Abstract
The development of functional Ca2+-activated K+ channels (KCa) in chick ciliary ganglion (CG) neurons requires interactions with afferent preganglionic nerve terminals. Here we show that the essential preganglionic differentiation factor is an isoform of β-neuregulin-1. β-Neuregulin-1 transcripts are expressed in the midbrain preganglionic Edinger–Westphal nucleus at developmental stages that coincide with or precede the normal onset of macroscopic KCa in CG neurons. Injection of β-neuregulin-1 peptide into the brains of developing embryos evoked a robust stimulation of functional KCa channels at stages before the normal appearance of these channels in CG neurons developing in vivo. Conversely, injection of a neutralizing antiserum specific for β-neuregulin-1 inhibited the development of KCa channels in CG neurons. Low concentrations of β-neuregulin-1 evoked a robust increase in whole-cell KCa in CG neurons cocultured with iris target tissues. By contrast, culturing CG neurons with iris cells or low concentrations of β-neuregulin-1 by themselves was insufficient to stimulate KCa. These data suggest that the preganglionic factor required for the development of KCa in ciliary ganglion neurons is an isoform of β-neuregulin-1, and that this factor acts in concert with target-derived trophic molecules to regulate the differentiation of excitability.
Keywords: ciliary ganglion, slowpoke, trophic factor, neuregulins, transforming growth factor β
The neuregulins comprise a class of growth factors that regulate the differentiation of many tissues by acting on cellular erbB receptors (1, 2). The products of the neureuglin-1 gene can be divided into two large groups, known as α- and β-neuregulins, on the basis of differences in the structure of their essential epidermal growth factor (EGF)-like domains (1). Of these two groups, the β-neuregulin-1 (β-NEU1) isoforms are considerably more potent in neural systems and have diverse functions in neural development. These include regulation of nicotinic ACh receptor expression in peripheral targets (3–5) and regulation of N-methyl-d-aspartate (6) and γ-aminobutyric acid type A (7) receptors in the cerebellum. In addition, β-neuregulins regulate proliferation and differentiation of glial and Schwann cells (8, 9), neuronal migration (10), cell number, and brain morphogenetic pattern (2, 11, 12).
Large-conductance Ca2+-activated K+ channels (KCa) are major determinants of the action potential waveform and the temporal pattern of repetitive discharge in many neurons (e.g., see refs. 13 and 14), including chick ciliary ganglion (CG) neurons (15). The development of KCa in chick CG neurons is regulated by interactions with target tissues and preganglionic innervation (16, 17). Functional KCa are not present in CG neurons before synapse formation with target tissues (18) or in later-stage CG neurons that develop in vivo in the absence of either target tissues or preganglionic input (16). The inductive effect of the afferent input is not activity-dependent and appears to be mediated by a differentiation factor derived from preganglionic cells (19). Three separate observations suggest that this factor could be an isoform of β-NEU1. First, β-Neu1 transcripts are expressed in the chick Edinger–Westphal nucleus, the source of midbrain preganglionic neurons that innervate the CG (20). Second, functional erbB receptors are expressed in chick CG neurons (20). Finally, application of recombinant β-NEU1 can increase the density of functional KCa in cultured CG neurons, at least at high concentrations (21). However, this effect also can be evoked by high concentrations of other erbB ligands such as EGF and transforming growth factor α (TGFα), but not by α-neuregulin (21). In addition, it is not known whether β-Neu1 transcripts are expressed in the Edinger–Westphal nucleus at developmental stages that coincide with the normal development of KCa. Thus, these observations, although consistent with the hypothesis, fall short of demonstrating a physiological role for β-NEU1 in the regulation of KCa. We now show that β-NEU1 plays an essential role in the development of functional KCa in CG neurons developing in vivo.
Materials and Methods
β-Neuregulin-1 in Situ Hybridization.
