Abstract
Dosage-sparing strategies, adjuvants and alternative substrates for vaccine production are being explored for influenza vaccine development. We assessed the safety and immunogenicity of a Vero cell culture-grown inactivated whole virus influenza A/H5N1 vaccine with or without aluminum hydroxide adjuvant [Al(OH)3] in healthy young adults. Vaccines were well tolerated, but injection site discomfort was more frequent in groups receiving Al(OH)3. Dose-related increases in serum antibody levels were observed. Neutralizing antibody titers varied significantly when tested by two different laboratories. Al(OH)3 did not enhance HAI or neutralizing antibody responses, and contributed to increased injection site pain. Because influenza antibody titers vary significantly between different laboratories, international standardization of assays is warranted.
Keywords: Influenza A/H5N1 vaccines, cell culture, adjuvant, aluminum hydroxide
1. INTRODUCTION
Global efforts are underway to develop vaccines for a potential influenza pandemic, particularly for influenza A/H5N1 [1]. In the decade since the emergence of the H5N1 avian strain, clinical vaccine trials have demonstrated the need for large quantities of H5 hemagglutinin (HA) and/or inclusion of an adjuvant to generate adequate immune responses in humans [2]. This observation has stimulated the search for antigen-sparing strategies such as use of more potent adjuvants, alternative methods of vaccine administration (e.g., intradermal injection), and use of whole virus (WV) preparations. Furthermore, efforts continue to develop methods other than growth in eggs for more efficient and controlled production of both pandemic and interpandemic influenza vaccines.
The potential of conventional aluminum hydroxide adjuvant [Al(OH)3] to enhance immunogenicity has been evaluated repeatedly in clinical trials of H5N1 vaccines [3-5]. In earlier trials, Al(OH)3 failed to confer clinically significant increases in immune responses when formulated with egg-grown subvirion (SV) inactivated vaccines. However, promising results with Al(OH)3 have been reported with WV H5N1 vaccines [6-8]. Earlier studies suggested that some, but not all WV vaccines were more immunogenic than SV vaccines [9, 10].
Results of a recent trial of a WV influenza A/H5N1 vaccine demonstrated that most subjects given a standard dosage developed detectable neutralizing (Neut) antibody responses, and that the inclusion of Al(OH)3 did not enhance responses [11]. The purpose of our study was to assess safety and immunogenicity of the same WV H5N1 vaccine in a placebo-controlled trial in which a higher dose of vaccine antigen was evaluated. The vaccine evaluated in this report was also unique since it was constructed using a wild type (wt) seed virus grown in tissue cell culture, as opposed to using genetically altered recombinant viruses grown in eggs or purified recombinant hemagglutinin (HA) produced in cell culture systems [12, 13]. In addition, neutralizing antibody responses were determined in a subset of subjects by two different laboratories in order to compare assay results directly.
2. MATERIALS AND METHODS
2.1 Vaccines
Ultraviolet and formalin-inactivated WV influenza A/H5N1 vaccine was prepared using wt A/Vietnam/1203/04 grown in Vero cells [14; Baxter]. Six study groups were compared. Two dosage levels (7.5 and 15μg of HA/0.5mL dose) were pre-formulated with or without aluminum hydroxide [Al(OH)3] adjuvant; a dose of 45μg of HA/0.5mL was formulated without Al(OH)3. The Al(OH)3 content in the adjuvanted vaccines was 350μg per dose. Saline placebo was used as the control vaccine. Vaccines were prepared in single-dose prefilled syringes.
2.2 Study Design and Subjects
A multicenter, randomized, double-blind, placebo-controlled clinical trial was conducted at four NIH-funded Vaccine Treatment and Evaluation Unit sites. Written informed consent was obtained from potential subjects prior to screening. Healthy non-pregnant females and males between the ages of 18 and 40 years who had no history of severe reactions to influenza vaccines, no known suppression of the immune system, and who had not previously received an influenza A/H5 vaccine were eligible. The study was conducted in accordance with protocols approved by Institutional Review Boards at each of the participating study sites.
2.3 Study Procedures
The study was conducted in two stages. During stage 1, prior to vaccination, eligible subjects had to demonstrate normal laboratory assessments, including total white blood cell count, hemoglobin, platelet count, alanine amino-transferase, and creatinine. Subjects were randomized to one of the 6 vaccine groups (approximately 15 subjects per group). Subjects received two doses of vaccine in the deltoid muscle approximately 28 days apart. Vaccinations were administered by unblinded vaccinators who were not involved in safety assessments. Subjects were observed for 30 minutes after each immunization. For seven days after each immunization, subjects recorded their oral temperature and the presence and severity of injection site reactions (pain, tenderness, redness and swelling) and systemic symptoms (fever, malaise, myalgia, headache, and nausea) on a memory aid. The severity of solicited adverse events (AEs) was scored on a scale from 0 to 3: 0=absence of the symptom; 1=mild symptom that did not interfere with activity; 2=moderate symptom that interfered with activity; and 3=severe, prevented daily activity. Injection site redness and swelling were graded on the diameter of measurement, with 0=none; 1=small (<2 cm); 2=medium (2-5 cm); and 3=large (>5 cm). Fever was defined as an oral temperature ≥100°F. Serious adverse events (SAEs) were defined as life-threatening AEs, or AEs that resulted in significant or persistent disability, congenital anomalies, hospitalizations, or death. All AEs reported during the first 2 months after enrollment were recorded, and SAEs were reported during the entire 7-month study period.
Subjects were seen in the clinic on days 2 and 8 after each immunization to review their memory aids. Blood samples for laboratory safety assessments (as described above for screening) were repeated on day 7 after each immunization in Stage 1. One month after each immunization and 6 months after the second dose, interim medical history was reviewed and serum samples for antibody assays were collected
Seven-day clinical and laboratory safety data in Stage 1 participants were reviewed by the Safety Monitoring Committee prior to enrollment of subjects into Stage 2. In Stage 2 of the study (approximately 35 subjects per group), identical procedures were performed, with the exception that blood samples for laboratory screening were not collected.
