Abstract
In the 1993 edition of this book, we described 4 major initiatives in liver transplantation: First, the evaluation of the new immunosuppressive drug FK506 (tacrolimus); second, the feasibility of combined liver-intestinal and multivisceral transplantation; third, 2 clinical attempts at hepatic xenotransplantation; and fourth, beginning attempts to enhance donor-specific nonreactivity with adjuvant bone marrow infusion. These and other new clinical studies during the last 12 months are the concerns of this update. The topics will be considered separately because of the unique design of each and the heterogeneity of the enrolled patient population.
The patient and graft survival curves were estimated by the Kaplan-Meier method and the comparisons were done by the log-rank test. Survival time for patients was defined as the time that elapsed from the transplantation date until death, or the date of the last follow-up evaluation. For calculating graft survival, the date of graft removal was also considered. Cox’s proportional hazards model was used to analyze different causes of mortality and graft failure. Single variable comparison for qualitative data was made by chi-square analysis. The one-way analysis of variance was used for 3-way comparison.
THE ADVANTAGES OF TACROLIMUS “FK506”
Following several years of extensive preclinical research (1–15), FK506 was first used in February 1989 (16). The drug was able to rescue patients who were undergoing intractable rejection of their hepatic allograft despite maximum cyclosporine (CyA)-based immunosuppressive therapy (16–18). Six months later, prospective nonrandomized and then randomized trials were begun with FK506 as the primary immunosuppressant for liver and other organ allograft recipients (19–21).
We report here our clinical experience in the 1,391 consecutive adult/pediatric primary hepatic allograft recipients who were treated from the outset with tacrolimus starting with the first case on August 18, 1989 and until December 30, 1993. Of these 1,391 cases, an internal cohort of 79 optimum-risk patients was enrolled in a randomized trial of FK506 versus CyA and the results have been reported elsewhere (21). The present analysis included a total number of cases which is 2.6 times more than the combined number in the recently published European and American multicenter trials (22–23).
PATIENTS AND METHODS
Patient Population
To evaluate the impact of FK506 on our liver transplant program, the cumulative patient and hepatic allograft survival were retrospectively studied back to the first attempted liver replacement in 1963 (24). The historical experience was step analyzed according to the breakthrough achievements that finally made hepatic transplantation the most practical treatment for patients with end-stage liver disease (25). From 1963 until 1979, 168 patients (Group I) received liver transplantation under immunosuppression with regimens of azathioprine (or cyclophosphamide), prednisone, and usually antilymphocyte globulin (ALG). After the clinical introduction of CyA in 1980 and up to 1987, 623 patients (Group II) had liver replacement under CyA prednisone-based immunosuppression to which azathioprine, ALG (or OKT3) were added as clinically indicated. Eurocollins (EC) solution which had been used for organ preservation since 1976 was the standard flush throughout the 1980–87 period. From 1987 until 1989, another 1,212 patients (Group III) were transplanted under the same immunosuppressive cocktail as Group II, but using University of Wisconsin (UW) solution for allograft preservation. From 1989 until 1993, 1,391 patients (Group IV) received UW-preserved grafts but were treated with FK506 instead of CyA.
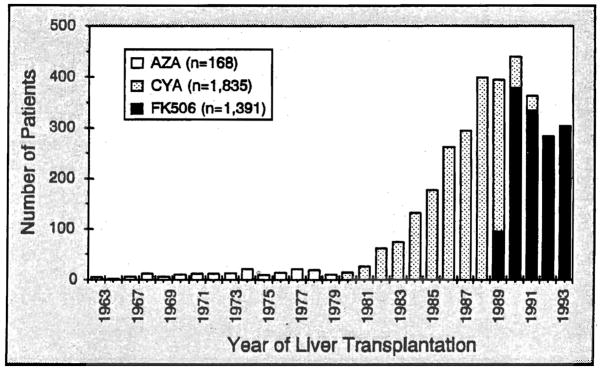
The yearly number of liver allograft recipients and the baseline immunosuppressive drugs are summarized in Figure 1. For a more detailed comparison between the CyA and FK506-based immunosuppressive regimens, Group III (n=1,212) was analyzed in the same depth as Group IV (n=1,391). The patient clinical profiles were similar except for trends in the FK506 era to older candidates (p <0.0001), a higher percentage of postnecrotic cirrhosis (p<0.00001), and more high-risk patients (p<0.00001) (Table 1). The urgency for transplantation was defined by the United Network for Organ Sharing (UNOS) criteria that existed at the time: a) working, b) at home or still working but requiring close medical supervision and/or sporadic hospital care, c) hospital-bound continuously or the majority of the time, or d) ICU-bound. All of the survival analyses were based upon follow-ups until March 1994.
Figure 1.
The immunosuppressive regimen used over the 3 decades of our Continuously functioning liver transplantation program. A few patients received cyclophosphamide instead of azathioprine in 1971–1972 (not Shown) (Modified from Todo S, Fung J, Starzl TE, et al. Single center experience with primary orthotopic liver transplantation under FK 506 immunosuppression. Ann Surg 1994;220:297, used by permission)
Table 1.
Patient characteristics, indications, and urgency for liver transplantation.
| Total Population | Adult (>18 yrs) | Pediatric (<18 yrs) | ||||
|---|---|---|---|---|---|---|
| FK506 | CyA | FK506 | CyA | FK506 | CyA | |
| Number of patients | 1391 | 1212 | 1188 | 971 | 203 | 241 |
| Age (mean±SD yr) | 43.6±19.4 | 37.9±19.8a | 50.1±12.0 | 46.7±12.6a | 5.4±5.5 | 4.8±4.8 |
| Number of grafts | 1582 | 1549 | 1356 | 1224 | 226 | 325 |
| Primary transplantation | 1391 | 1212 | 1188 | 971 | 203 | 241 |
| Retransplantation | 191 (13.7%) | 337 (27.8%)a | 168 (14.1%) | 253 (26.1 %)a | 23 (11.3%) | 84 (34.9%)a |
| Indications | ||||||
| Postnecrotic cirrhosis | 770 (55.4%) | 526 (43.4%)a | 749 (63.0%) | 508 (52.3%)a | 21 (10.3%) | 18 (7.5%) |
| Biliary atresia | 103 (7.4%) | 144 (11.9%) | 4 (0.3%) | 6 (0.6%) | 99 (48.8%) | 138 (57.3%) |
| Cholestatic disease | 198 (14.2%) | 225 (18.6%) | 196 (16.5%) | 223 (23.0%) | 2 (1.0%) | 2 (0.8%) |
| Metabolic disease | 65 (4.7%) | 70 (5.8%) | 38 (3.2%) | 39 (4.0%) | 27 (13.3%) | 31 (12.9%) |
| Primary malignancy | 90 (6.5%) | 73 (6.0%) | 89 (7.5%) | 69 (7.1%) | 1 (0.5%) | 4 (1.7%) |
| Fulminant failure | 43 (3.1%) | 62 (5.1%) | 30 (2.5%) | 44 (4.5%) | 13 (6.4%) | 18 (7.5%) |
| Others | 122 (8.8%) | 112 (9.2%) | 82 (6.9%) | 82 (8.4%) | 40 (20.7%) | 30 (12.4%) |
| UNOS statusb | ||||||
| 1 | 8 (0.6%) | 67 (6.7%)a | 5 (0.4%) | 56 (6.8%)a | 3 (1.5%) | 11 (6.1 %)a |
| 2 | 246 (17.7%) | 208 (20.7%) | 189 (15.9%) | 156 (18.9%) | 57 (28.1%) | 52 (28.9%) |
| 3 | 548 (39.4%) | 401 (39.8%) | 476 (40.1%) | 354 (42.8%) | 72 (35.5%) | 47 (26.1%) |
| 4 | 589 (42.3%) | 331 (32.9%)a | 518 (43.6%) | 261 (31.6%)a | 71 (35%) | 70 (38.9%) |
| Median follow-up, months (range) | 30 (3–55) | 68 (25–99) | 29 (3–55) | 66 (25–99) | 34 (3–53) | 70 (53–98) |
CyA=cyclosporine, UNOS=United Network for Organ Sharing.
p<0.05.
UNOS status of 144 adult patients and 61 children in cyclosporine group could not be determined.
For both FK506 and CyA-immunosuppressed patients, ABO-identical donors were routinely used with only a handful of exceptions. HLA matching was random. The lymphocytotoxic crossmatches were positive in about 10% of cases (26). The different techniques used for both the donor and recipient operations have been described elsewhere (27).
Immunosuppression
The management policies were essentially the same as previously reported for FK506 (19) and CyA (28). The nephrotoxicity, neurotoxicity, and diabetogenicity of FK506 were recognized and shown to be dose related from the outset (19,20,29,30). It was also promptly observed and documented that hepatic graft dysfunction was associated with defective clearance of FK506 which necessitated downward dose adjustments (30,31). Consequently, dose changes were guided by the balance between evidence of drug toxicity and graft rejection, as well as by the FK506 trough plasma levels which were targeted to 1–1.5 ng/ml. During the first 10 months of the study, the initial intravenous doses of FK506 were 0.15 mg/kg/day given in divided doses every 12 hours over 4 hours. In May 1990, after obtaining a better understanding of the pharmacokinetics of FK506, the intravenous doses were reduced to 0.1 mg/kg/day and administered by continuous infusion until oral administration was started (32). When these intravenous induction doses were still found to achieve toxic drug plasma levels in a significant number of patients, further reduction was adopted in August 1991 to a daily dose of 0.05 mg/kg. The oral doses which originally were 0.3 mg/kg/day also were scaled down according to the daily available trough plasma levels. Interestingly, children required average FK506 doses per kg one and a half times greater than those of adults to achieve equivalent plasma trough levels (25).
With these improvements in management policy and consequent avoidance of the early postoperative FK506 plasma spike, the toxicity problems that plagued our initial trial (as well as the early multicenter European and American trials) were largely eliminated. Importantly, the incidence of rejection did not increase as the FK506 doses were reduced (25). The penalty was the need for compensatory increases in maintenance prednisone doses and consequent greater incidence of early hyperglycemia (25). However, progressive weaning to lower doses of Prednisone as well as FK506 throughout the first year and thereafter made management easier, reduced carbohydrate side effects and other toxicity, and assured a high quality of life among both pediatric and adult recipients.
Two regimens of steroid co-therapy were systematically tested. In both, 1 gm of methylprednisolone was administered intravenously immediately after graft reperfusion. In the low-dose steroid induction variation, a daily dose of 20 mg of prednisone was begun and reduced in 2 or 4 weeks in the absence of rejection. Thereafter, prednisone was weaned and frequently discontinued. The high-dose regimen used for the first 63 patients and at the end of the trial consisted of a 5-day prednisone taper beginning at 200 mg/day for the first Postoperative day with reduction of 40 mg/day until 20 mg/day was reached on the sixth day (33,34). Doses were scaled down for children with both high- and low-dose induction. Subsequent weaning was the same with both steroid variations and nearly half of the FK506 patients were steroid free by 3 months after transplantation.