A 497-bp fragment of chicken β-Neu1 cDNA was isolated by reverse transcription–PCR (RT-PCR). In brief, 10 ng of embryonic day 13 (E13) chick brain total RNA was random transcribed (RT-PCR kit, Stratagene) and first-strand cDNAs were amplified by using the following matched primers: 5′-GGA-GAG-TGC-TAC-ATG-GTT-AAA-GA-3′ (forward) and 5′-GCT-GTG-GCT-AGG-CGT-CTG-GG-3′ (reverse), which span the conserved EGF-like domain in all known chick β-NEU1 isoforms. A fragment of the expected size was cloned into a TA vector (PCR 2.1, Invitrogen), and sequenced. 35S-labeled antisense and sense riboprobes were prepared by in vitro transcription (Stratagene in vitro transcription kit no. 200340) according to the manufacturer's instructions. Brains were removed from E9 and E13 chick embryos, fixed by immersion in 4% paraformaldehyde, dehydrated, and embedded in paraffin. In situ hybridization was carried out on 10-μm sections as described (22). Sections were counterstained with cresyl violet and photographed under bright-field illumination.
Cell Culture and Electrophysiology.
Chick CG neurons were dissociated at E9 or E11 as described previously (16, 21, 23–25). For coculture experiments, iris tissue was dissociated at E13 and cultured for 2 days resulting in formation of a confluent layer of myotubes. Dissociated E9 CG neurons were then plated directly onto the iris myotubes. Recombinant β-NEU1 and α-neuregulin peptides were obtained from R & D Systems and added to culture media as indicated. Note that in our previous studies (21, 24), recombinant β-NEU1 peptide was provided by Gerald Fischbach of the Harvard Medical School. This peptide is no longer available. The commercial preparation of β-NEU1 used in the present study is considerably less potent, as indicated in the technical specification sheet. Whole-cell recordings of KCa and voltage-activated Ca2+ currents were made by using standard methods as described previously (16, 18, 23–26). Data were normalized for cell size and analyzed by one-way ANOVA followed by Scheffé's post hoc multiple range test by using statistica software (version 5.1, StatSoft, Tulsa, OK) with P < 0.05 regarded as significant.
Preparation of ex Ovo Chick Embryos and Injections of Peptides and Antibodies.
Shell-less (ex ovo) chicken embryo cultures were prepared exactly as described elsewhere (24, 25, 27, 28). Embryos developing under these conditions exhibit normal gross morphology between E8 and E13 (29). Recombinant β-NEU1 or recombinant α-neuregulin was reconstituted in PBS at a concentration of 10 μM. A fine glass micropipette was used to inject approximately 10 μl of this solution, or 10 μl of saline vehicle, into the brains of E8 chick embryos developing ex ovo. Pilot studies with methylene blue dye indicate that this injection volume allows for solute diffusion throughout the brain and adjacent structures. CG neurons were isolated acutely at E9 and KCa and Ca2+ currents were quantified by whole-cell recording. In other experiments, a specific β-NEU1-neutralizing antiserum (R & D Systems) was dissolved in PBS at a concentration of 5 μg/ml. This antibody is directed against the EGF-domain of human β-neuregulins, which is essential for biological activity and conserved in all β-NEU1 isoforms (1). This antibody shows no crossreactivity against more than 100 other cytokines tested, including several other erbB ligands, α-neuregulins, and all known isoforms of TGFβ. The ability of this antibody to recognize avian β-NEU1 was confirmed by immunoblot analysis (data not shown). Approximately 10 μl of the antibody solution was injected into the brains of E9 embryos. Control embryos were injected with 10 μl of PBS or 10 μl of nonimmune serum. Embryos were then allowed to develop until E11, at which time CG neurons were isolated acutely and ionic currents determined by whole-cell recordings.
Results
β-Neu1 Transcript Expression in the Embryonic Chick Edinger–Westphal Nucleus.
