Abstract
Background
Transplantation of untreated rat bone marrow into mouse recipients conditioned by total-body irradiation results in fully xenogeneic chimerism (rat → mouse). The chimerism is stable for up to 70 months, survival is excellent, and there is no evidence for graft-versus-host disease. We recently reported the long-term survival (> 180 days) of donor-specific pancreatic islet xenografts in these fully xenogeneic chimeras.
Methods
Chimeras were prepared and typed for chimerism at 6 weeks, and diabetes was induced by streptozocin injection. Donor-specific pancreatic islets were placed under the renal capsule and recipient blood glucose levels were followed biweekly. The aim of this study was to examine whether the transplanted pancreatic islets exhibited normal function in a xenogeneic environment and assess whether the islet xenografts were not only sufficient to support euglycemia but also regulated in function in response to a glucose challenge.
Results
We report for the first time that donor-specific rat islet xenografts were capable of producing normal basal and peak levels of insulin and responding to a glucose challenge in a manner similar to that of normal mouse islets.
Conclusions
These data indicate that donor-specific rat islet xenografts are functional and regulated normally in fully xenogeneic (rat → mouse) chimeras.
Type I diabetes is a systemic autoimmune disease state that results in destruction of the insulin-producing β-islets and other severe complications. It has been demonstrated that the severity and incidence of diabetes-associated complications (microangiopathy, blindness, and renal failure) is influenced by the metabolic control of glucose homeostasis, in that hyperglycemia results in accelerated progression of the complications associated with diabetes. Attempts to achieve normoglycemia with exogenous insulin have been limited by hypoglycemia and patient noncompliance. It is now believed that transplantation of the insulin-Producing β-cells as a whole pancreas, or as a free cellular graft, is the most physiologic approach to achieve complete glucose homeostasis. Cellular transplantation offers the following advantages: (1) the ease of the surgical approach, (2) ability for cryopreservation or in vitro manipulation with transplantation at a later date, and (3) avoidance of the requirement for anastomoses and exocrine drainage associated with whole-pancreatic graft placement.1-3 However, this approach has been limited by graft rejection that has not been controlled by conventional immunosuppressive agents and a shortage of pancreases available for transplantation.4 Attempts to modify the immunogenicity of transplanted islets by a variety of approaches have achieved only limited success.5-12 The induction of donor-specific transplantation tolerance across a species barrier has been suggested as one approach to overcome both graft rejection and the shortage of donor organs.
We recently developed a model to induce donor-specific transplantation tolerance through preparation of bone marrow chimeras (rat → mouse). Transplantation of untreated rat bone marrow stem cells into recipient mice conditioned by total-body irradiation resulted in donor-specific transplantation tolerance in vivo and in vitro, excellent survival, and stable multilineage chimerism.13,14 Donor-specific pancreatic islets were permanently accepted (> 180 days) by the chimeric recipients, whereas major histocompatibility complex (MHC)–disparate grafts were rejected rapidly.15 We have now examined whether regulation of function and maintenance of glucose homeostasis could be achieved in a xenogeneic environment. We report here that donor-specific rat islet xenografts are functional and normally regulated in function in a xenogeneic mouse environment in fully xenogeneic (rat → mouse) chimeras.
MATERIAL AND METHODS
Animals
Six- to eight-week-old male C57BL/10SnJ (B10), B10.BR/Sgn (B10.BR) mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). Four- to 8-week-old male Fisher 344 (F344; RtIA1) and Wistar-Furth (WF; RtIAu) male rats were purchased from Harlan Sprague Dawley Inc. (Indianapolis, Ind.).
Fully xenogeneic reconstitution (WF rat → B10 mouse)
Fully xenogeneically reconstituted animals were prepared as described previously.15 After 28 days animals were typed to document chimerism by flow cytometry with anti-class I rat and mouse monoclonal antibody staining.13
Diabetes
Six weeks after fully xenogeneic bone marrow reconstitution, chimeras were made diabetic as described previously.15
Rat islet isolation and transplantation
Rat islets were separated by collagenase digestion as described previously.15, l6
Intraperitoneal glucose tolerance test (IPGTT)
IPGTTs were performed in the chimeras 8 months after the islet transplantation. The animals were fasted overnight and then injected intraperitoneally with 25% glucose (2 gm glucose/kg body weight).17 Blood samples were obtained before and 15, 30, and 60 minutes after glucose injection and analyzed for plasma glucose and insulin levels. The K value, which represents the kinetics of return to normoglycemia after a glucose challenge, was calculated from the straight line describing the change in glucose over time according to the standard formula.18 Statistics are presented as mean ± SEM and significance of different was assessed by the Student t test.