2.4 Laboratory assays
Hemagglutination-inhibition (HAI) and neutralization assays were performed at Southern Research Institute (SR), as described previously [15, 16]. For both assays, a significant antibody response, or seroresponse, was defined as a four-fold or greater increase in antibody titer after immunization (if antibody was detectable in the preimmunization sample) or an increase in titer from <10 before immunization to ≥40 after immunization [17]. Neutralization antibody assays were performed at both SR and Baxter [11]. The SR reference laboratory assayed all serum samples, while the Baxter laboratory assayed only a subset of 100 subjects who had consented to the future use of their specimens and who were randomly selected based on the SR results to be representative of the SR titer distribution, specifically limiting the selection to include only 5 placebo recipients. Both laboratories were blinded to sample identity. A brief overview of the two neutralizing antibody assay procedures is provided in Table 1.
Table 1.
Comparison of SR Laboratory and Baxter Laboratory Neutralizing Antibody Assay Methods
| Parameter | Baxter Lab | SR Lab |
|---|---|---|
| Virus Type, Growth Substrate | Wild type, Vero cells | Reverse Genetics, Egg-derived |
| Initial Serum Dilution | 1:5 | 1:10 |
| Dilution Factor | 2-fold | 2-fold |
| Diluent | Cell culture medium | DMEM |
| Virus Inoculum | 100 TCID50 | 100 TCID50 |
| Initial Incubation Time* and Temperature | 1 hour (Room temperature) | 2-2.5 hours (37° C) |
| Assay Substrate | Vero cells; monolayer | MDCK cells; suspension |
| Second Incubation | 5 days (37° C) | 19-21 hours (37° C) |
| Assay Endpoint | Cytopathic effect (50%) | ELISA: Detection of nucleoprotein |
| Replicates | 8/sample | 2/sample |
Neutralization time with virus-serum mix
2.5 Statistical analyses
The primary objectives of the study were to determine the dose-related safety of a Vero cell culture-grown WV inactivated H5N1 vaccine with or without Al(OH)3 in healthy adults; and to assess the potential for Al(OH)3 to enhance immune responses after immunization. The primary reactogenicity endpoints were the frequency and severity of AEs and SAEs after immunization. The primary immunogenicity endpoints included the proportion of subjects in each dose group achieving a serum HAI or neutralizing antibody titer of ≥40 against the influenza A/H5N1 virus 28 days after receipt of the second dose of vaccine; and the geometric mean titer (GMT) and frequency of ≥4-fold rises in HAI and neutralizing antibody titers in each group one month after receipt of dose 2. Secondary endpoints were immune responses at 1 month after receipt of the first dose and six months after the second dose.
Solicited reactogenicity was analyzed by taking the most severe response over the follow-up period and dichotomizing into a binary variable: none versus mild/moderate/severe. Overall comparisons among treatment groups were made using Fisher’s exact test. Further analyses were conducted using multivariate logistic regression separately for each vaccination and each symptom.
Immune responses were summarized in terms of H5-specific neutralization and HAI antibody titers, transformed to a logarithmic scale for analyses, and the proportion of subjects achieving a neutralization titer ≥40 at 28, 56, and 208 days after the initial vaccination was noted. Fisher’s exact test and ANOVA (F test) were used to compare differences among treatment groups for 4-fold rise and GMT, respectively. Univariate and multivariate logistic regression and linear regression were conducted for 4-fold responses and GMT, respectively. The Cochran-Armitage trend test was performed to explore the dose response. The scores of the dosages for this test were defined as 7.5, 15 and 45. One-sided exact p values were selected.
Neutralization assays were performed at both SR and Baxter, and comparisons of GMT and 4-fold rises were made. A paired t-test was utilized to determine the confidence intervals and p-values of the GMTs; while for ≥4-fold rises, Fisher’s exact test was performed. Pearson and Spearman correlation coefficients were also calculated with no adjustments made for multiple comparisons.
3. RESULTS
Enrollment into the trial was rapid, with stage 1 beginning in October 2006 and stage 2 enrollment completed during January 2007. A total of 308 subjects were enrolled (93 in stage 1, and 215 in stage 2), with 299 (98%) receiving both doses of vaccine and providing a blood sample 1 month after the second dose, and 293 providing a blood sample 6 months after receipt of the second dose. Baseline demographic characteristics and preimmunization serum HAI and neutralizing antibody GMTs of enrolled subjects are shown in Table 2 and did not differ among the six vaccine groups.
Table 2.