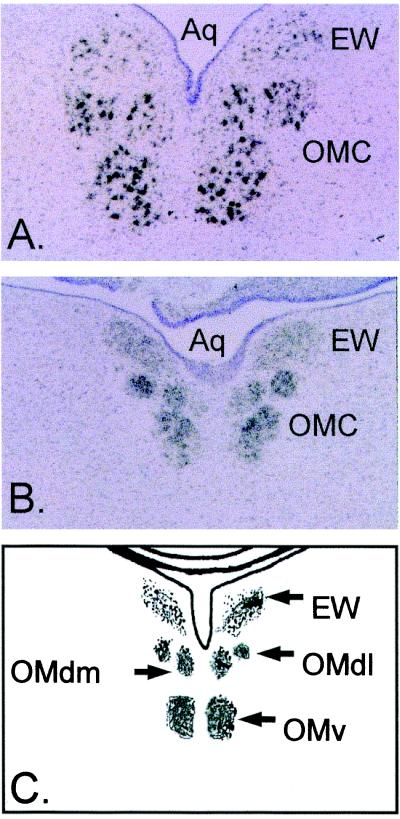
Functional whole-cell KCa is normally undetectable or present at low levels at E9 and reaches maximum current density by E13 in chick CG neurons developing in vivo (18, 24, 25). Therefore, if β-NEU1 is a critical factor regulating the development of these channels, then it should be expressed at corresponding developmental stages in the preganglionic neurons that innervate the chick CG. Corfas et al. (20) have shown that β-Neu1 transcripts are expressed in the chick Edinger–Westphal nucleus at E13. Here we have examined expression of these transcripts at earlier developmental stages by means of radioactive in situ hybridization. Expression of β-Neu1 transcripts was detected in the Edinger–Westphal nucleus and adjacent oculomotor nuclei at E13 (Fig. 1A) and at E9 (Fig. 1B). The signal appeared somewhat more intense in the oculomotor nuclei than in the Edinger–Westphal nuclei but was robust in both regions and at both developmental stages. No signal was obtained by using radiolabeled sense probes (data not shown). These results indicate that one or more isoforms of β-NEU1 are expressed at the right location and at the appropriate developmental stages to play a role in the regulation of KCa in CG neurons.
Figure 1.
Expression of β-Neu1 transcripts in developing chick oculomotor nuclei. Bright-field micrographs of in situ hybridization histochemistry with antisense riboprobes directed against EGF-domains conserved in all β-NEU1 isoforms. Sections were counterstained with cresyl violet. Note robust hybridization signal in the Edinger–Westphal nucleus (EW) and oculomotor complex (OMC) of E13 (A) and E9 (B) chick embryos. (C) The relative locations of midbrain oculomotor nuclei. The dorsal lateral oculomotor nucleus (OMdl), dorsal medial oculomotor nucleus (OMdm), ventral oculomotor nucleus (OMv), and mesencephalic aqueduct (Aq).
β-NEU1 Accelerates the Appearance of KCa in CG Neurons Developing in Vivo.
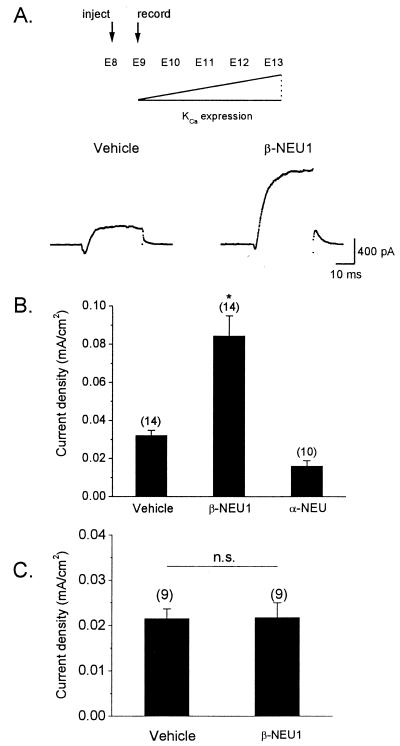
We have previously shown that β-NEU1 can stimulate the development of functional KCa in CG neurons developing in vitro (21, 25). However, experiments carried out in dissociated cell culture must be interpreted with caution. If preganglionic β-NEU1 contributes to the normal development of KCa in CG neurons, then early in vivo exposure to high concentrations of this factor should accelerate the appearance of KCa. To test this hypothesis, we used an ex ovo chicken embryo culture system that facilitates pharmacological manipulation of chick embryos. We have previously shown that embryos developing under these conditions exhibit normal developmental increases in whole-cell KCa (24, 25). β-NEU1 or saline-vehicle was injected into the forebrains of E8 embryos at a high volume so as to ensure diffusion of this factor to the vicinity of CG neurons. CG neurons were isolated acutely on the next day (E9) and KCa was quantified by whole-cell recordings. Recall that macroscopic KCa is normally undetectable or present at very low levels at E9 (18, 24, 25). Injection of β-NEU1 caused a robust stimulation of KCa in E9 CG neurons developing in vivo compared to neurons isolated from saline-injected control embryos (Fig. 2 A and B). Moreover, we observed that injection of α-neuregulin peptide had no effect on KCa (Fig. 2B), consistent with our earlier in vitro studies (21). The amino acid sequence of α-neuregulin is similar to that of β-NEU1, and this experiment provides an additional control for nonspecific effects. Injection of β-NEU1 had no effect on the gating or density of voltage-activated Ca2+ currents (Fig. 2C). Together, these results indicate that β-NEU1 can accelerate the development of macroscopic KCa and that this stimulation is not an artifact of cell culture.