After it was demonstrated that the adverse effect of the cytotoxic crossmatch (about 10% of cases but usually not known in advance) could be abrogated with the combination of an early prednisone cycle when combined with a 1–2 week course of prostaglandin E1 (ProstinR) (34), we adopted high-dose steroid induction for all patients and this is our current policy. A low dose of azathioprine (0.5–2 mg/kg/day) was given to about 10% of the patients at some time during the postoperative period.
When rejection occurred, it was treated with an increased maintenance dose of FK506 and a 1 gm burst of either methylprednisolone or hydrocortisone. A steroid recycle and/or a 5-day course of OKT3 (5–10 mg/day) was given to patients with moderate to severe rejection episodes.
RESULTS
Total Population
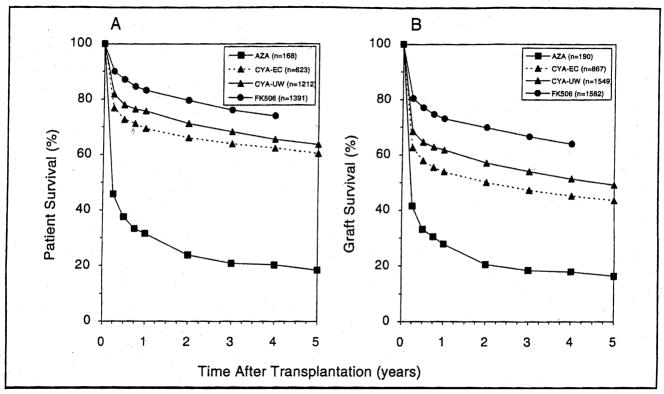
The significant improvement in patient and graft survival over the 30-year history of clinical hepatic transplantation is shown in Figure 2A and 2B, respectively.
Figure 2.
The Kaplan-Meier actuarial survival of 3,394 patients (A) that received 4, 188 liver grafts (B) during the 30-year history of the program at the Universities of Colorado (1963–1980) and Pittsburgh (1981–1993). The dates of case accrual from bottom to top curves were 1963–1979 (Group I), 1980–1987 (Group II), 1987–1989 (Group III), and 1989–1993 (Group IV). The difference in survival between Group III (CyA) and Group IV (FK506) was statistically signficant (p<0.001) (From Todo S, Fung J, Starzl TE, et al. Single center experience with primary orthotopic liver transplantation under FK 506 immunosuppression. Ann Surg 1994;220;297, used by permission)
Patient Survival
The greatest increment in survival was achieved with the use of CyA (Group II). A small additional gain came with the introduction of UW solution (Group III). Further improvement relative to what had been achievable previously already was evident with our initial experience with FK506 (35) and has been confirmed with our current long-term survival data (25).
The patient survival advantage of FK506 was evident within the first 3 postoperative months (8%) and was maintained at 9% out to 4 years [compare Group III (CyA-UW) and Group IV (FK506) Fig. 2A]. The causes of mortality were the same in both groups, but the early and late incidence of each of the principal lethal complications was less with FK506 (Table 2). The difference was statistically significant for 3 of the 7 categories: technical failure (vascular or biliary), sepsis, and the umbrella of “immunologic reasons” [which included rejection, graft-versus-host disease (GvHD), and posttransplant lymphoproliferative disorders (PTLD)]. In later follow-up, the lower rate of death with FK506 in 2 other categories also became statistically significant: disease recurrence and extrahepatic events.
Table 2.
Early and late cause of death among FK506- and CyA-based immunosuppression primary liver allograft recipients.
| Cause | Early Mortality (<3 months) | Late Mortality (>3 months) | ||
|---|---|---|---|---|
| FK506 (n=1,391) | CyA (n=1,212) | FK506 (n=1,254) | CyA (n=992) | |
| Technical failure | 28 (2.0%) | 69 (5.7%)a | 25 (2.0%) | 22 (2.2%) |
| Sepsis | 34 (2.4%) | 52 (4.3%)a | 27 (2.2%) | 35 (3.5%)a |
| Immunosuppression related | 10 (0.7%) | 23 (1.9%)a | 17 (1.4%) | 36 (3.6%) |
| Graft failure | 40 (2.9%) | 43 (3.5%) | 3 (0.2%) | 2 (0.2%) |
| Disease recurrence | 1 (0.1%) | 3 (0.2%) | 54 (4.3%) | 84 (8.5%)a |
| Extrahepatic event | 23 (1.7%) | 26 (2.1%) | 14 (1,1%) | 32 (3.2%)a |
| Others | 1 (0.1%) | 4 (0.3%) | 16 (1.3%) | 31 (3.1%)a |
| Total | 137 (9.8%) | 220 (18.2%)a | 156 (12.4%) | 242 (24.3%)a |
CyA=Cyclosporine
p<0.05
In Table 3, the 7 categories of causes of death under CyA and FK506 are stratified for adults and children. By Cox regression analysis, the relative risk of fatal technical complications or sepsis in the pediatric group was more than 4 times higher under CyA (p<0.05). For adults, immunologic problems, sepsis, and disease recurrence (most commonly malignancy or viral hepatitis) ranged from 1.6 to 2 times higher with CyA than with FK506 (p<0.05).
Table 3.
Cause of death after primary liver transplantation under CyA- and FK506-based immunosuppression for both adults and children
| Cause | Adult (n=2159) | Pediatric (n=444) | ||
|---|---|---|---|---|
| FK506 (n=1188) | CyA (n=971) | FK506 (n=203) | CyA (n=241) | |
| Technical failure | 47 (4.0%) | 58 (6.0%)a | 6 (3.0%) | 33 (13.7%)a |
| Sepsis | 58 (4.9%) | 74 (7.6%)a | 3 (1.5%) | 13 (5.4%)a |
| Immunosuppression related | 21 (1.8%) | 43 (4.4%)a | 6 (3.0%) | 16 (6.6%) |
| Graft failure | 36 (3.0%) | 36 (3.7%) | 7 (3.4%) | 9 (3.7%) |
| Disease recurrence | 54 (4.5%) | 84 (8.7%)a | 1 (0.5%) | 3 (1.2%) |
| Extrahepatic event | 34 (2.9%) | 49 (5.0%)a | 3 (1.5%) | 9 (3.7%) |
| Others | 17 (1.4%) | 28 (2.9%)a | 0 (0.0%) | 7 (2.9%)a |
| Total | 267 (22.5%) | 372 (38.3%)a | 26 (12.8%) | 90 (37.3%)a |
CyA=Cyclosporine
p<0.05
Graft Survival
The depicted survival curves of grafts in the different eras (Fig. 2B) and their degree of separation from each other were parallel to those of patient survival (Fig. 2A) but about 10% lower. The difference between patient and graft survival emphasizes the survival benefit of retransplantation. The improvement of graft survival when UW solution was added to CyA-based immunosuppression was significant (p=0.006) as was the further improvement with FK506 (p<0.001).
Survival of Subgroups
By Age Groups
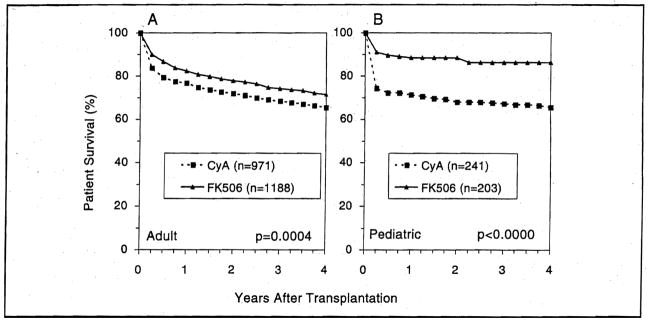
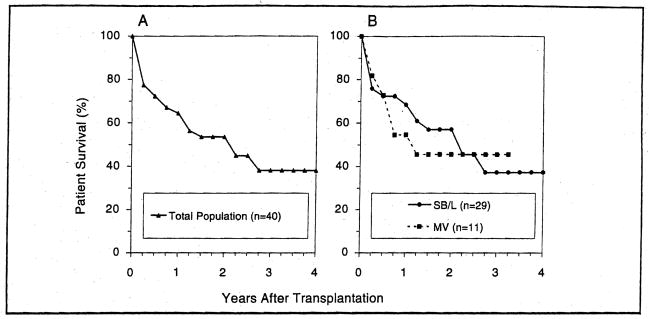
Infants and children of all ages, with all diagnoses and of all urgency categories including the high-risk UNOS Status 4, had a 20.7% improvement in survival under FK506 (p=0.001) up to 4 years (Fig. 3). The long-term survival benefit was greater in the 0–2 years (24.3%) and 12–18 years (27.1 %) age range than in the 2–12 years age group (14.6%).
Figure 3.
The actuarial patient survival for both adult (A) and pediatric (B) primary liver recipients who received either CyA or FK506 as the primary immunosuppressant agent.
For adults, the survival benefit of FK506 was 6.1 % at 4 years (p=0.0005) — higher in the young adults (7.4%) than in the senior (>60 years) group (4.5%).
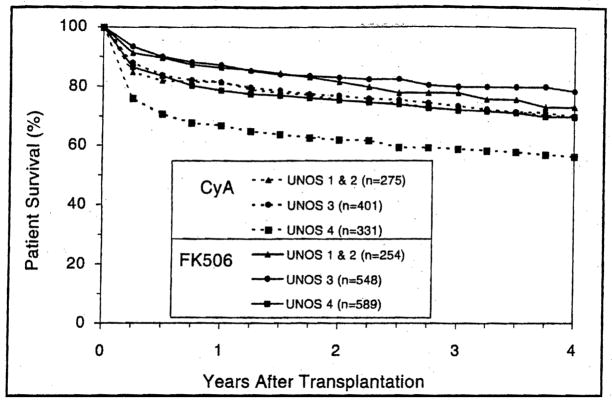
By Medical Urgency
Disease gravity significantly affected the survival of both FK506 and CyA liver allograft recipients (p<0.05) (Fig. 4). FK506 permitted better short-and long-term survival than CyA among all risk categories with a highly significant benefit among both UNOS Status 3 (p=0.004) and UNOS Status 4 patients (p<0.001). In UNOS Status 4 patients, the 4-year survival advantage was 18% among pediatric recipients (p=0.01) and 12.9% among adults (p=0.0002).
Figure 4.
Patient survival after liver transplantation stratified according to the primary immunosuppressant and the medical urgency for surgery as defined by the standard criteria of the United Network for Organ Sharing (UNOS).