Baseline Characteristics of Enrolled Subjects
| VACCINE GROUPS | |||||||
|---|---|---|---|---|---|---|---|
| 7.5 μg (N= 49) | 7.5 μg +Al(OH)3 (N= 51) | 15 μg (N= 51) | 15 μg + Al(OH)3 (N= 50) | 45 μg (N= 54) | Placebo (N= 51) | p-value | |
| Gender – N (%) | 0.079 | ||||||
| Male | 19 (39) | 27 (53) | 25 (49) | 23 (46) | 24 (44) | 22 (43) | |
| Female | 30 (61) | 24 (47) | 26 (51) | 27 (54) | 30 (56) | 29 (57) | |
| Ethnicity – N (%) | 0.45 | ||||||
| Non-Hispanic | 38 (78) | 42 (82) | 41 (80) | 46 (92) | 46 (85) | 44 (86) | |
| Hispanic | 11 (22) | 9 (18) | 10 (20) | 4 (8) | 8 (15) | 7 (14) | |
| Race – N (%) | 0.23 | ||||||
| Asian | 5 (10) | 8 (16) | 4 (8) | 3 (6) | 8 (15) | 8 (16) | |
| Hawaiian/Pacific Islander | 0 | 0 | 1 (2) | 1 (2) | 2 (4) | 0 | |
| Black/African American | 3 (6) | 3 (6) | 5 (10) | 5 (10) | 6 (11) | 6 (12) | |
| White | 31 (63) | 36 (71) | 39 (76) | 37 (74) | 31 (57) | 35 (69) | |
| Multi-Racial | 3 (6) | 1 (2) | 1 (2) | 3 (6) | 5 (9) | 0 | |
| Other/Unknown | 7 (14) | 3 (6) | 1 (2) | 1 (2) | 2 (4) | 2 (4) | |
| Age | 0.28 | ||||||
| Mean (STD) | 26.5 (4.6) | 28.7 (5.9) | 27.5 (5.4) | 27.4 (6.3) | 28.2 (5.8) | 26.6 (5.6) | |
| Antibody Level* | |||||||
| HAI GMT (CI) | 5.0 (5.0,5.1) | 5.0 (-) | 5.3 (4.7,5.9) | 5.5 (4.8,6.2) | 5.0 (-) | 5.1 (4.9,5.9) | 0.42 |
| Neut GMT (CI) | 5.0 (-) | 5.1 (4.9,5.2) | 5.4 (4.8,6.1) | 5/5 (4.9,6.1) | 5.2 (4.9,5.6) | 5.4 (4.9, 5.9) | 0.57 |
As determined in the SR Laboratory
Abbreviations: AlOH3=aluminum hydroxide; HAI=hemagglutination inhibition; Neut=neutralizing; GMT=geometric mean titer
3.1 Safety and Reactogenicity
Safety
All 6 vaccine formulations were safe and well tolerated; no subjects refused the second dose as a result of injection site or systemic reactions following receipt of the first dose. Only three SAEs were reported during the study period, and none was considered vaccine-related (one subject was hospitalized for multiple traumatic fractures; one for surgical repair of a foot fracture, and one for histoplasmosis in an immunocompetent subject).
Injection Site Reactogenicity
One subject in the 45μg without Al(OH)3 vaccine group developed a large area of injection site redness (maximum diameter of 5.4 cm) on days 1 and 2 after receipt of the second dose of vaccine; this subject had previously developed an area of injection site erythema of 3.8cm in diameter after the first dose. No other grade 3 injection site reactions were reported.
Injection site pain and tenderness were the most common solicited AEs during the week after each vaccination. The percentages of subjects reporting any injection site tenderness after receipt of dose 1 are shown in Figure 1. Although the frequency of injection site tenderness was significantly lower in the group given placebo when compared with the other vaccine groups combined (p<0.05; Fisher’s exact test), there were no significant differences among the other vaccine groups (p=0.28; Fisher’s exact test). Most reactions were mild, peaked on day 1-2 after vaccination, and resolved within a day or two (data not shown). In multivariate analyses, increasing dosage level was associated with significantly more injection site swelling after dose 1 only. Female gender and younger age were also associated with more injection site tenderness after the first dose (p<0.05 for each; data not shown), and after dose 2, dosage level (p<0.05), and female gender and younger age (p<0.01 for each) were associated with a higher frequency of injection site tenderness (data not shown).
Figure 1.
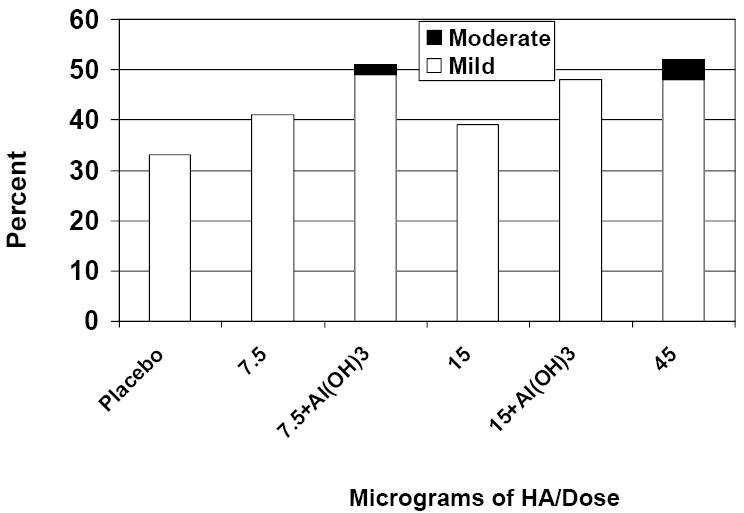
Percent of Subjects with Injection Site Tenderness after the First Dose
Systemic Reactogenicity
Three subjects experienced grade 3 systemic reactions during the week after receipt of dose 1, but only 1 was considered vaccine-related (malaise on day 0 in a subject given the 15μg without Al(OH)3 vaccine formulation). Three other subjects experienced grade 3 systemic reactions during the week after dose 2, and two of these were considered vaccine-related: 1 reported malaise, headache and myalgia on days 2, 3 and 6 after vaccination; and the other developed headache on day 2 after vaccination. Both of these subjects were in the 7.5μg with Al(OH)3 group. Fever during the week after immunization was uncommon (0-3 persons/vaccine group after each dose). In multivariate analyses, younger age was associated with a higher frequency of nausea after the first dose of vaccine, and female gender was associated with a higher frequency of nausea after the second dose of vaccine (p<0.05 for both; data not shown).