Figure 2.
Stimulation of functional KCa by β-NEU1 in CG neurons developing in vivo. (A) Recombinant β-NEU1 peptide, recombinant α-neuregulin peptide, or saline vehicle was injected into the brains of chick embryos at E8. KCa was determined by whole-cell recordings from CG neurons isolated acutely at E9, at stage at which functional KCa is normally present at low levels (Upper). Representative whole-cell KCa currents in E9 CG neurons dissociated from vehicle-injected (Lower Left) and β-NEU1-injected (Lower Right) embryos are shown below. Traces shown are net Ca2+-dependent K+ currents obtained by digital subtraction of currents evoked in normal and Ca2+-free bath salines. (B) Summary of data obtained from several experiments. Injection of β-NEU1 evoked a robust and significant increase in mean KCa density compared to CG neurons isolated from embryos injected with either α-neuregulin or saline vehicle. In this and subsequent figures, bar graphs denote mean ± SEM, numbers above bars denote number of cells tested, and asterisk denotes statistical significance (P < 0.05). (C) Injection of β-NEU1 has no effect on the density or gating of voltage-activated Ca2+ currents.
β-NEU1 Neutralizing Antiserum Prevents the Normal Development of KCa in CG Neurons Developing in Vivo.
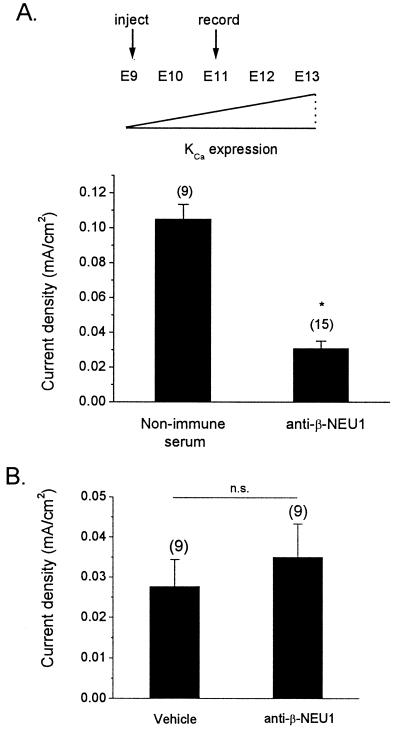
The experiments described above indicate that β-NEU1 can stimulate the appearance of functional KCa in vivo. However, if endogenous β-NEU1 is required for the normal development of KCa, then in vivo blockade of β-NEU1 function should inhibit the appearance of these channels. To test this hypothesis, a specific β-NEU1-neutralizing antibody was injected into the forebrains of E9 chick embryos developing ex ovo. Again, a large injection volume was used to ensure widespread diffusion to the vicinity of the CG. Control embryos were injected with the same volumes of either saline vehicle or nonimmune serum. The embryos were allowed to develop undisturbed until E11, a developmental stage at which there is normally substantial KCa. CG neurons were then isolated acutely, and KCa was quantified by whole-cell recordings. Injection of the neutralizing antiserum caused a nearly complete inhibition of the development of KCa in CG neurons compared to neurons isolated from embryos injected with nonimmune serum (Fig. 3A). In addition, injection of β-NEU1 antiserum had no effect on whole-cell voltage-activated Ca2+ currents in CG neurons developing in vivo (Fig. 3B). Finally, it bears noting that this experimental paradigm did not cause any visible change in the size or morphology of the CG. In particular, pre- and postganglionic nerve trunks of normal diameter were apparent at the time of dissection. Thus, antibody injections did not cause deafferentation or blockade of synaptic activation of the ganglion neurons, both of which result in substantial and easily visible reductions in ganglion size and morphology (16, 19, 30). These results indicate that endogenous β-NEU1 regulates the normal development of KCa.