By Original Liver Disease
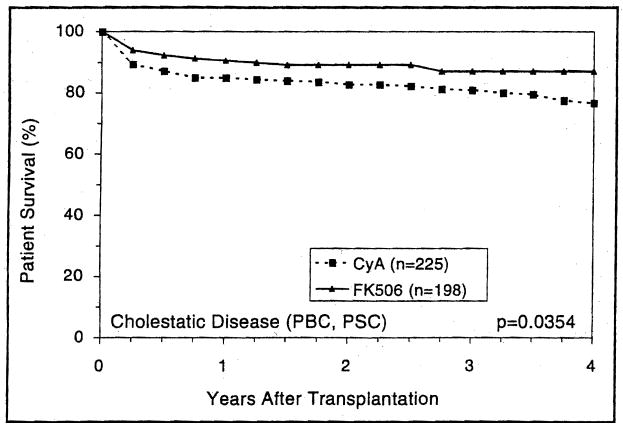
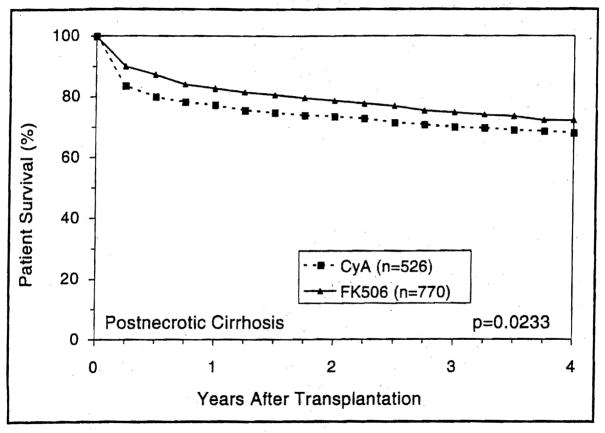
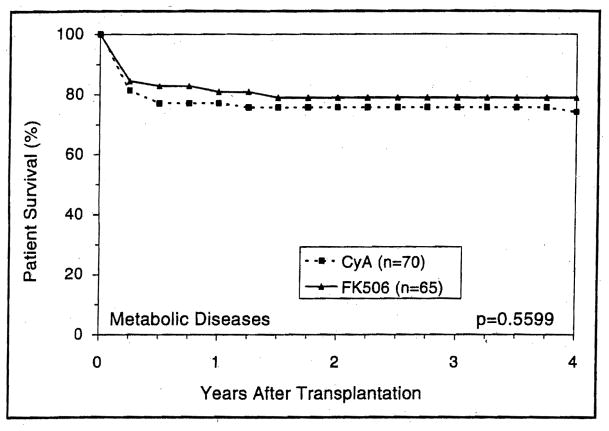
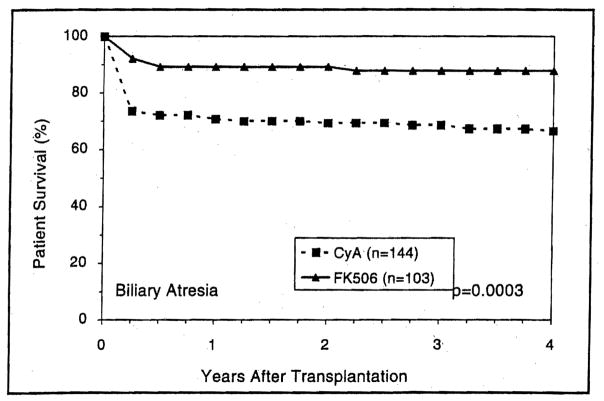
Patients with either cholestatic or parenchymal liver disease had higher short- and long-term survival with FK506-based immunosuppression than with CyA (Figs. 5 and 6) (p=0.03 and 0.02, respectively). However, the trend toward better 4-year survival with FK506 in recipients with metabolic liver diseases was not statistically significant (p=0.5) (Fig. 7). Patients with cholestatic liver disease experienced a 4-year survival increment of 7.2% for primary biliary cirrhosis (p=0.5) and 15% for primary sclerosing cholangitis (p=0.01). Interestingly, patients with biliary atresia demonstrated the highest survival advantage of FK506 (Fig. 8).
Figure 5.
The actuarial survival of primary liver allograft recipients who had cholestatic liver diseases (PBG, PSC) before transplantation and received GyA- and FK506-baseline immunosuppression.
Figure 6.
The actuarial patient survival after primary liver transplantation among recipients with perioperative diagnosis of postnecrotic cirrhosis.
Figure 7.
The estimated survival of the metabolic disease primary liver recipients under CyA and FK506.
Figure 8.
The survival advantage of FK506 over CyA among primary liver recipients with perioperative diagnosis of biliary atresia.
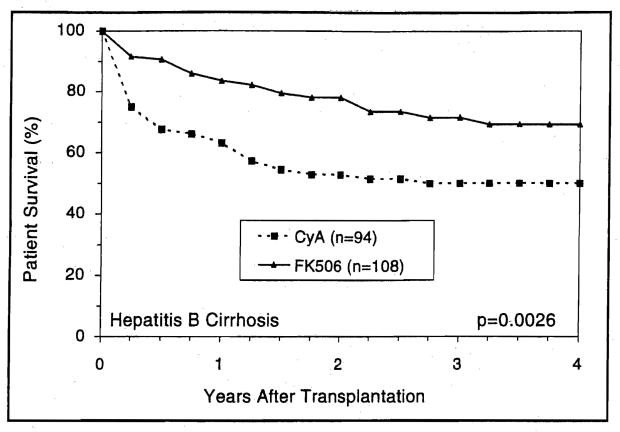
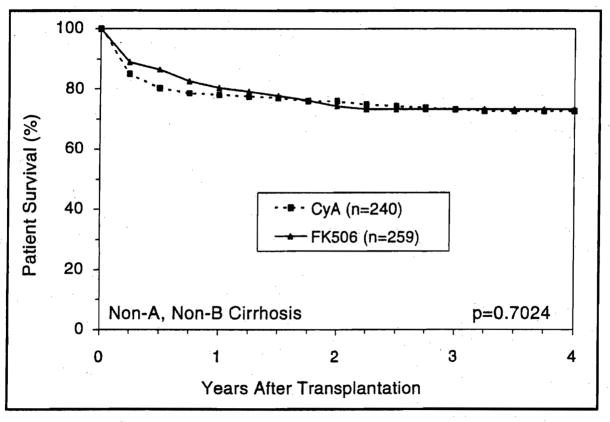
In the postnecrotic cirrhosis population, recipients with preoperative diagnosis of viral B hepatitis achieved a significantly (p=0.003) better survival with FK506 than with CyA (Fig. 9). This survival benefit was not observed among recipients with non-A, non-B hepatitis (Fig. 10).
Figure 9.
The actuarial (Kaplan-Meier) survival of hepatitis B cirrhotics following liver transplantation under CyA and FK506.
Figure 10.
The estimated survival for patients who received primary liver transplantation under CyA and FK506 because of non-A, non-B liver cirrhosis.
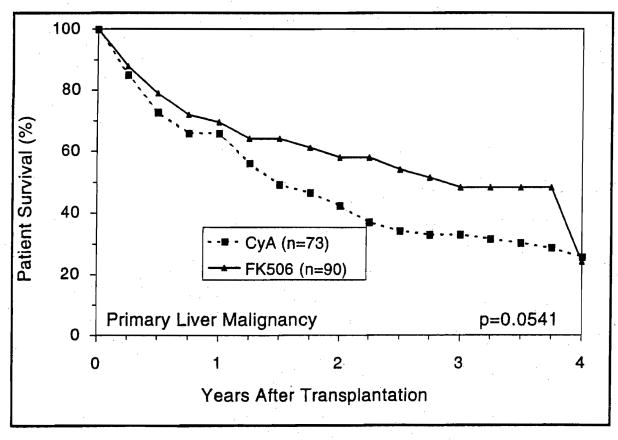
Survival at 4 years of recipients with the diagnosis of primary hepatobiliary malignancy was eroded by tumor recurrence in both the FK506 and CyA limbs (Fig. 11), despite the frequent use of pretransplant intra-arterial chemotherapy in many with or without post-transplant systemic chemotherapy.
Figure 11.
The detrimental effect of primary liver malignancy on long-term patient survival after orthotopic liver transplantation.
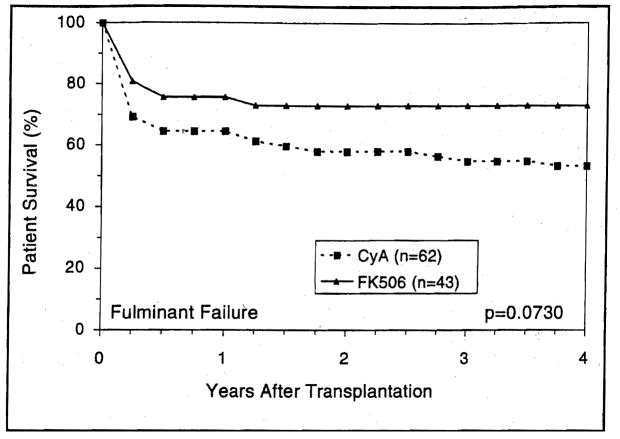
Patients who were transplanted for fulminant hepatic failure showed significantly better survival with FK506 compared with CyA (Fig. 12).
Figure 12.
The survival advantage of FK506 over CyA among patients who received liver transplantation due to fulminant hepatic failure.
The Retransplantation Index
Under FK506, retransplantation was required in 13.7% of cases compared to 27.8% in those treated with CyA. The reduction with FK506 was statistically significant (p<0.00001) in the total, pediatric, and adult populations (Table 1). The survival of the second and subsequent grafts was also significantly better with FK506 recipients, even when crediting success to CyA in 125 patients rescued with FK506 either immediately before or after retransplantation (Fig. 13).
Figure 13.
The Kaplan-Meier graft survival after hepatic retransplantation under CyA and FK506.
The early and late causes of retransplantation under FK506 and CyA are given in Table 4. Among causes of early retransplantation, technical failure and intractable rejection were significantly less under FK506 (p<0.00001 and 0.006, respectively). The reduction of need for late retransplantation under FK506 was mainly due to fewer examples of irreversible rejection and disease recurrence (Table 4). By Cox regression analysis, the risk of retransplantation because of rejection over 4 years of follow-up was 2.4 times greater with CyA than with FK506. While technical failure and rejection accounted for the significant difference in the retransplantation rate among both adults and children, disease recurrence was a significant variable in the adult recipients only (Table 5).
Table 4.
Causes of liver retransplantation in FK506 and CyA primary allograft recipients early and late after operation.
| Cause | Early Retransplant (<3 months) | Late Retransplant (>3 months) | ||
|---|---|---|---|---|
| FK506 (n=1391) | CyA (n=1212) | FK506 (n=1254) | CyA (n=992) | |
| Technical failure | 34 (2.4%) | 69 (5.7%)a | 14 (1.1%) | 18 (1.8%) |
| Rejection | 7 (0.5%) | 19 (1.6%)a | 11 (0.9%) | 37 (3.7%)a |
| Graft infection | 4 (0.3%) | 8 (0.7%) | 3 (0.2%) | 3 (0.3%) |
| Graft failure | 89 (6.4%) | 92 (7.6%) | 1 (0.1%) | 0 (0.0%) |
| Disease recurrence | 0 (0.0%) | 0 (0.0%) | 8 (0.6%) | 25 (2.5%)a |
| Others | 2 (0.1%) | 0 (0.0%) | 2 (0.2% | 4 (0.4%) |
| Total | 136 (9.8%) | 188 (15.6%)a | 39 (3.1%) | 87 (8.7%)a |
CyA=Cyclosporine
p<0.05
Table 5.