3.2 Immunogenicity
Dose-Response Relationships
Serum antibody responses one month after each dose of vaccine, determined in the SR lab, are shown in Table 3 and Figure 2. The highest responses after the second dose of vaccine were seen in the group given 45μg without Al(OH)3, with 40% and 44% of subjects in this group developing four-fold or greater increases in HAI and neutralizing antibody titers, respectively. One month after each dose of vaccine, statistically significant increases in HAI and neutralizing antibody responses from baseline values in terms of GMTs, proportions of subjects with a ≥4-fold increase in titer, and the proportions of subjects with a titer ≥40 were noted among all vaccine groups (p≤ 0.01 for each comparison).
Table 3.
Serum HAI and Neutralizing Antibody Responses One Month after Each Dose of Vaccine or Placebo and Six Months after the Second Dose
| Parameter | Dosage Level (μg of HA) | ||||||
|---|---|---|---|---|---|---|---|
| 7.5 | 15 | 45 | Placebo | ||||
| Al(OH)3 Adjuvant | No (N=48) | Yes (N=50) | No (N=49) | Yes (N=48) | No (N=54) | NA (N=50) | |
| HAI Antibody | GMT-dose 1* | 6.2 (5.0,7.7) | 5.0 (-) | 8.2 (5.9,11.3) | 6.1 (5.0,7.4) | 10.1 (7.1,14.2) | 5.0 (5.0,5.1) |
| GMT-dose 2 | 10.5 (7.0,15.7) | 6.7 (5.4,8.4) | 9.4 (6.6,13.4) | 8.1 (6.2,10.6) | 18.1 (12.1,27.0) | 5.0 (5.0,5.1) | |
| GMT-6 months | 5.5 (4.8,6.2) | 5.3 (4.8,5.8) | 6.4 (5,2,8.0) | 6.5 (5.1,8.2) | 7.3 (5.8,9.2) | 5 (-) | |
| % Responding-dose 1** | 8 | 0 | 12 | 2 | 17 | 0 | |
| % Responding-dose 2 | 19 | 8 | 18 | 8 | 40 | 0 | |
| % Responding-6 months | 2 | 2 | 8 | 4 | 13 | 0 | |
| Neut Antibody | GMT-dose 1* | 6.8 (5.5,8.5) | 5.2 (4.9,5.5) | 9.3 (7.0,12.3) | 6.1 (5.1,7.2) | 10.6 (7.6,14.7) | 5.4 (4.9,5.8) |
| GMT-dose 2 | 13.4 (9.6,18.9) | 8.3 (6.8,10.1) | 19.7 (14.9,26.2) | 12.7 (9.8,16.4) | 31.3 (23.9,40.9) | 5.4 (5.0,5.9) | |
| GMT-6 months | 9.1 (7.0,11.7) | 5.8 (5.2,6.4) | 11.3 (8.8,14.4) | 7.6 (5.9,9.6) | 19.2 (15.7,23.5) | 5.5 (4.9,6.0) | |
| % Responding-dose 1** | 10 | 0 | 10 | 0 | 19 | 0 | |
| % Responding-dose 2 | 17 | 6 | 27 | 17 | 44 | 0 | |
| % Responding-6 months | 6 | 0 | 10 | 6 | 25 | 0 | |
Geometric mean titer (95% confidence intervals)
Four-fold or greater increase in titer when compared with preimmunization titer and titer ≥40 after immunization.
Figure 2.
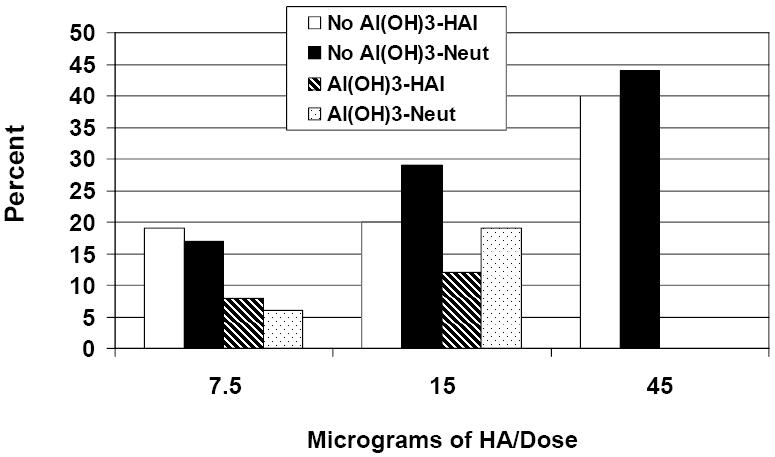
Percent of Subjects with Serum HAI and Neutralizing Antibody Titer ≥40 after Two Doses
In the groups given vaccine containing Al(OH)3, dose-related increases in the GMTs of serum HAI antibody after the first dose (p<0.05), and increases in GMTs of neutralizing antibody after the second dose (p<0.01) were observed. For the non-adjuvanted vaccine groups, significant dose-responses for HAI and neutralizing antibody were seen after the second dose of vaccine in terms of GMTs, proportions of subjects with a ≥4-fold increase in titer, and the proportions of subjects with a titer ≥40) (p<0.02 for all comparisons). At six months after receipt of the second dose, dose-related increases in antibody responses persisted among groups receiving non-adjuvanted vaccines for all HAI and neutralizing antibody response parameters, with the exception of GMT of HAI antibody (p<0.05 for all comparisons). In contrast, dose-response relationships among groups given adjuvanted vaccine persisted only for GMT of neutralizing antibody (p<0.05).
Effect of Al(OH)3 Adjuvant on Immune Responses
HAI and neutralizing antibody GMTs were significantly higher (p<0.05 for both) after the first dose containing 7.5μg without Al(OH)3 when compared with group given 7.5μg with Al(OH)3. Neutralizing antibody GMTs were significantly higher in the group given 15μg without Al(OH)3 when compared with group given 15μg with Al(OH)3 after the first and second doses (p<0.03 for each), and in the group given 7.5μg without Al(OH)3 when compared with the group given 7.5μg with Al(OH)3 after the second dose (p<0.02). At six months after receipt of the second dose of vaccine, GMTs of neutralizing antibody were significantly greater among groups given 7.5 or 15μg without Al(OH)3 when compared with subjects given the same dose with Al(OH)3.