Figure 3.
In vivo injection of β-NEU1-neutralizing antiserum inhibits the normal development of KCa in CG neurons. (A) β-NEU1-neutralizing antiserum, saline vehicle, or nonimmune serum were injected into the brains of E9 chick embryos. Density of ionic currents was determined by whole-cell recordings at E11, a stage at which KCa density is normally increased in CG neurons (Upper). CG neurons from embryos injected with β-NEU1-neutralizing antiserum exhibited significantly reduced mean KCa density compared to neurons from embryos injected with nonimmune serum (Lower). (B) In contrast, injection of neutralizing antiserum had no effect on voltage-activated Ca2+ currents in CG neurons.
β-NEU1 Stimulates KCa in CG Neurons Developing in Coculture with Target Tissue Cells.
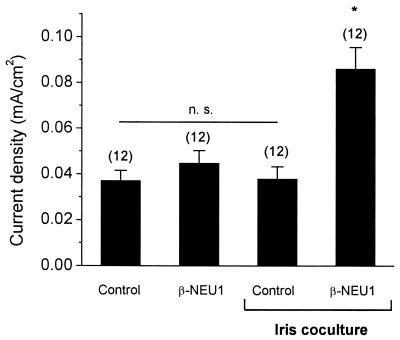
Macroscopic KCa density can be stimulated by application of target-derived factors in CG neurons developing in vitro (23, 24). However, we also have observed that coculture with target tissue (iris) cells or target tissue-conditioned medium is insufficient to reconstitute normal KCa in cultured CG neurons. One possible explanation is that biologically active TGFβ1/4 is not secreted from target tissues in sufficient quantities to stimulate KCa in the absence of other stimulatory factors. This theory predicts that a combination of target tissues and preganglionic factors is necessary for normal development of KCa. To test this hypothesis, E9 CG neurons were cocultured for 12 h with iris myotubes in the presence or absence of 1 nM recombinant β-NEU1 peptide. Two additional control groups included CG neurons cultured in β-NEU1 but without iris myotubes and CG neurons cultured in the absence of either iris myotubes or β-NEU1. Robust and statistically significant stimulation of macroscopic KCa was observed only in CG neurons cultured in the presence of both myotubes and β-NEU1 (Fig. 4). Iris myotubes by themselves, or 1 nM β-NEU1 in the absence of myotubes, did not induce significant expression of KCa in CG neurons. These results provide additional evidence that the development of KCa is controlled by synergistic actions of multiple growth factors secreted from different tissues.
Figure 4.
β-NEU1 permits KCa stimulation by target tissue myotubes in CG neurons developing in vitro. E9 CG neurons were cocultured with iris myotubes for 12 h in the presence or absence of 1 nM β-NEU1 peptide. Additional control neurons were grown in the absence of iris myotubes but in the presence or absence of 1 nM β-NEU1. Robust macroscopic KCa was observed only in CC neurons grown in the presence of iris myotubes and β-NEU1. Iris myotubes or 1 nM β-NEU1 by themselves were insufficient to promote significant macroscopic KCa. CG neurons cultured in the absence of added factors also failed to express significant whole-cell KCa.