Causes of hepatic retransplantation among adult and pediatric recipients under FK506-and CyA-based immunosuppression.
| Cause | Adult | Pediatric | ||
|---|---|---|---|---|
| FK506 (n=1,188) | CyA (n=971) | FK506 (n=203) | CyA (n=241) | |
| Technical failure | 42 (3.5%) | 52 (5.4%)a | 6 (3.0%) | 35 (14.5%)a |
| Graft Failure | 77 (6.5%) | 80 (8.2%) | 13 (6.4%) | 12 (5.0%) |
| Rejection | 18 (1.5%) | 40 (4.1 %)a | 0 (0.0%) | 16 (6.6%)a |
| Graft infection | 4 (0.3%) | 4 (0.4%) | 3 (1.5%) | 7 (2.9%) |
| Disease recurrence | 8 (0.7%) | 24 (2.5%)a | 0 (0.0%) | 1 (0.4%) |
| Others | 4 (0.3%) | 3 (0.3%) | 0 (0.0%) | 1 (0.4%) |
| Total | 153 (12.9%) | 203 (20.9%)a | 22 (10.8%) | 72 (29.9%)a |
CyA=Cyciosporine
p<0.05
The Common Hepatidites
Viral B hepatitis
Of 124 patients with prior hepatitis B infections (all HBsAg+), 65 were treated with FK506 and 59 with CyA (Table 6). Antihepatitis delta antibody (anti-HD) was positive in 17.1% of the FK506 group and 27.3% of the CyA group. With a similar follow-up period, the mortality rate and frequency of retransplantation were higher among the CyA group (Table 6). Also, recurrence of viral B hepatitis in the transplanted liver was significantly (p=0.02) higher among the CyA group (82%) compared with the FK506 group (60.7%). The reduction in the incidence of disease recurrence with FK506 could be related to the more extensive use of prophylactic HBIG therapy in the later cohort and/or the lesser use of steroids (25).
Table 6.
Liver transplant recipients with HBV infection who received either FK506 or CyA as the baseline immunosuppressive drug.
| Characteristics | FK506 | CyA |
|---|---|---|
| No. of patients | 65 | 59a |
| HBV serology | ||
| HBsAg | 100% | 100% |
| HBeAg | 53% | 46% |
| Anti-delta | 17.1% | 27.3% |
| Patients with tumor | 21.5% | 19% |
| Follow-up (range-months) | 26–64 | 19–68 |
| Death | ||
| Disease related | 13 (20%) | 14 (24%) |
| Tumor related | 2 (3%) | 3 (5%) |
| Others | 7 (10.8%) | 12 (20%) |
| Total | 22 (33.8%) | 29 (49%) |
| Retransplantation | ||
| Disease related | 5 (7.7%) | 7 (11.9%) |
| Nondisease related | 8 (12.3%) | 10 (16.9%) |
| Total | 13 (20%) | 17 (28.8%) |
| Disease recurrenceb | ||
| 6 months HBIG | 20/38 (52.6%) | — |
| ≤3 months HBIG | 12/15 (80%) | 19/25 (76%) |
| No HBIG | 5/8 (62.5%) | 18/20 (90%) |
| Overall | 37/61 (60.7%) | 37/45 (82%)c |
8 patients had fulminant hepatic failure
Patients who survived beyond 60 days, HBIG=hepatitis B immune globulin
p<0.05
Viral C hepatitis
This diagnosis was made perioperatively in 157 of our adult liver recipients without concomitant hepatitis B infection or hepatobiliary malignancy. With a mean follow-up of 23 months, 32 of these patients (20.4%) required retransplantation. However, disease recurrence was responsible in only 4 (2.5%). Retransplantation carried a 53.1% mortality. FK506 versus CyA comparisons could not be made because sophisticated serologic and molecular diagnostic tools were not routinely available in the earlier era.
Autoimmune Disorders
Theoretically, the liver allografts given to patients with perioperative diagnosis of autoimmune hepatic disease; primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune chronic active hepatitis, should be at risk to develop disease recurrence in the graft. The preliminary results of an ongoing study at our center showed that some of these patients developed clinical, radiologic, immunologic, and histopathologic changes supportive of disease recurrence in the liver allograft despite the use of maintenance immunosuppression (unpublished data).
HEPATIC-INTESTINAL AND MULTIVISCERAL TRANSPLANTATION
PATIENTS AND METHODS
During the period from July 24, 1990 until May 14, 1994, a total of 40 consecutive patients were given liver allografts in continuity with the intestine (n=29) or as part of a multivisceral composite graft (n=11). Twenty-six recipients were children with a mean age of 3.4 years (range: 0.5–15.5) and the remaining 14 were adults with a mean age of 31 years (range: 19–45). The causes of intestinal failure for both children and adults and the indications for the two different transplant operations are shown in Table 7.
Table 7.
Causes of intestinal failure and indications for combined liver-intestinal and abdominal multivisceral transplantation.
| Cause | Adults (n=14) | Cause | Children (n=26) | ||
|---|---|---|---|---|---|
| Liver-intestine | Multivisceral | Intestine-Liver | Multivisceral | ||
| Crohn’s disease | 1 | 0 | Gastroschisis | 5c | 0 |
| Abdominal trauma | 2 | 0 | Necro-enterocolitis | 6 | 0 |
| Celiac A occlusion | 0 | 3a | Volvulus | 5 | 1 |
| SMA thrombosis | 3 | 0 | Intestinal atresia | 4 | 1 |
| Desmoid tumor | 1 | 1 | Microvillus disease | 1 | 0 |
| Metastatic gastrinoma | 0 | 1 | Pseudo-obstruction | 0 | 2d |
| Budd-Chiari syndrome | 0 | 1 | Hirschsprung’s disease | 1 | 0 |
| Pseudo-obstruction | 0 | 1b | |||
These patients developed short-gut syndrome due to concomitant superior mesenteric artery (SMA) thrombosis because of protein S deficiency (n=1) antithrombin III deficiency (n=1) or unknown (n=1).
The patient received the multivisceral graft after failure of the primary isolated intestinal graft due to refractory rejection.
One patient required multivisceral retransplantation 15 months after receiving the liver-intestinal graft because of graft dysfunction.
One patient had pseudo-obstruction after birth that was not diagnosed and received isolated liver allograft 50 months before the multivisceral graft.
Liver-Intestinal Transplantation
All 29 patients who received combined liver-intestinal transplantation (7 adults and 22 children) were primary recipients who were suffering from irreversible intestinal and hepatic failure. Liver failure was precipitated in most cases by the cholestatic effect of long-term total parenteral nutrition (TPN). One of the liver-intestinal recipients required retransplantation with a multivisceral graft 15 months after the first operation because of persistent graft and native gastrointestinal dysfunction. The lymphocytotoxic crossmatch was positive in 3 recipients; two are still alive at 6 and 18 months and the third died of chronic rejection 2 years after transplantation. The colon was included in 9 of the 29 primary grafts and 10 patients received grafts from cytomegalovirus (CMV) seropositive donors.
Multivisceral Transplantation
The primary 11 multivisceral recipients included 7 adults and 4 children (Table 7). Two of these had already failed a lesser intraabdominal transplant procedure. One adult had an intractibly rejected intestinal graft removed 2 months previously. The other was a pediatric liver recipient with intestinal pseudo-obstruction since birth who should have undergone a multivisceral transplantation on the first occasion.
Although combined liver and intestine grafting was initially considered for some of the 11 primary recipients, multivisceral transplantation was chosen at the time of surgery because of significantly ischemic or diseased native stomach and/or pancreas. A lymphocytotoxic crossmatch was strongly positive in one patient who died of intractable cellular rejection after 58 days. The colon was part of the multivisceral graft in 8 recipients and 4 received grafts from CMV-seropositive donors.
Operative Procedures
All grafts were obtained from ABO blood type-identical cadaveric donors. HLA matching was random and uniformly poor. Immunomodulation of donors or grafts by either irradiation, antilymphocyte preparations (ALG, OKT3), or other modalities was not attempted. The techniques for composite graft harvesting have been described elsewhere (36,37).
Modification of the recipient procedure was frequently required to accommodate anatomic and pathologic circumstances (38–41). In one patient with Budd-Chiari syndrome and mesenteric venous thrombosis, fatal hemorrhage caused by portal hypertension was prevented by occluding the celiac axis and the superior mesenteric artery with intraaortic balloons placed under radiographic guidance before starting the operation. This multivisceral recipient survived the operation with a blood loss of 26 units (41). A pediatric patient with Hirschsprung’s disease had rectal reconstruction by a pull-through technique following a liver-intestine transplantation procedure. In another pediatric patient who had renal insufficiency following a failed liver-intestinal transplantation, both kidneys were included enbloc with the multivisceral graft (41).
Postoperative Management
The postoperative management of these unique recipients is described in full detail elsewhere (42,43). Immunosuppression was the same as described under the FK506 trials for liver transplantation, using the high-dose steroid induction. Routine liver function tests were used to monitor liver rejection, and liver biopsies were performed if indicated. Amylase and lipase levels in blood and/or abdominal fluids were obtained to monitor preservation injury and/or rejection of the pancreas. In long-term survivors, blood glucose and c-peptide levels were determined after intravenous injection of 0.5 g/kg glucose.
Intestinal graft rejection was diagnosed by clinical findings, endoscopic examination, and histopathological study of endoscopic-guided biopsies. Intestinal graft function was assessed by body weight, volume of stomal output, frequency and nature of the stool, and dependency on TPN, enteral feeding, and/or oral diet. In addition, absorptive functions were directly measured by d-xylose tests and by 72-hour fecal fat excretion. Gastrointestinal motility was evaluated by measurements of gastric emptying after radio-labeled test meals, intestinal transit time of a barium meal, and manometric measures of contractile activity.
Systemic antibiotics were given prophylactically for the first 5 days, as well as subsequently, if indicated by the results of blood and body fluid cultures. Selective gut decontamination was used for 4–6 weeks after transplantation and resumed later during moderate to severe rejection episodes. Chronic viral and protozoal prophylaxis was with acyclovir for CMV and bactrim for pneumocystis carinii. Because of the high incidence of CMV disease among most of the early recipients who received CMV-positive grafts, ganciclovir was given prophylactically for 2–3 weeks in children and for 3 weeks to 3 months in adults based upon the CMV status of both donors and recipients. If severe CMV infection occurred despite prophylaxis, Foscarnet or CMV immunoglobulin or both, were added to or replaced the ganciclovir treatment (44,45).