Serum Antibody Response Frequencies Using an Alternative Definition
While the EMEA and FDA have harmonized definitions of serum HAI antibody responses as outlined in the Methods section [17, 18], traditional definitions of a four-fold or greater increase in antibody titer did not require attainment of a titer of at least 40 after immunization. Therefore, an increase in titer from undetectable before immunization (<10) to ≥ 20 after immunization was considered a seroresponse. To bridge results from prior influenza vaccine studies (including earlier pandemic vaccine evaluations) using more traditional definitions of seroresponse, the frequencies of HAI and/or neutralizing antibody responses were recalculated such that no minimum post-immunization titer was required: 0, 38%, 53% and 77% of subjects given 0, 7.5, 15 and 45μg doses without Al(OH)3 responded, compared with 16% and 38% of subject given 7.5 and 15μg doses with Al(OH)3 (Figure 3). Once again, dose-related increases in antibody responses were observed, and the inclusion of Al(OH)3 reduced the frequencies of all immune responses.
Figure 3.
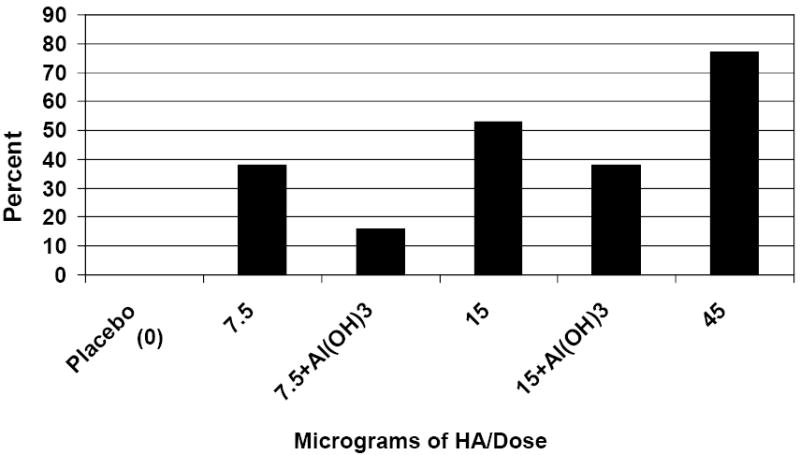
Percent of Subjects with ≥ 4-Fold Increase in Serum HAI and/or Neutralizing Antibody Titer after Two Doses (No Minimum Post Vaccination Titer Requirement)
Comparison of Neutralizing Antibody Results Obtained from Two Different Laboratories
A subset of sera collected from 100 subjects before and 1-month after each dose of vaccine was assayed in both a reference laboratory (SR) and in the laboratory of the vaccine manufacturer to bridge the results of clinical trials being conducted in the U.S to those conducted in Europe [11, current study]. Sera collected from 5 placebo subjects and 14-22 subjects from each of the 5 vaccine groups were tested.
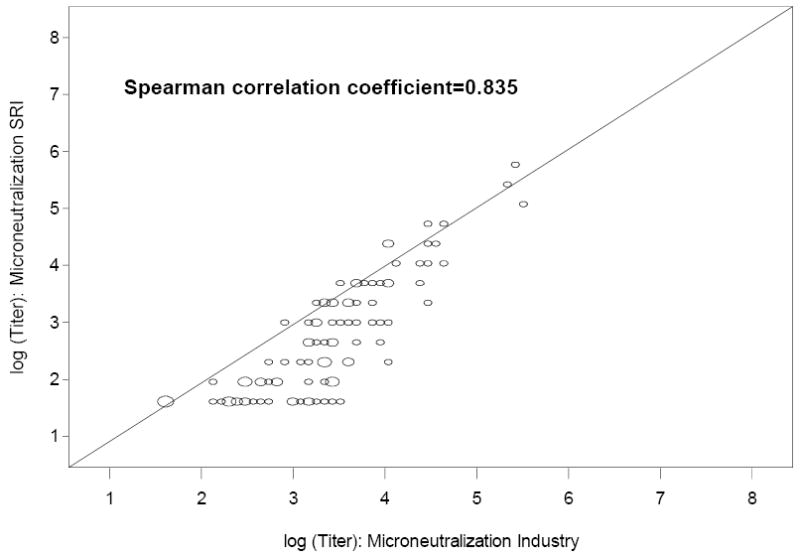
Clear dose-response relationships in GMTs were observed in assays performed in both the SR and Baxter laboratories (data not shown); however, a significant dose-response relationship in ≥4-fold responses was observed in the SR assay only. For example, among subjects given 7.5, 15 or 45μg of vaccine without Al(OH)3, 5.9 (0.1, 28.7), 28.6 (8.4, 58.1) and 45.5 (24.4, 67.8) percent (95% C.I.) developed a 4-fold or greater rise in neutralizing antibody titer after 2 doses in the SRI assay (p<0.01; Cochran-Armitage trend test). The corresponding percentages and 95% C.I. for the Baxter assay were 23.5 (6.8, 49.9), 42.9 (17.7, 71.1) and 50.0 (28.2, 71.8) (p=0.08; Cochran-Armitage trend test). Neutralizing antibody GMTs were significantly higher (p<0.05; paired t-test) using the Baxter assay when compared with the SR assay at all timepoints (before and 1 month after each immunization), with the exception of the pre-immunization titer in the group given 45μg without Al(OH)3 (data not shown). When results for all 100 subjects are considered, the titer ratios (Baxter/SR) for GMT (95% CI) were 1.3 (1.2, 1.4); 2.23 (1.9, 2.6); and 1.89 (1.7, 2.1) before and 1 month after dose 1, and after dose 2, respectively (p<0.01 for all comparisons; paired t test). In general, the assay results were highly correlated for GMT (Spearman correlation coefficient=0.835 after 2 doses; Figure 4).