Discussion
The development of functional KCa in chick CG neurons depends on trophic factors released from preganglionic nerve terminals and ocular target tissues (16, 17). We have previously demonstrated that KCa are regulated by target-derived TGFβ1/4, which stimulates the development of KCa (24), and target-derived TGFβ3, which inhibits the development of KCa (25). Here we show that β-Neu1 transcripts are expressed in the preganglionic Edinger–Westphal nucleus at developmental stages that coincide with the appearance of macroscopic KCa. Early in vivo exposure to high concentrations of β-NEU1 accelerates the appearance of macroscopic KCa, whereas in vivo blockade of β-NEU1 function inhibits the normal development of KCa in CG neurons. These effects are not associated with changes in Ca2+ channel density. Finally, β-NEU1 is required to observe normal KCa density in CG neurons cocultured with target tissue cells. Macroscopic KCa are the largest macroscopic currents in CG neurons and regulate the late phases of spike repolarization and the amplitude of the hyperpolarizing after potential (15). Moreover, the kinetics of KCa play a role in determining the pattern of repetitive spike discharge in different populations of CG neurons (33).
CG neurons cultured in vitro in the presence of high concentrations of either recombinant β-NEU1 or TGFβ1 alone exhibit a robust increase in the density of functional KCa (21, 24) and the stimulatory actions of these factors are highly synergistic (24). In addition, the appearance of KCa in CG neurons developing in vivo is accelerated by the injection of either factor (ref. 24 and data presented here). However, ablation of either preganglionic tissues or target tissue primordia before the formation of the CG prevents the normal development of KCa (16). These data suggest that TGFβ1/4 and β-NEU1 are not available to CG neurons developing in vivo in concentrations sufficient to stimulate KCa by themselves. The mechanisms that control the availability of these differentiation factors are not known. However, TGFβs and β-neuregulins both require extensive posttranslational processing to produce biologically active peptides. All known TGFβ isoforms are secreted as latent complexes that require proteolytic cleavage to release the active factor from its latency-associated peptide, a process that is highly regulated in certain systems (31). Similarly, at least some β-NEU1 isoforms are synthesized as inactive transmembrane precursors that require proteolytic cleavage of extracellular domains for release of the active peptide (1, 32). This raises the possibility that the electrophysiological differentiation of CG neurons is ultimately controlled by extracellular processing of multiple neurotrophic factors.
The neuregulin-1 gene encodes a large number of bioactive proteins that control the differentiation of many different tissues. These proteins can be broadly classified as α- or β-neuregulins based on the structure of the EGF-like domains. The β-NEU1 isoforms are much more effective in neural systems and are in fact the only forms that can stimulate KCa in CG neurons (21). However, there are many different isoforms of βNEU1, which can be subdivided into type I, type II, or type III based on sequence differences on the N-terminal side of the EGF-like domains (1, 12). For example, type I and type II β-NEU1 isoforms contain Ig domains, which may allow for binding to extracellular heparin proteoglycans resulting in a more gradual and sustained release onto target cells (32). These isoforms may be significant for maintaining a phenotype that has already been established. The type III β-NEU1 isoforms contain cysteine-rich domains and may be more free to diffuse after secretion. The distributions and biological activities of these isoforms do not completely overlap (5, 11, 12). However, there is strong evidence that Ig-containing and cysteine-rich isoforms are both important for synapse formation and maintenance at the mouse neuromuscular junction (4, 12). In the present experiments, we used antibodies and riboprobes directed against the EGF-like domain, and consequently, we cannot state which isoforms are essential. Indeed, it is possible that KCa development in CG neurons is regulated by multiple isoforms of β-NEU1, as at the neuromuscular junction.
The mechanism of β-NEU1 stimulation of KCa in CG neurons is unknown, except that a component of this effect clearly entails posttranslational mechanisms (21). The acute stimulatory effects of TGFβ1/4 on KCa are also posttranslational, at least in part (24, 26), and we have obtained preliminary evidence suggesting that this effect entails insertion of preexisting KCa into the plasma membrane (L. Lhuillier and S.E.D., unpublished data). It is possible that β-NEU1 works through a similar mechanism, albeit through partially distinct transduction cascades that allow for synergistic stimulation of KCa (24).
