by Weiland et al. [11], showing that 45% of patients with positive cultures had invasive aspergillosis. In our study, 72% of patients with respiratory isolation of Aspergillus species and 50% with positive abdominal wound cultures had invasive aspergillosis. This indicates that not only isolation of Aspergillus species from respiratory secretions but also isolation from wound drainage may represent invasive disease after liver transplantation.
Of 29 patients with invasive pulmonary aspergillosis, 23 (79%) had positive culture and 6 (21 %) had negative culture. This suggests that invasive aspergillosis should be considered not only in patients with positive culture but also in high-risk patients with negative culture. The issue of fungal prophylaxis in such patients is unclear but may be considered.
We previously found, in a large series of liver transplant patients [1], an association between invasive fungal infection and the following variables: longer courses of nonprophylactic intravenous antibiotics, longer cumulative surgical time and higher number of laparotomies, and increased number of units of red blood cells transfused. There was also a trend between the use of OKT3 and the development of invasive fungal infections. It is interesting that the same variables were found to be associated with invasive aspergillosis in the present series according to univariate analysis. However, the only two variables significantly associated with pulmonary invasive aspergillosis in our multifactor model were the use of OKT3 monoclonal antibody and the creatinine level at time of diagnosis. The odds of invasive disease in patients who were treated with OKT3 adjusted for creatinine level was 6.29 (95% confidence interval, 0.93–42.65) times that of patients who did not receive OKT3. Because of the known association between CMV infection and OKT3, we examined the possibility that OKT3 was not directly associated with invasive aspergillosis. OKT3 could have been associated with a higher rate of CMV infection (symptomatic or asymptomatic) and indirectly associated with aspergillosis. We did not find any association between the use of OKT3 and CMV infection or an association between CMV infection and invasive aspergillosis. Therefore, we concluded that OKT3 was an independent factor associated with invasive aspergillosis.
OKT3 is known to affect circulating T cells by blocking their function through interaction with the T cell receptor [15]. T cell immunity is an important host defense mechanism against fungal infections such as cryptococcosis, histoplasmosis, and coccidioidomycosis. In contrast, it is thought that neutrophils and macrophages are the main defense mechanism against aspergillosis [16]. Recent reports show that AIDS patients may also develop invasive aspergillosis [5, 17]. It has been demonstrated that AIDS patients can have leukocyte and macrophage dysfunction without neutropenia or corticosteroid intake. It has been suggested that the abnormal T cell function in AIDS patients leads to neutrophil and macrophage impairment [18]. It is possible that in liver transplant recipients, OKT3, by reducing T cell immunity, increases further the impairment of leukocyte and macrophage function already caused by chronic corticosteroid treatment and raises the risk of invasive aspergillosis.
Cuervas-Mons et al. [19] demonstrated that pretransplant serum creatinine level was the best short-term prognostic value in liver transplantation. In our model, we found that higher serum creatinine level at time of diagnosis was significantly associated with invasive disease. Serum creatinine level may reflect the overall clinical status after liver transplantation, and a higher creatinine level may select patients with a complicated course who may be more likely to develop invasive aspergillosis.
Previous studies have shown that two or more positive cultures for Aspergillus species can discriminate between disease and colonization [9, 20]. We could not do the same analysis here. The limitation of our study was in the fact that the respiratory cultures were not taken routinely but only when there was clinical indication. This creates a bias, as patients with a more severe clinical picture would have had a higher chance to be sampled than asymptomatic patients. This explains the higher number of fungal cultures submitted to the laboratory for patients with invasive aspergillosis in this study. Since this was a retrospective study, we had no control on such potential bias.
We and others have observed [1–4] that the use of nonprophylactic intravenous antibiotics increases the risk of fungal infection after liver transplantation. Although this factor was included in the multivariate analysis, it was not statistically associated with invasive aspergillosis. This suggests that after solid organ transplantation, the impairment of innate host immune responses are more important than the length of antibiotic treatment in predicting invasive aspergillosis [11].
In summary, positive cultures for Aspergillus species after liver transplantation should never be ignored, as frequently they indicate invasive disease. We suggest the following approach to management in patients with positive Aspergillus cultures of respiratory secretions after liver transplantation: A patient with chest radiography abnormalities should be started on amphotericin B without delay. At the same time, further diagnostic procedures should be done to obtain tissue specimens for confirmation of invasive aspergillosis. In some cases, in patients who have received OKT3 and who have high creatinine levels, invasive aspergillosis should be considered even without positive culture. Each case should be assessed separately, but the use of empiric amphotericin B in some cases does not seem unwise. Patients with positive Aspergillus wound culture should be investigated for local invasion and should not be assumed to be just colonized with the fungus.
Acknowledgments
Grant support: Department of Veterans Affairs; National Institutes of Health (DK-29961).
Footnotes
Presented in part: 31st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, September 1991 (abstract 736).