Figure 4.
Correlation between Serum Neutralizing Antibody Titers When Comparing Results Using Two Different Assays
Because starting dilutions for the 2 assays differed, direct comparisons between the proportions of subjects who achieved a neutralizing antibody titer of at least 20 after receipt of 2 doses were made (Figure 5). Although no placebo recipient achieved a titer ≥20 after 2 doses in either assay, significant dose-related increases in the proportions of subjects with a titer ≥20 after 2 doses were observed with the SR assay only (p< 0.01; Cochran-Armitage trend test). The proportions of subjects who achieved a titer ≥20 using the Baxter assay were higher when compared with the SR assay (p<0.01; Fisher’s Exact test), and a marginal dose-response relationship was observed (p=0.054; Cochran-Armitage trend test). After 2 doses of vaccine or placebo, 30 subjects achieved a titer ≥20 in the Baxter assay but not the SR assay; 1 achieved a titer ≥20 in the SRI assay but not the Baxter assay; 42 achieved a titer ≥20 in both assays; and 27 failed to achieve a titer ≥20 in either assay. These responses were highly non-concordant between the two laboratories (p<0.01; McNemar’s test).
Figure 5.
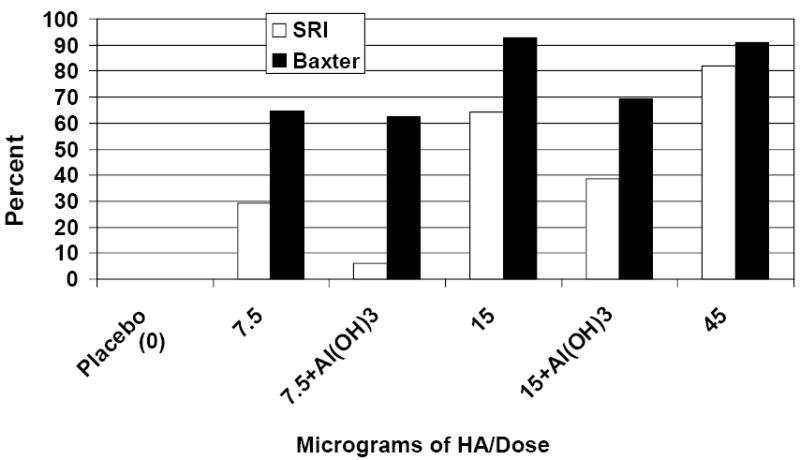
Percent of Subjects with a Neutralizing Antibody Titer ≥20 after Two Doses: SR vs. Baxter Results
4. DISCUSSION
Our data confirm that this novel inactivated whole virus vaccine prepared in Vero cells using a wild type influenza A/H5N1 virus was safe and immunogenic when administered to healthy adults. Dose-related increases in injection site reactogenicity were observed; however, all dosage levels were well tolerated. Inclusion of AlOH was associated with a somewhat higher frequency of injection site reactogenicity in the absence of increased systemic reactogenicity, as noted in previous studies using this preparation, as well as other adjuvanted subunit influenza vaccines [3, 4, 11]. Dose-related increases in serum antibody responses were observed, with the 45 μg dose without Al(OH)3 providing the highest responses. Responses were significantly lower among subjects given vaccines containing Al(OH)3 when compared with those given the same dosage of vaccine without adjuvant. Other reports have shown similar suppression of immune responses with the addition of Al(OH)3 [3, 4, 11].
Serum HAI and neutralizing antibody response frequencies were moderate, even at the highest dosage level using current definitions, similar to results from other recent trials of SV influenza A/H5N1 vaccines [4, 12]. Nevertheless, more than 50% of subjects given two-15μg doses without Al(OH)3 responded using a more traditional definition of seroresponse, and 77% of subjects given two 45μg doses without Al(OH)3 had a 4-fold or greater increase in HAI and/or neutralizing antibody. In spite of these immune responses, fewer than half of subjects receiving the highest amount of antigen achieved the putative protective HAI titer of 40 after two doses. Antibody responses against drifted H5 variants were not assessed in this study; however, it is likely these responses would be lower than those seen when measured against the homologous vaccine antigens [19]. Similar trends have been observed with candidate H5N1 vaccines where higher levels of antibody to the vaccine virus are associated with somewhat lower levels of antibody directed against drifted variants [11, 20, 21]. Because the precise pandemic strain is unknown, efforts must continue to develop prepandemic vaccines capable of eliciting antibody against both homologous and drifted variants.
The results of our study extend those reported by Ehrlich and colleagues using the same vaccine [11]. However, in contrast to the previous report, definite dose-responses were observed in both HAI and neutralizing antibody assays. In order to bridge the results between clinical trials conducted in different parts of the world, neutralizing antibody assays were performed on a subset of sera in the two laboratories responsible for assessing vaccine immunogenicity for the two clinical trials. Interestingly, the Baxter assay appeared somewhat more sensitive than the SR assay. The reasons for this are not known, but there are significant differences in assay methods. For example, the virus used in the Baxter assay is the wild type (wt) virus, in contrast to the SR assay, which uses a recombinant virus. As the vaccine was prepared using wild type virus, the match between test antigen and vaccine antigen is likely closer for the Baxter assay. Hoschler et al. previously demonstrated that use of wt virus yielded higher GMT values when compared with rg viruses (22). Another major difference is the longer duration of the incubation period with the Baxter assay, likely contributing to greater sensitivity. Nevertheless, clear dose-response relationships for GMTs were noted in both assays (data not shown). Our results underscore the complex issues related to comparing serologic results obtained in different laboratories. Conclusions regarding the immunogenicity of a vaccine are highly dependent on the assays used to assess endpoints used, and the definitions of significant response and putative protective titer. Stephenson et al. recently reported comparisons of serum HAI and neutralizing antibody assays against interpandemic influenza strains in different laboratories using aliquots of the same serum samples and noted considerable inter-laboratory variability with greatest differences in the neutralizing antibody values [23]. With the necessity of comparing results of clinical trials of candidate pandemic influenza vaccines around the world, an international collaborative effort is in progress to develop an international H5N1 antibody standard that should reduce inter-laboratory variability. Alternatively, head-to-head comparative trials of various vaccines with serology measured in a single lab could be performed. Differences in assay methods and results obtained in different laboratories pose challenges to regulatory authorities who must determine which vaccines to license, purchase and stockpile.