RESULTS
Patient Survival
During potential follow-ups of 7–52 months (to December 1, 1994) 21 (52.5%) of the 40 patients died: 15/29 liver plus intestine and 6/11 multivisceral. The causes of death were technical complications (n=3) opportunistic infections (n=4), uncontrolled graft rejection (n=4), disseminated posttransplant lymphoproliferative disease (PTLD) (n=5), liver failure from cholestatic viral C hepatitis (n=2), and others (n=3).
Two of the 6 deaths in the multivisceral group were caused by PTLD, one by respiratory failure, one by rejection, one by sepsis, and one by liver failure from cholestatic viral C hepatitis. In addition to the 2 primary multivisceral recipients who died from PTLD 49 and 198 days after transplantation, a third patient succumbed to this complication 57 days after multivisceral retransplantation of a failed primary liver-intestinal graft.
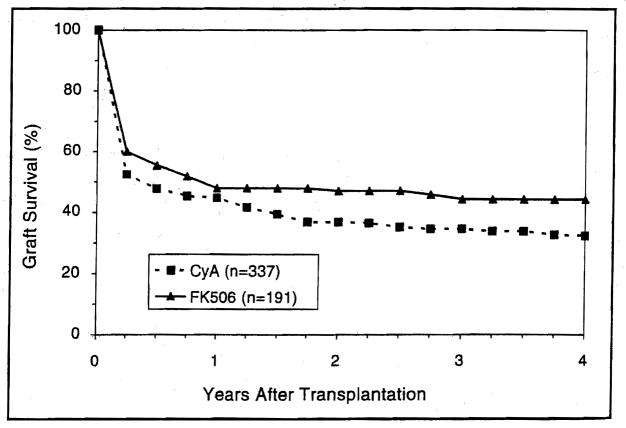
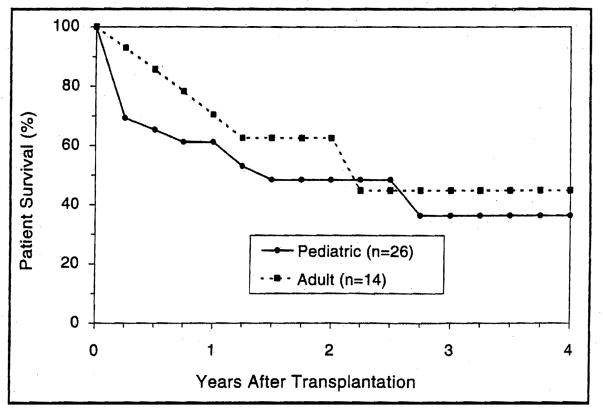
The actuarial survival rate for the 40 patients in the combined groups, at 6,12,24, and 48 months after transplantation, was 72%, 65%, 54%, and 38%, respectively (Fig. 14A). With a mean follow up of 15±12 months for liver-intestine recipients and 20±15 for multivisceral recipients, the 6-month Kaplan-Meier estimated survival was similar for both cohorts with a rate of 72.3% and 72.7%, respectively (Fig. 14B). At one year following transplantation, these estimates were 68.5% and 54.6%, respectively. At 3 years, the actuarial survival rate was 37.2% and 45.5% for the liver-intestine and multivisceral recipients, respectively (Fig. 14B). The adult liver-intestine and multivisceral recipients had better early and late survival than those in the pediatric population (Fig. 15).
Figure 14.
The actuarial survival of the combined liver-intestinal and multivisceral recipients. A) all 40 patients, B) according to procedure.
Figure 15.
The actuarial patient survival after combined liver-intestinal and multivisceral transplantation among both adult and pediatric recipients.
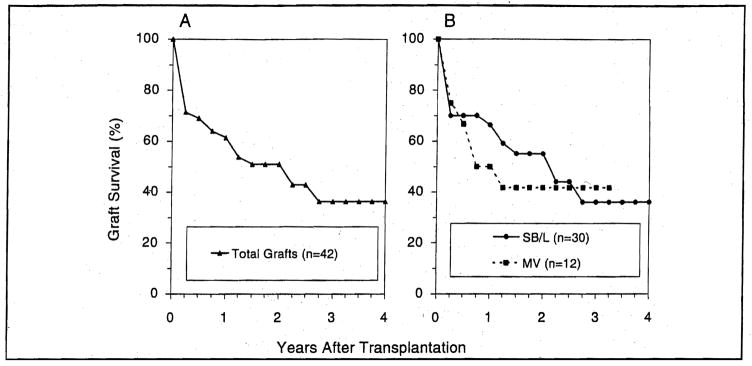
Graft Survival
Actuarial survival for the grafts (n=42) closely mirrored patient survival (Fig. 16A and B) because all but 2 grafts were lost due to patient’s death. The only 2 grafts removed at retransplantation were liver-intestinal, of which one had been transplanted to a child with a strongly positive cytotoxic crossmatch. Replacement was with another liver-intestinal graft in one and a multivisceral graft in the other—both in pediatric recipients. Although these procedures were technically successful, the children died 60 and 57 days later from refractory rejection and PTLD, respectively.
Figure 16.
Survival of the combined hepatic-intestinal and multivisceral grafts. A) all 42 attempts including 2 retransplantations; B) according to procedure.
Two other recipients had loss of parts of their composite grafts because of severe preservation injury of the pancreas in one instance necessitating pancreatectomy, and hepatic artery thrombosis in the other requiring replacement of the allograft liver. These patients died 621 and 29 days, respectively, after their primary transplantation.
Risk Factors
Two correctable factors eroded the optimistic expectations from our early experience with intestinal transplantation, either alone or as part of a composite graft (38,39,46): inclusion of the colon with the small bowel (47) and acceptance of the grafts from CMV-seropositive donors (44,45,47). The one-year survival was 78% for patients (n=23) who did not receive the colon and 47% for those (n=17) who did (p=0.04). The presence of the colon appeared to increase difficulties with rejection which was a common cause of graft loss.
Liver-intestinal and multivisceral recipients of CMV-positive grafts (n=14) had 2- and 3-year survival of 33% and 16% compared to 64.5% and 49% when the donors were seronegative (n=26) (p=0.01). Although the immediate cause of death often was something other than CMV, the pervasively harmful influence of this transplanted virus, and the necessity for its intensive treatment, particularly in preoperatively CMV-negative recipients, has been documented by Manez et al (44).
Patients who did not receive the colon and had CMV-negative donors had a 2-year survival rate of 75.6%.
Long-Term Rehabilitation
This experience has established feasibility, although not the practicality of both liver-intestinal and multivisceral transplantation. All of the 19 current survivors are at home, free of TPN, and on unrestricted oral diets.
DRUG-FREE GRAFT ACCEPTANCE
It has long been assumed that clinical organ transplantation implies a lifetime commitment to immunosuppression. Our recent experience in weaning drugs in long-surviving hepatic allograft recipients has shown that many of these patients have achieved donor-specific tolerance (48).
MATERIALS AND METHODS
Between June, 1992, and March, 1994, 59 liver allograft recipients (20 children and 39 adults) who had survived more than 5 years were thoroughly evaluated for weaning of their maintenance immunosuppression. Another 4 patients were self weaned (total 63). Excluded from the study were patients with histological proven chronic rejection and/or hepatitis. The age of the enrolled patients ranged from 12–68 years and the spectrum of the native liver disease was diverse. Most of the patients (97%) had experienced one or more long-term adverse effects of one or more of the immunosuppressive drugs including impending renal failure (n=8), skin cancer (n=2), verruca vulgaris of skin (n=19), significant osteoporosis and arthropathy (n=12), morbid obesity (n=3), refractory systemic hypertension (n=11), and recurrent opportunistic infections (n=2). Eighteen of these recipients had been reported previously to be chimeric (49).
Weaning was gradually done in 2 controlled steps; first, gradual reduction of the daily steroid doses with total withdrawal guided by the results of corticotropin- stimulation tests and second, gradual stepwise withdrawal of azathioprine, CyA, or FK 506. Further reductions in immunosuppression were made on a monthly basis and biochemical monitoring of liver cell injury was done every 1–2 weeks and when it was clinically indicated. Specific details of the weaning protocol and management policy have been published elsewhere (48).
RESULTS
Four of the first 18 patients with unequivocally proven chimerism have been completely off immunosuppressive drug therapy for 5–11 years. Another 16 liver recipients have been successfully weaned of immunosuppression with a mean (±SD) drug-free period of 11±6 months (range 1–16), giving a. total incidence of drug-free graft acceptance of 31.7%. In addition, 28 recipients are currently at various stages of drug withdrawal without significant changes in the biochemical battery of liver functions. In 15 (24%) cases, however, immunosuppression,. was resumed because of biochemical and histopathologic evidence of rejection; mild to moderate in 13 and moderate to severe in 2.
Patients who failed weaning resumed their baseline therapy and in addition were. given a 1 gm steroid bolus followed by a 5-day prednisone taper if indicated. Of interest, 4 of these 15 patients who developed rejection due to withdrawal of immunosuppression had previously been shown to have donor leukocyte chimerism. There were no graft losses or demonstrable loss of graft function nor any penalty of increased chronic need for immunosuppression.
EFFORTS TO INDUCE TOLERANCE
The foregoing weaning trials were undertaken following our demonstration in 1992 of migration of donor leukocytes from the transplanted organs and persistence of these cells in recipient tissues (microchimerism). We have postulated that these events explain the long-term acceptance of organ allografts and is the first stage in the development of donor-specific tolerance (49–53). As a natural extension of this concept, we attempted to increase the chimerism by augmenting the leukocyte load from the donor with perioperative infusion of unmodified donor bone marrow. The first 18 cases have been reported in detail (54).
MATERIALS AND METHODS
Between December 1992 through October 1994, 80, organ recipients (Table 8) were given 3–5×108 per kg of unmodified donor bone marrow cells intravenously. They were treated with the same regimen of conventional immunosuppressional FK506 and prednisone described earlier in this chapter (Fig. 17). The whole organs were 32 livers, 37 kidneys, 8 hearts, and 3 lungs (Table 8). Probes that detect donor leukocyte HLA antigens (chromosome 6) in venous blood have been routinely used to detect chimerism. In female recipients of male organs, systemic chimerism has been detected by Y chromosome in situ hybridization. Monoclonal antibodies, in situ hybridization, and polymerase chain reaction (PCR) were used to study both chromosome 6 and the Y chromosome (with cross-sex transplantation) as described else where (49,50,53,54); The immunological status of patients pre- and posttransplantation was determined in vitro by the response of patient’s peripheral blood mononuclear cells to conconavalin A and phytohemagglutinin (PHA) mitogens and recall antigen, mixed lymphocyte reaction (MLR, Fig. 18), and cell-mediated lymphocytotoxicity (CML) (54).
Table 8.