Insulin assay
Insulin concentration in the serum was determined by a double-antibody radioimmunoassay with 125I-labeled human insulin and a human insulin standard.17
RESULTS
Characterization of fully xenogeneic chimeras (WF → B10 mouse or F344 rat → B10 mouse)
Fully xenogeneic chimeras (WF → B10, n = 8) were prepared and typed for engraftment 6 weeks after reconstitution. A second group of chimeras was prepared with F344 rat bone marrow donors (n = 6). Diabetes was induced in the chimeras with streptozocin (Fig. 1). Daily blood glucose monitoring was performed. All mice had diabetes as evidenced by serum glucose levels greater than 300 mg/dl. Mice that remained hyperglycemic for greater than 7 days were transplanted with 800 donor-specific islet xenografts placed under the renal capsule. As in our past experience, survival of the donor-specific islet xenografts was prolonged significantly (median survival time >8 months), whereas MHC-disparate third-party grafts were rejected rapidly(Fig.2).
Fig. 1.

Protocol for preparation of diabetic fully xenogeneic chimeras (rat → mouse). Recipients were typed for chimerism at 6 weeks and diabetes was then induced by streptozocin. Donor-specific or third-party pancreatic islet xenografts were placed under left renal subcapsular space.
Fig. 2.

Life-table survival of pancreatic islet xenografts in fully xenogeneic (rat → mouse) chimeras. Minimum follow-up was 8 months.
Xenogeneic rat islets: Evidence for regulation of function
Plasma glucose levels normalized within 1 week after subcapsular islet xenotransplantation. However, nonfasting plasma glucose levels do not provide an accurate assessment of regulation of glucose homeostasis in recipients of islet transplants. For this reason, IP-GTTS were performed in selected chimeras 8 months after graft placement (Table I). K values were calculated to determine the kinetics of return to normoglycemia after glucose challenge. Fasting plasma glucose levels were determined in individual animals (n = 3) before and after intraperitoneal injection of 25% glucose (2 gm/kg body weight). No significant difference was observed in the fasting plasma glucose levels of chimeras compared with normal controls. More important, comparable K values were present in the two groups, indicating normal regulation of islet function in a xenogeneic environment.
Table I.
Intraperitoneal glucose tolerance testing
| Group | Fasting plasma glucose (mg/dl) | K value (%/min) | Insulin level (ng/ml) |
|
|---|---|---|---|---|
| Basal | Peak | |||
| Rat → mouse chimera | 92 ± 10 | 2.5 ± 0.34 | 0.02 ± 0.02 | 5.81 ± 2.43 |
| Normal B10 mouse | 89 ± 12 | 2.3 ± 0.24 | 0.03 ± 0.02 | 4.21 ± 2.21 |
Values represent mean ± SD for three animals per group.
Basal and peak insulin determinations were made in the chimeras during the IPGTTs (Table I). The chimeric recipients of xenogeneic rat islets demonstrated production of insulin levels similar to those for normal controls. Stimulated insulin release (peak insulin level [nanograms per milliliter]) was higher in chimeras (5.81 ± 2.43 ng/ml) than in normal mice (4.21 ± 2.21 ng/ml). However, this result was not statistically significant.
The assay for rat insulin is cross-reactive with that for the mouse. To confirm that the insulin was produced by the islet xenografts in the chimeras and not caused by regeneration of native pancreatic islet tissue, a transplant nephrectomy was performed 1 day after the IPGTT. Blood glucose determinations were made daily. There was a prompt return of hyperglycemia in all chimeras (n = 3), confirming that the transplanted islets were, in fact, the source of the insulin produced.