Our study did not explore several issues that are relevant for the use of WV vaccines. First, although the vaccine formulations were well tolerated in the adults studied, safety and reactogenicity issues still need investigation in pediatric age groups, especially young children. Previous administration of egg-grown A/ New Jersey/76 (H1N1) WV vaccines to young children resulted in a high frequency of fever and systemic reactions [24]. Another area needing investigation is a direct comparison of the safety and immunogenicity of the WV vaccine with that of a corresponding subvirion (SV) preparation. Although WV vaccines have been considered more immunogenic than SV vaccines, comparisons of the immunogenicity of the two products have not been performed using well-standardized assays.
The results of this and other recent clinical trials of candidate pandemic influenza vaccines raise concern about the value of including Al(OH)3 adjuvant in WV and SV influenza vaccines. While modest enhancement of immunogenicity has been observed in some trials at some antigen dosage levels, the lack of an adjuvant effect in this trial as well as the increase in local reactions are problematic. Other novel adjuvants, including ASO3 and MF59, have markedly enhanced immune responses to H5 and H9 vaccines in earlier studies [20, 21, 23, 25, 26] and would be interesting to assess with this vaccine. Cautious evaluation of this and other WV preparations in other age groups, particularly children, is warranted.
Acknowledgments
The authors gratefully acknowledge the participants and the following persons for their contributions to the study: Chianti Wade-Bowers, R.N., Alan Jewell, and the staff of the BCM Vaccine Research Center (BCM); Sally Mackey, M.S., Susan Swope, R.N. and the Stanford-Lucile Packard Vaccine Program staff (Stanford); Susan Partridge, R.N., M.B.A., Patricia Chatfield, P.N.P. and the Center for Vaccine Research Staff (UCLA); MaryLou Mullen, Lisa Chrisley, and the Center for Vaccine Development staff (U Md); Shanda Hand, R.N., Deborah Hunter R.N., and Deborah Myers (Vanderbilt); Megan Sanza (EMMES); Amy Milling and Jill Milnamow (PPD); and Tracy Williams and E. Lucile White (SR). The authors would also like to thank the members of the Safety Monitoring Committee for their valuable input (Rebecca Brady, MD, Chair; Wilbur Chen, MD; Thomas Talbot, MD; Barbara Trautner, MD; Brian Blackburn, MD; and Brad Spelling, MD) and their colleagues at the NIH/NIAID/DMID (Sonnie Kim-Grossman, Jean Hu-Primmer, Roland Levandowski, MD, Rosemary McCown, Katherine Muth, and Shy Shorer, MD).
Financial Support: National Institute of Allergy and Infectious Diseases; Division of Microbiology and Infectious Diseases (contracts NO1-AI-25465, NO1-AI-25461, NO1-AI-25462, NO1-AI-25463, NO1-AI25462); the Stanford University General Clinical Research Center (grant M01 RR-00070); the University of Maryland General Clinical Research Center (grant M01 RR165001); and the Vanderbilt University General Clinical Research Center (grant M01 RR-00095).
Footnotes
Presented in part at the 11th Annual Conference on Vaccine Research, Baltimore, MD, May 5-7, 2008; Abstract S2.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials.gov identifier: NCT00382980
http://clinicaltrials.gov/ct2/show/NCT00382980?term=H5N1%2C+adjuvant&rank=5
References
- 1.Dennis C. Flu-vaccine makers toil to boost supply. Nature. 2006;440:1099. doi: 10.1038/4401099a. [DOI] [PubMed] [Google Scholar]
- 2.Keitel W, Atmar R. Preparing for a possible pandemic; influenza A/H5N1 vaccine development. Curr Opin Pharmacol. 2007;7:484–90. doi: 10.1016/j.coph.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, Wood J, Hoschler K, Zambon MC. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006;367:1657–1664. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- 4.Keitel WA, Campbell JD, Treanor JJ, Walter EB, Patel SM, He F, Noah DL, Hill H. Safety and immunogenicity of an inactivated influenza A/H5N1 vaccine given without or with aluminum hydroxide to healthy adults: Results of a phase I-II randomized clinical trial. J Infect Dis. 2008;198:1309–16. doi: 10.1086/592172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nolan T, Richmond P, Skeljo M, Pearce G, Hartel G, Formica N, Hoschler K, Bennet J, Ryan D, Papanaoum K, Basser R, Zambon M. Phase I and II randomised trials of the safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in healthy adults. Vaccine. 2008;26:4160–7. doi: 10.1016/j.vaccine.2008.05.077. [DOI] [PubMed] [Google Scholar]