Patient and graft survival of primary allograft recipients who received perioperative bone marrow infusion and were treated with the conventional FK506-prednisone therapy.
| Recipient | No. of Patients | Survival | |
|---|---|---|---|
| Patient | Graft | ||
| Liver. | 31 | 30a | 30 |
| Liver islets | 1 | 1 | 1 |
| Kidney | 21 | 21 | 19b |
| Kidney-islets | 6 | 6 | 6 |
| Kidney-pancreas | 10 | 10 | 10 |
| Heart | 8 | 7c | 7 |
| Lung | 3 | 3 | 3 |
| Total | 80 | 78 | 76 |
One patient died 23 days after transplant due to sepsis and multiple organ failure
Two kidneys were lost to rejection on postoperative day 499 and 456, respectively
One patient died 267 days after transplant due to pulmonary embolism
Figure 17.
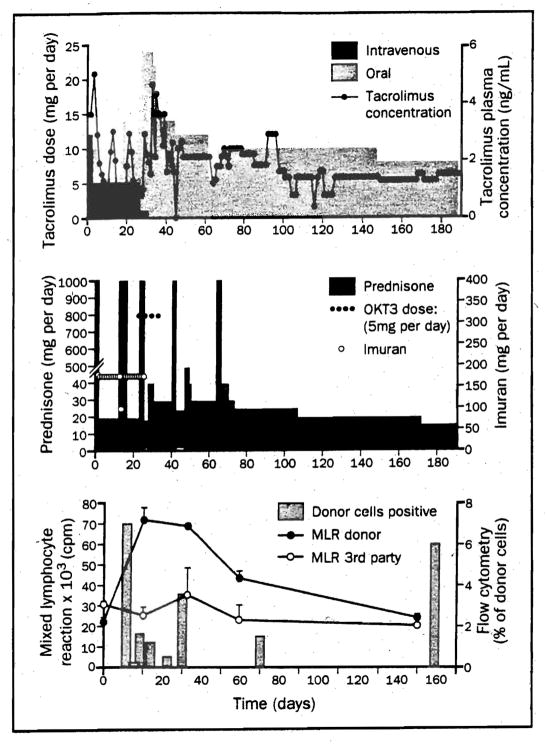
Immunosuppression and MLR responses to irradiated donor and third-party cells, and donor cell chimerism by flow cytometry during the first 6 months after heart-bone marrow transplantation. (From Fontes P, Rao A, Demetris A, et al. Bone marrow augmentation of donor-cell chimerism in kidney, liver, heart, and pancreas islet transplantation. Lancet, 1994; 344:151, Used by permission)
Figure 18.
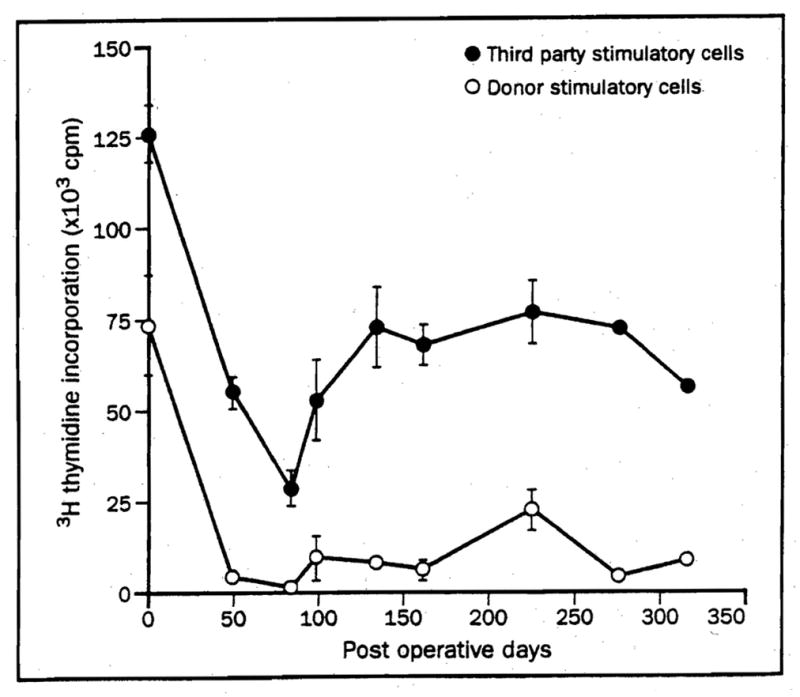
MLR of a kidney-bone marrow recipient Mean (SD) of [3H] thymidine incorporation. (From Fontes P, Rao A, Demetris A, et al. Bone marrow augmentation of donor-cell chimerism in kidney, liver, heart and pancreas islet transplantation. Lancet, 1994;344:151, used by permission).
RESULTS
With follow-up to January 10, 1995, patient and graft survival in the bone marrow-augmented cases is 98% and 95%, respectively (Table 8). Neither the 2 patient nor 4 graft losses were attributable to bone marrow infusion. The density of the resulting chimerism was 1,000 times higher than that occurring spontaneously (54). The postoperative clinical course and the chimeric and immunologic tests were fully described for the first 18 patients elsewhere (54). Observations in later cases have been similar.
The bone marrow infusions were uneventful. There were no clinically significant examples of GvHD and no other complications attributable to the bone marrow.
The rejection-free interval and cumulative incidence of rejection was not different in contemporaneous control (nonmarrow) recipients. Histopathologically, the organ rejections were indistinguishable from those in the bone marrow-augmented groups. These were expected findings. Although the benefit from leukocyte augmentation is expected to be long term, not in the early convalescence, the early results have been superior to those in the nonmarrow controls.
CLINICAL XENOTRANSPLANTATION
Our experience with clinical xenotransplantation was summarized in the 1993 edition of this book and fully reported elsewhere (55,56). Survival of our 2 human recipients of orthotopic baboon livers for only 26 and 70 days prompted a self-imposed moratorium on further trials until new treatment strategies can be devised that deal effectively with the complement activation syndromes that have prevented clinical success, even using subhuman primate donors. Our analysis of this problem has been published elsewhere (57) and is discussed in the next section.
PAST LESSONS AND FUTURE PROSPECTS
Survival after hepatic transplantation has improved incrementally with more effective immunosuppressive agents, better graft preservation, and greater surgical experience. The recent introduction of tacrolimus (FK506) has further increased the survival and welfare of liver and other organ transplant recipients. Our experience indicates that FK506 is superior to CyA. In our abbreviated randomized liver transplantation trial (21), as in the multicenter trials conducted in Europe (22) and the United States (23), crossover from CyA to FK506 because of intractable rejection was a common event that frequently prevented death or the need for graft replacement.
Analyses of the results in the multicenter trials by “intent to treat,” obscured the superiority of FK506 and made it appear that patient and graft survival was similar on both treatment limbs (22,23). However, the parity was made possible by the rescue of a large number of patients denoted as CyA “successes” for whom treatment had been changed permanently to FK506. The obfuscation of results by introduction of this analytic artefact is currently under examination by biostatisticians of unquestioned competence, using the multicenter liver trials as the case material to examine the appropriateness of the methodology. Their conclusions will be important for the field of transplantation, if only to prevent repetition of the same potential errors of evaluation as new immunosuppressive (and other) treatment modalities are introduced to the clinic.
In the 1993 edition of this book, we presented our first 27 combined liver-intestinal and multivisceral recipients with more encouraging actuarial 2-year patient and graft survival than has been realized in subsequent experience. The procedure was modified in 4 of the previously reported 27 recipients and the next 13 cases by retaining the donor colon with the small bowel graft in an attempt to ameliorate the morbidity related to high stomal output and dehydration (58). Significant deterioration of patient and graft survival was observed during the early postoperative period or later because of rejection and/or infection. In addition, some of the previous 27 patients who were given colons were lost late for similar or different reasons.
Another factor — seemingly more detrimental in intestinal transplantation than with other kinds of allografts — was the use of CMV-seropositive grafts to recipients who were CMV seronegative or to a lesser degree even those who were CMV positive preoperatively.
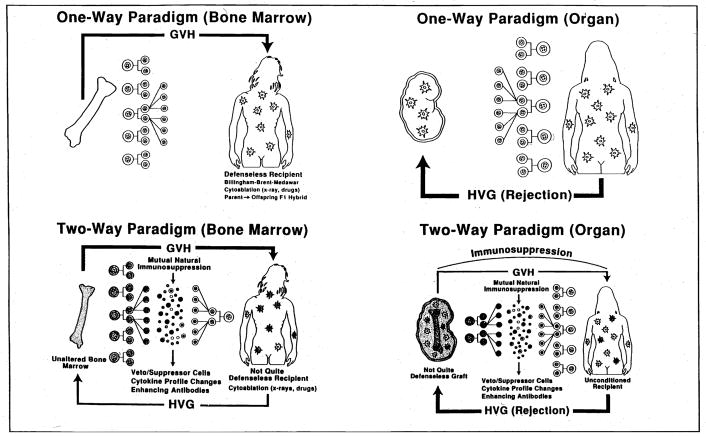
Three new patients have been treated with avoidance of both the colonic and CMV risk factors. Two are at home and well with functioning grafts. The third died of intracranial hemorrhage with a functioning graft 53 days postoperatively. In an additional management change, simultaneous bone marrow infusion from the donor to augment microchimerism has been added to the protocol for future intestinal transplant cases (46). The use of adjuvant bone marrow would have been unthinkable in intestinal cases, even as recently as 2 years ago, because of the fear of GvHD. Historically, an intestinal allograft has been envisioned either as a defenseless target for immunologic attack or alternatively as the aggressor capable of reversing roles and overwhelming the recipient with GvHD. This dogma defined transplantation immunology in terms of a unidirectional immune reaction in which the balance tipped all or none against the host (Fig. 19A) or against the graft (Figure 19B). This one-way paradigm took root several years not long after the description in 1953 of acquired immunologic tolerance by Billingham, Brent, and Medawar (59) and remained unchallenged for more than 3 decades.
Figure 19.
Evolution of the dogma of transplantation immunology from one-way paradigm (A, B) to 2-way paradigm (C, D), with mutual engagement migratory immunocytes from the graft and the recipient under potent pharmacological immunosuppression.
When, in 1992, ubiquitous low-level donor leukocyte chimerism was found in our human recipients of whole organs as long as 30 years posttransplantation (49–53,60), we postulated that the interaction of 2 coexisting donor and recipient leukocyte populations, each to the other, was the fundamental explanation of both bone marrow and organ allograft acceptance and of transplantation tolerance generally (49–53,60). Examination of this “2-way (bidirectional) paradigm” of transplantation immunology (Figs. 19C and D) with the objective of exploiting its mechanisms therapeutically for allo- and xenotransplantation is the theme which we believe is the clear pathway to improve results with all organ allografts including the intestine and ultimately with xenotransplantation.
As for xenotransplantation, the question whether cell migration and chimerism are associated with xenograft acceptance has already been answered. Chronically surviving rat recipients of hamster hearts and more dramatically of livers have graft (61) and systemic chimerism (62), allowing graft survival for several months after discontinuance of immunosuppression at 100 days (61). Similar striking microchimerism was demonstrated throughout life and at autopsy in both of our recent baboon liver recipients (55,63), the second of whom also was infused with 3x108/kg baboon bone marrow cells with no evidence of GvHD. Thus, as with allografts, it is evident that the persistence of the double-cell population is both inevitable and obligatory for success. Other chimerism-associated phenomena including hepatic tolerogenicity have been demonstrated after hamster → rat xenotransplantation (64).