Evidence for insulin and glucagon production by pancreatic islet xenografts
The isolated islet is a complex composed of acinar tissue including β-cells, glucagon-producing α-cells, δ-cells, lymphoid cells, and antigen-presenting cells. We previously characterized the presence of insulin in the rat islet xenografts.15 As a further assessment of graft viability and function, we analyzed the transplanted α-islets for evidence of glucagon present in all islet xenografts examined (n = 6), demonstrating that the α-cells can survive in a xenogeneic environment as well. As with the β-cells, there was no evidence of chronic rejection. As in our past experience, all islet xenografts had histochemical evidence for the presence of insulin. The islets were well granulated and had no evidence for inflammatory cell infiltrates suggestive of chronic rejection (Fig. 3).
Fig. 3.
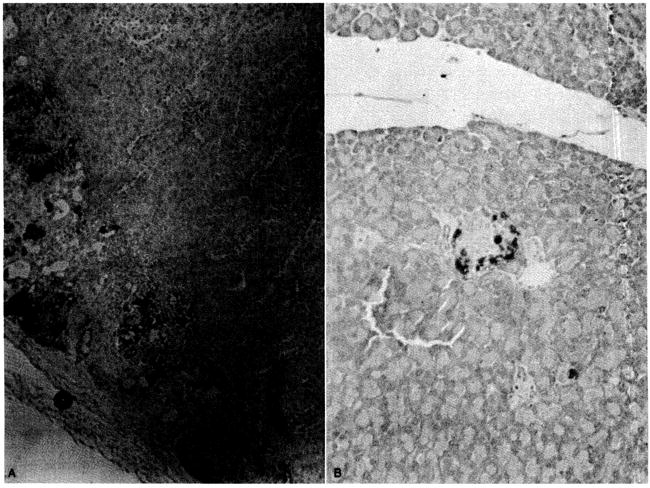
Representative immunoperoxidase stain to detect presence of glucagon in renal subcapsular pancreatic islet xenografts 8 months after transplantation (A). Note absence of inflammatory cell infiltrates. Comparison was made with normal mouse pancreas (B).
DISCUSSION
Type I diabetes is a multifactorial disease that affects 0.4% of white people in the United States. It is the single most common cause of renal failure that requires transplantation. In excess of 50% of patients with type I diabetes have renal failure in their lifetime. It has become clear that the underlying defect in type I diabetes is one of chronic systemic autoimmunity. Since the advent of insulin therapy, most acute deaths caused by insulin deficiency have been prevented, but the complications associated with the disease have not been eliminated. Glucose homeostasis is believed to exert a significant influence on the development and severity of the complications associated with diabetes. However, tight glucose homeostasis has been difficult to achieve, and patients are at constant risk for hypoglycemia caused by the pharmacologic administration of insulin. Even with tight glucose control, the majority of patients eventually have a constellation of progressive systemic complications, including renal failure, proliferative retinopathy leading to blindness, and peripheral neuropathy. Transplantation of the whole pancreas, or purified insulin-producing β-islets, is now believed to be the best approach to achieve complete glucose homeostasis. However, transplantation of whole pancreas, or even the isolated β-islets, which constitute at most 2% of the total pancreatic mass, has been limited by rejection and a shortage of organs available for transplantation.
The induction of tolerance across a species barrier has been suggested as one potential approach to overcome both of these limitations. To date, the only true state of donor-specific transplantation tolerance has been that associated with bone marrow chimerism.19-24 The first association between chimerism and tolerance was reported by Billingham et a1.19 in 1953, in which they demonstrated the induction of permanent and stable systemic donor-specific transplantation tolerance by transplantation of allogeneic bone marrow stem cells into newborn mouse recipients. The induction of similar medawarian tolerance was subsequently achieved in adult recipients of bone marrow from genetically different donors of the same species with a number of preparative approaches.20-24 We recently reported a model for preparation of Fully xenogeneic chimeras (rat → mouse) in which stable engraftment of rat donor bone marrow stem cells occurred in mouse recipients.14 Chimeras exhibited systemic donor-specific transplantation tolerance in vivo and in vitro and excellent survival and resisted graft-versus-host disease.
The model for fully xenogeneic chimeras was recently applied to examine whether rejection-free graft acceptance would occur for pancreatic islet xenografts.15 After the induction of diabetes in chimeras with streptozocin, doner-specific, or MHC-disparate third-party islet grafts were placed under the renal capsule. Although third-party islet grafts were rapidly rejected (mean survival time, 9 days), donor-specific grafts were permanently accepted and sufficient to maintain euglycemia when nonfasting blood glucose determinations were performed. To examine whether true regulation of islet function and insulin production could occur in a xenogeneic stromal environment, IPGTTs were performed in selected recipients, with grafts surviving for greater than 8 months. In all animals examined, the K value and peak and basal insulin levels were comparable to those for normal unmanipulated B10 mice in response to a glucose challenge.