- 6.Hehme N, Engelmann H, Kuenzel W, Neumeier E, Saenger R. Immunogenicity of a monovalent, aluminum-adjuvanted influenza whole virus vaccine for pandemic use. Virus Res. 2004;103:163–71. doi: 10.1016/j.virusres.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Lin J, Zhang J, Dong X, Fang H, Chen J, Su N, Gao Q, Zhang Z, Liu Y, Wang Z, Yang M, Sun R, Li C, Lin S, Ji M, Liu Y, Wang X, Wood J, Feng Z, Wang Y, Yin W. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet. 2006;368:991–997. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- 8.Vajo Z, Kosa L, Visontay I, Jankovics M, Jankovics I. Inactivated whole virus influenza A (H5N1) vaccine. Emerg Infect Dis. 2007;13:807–8. doi: 10.3201/eid1305.061248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ennis FA, Mayner RE, Barry DW, Manischewitz JE, Dunlap RC, Verbonitz MW, Bozeman RM, Schild GC. Correlation of laboratory studies with clinical responses to A/New Jersey influenza vaccines. J Infect Dis. 1977;136(Suppl):S397–S406. doi: 10.1093/infdis/136.supplement_3.s397. [DOI] [PubMed] [Google Scholar]
- 10.LaMontagne JR, Noble GR, Quinnan GV, Curlin GT, Blackwelder WC, Smith JI, Ennis FA, Bozeman FM. Summary of clinical trials of inactivated influenza vaccine-1978. Rev Infect Dis. 1983;5:723–36. doi: 10.1093/clinids/5.4.723. [DOI] [PubMed] [Google Scholar]
- 11.Ehrlich HJ, Müller M, Oh HM, Tambyah PA, Joukhadar C, Montomoli E, Fisher D, Berezuk G, Fritsch S, Löw-Baselli A, Vartian N, Bobrovsky R, Pavlova BG, Pöllabauer EM, Kistner O, Barrett PN. Baxter H5N1 Pandemic Influenza Vaccine Clinical Study Team. A clinical trial of a whole virus H5N1 vaccine derived from a cell culture. New Engl J Med. 2008;358:2573–84. doi: 10.1056/NEJMoa073121. [DOI] [PubMed] [Google Scholar]
- 12.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 13.Treanor JJ, Wilkinson BE, Masseoud F, Hu-Primmer J, Battaglia R, O’Brien D, Wolff M, Rabinovich G, Blackwelder W, Katz JM. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine. 2001;19:1732–1737. doi: 10.1016/s0264-410x(00)00395-9. [DOI] [PubMed] [Google Scholar]
- 14.Kistner O, Barrett PN, Mundt W, Reiter M, Schober-Bendixen S, Eider G, Dorner F. Development of a Vero cell-derived influenza whole virus vaccine. In: Brown F, Robertson JS, Schild GC, Wood JM, editors. Inactivated Influenza Vaccines Prepared in Cell Culture. Dev Biol Stand. Vol. 98. 1999. pp. 101–10. [PubMed] [Google Scholar]
- 15.Stephenson I, Wood JM, Nicholson KG, Charlett A, Zambon MC. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Research. 2004;103:91–95. doi: 10.1016/j.virusres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim WL, Fukuda K, Cox NJ, Katz JM. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Micro. 1999;37:937–943. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Food and Drug Administration: Draft guidance for industry on clinical data needed to support the licensure of trivalent inactivated influenza vaccines; availability. Fed Reg. 2006;71:12367. [Google Scholar]
- 18.European Committee for Proprietary Medicinal Products: Note for guidance on harmonization of requirements for influenza vaccines. March 1997 (CPMP/BWP/214/96) European Agency for the Evaluation of Medicinal Products. 1997 March 12; [Google Scholar]
- 19.Keitel WA, Atmar RL, Nino D, Cate TR, Couch RB. Increasing dosages of an inactivated influenza A/H1N1 vaccine induce increasing cross-reacting antibody to subsequent antigenic variants. J Infect Dis. 2008;198:1016–8. doi: 10.1086/591465. [DOI] [PubMed] [Google Scholar]
- 20.Leroux-Roels I, Borkowski A, Vanwolleghem T, Dramé M, Clement F, Hons E, Devaster JM, Leroux-Roels G. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomized controlled trial. Lancet. 2007;370:580–589. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, Zambon MC. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–43. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- 22.Hoschler K, Gopal R, Andrews N, Saville M, Pepin S, Wood J, Zambon M. Cross-neutralization of antibodies elicited by an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine in healthy adults against H5N1 clade 2 strains. Influenza and Other Respiratory Viruses. 2008;1:199–206. doi: 10.1111/j.1750-2659.2007.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephenson I, Das RG, Wood JM, Katz JM. Comparison of neutralising antibody assays for detection of antibody to influenza A/H3N2 viruses: An international collaborative study. Vaccine. 2007;25:4056–63. doi: 10.1016/j.vaccine.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 24.Wright PF, Thompson J, Vaughn WK, Folland DS, Sell SHW, Karzon DT. Trials of influenza A/New Jersey/76 virus-vaccine in normal children - Overview of age-related antigenicity and reactogenicity. J Infect Dis. 1977;136:S731–S741. doi: 10.1093/infdis/136.supplement_3.s731. [DOI] [PubMed] [Google Scholar]
- 25.Atmar RL, Keitel WA, Patel SM, Katz JM, She DW, El Sahly H, Pompey J, Cate TR, Couch RB. Safety and immunogenicity of nonadjuvanted and MF59-adjuvanted influenza A/H9N2 vaccine preparations. Clin Infect Dis. 2006;43:1135–1142. doi: 10.1086/508174. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein DI, Edwards KM, Dekker CL, Belshe R, Talbot HK, Graham IL, Noah DL, He F, Hill H. Effects of adjuvants on the safety and immunogenicity of an avian influenza (H5N1) vaccine in adults. J Infect Dis. 2008;197:667–75. doi: 10.1086/527489. [DOI] [PubMed] [Google Scholar]