The concept of a reciprocal host-versus-graft (HvG) and graft-versus-host (GvH) reaction has been developed exclusively in terms of cellular immunity, but the probability of a bidirectional humoral reaction is self evident, in which xenografts may have an ability to mount a weak attempt to hyperacutely reject the recipient. In either direction, this kind of rejection is characterized by multiple secondary inflammatory and coagulation events. It is most commonly activated by the interaction of antigen and antibody (classical complement pathway), but the process also can be independent of antibodies (alternative pathway).
The intractability of the humoral component of xenograft rejection to intervention has been rediscovered over and over in terms of the recipient response (65–71). The new cycles of rediscovery have been driven by the availability of better technologies for antibody removal or drugs that allowed intervention at various levels of the “humoral rejection” pathogenesis: staphylococcal protein A adsorbent columns (72) or monoclonal antibodies (73) for antibody removal, the modern anticomplement agents [modified snake venom (74,75); soluble type I complement receptor (76,77); the sesquiterpene compound K76 (63,78)], and antimetabolite drugs that inhibit the clonal expansion of antibody producing B cells (74,79–87). A different kind of drug or other modality than anything previously tried will be required before we will contemplate further clinical xenotransplant trials.
CONCLUSIONS
The survival advantage of hepatic allotransplantation and patient welfare has been significantly improved by the clinical use of tacrolimus (FK506, PrografR). Although hepatic-intestinal and multivisceral transplantation has become a feasible therapy for patients with combined hepatic and intestinal failure, revised treatment strategies are required to achieve better outcome and to increase its practicality.
Weaning to a drug-free state in selected long-term liver allograft survivors is frequently feasible but should never be attempted without a stepwise protocol and careful monitoring of graft function. Augmentation of natural chimerism after whole organ transplantation with perioperative donor bone marrow infusion has been shown to be free of significant complications (including GvHD) in liver, kidney, heart, lung, and pancreas recipients. The persistence of increased microchimerism in these patients along with exceptionally high graft survival and function justifies optimism and further trials.
The future of clinical xenotransplantation has to be projected in the same perspective of the 2-way immunologic paradigm as allotransplantation but with the additional barrier of the complement activation syndromes that also can cause hyperacute rejection of human allografts. Measures to effectively control this kind of rejection have so far eluded us.
Acknowledgments
I gratefully acknowledge the following members of the Pittsburgh Transplantation Institute for their support during preparation of this chapter:
Adrian Casavilla, M.D., Forrest Dodson, M.D., Howard Doyle, M.D., Bijan Eightesad, M.D., Paulo Fontes, M.D., Hiroyuki Furukawa, M.D., Timothy Gayowski, M.D., William Irish, M.Sc., Nicolas Jabbour, M.D., Ashok Jain, M.D., Juan Madariaga, M.D., Ignazio Marino, M.D., Wallis Marsh, M.D., George Mazariegos, M.D., Steve Miller, Antonio Pinna, M.D., Hector Ramos, M.D., Abdul Rao, M.D., Jorge Reyes, M.D., Rick Selby, M.D., and Andreas Tzakis, M.D.
Aided by Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, Maryland.
References
- 1.Ochiai T, Nakajima K, Nagata M, et al. New immunosuppressive drugs. Effect of a new immunosuppressive agent, FK 506, on heterotopic cardiac allotransplantation in the rat. Transplant Proc. 1987;19:1284. [PubMed] [Google Scholar]
- 2.Ochiai T, Nakajima K, Nagata M, et al. Studies of the induction and maintenance of long-term graft acceptance by treatment with FK 506 in heterotopic cardiac allotransplantation in rats. Transplantation. 1987;44(6):734. doi: 10.1097/00007890-198712000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Ochiai T, Nagata M, Nakajima K, et al. Studies of the effect of FK 506 on renal allograft in the beagle dog. Transplantation. 1987;44:729. doi: 10.1097/00007890-198712000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Ochiai T, Hamaguchi K, Isono K. Histopathologic studies in renal transplant recipient dogs receiving treatment with FK 506. Transplant Proc. 1987;19:93. [PubMed] [Google Scholar]
- 5.Ochiai T, Sakamoto K, Gunji Y, et al. Effects of combination treatment with FK 506 and cyclosporine on survival time and vascular changes in renal allograft recipient dogs. Transplantation. 1989;48(2):193. doi: 10.1097/00007890-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Ochiai T, Gunji Y, Sakamoto K, et al. Optimal serum trough levels of FK 506 in renal allotransplantation of the beagle dog. Transplantation. 1989;48(2):189. doi: 10.1097/00007890-198908000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Murase N, Todo S, Lee P-H, et al. Heterotopic heart transplantation in the rat under FK 506 alone or with cyclosporine. Transplant Proc. 1987;19:71. [PMC free article] [PubMed] [Google Scholar]
- 8.Todo S, Demetris AJ, Ueda Y, et al. Canine kidney transplantation with FK 506 alone or in combination with cyclosporine and steroids. Transplant Proc. 1987:57. [PMC free article] [PubMed] [Google Scholar]
- 9.Todo S, Ueda Y, Demetris JA, et al. Immunosuppression of canine, monkey, and baboon allografts by FK 506 with special reference to synergism with other drugs, and to tolerance induction. Surgery. 1988;104:239. [PMC free article] [PubMed] [Google Scholar]
- 10.Todo S, Demetris A, Ueda Y, et al. Renal transplantation in baboons under FK 506. Surgery. 1989;106:444. [PubMed] [Google Scholar]
- 11.Zeevi A, Duquesnoy R, Eiras G, et al. Immunosuppressive effect of FK 506 on in vitro lymphocyte alloactivation: Synergism with cyclosporine A. Transplant Proc. 1987;19:40. [PMC free article] [PubMed] [Google Scholar]
- 12.Warty V, Diven W, Cadoff E, Todo S. FK 506: a novel immunosuppressive agent. Characteristics of binding and uptake by human lymphocytes. Transplantation. 1988;46:453. doi: 10.1097/00007890-198809000-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim SML, Thiru S, White DJG. Heterotopic heart transplantation in the rat receiving FK 506. Transplant Proc. 1987;19:68. [PubMed] [Google Scholar]
- 14.Collier DS, Thiru S, Calne R. Kidney transplantation in the dog receiving FK 506. Transplant Proc. 1987;19:62. [PubMed] [Google Scholar]
- 15.Thiru S, Collier DS, Calne R. Pathological studies in canine and baboon renal allograft recipients immunosuppressed with FK 506. Transplant Proc. 1987;19:198. [PubMed] [Google Scholar]
- 16.Starzl TE, Todo S, Fung J, et al. FK 506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fung J, Todo S, Jain A, et al. Conversion from cyclosporine to FK 506 in liver allograft recipients with cyclosporine-related complications. Transplant Proc. 1990;22:6. [PMC free article] [PubMed] [Google Scholar]
- 18.Fung J, Todo S, Tzakis A, et al. Conversion of liver allograft recipients from cyclosporine to FK 506-based immunosuppression. Benefits and pitfalls. Transplant Proc. 1991;23:14. [PMC free article] [PubMed] [Google Scholar]
- 19.Todo S, Fung JJ, Starzl TE, et al. Liver, kidney and thoracic organ transplantation under FK 506. Ann Surg. 1990;212:295. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Starzl TE, Fung J, Jordan M, et al. Kidney transplantation under FK 506. JAMA. 1990;164:63. [PMC free article] [PubMed] [Google Scholar]
- 21.Fung J, Todo S, Abu-Elmagd K, et al. A randomized trial in primary liver transplantation under immunosuppression with FK 506 or cyclosporine. Transplant Proc. 1993;25:1130. [PMC free article] [PubMed] [Google Scholar]
- 22.European FK 506 multicenter liver study group. Randomized trial comparing tacrolimus (FK 506) and cyclosporine in prevention of liver allograft rejection. Lancet. 1994;344:423. [PubMed] [Google Scholar]
- 23.The U S. multicenter FK 506 liver study group. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med. 1994;331:1110. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 24.Starzl TE, Marchioro T, Waddell W. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385. [PMC free article] [PubMed] [Google Scholar]
- 25.Todo S, Fung J, Starzl TE, et al. Single center experience with primary orthotopic liver transplantation under FK 506 immunosuppression. Ann Surg. 1994;220:297. doi: 10.1097/00000658-199409000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takaya S, Bronsther O, Iwaki Y, et al. The adverse impact on liver transplantation of using positive cytotoxic crossmatch donors. Transplantation. 1992;53:400. doi: 10.1097/00007890-199202010-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starzl TE, Demetris A. Liver Transplantation. Year Book Medical Publishers, Inc; Chicago, IL: 1990. pp. 1–194. [Google Scholar]
- 28.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starzl TE. Contempo 89: Transplantation. JAMA. 1989;261:2894. [PMC free article] [PubMed] [Google Scholar]
- 30.Starzl TE, Abu-Elmagd K, Tzakis A, et al. Selected topics on FK 506: with special references to rescue of extrahepatic whole organ grafts, transplantation of “forbidden organs”, side effects, mechanisms, and practical pharmacokinetics. Transplant Proc. 1991;23:914. [PubMed] [Google Scholar]
- 31.Abu-Elmagd K, Fung JJ, Alessiani M, et al. The effect of graft function on FK 506 plasma levels, doses, and renal function with particular reference to the liver. Transplantation. 1991;52:71. doi: 10.1097/00007890-199107000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abu-Elmagd K, Fung J, Draviam R, et al. Four-hour versus 24 hour intravenous infusion of FK 506 in liver transplantation. Transplant Proc. 1991;23:2767. [PMC free article] [PubMed] [Google Scholar]
- 33.Jain A, Fung J, Todo S, et al. Incidence and treatment of rejection episodes in primary orthotopic liver transplantation under FK 506. Transplant Proc. 1991;23:928. [PMC free article] [PubMed] [Google Scholar]
- 34.Takaya S, Iwaki Y, Starzl TE. Liver transplantation in cytotoxic crossmatch cases using FK 506, high dose steroids, and prostaglandin E1. Transplantation. 1992;54:927. doi: 10.1097/00007890-199211000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Todo S, Fung JJ, Demetris AJ, et al. 110 consecutive primary orthotopic liver transplantation under FK 506 in adults. Transplant Proc. 1991;23:1397. [PubMed] [Google Scholar]
- 36.Starzl TE, Todo S, Tzakis A, et al. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335. [PMC free article] [PubMed] [Google Scholar]