Stimulated insulin release (peak insulin level) was slightly higher in the chimeras than in the normal mice, but this difference was not significant. This finding could be the result of the heterotopic site for implantation, resulting in endocrine drainage into the systemic circulation rather than the anatomic portal system. The higher stimulated insulin release in mice that received rat islet xenografts could also represent the high volume of transplanted islets (800), because it is well known that up to 600 rat or human islets are sufficient to induce normoglycemia in diabetic nude mice.
The islet isolation procedure results in harvest of a complex containing acinar tissue, the glucagon-producing α-cells, insulin-producing β-cells, δ-cells, lymphocytes, and antigen-presenting cells. To confirm the presence of expected α-cells, immunohistochemical analysis for glucagon was performed. As we previously identified for β-cells, the α-cells were healthy, had evidence of the presence of glucagon, and had no evidence for chronic rejection even 8 months after transplantation into the xenogeneic chimeras. Interestingly, glucagon staining was significantly greater than that observed in the native pancreas of unmanipulated rats of the same donor strain. The overexpression of glucagon in transplanted islets has been observed by others in human, rodent, and canine allograft models.25 The mechanism for this effect is not understood but may represent a regulatory effect because the glucagon produced by the α-cells has a known counterregulatory role in glucose homeostasis. If the glycemic state were more variable after islet transplantation, with fluctuation in insulin production and therefore plasma glucose level, one might predict α-cell hypertrophy.
In conclusion, the preparation of fully xenogeneic chimeras resulted in the induction of MHC-specific donor-specific transplantation tolerance to pancreatic islet xenografts. Donor-specific islet xenografts exhibited rejection-free graft survival for 8 months or longer after transplantation. Most important, the grafts were functional to maintain glucose homeostasis to a glucose challenge. Basal and peak insulin levels in chimeras were comparable to those of normal mouse controls. Taken together, these data demonstrate that pancreatic islet xenografts are regulated in function in a xenogeneic stromal environment.
Acknowledgments
We thank Richard James and Cathie Carr for technical support and Drs. Sherry M. Wren, Christina Kaufman, and Ashraf Y. Abou El-Ezz for manuscript review.
Supported in part by American College of Surgeons Fellowship Award 1990-1992, Juvenile Diabetes Foundation grant 1911433, National Institutes of Health grant A130615, Shannon Award lROlDK43901, National Institutes of Health grant DK29961, and the National Kidney Foundation of Western Pennsylvania.
Footnotes
Presented at the Fifty-third Annual Meeting of The Society of University Surgeons, Cincinnati, Ohio, Feb. 13-15, 1992.
References
- 1.Scharp DW, Lacy PE, Santiago JV, et al. Insulin independence after islet transplantation into type I diabetic patient. Diabetes. 1990;39:515–8. doi: 10.2337/diab.39.4.515. [DOI] [PubMed] [Google Scholar]
- 2.Warnock GL, Kneteman NM, Ryan E, et al. Normoglycemia after transplantation of freshly isolated and cryopreserved pancreatic islet in type I (insulin-dependent) diabetes mellitus. Diabetologia. 1991;34:55–8. doi: 10.1007/BF00404026. [DOI] [PubMed] [Google Scholar]
- 3.Sharp DW, Lacy PE, Santiago J, et al. Results of our first nine intraportal islet allografts in type 1, insulin-dependent diabetic patients. Transplantation. 1991;51:76–85. doi: 10.1097/00007890-199101000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Unos update. National organ procurement and transplantation network. 1991;7:22–33. [Google Scholar]