References
- 1.Kusne S, Dummer JS, Singh N, et al. Fungal infections after liver transplantation. Transplant Proc. 1988;20:650–651. [PMC free article] [PubMed] [Google Scholar]
- 2.Wajszczuk CP, Dummer JS, Ho M, et al. Fungal infections in liver transplant recipients. Transplantation. 1985;40:347–353. doi: 10.1097/00007890-198510000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castaldo P, Stratta RJ, Wood RP, et al. Clinical spectrum of fungal infections after orthotopic liver transplantation. Arch Surg. 1991;126:149–156. doi: 10.1001/archsurg.1991.01410260033005. [DOI] [PubMed] [Google Scholar]
- 4.Kusne S, Dummer JS, Singh N, et al. Infections after liver transplantation: an analysis of 101 consecutive cases. Medicine. 1988;67:132–143. doi: 10.1097/00005792-198803000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denning DW, Follansbee SE, Scolaro M, Norris S, Edelstein H, Stevens DA. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:654–662. doi: 10.1056/NEJM199103073241003. [DOI] [PubMed] [Google Scholar]
- 6.Starzl TE, Demetris AJ, Van Thiel D. Liver transplantation. N Engl J Med. 1989;321:1014–1022. doi: 10.1056/NEJM198910123211505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosmer DW, Lemeshow S. Applied logistic regression. New York: John Wiley & Sons; 1989. [Google Scholar]
- 8.Bodey GP, Vartivarian S. Aspergillosis. Eur J clin Microbiol Infect Dis. 1989;8:413–437. doi: 10.1007/BF01964057. [DOI] [PubMed] [Google Scholar]
- 9.Treger TR, Visscher DW, Bartlett MS, Smith JW. Diagnosis of pulmonary infection caused by Aspergillus: usefulness of respiratory cultures. J Infect Dis. 1985;152:572–576. doi: 10.1093/infdis/152.3.572. [DOI] [PubMed] [Google Scholar]
- 10.Pepys J, Riddell RW, Citron KM, Clayton YM, Short EI. Clinical and immunologic significance of Aspergillus fumigatus in the sputum. Am Rev Respir Dis. 1959;80:167–180. doi: 10.1164/arrd.1959.80.2.167. [DOI] [PubMed] [Google Scholar]
- 11.Weiland D, Ferguson RM, Peterson PK, Snover DC, Simmons RL, Najarian JS. Aspergillosis in 25 renal transplant patients. Ann Surg. 1983;198:622–629. doi: 10.1097/00000658-198311000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu VL, Muder RR, Poorsattar A. Significance of isolation of Aspergillus from the respiratory tract in diagnosis of invasive pulmonary aspergillosis. Results from a three-year prospective study. Am J Med. 1986;8:249–254. doi: 10.1016/0002-9343(86)90259-7. [DOI] [PubMed] [Google Scholar]
- 13.Gerson SL, Talbot GH, Hurwitz S, et al. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345–351. doi: 10.7326/0003-4819-100-3-345. [DOI] [PubMed] [Google Scholar]
- 14.Gustafson TL, Schaffner W, Lavely GB, Stratton CW, Johnson HK, Hutcheson RH., Jr Invasive aspergillosis in renal transplant recipients: correlation with corticosteroid therapy. J Infect Dis. 1983;148:230–238. doi: 10.1093/infdis/148.2.230. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein G. Overview of the development of orthoclone OKT3: monoclonal antibody for therapeutic use in transplantation. Transplant Proc. 1987;19:1–6. [PubMed] [Google Scholar]
- 16.Murphy JW. Immunity to fungi. Curr Opinion Immunol. 1990;2:360–367. doi: 10.1016/0952-7915(89)90142-8. [DOI] [PubMed] [Google Scholar]
- 17.Pursell KJ, Telzak EE, Armstrong D. Aspergillus species colonization and invasive disease in patients with AIDS. Clin Infect Dis. 1992;14:141–148. doi: 10.1093/clinids/14.1.141. [DOI] [PubMed] [Google Scholar]
- 18.Murray HW. Interferon-gamma, the activated macrophage, and the host defense against microbial challenge. Ann Intern Med. 1988;108:595–608. doi: 10.7326/0003-4819-108-4-595. [DOI] [PubMed] [Google Scholar]
- 19.Cuervas-Mons V, Millan I, Gavaler JS, Starzl TE, Van Thiel DH. Prognostic value of preoperatively obtained clinical and laboratory data in predicting survival following orthotopic liver transplantation. Hepatology. 1986;6:922–927. doi: 10.1002/hep.1840060519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nalesnik MA, Myerowitz RL, Jenkins R, Lenkey J, Herbert D. Significance of Aspergillus species isolated from respiratory secretions in the diagnosis of invasive pulmonary aspergillosis. J Clin Microbiol. 1980;11:370–376. doi: 10.1128/jcm.11.4.370-376.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]