- 37.Casavilla A, Selby A, Abu-Elmagd K, et al. Logistics and technique for combined hepatic-intestinal retrieval. Ann Surg. 1992;216:605. doi: 10.1097/00000658-199211000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Todo S, Tzakis A, Abu-Elmagd K, et al. Cadaveric small bowel and small bowel-liver transplantation in humans. Transplantation. 1992;53:369. doi: 10.1097/00007890-199202010-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Todo S, Tzakis A, Abu-Elmagd K, et al. Intestinal transplantation in composite visceral grafts or alone. Ann Surg. 1992;216:223. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furukawa H, Abu-Elmagd K, Reyes J, et al. Technical aspects of intestinal transplantation. In: Braverman MH, Tawes RL, editors. Surgical Technology International II. San Francisco: Surgical Technology International; 1993. p. 165. [PubMed] [Google Scholar]
- 41.Todo S, Tzakis A, Abu-Elmagd K, et al. Abdominal multivisceral transplantation. Transplantation. doi: 10.1097/00007890-199501270-00015. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abu-Elmagd K, Fung JJ, Reyes J, et al. Management of intestinal transplantation in humans. Transplant Proc. 1992;24:1243. [PMC free article] [PubMed] [Google Scholar]
- 43.Reyes J, Todo S, Tzakis A, et al. Transplantation of the small bowel and other abdominal organs in humans. In: Makowka L, Sher L, editors. Intra-abdominal Organ Transplantation 2000. Georgetown, TX: R.G. Landes Co; 1994. p. 165. [Google Scholar]
- 44.Manez R, Kusne S, Green M, et al. Incidence and risk factors associated with the development of cytomegalovirus disease after intestinal transplantation. Transplantation. doi: 10.1097/00007890-199504150-00016. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furukawa H, Manez R, Kusne S, et al. Cytomegalovirus disease in intestinal transplantation. Transplant Proc. (in press) [PMC free article] [PubMed] [Google Scholar]
- 46.Abu-Elmagd K, Todo S, Tzakis A, et al. Three years clinical experience with intestinal transplantation. J Am Col Surg. 1994;179:385. [PMC free article] [PubMed] [Google Scholar]
- 47.Todo S, Tzakis A, Reyes J, et al. Intestinal transplantation: four-year experience. Transplant Proc. (in press) [PMC free article] [PubMed] [Google Scholar]
- 48.Ramos H, Reyes J, Abu-Elmagd K, et al. Weaning of immunosuppression in long term liver transplant recipients. Transplantation. doi: 10.1097/00007890-199501270-00010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Starzl TE, Demetris A, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation. The basis of graft acceptance. Hepatology. 1993;17:1127. [PMC free article] [PubMed] [Google Scholar]
- 50.Starzl TE, Demetris A, Murase N, et al. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Starzl TE, Demetris A, Trucco M, et al. Systemic chimerism in human female recipients of male livers. Lancet. 1992;340:876. doi: 10.1016/0140-6736(92)93286-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Starzl TE, Demetris A, Trucco M, et al. Chimerism after liver transplantation for Type IV glycogen storage disease and Type I Gaucher’s disease. N Engl J Med. 1993;328:735. doi: 10.1056/NEJM199303183281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Starzl TE, Demetris A, Murase N, et al. Donor cell chimerism permitted by immunosuppressive drugs: a new view of organ transplantation. Immunol Today. 1993;14:326. doi: 10.1016/0167-5699(93)90054-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fontes P, Rao A, Demetris A, et al. Bone marrow augmentation of donor cell chimerism in kidney, liver, heart and pancreas islet transplantation. Lancet. 1994;344:151. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Starzl TE, Fung JJ, Tzakis A, et al. Baboon to human liver transplantation. Lancet. 1993;341:65. doi: 10.1016/0140-6736(93)92553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Starzl TE, Tzakis A, Fung JJ, et al. Human liver xenotransplantation. Xeno. 1993;1:4. [PMC free article] [PubMed] [Google Scholar]
- 57.Starzl TE, Valdivia L, Murase N, et al. The biological basis of and strategies for clinical xenotransplantation. Immunological Reviews. 1994;141:213. doi: 10.1111/j.1600-065x.1994.tb00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Todo S, Tzakis A, Reyes J, et al. Small intestinal transplantation in humans with or without colon. Transplantation. 1994;57:840. doi: 10.1097/00007890-199403270-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Billingham RE, Brent L, Medawar PB. “Actively acquired tolerance” of foreign cells. Nature. 1953;172:603. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 60.Starzl TE, Demetris A, Trucco M, et al. Chimerism and donor specific nonreactivity 27 to 29 years after kidney allotransplantation. Transplantation. 1993;55:1272. doi: 10.1097/00007890-199306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valdivia LA, Demetris AJ, Langer AM, et al. Dendritic cell replacement in long-surviving liver and cardiac xenografts. Transplantation. 1993;56:482. doi: 10.1097/00007890-199308000-00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Starzl TE, Murase N, Demetris AJ, et al. Drug development and testing in relation to cell migration and chimerism. Transplant Proc. 1993;25:469. [PMC free article] [PubMed] [Google Scholar]
- 63.Starzl TE, Tzakis A, Fung JJ, et al. Prospects of clinical xenotransplantation. Transplant Proc. 1994;26:3071. [PMC free article] [PubMed] [Google Scholar]
- 64.Valdivia LA, Demetris AJ, Fung JJ, et al. Successful hamster to rat liver xenotransplantation under FK506 immunosuppression induces unresponsiveness to hamster heart and skin. Transplantation. 1993;55:659. [PMC free article] [PubMed] [Google Scholar]
- 65.Makowka L, Cramer DV. The pathogenesis of xenograft rejection. Clin Transpl. 1994;8:145. [PubMed] [Google Scholar]
- 66.Auchiricloss H., Jr Xenogeneic transplantation. Transplantation. 1988;46:1. doi: 10.1097/00007890-198807000-00001. [DOI] [PubMed] [Google Scholar]
- 67.Platt JL, Vercellotti GM, Dalmasso AP. Transplantation of discordant xenografts: a review of progress. Immunol Today. 1990;11:450. doi: 10.1016/0167-5699(90)90174-8. [DOI] [PubMed] [Google Scholar]
- 68.Platt JL, Bach FH. The barrier to xenotransplantation. Transplantation. 1991;52:937. doi: 10.1097/00007890-199112000-00001. [DOI] [PubMed] [Google Scholar]
- 69.Weill B, Houssin D. Xenotransplantation: The present and the future. In: Touraine JL, et al., editors. Rejection and Tolerance. Kluwer Academic Publishers; The Netherlands: 1994. p. 71. [Google Scholar]
- 70.Bach FH, Van der Werf WJ, Blakely ML, et al. Xenotransplantation: The current status of understanding. In: Touraine JL, et al., editors. Rejection and Tolerance. Kluwer Academic Publishers; The Netherlands: 1994. p. 65. [Google Scholar]
- 71.Dalmasso AP. The complement system in xenotransplantation. Immunopharmacology. 1992;24:149. doi: 10.1016/0162-3109(92)90020-d. [DOI] [PubMed] [Google Scholar]
- 72.Bygren P, Freiburghaus C, Lindholm T, et al. Goodpasture’s syndrome treated with staphylococcal protein A immunoadsorption. (Letter) Lancet. 1985;2:1295. doi: 10.1016/s0140-6736(85)91571-5. [DOI] [PubMed] [Google Scholar]
- 73.Soares M, Lu X, Havaux X, et al. In vivo IgM depletion by anti-Â monoclonal antibody therapy: The role of IgM in hyperacute vascular rejection of discordant xenografts. Transplantation. 1994;57:1003. [PubMed] [Google Scholar]
- 74.Van Den Bogaerde J, Aspinall R, Wang M-W, et al. Induction of long-term survival of hamster heart xenografts in rats. Transplantation. 1991;52:15. doi: 10.1097/00007890-199107000-00003. [DOI] [PubMed] [Google Scholar]
- 75.Leventhal JR, Dalmasso AP, Cromwell JW, et al. Prolongation of cardiac xenograft survival by depletion of complement. Transplantation. 1993;55:857. doi: 10.1097/00007890-199304000-00033. [DOI] [PubMed] [Google Scholar]
- 76.Xia W, Fearon DT, Kirkman RL. Effect of repetitive doses of soluble human complement receptor type I on survival of discordant cardiac xenografts. Transplant Proc. 1993;25:410. [PubMed] [Google Scholar]
- 77.Pruitt SK, Kirk AD, Bollinger AR, et al. The effect of soluble complement receptor type I on hyperacute rejection of porcine xenografts. Transplantation. 1994;57:363. doi: 10.1097/00007890-199402150-00009. [DOI] [PubMed] [Google Scholar]
- 78.Miyagawa S, Shirakura R, Matsumiya G, et al. Prolonging discordant xenograft survival with anticomplement reagents K76COOH and FUT175. Transplantation. 1993;55:709. doi: 10.1097/00007890-199304000-00004. [DOI] [PubMed] [Google Scholar]
- 79.Hasan RIR, Bogaerde van den J, Wallwork J, et al. Evidence that long-term survival of concordant xenografts is achieved by inhibition of antispecies antibody production. Transplantation. 1992;54:408. doi: 10.1097/00007890-199209000-00004. [DOI] [PubMed] [Google Scholar]
- 80.Murase N, Starzl TE, Demetris AJ, et al. Hamster to rat heart and liver xenotransplantation with FK506 plus antiproliferative drugs. Transplantation. 1993;55:701. doi: 10.1097/00007890-199304000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Valdivia LA, Monden M, Gotoh M, et al. Prolonged survival of liver xenografts from hamster to rat by splenectomy and cyclosporine administration. Transplantation. 1987;44:759. doi: 10.1097/00007890-198712000-00007. [DOI] [PubMed] [Google Scholar]
- 82.Yamaguchi Y, Halperin EC, Harland RC, et al. Significant prolongation of hamster liver transplant survival in Lewis rats by total lymphoid irradiation, cyclosporine, and splenectomy. Transplantation. 1990;49:13. doi: 10.1097/00007890-199001000-00003. [DOI] [PubMed] [Google Scholar]
- 83.Miyazawa H, Murase N, Demetris AJ, et al. Hamster to rat kidney xenotransplantation: Effects of FK 506, cyclophosphamide, organ perfusion, and complement inhibition. Transplantation. doi: 10.1097/00007890-199504270-00018. (in Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knechtle S, Kolbeck PC, Tsuchimoto S, et al. Hepatic transplantation into sensitized recipients. Transplantation. 1987;43:8. doi: 10.1097/00007890-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 85.Monden M, Valdivia LA, Gotoh M, et al. A crucial effect of splenectomy on prolonging caridac xenograft survival in combination with cyclosporine. Surgery. 1989;105:535. [PubMed] [Google Scholar]
- 86.Cramer DV, Chapman FA, Jaffee BD, et al. The prolongation of concordant hamster-to-rat cardiac xenografts by Brequinar sodium. Transplantation. 1992;54:403. doi: 10.1097/00007890-199209000-00003. [DOI] [PubMed] [Google Scholar]
- 87.Langer A, Valdivia LA, Murase N, et al. Humoral and cellular immunopathology of hepatic and cardiac hamster-into-rat xenograft rejection. Am J Pathol. 1993;143:85. [PMC free article] [PubMed] [Google Scholar]