- 5.Starzl TE, Todo S, Fung JJ, Demetris DA, Vankataraman R, Jain A. FK-506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000–4. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faustman D, Hauptfeld V, Davie JM, Lacey PE. Prolongation of murine islet allograft survival by pretreatment of islets with antibody directed to Ia determinants. Proc Natl Acad Sci USA. 1981;78:5156–9. doi: 10.1073/pnas.78.8.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faustman D, Steinmann RM, Geble HM, Hauptfeld V, Davie JM, Lacy PE. Prevention of rejection of murine islet allografts by pretreatment with anti-dendritic cell antibody. Proc Natl Acad Sci USA. 1984;81:3864–8. doi: 10.1073/pnas.81.12.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacy PE, Davie JM, Finke EH. Prolongation of islet allograft survival following in vitro culture (24 C) and single injection of ALS. Science. 1979;204:312–3. doi: 10.1126/science.107588. [DOI] [PubMed] [Google Scholar]
- 9.Lau H, Reemtsma K, Hardy MA. Pancreatic islet allograft prolongation by donor-specific blood transfusion treated with ultraviolet irradiation. Science. 1983;221:754–5. doi: 10.1126/science.6410509. [DOI] [PubMed] [Google Scholar]
- 10.Lim F, Sun AM. Microencapsulated islet as bioartificial endocrine pancreas. Science. 1980;210:908–10. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 11.O’Shea GM, Sun AM. Encapsulation of rat islets of Langerhans prolongs xenograft survival in diabetic mice. Diabetes. 1986;35:943–6. doi: 10.2337/diab.35.8.943. [DOI] [PubMed] [Google Scholar]
- 12.Gotoh M, Maki T, Satomi S, Porter J, Monaco AP. Immunological characteristics of purified pancreatic islet grafts. Transplantation. 1986;42:387–90. doi: 10.1097/00007890-198610000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Ildstad ST, Wren SM, Boggs SS, Hronakes ML, Vecchini F, Van den Brink MRN. Cross-species bone marrow transplantation: evidence for tolerance, stem cell engraftment, and maturation of T-lymphocytes in a xenogeneic stromal environment. J Exp Med. 1991;174:467–78. doi: 10.1084/jem.174.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ildstad ST, Vacchio MS, Markus PM, Hronakes ML, Wren SM, Hodes RJ. Cross-species transplantation tolerance: rat hone marrow–derived cells can contribute to the ligand for negative selection of mouse T-cell receptor Vβ in chimeras tolerant to xenogeneic antigens (mouse + rat → mouse) J Exp Med. 1992;175:147–55. doi: 10.1084/jem.175.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng J, Ricordi C, Tzakis A, et al. Long-term survival of donor-specific pancreatic islet xenografts in fully xenogeneic chimeras (WF rat → B10 mouse) Transplantation. 1992;53:277–83. doi: 10.1097/00007890-199202010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–20. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 17.Wilson JD, Prowse SJ, Haynes SP. Pancreatic islet allograft function in nonimmunosuppressed conscious mouse. Metabolism. 1985;34:92–105. doi: 10.1016/0026-0495(85)90067-8. [DOI] [PubMed] [Google Scholar]
- 18.Alsever RN, Gotlin RW. Handbook of endocrine tests in adults and children. 2. Vol. 95 Chicago: Year Book; 1980. [Google Scholar]
- 19.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance to foreign cells. Nature. 1953;172:606–8. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 20.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:170–2. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 21.Slavin S, Strober S, Fukes Z, Kaplan HS. Induction of specific tissue transplantation tolerance using fractionated total lymphoid irradiation in adult mice: long term survival of allogeneic bone marrow and skin grafts. J Exp Med. 1977;146:34–51. doi: 10.1084/jem.146.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood ML, Monaco AP. Suppressor cells in specific unresponsiveness to skin grafts in ALS-treated, marrow-injected mice. Transplantation. 1980;29:196–202. doi: 10.1097/00007890-198003000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Thomas JM, Carber FM, Foil MB. Renal allograft tolerance induced with ATG and donor bone marrow in outhred rhesus monkeys. Transplantation. 1983;36:104–10. [PubMed] [Google Scholar]
- 24.Mayumi H, Himeno K, Tanaka K, Fan L, Nomoto K. Drug-induced tolerance to allografts in mice. Transplantatiorl. 1986;42:3417–25. [PubMed] [Google Scholar]
- 25.Alejandro R, Cutfield RG, Shienvold FL, et al. Natural history of intrahepatic canine islet cell autografts. J Clin Invest. 1986;78:1339–45. doi: 10.1172/JCI112720. [DOI] [PMC free article] [PubMed] [Google Scholar]