In summary, we have shown that endogenous β-NEU1 is essential for the normal development of functional KCa in chick CG neurons. The differentiation of excitability in these neurons therefore appears to be regulated by the coordinated actions of multiple trophic factors derived from separate cell–cell interactions.
Figure 5.
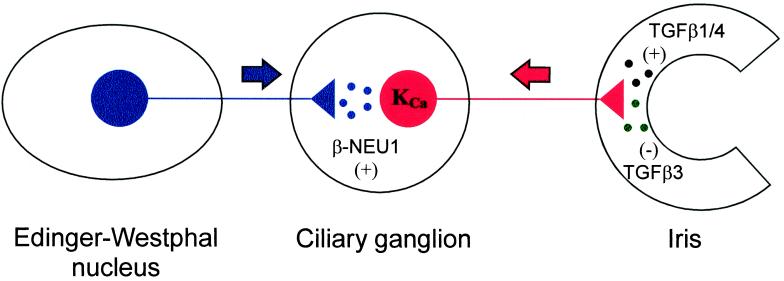
Model for the developmental regulation of CG neuron KCa by multiple cell–cell interactions. The data presented in this and previous studies are consistent with a model in which the development of KCa is regulated by the combined actions of at least three differentiation factors: β-NEU1 (stimulatory, derived from preganglionic nerve terminals); TGFβ1/4 (stimulatory, derived from ocular target tissues); and TGFβ3 (inhibitory, also derived from ocular target tissues). The balance of the in vivo activities of these factors is such that disruption of interactions with either the target tissues or the afferent input prevents the normal development of functional plasma membrane KCa.
Acknowledgments
We are grateful to Loic Lhuillier for helpful discussions. This paper was supported by National Institutes of Health Grants NS-32748 (to S.E.D.) and MH12758 (to J.S.C.).
Abbreviations
- KCa
Ca2+-activated K+ channels
- EGF
epidermal growth factor
- β-NEU1
β-neuregulin-1
- CG
ciliary ganglion
- TGF
transforming growth factor
- En
embryonic day n
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Fischbach G D, Rosen K M. Annu Rev Neurosci. 1997;20:429–458. doi: 10.1146/annurev.neuro.20.1.429. [DOI] [PubMed] [Google Scholar]
- 2.Gassmann M, Lemke G. Curr Opin Neurobiol. 1997;7:87–92. doi: 10.1016/s0959-4388(97)80125-0. [DOI] [PubMed] [Google Scholar]
- 3.Falls D L, Rosen K M, Corfas G, Lane W S, Fischbach G D. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- 4.Sandrock A W, Dryer S E, Rosen K M, Gozani S N, Kramer R, Theill L E, Fischbach G D. Science. 1997;276:599–603. doi: 10.1126/science.276.5312.599. [DOI] [PubMed] [Google Scholar]
- 5.Yang X, Kuo Y, Devay P, Yu C, Role L. Neuron. 1998;20:255–270. doi: 10.1016/s0896-6273(00)80454-7. [DOI] [PubMed] [Google Scholar]
- 6.Ozaki M, Sasner M, Yano R, Lu H S, Buonanno A. Nature (London) 1997;390:691–694. doi: 10.1038/37795. [DOI] [PubMed] [Google Scholar]
- 7.Rieff H I, Raetzman L T, Sapp D W, Yeh H H, Siegel R E, Corfas G. J Neurosci. 1999;19:10757–10766. doi: 10.1523/JNEUROSCI.19-24-10757.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchionni M A, Goodearl A D, Chen M S, Bermingham-McDonogh O, Kirk C, Hendricks M, Danehy F, Misumi D, Sudhalter J, Kobayashi K, et al. Nature (London) 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 9.Zorick T S, Lemke G. Curr Opin Cell Biol. 1996;8:870–876. doi: 10.1016/s0955-0674(96)80090-1. [DOI] [PubMed] [Google Scholar]
- 10.Rio C, Rieff H I, Qi P, Khurana T S, Corfas G. Neuron. 1997;19:39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- 11.Meyer D, Yamaai T, Garratt A, Riethmacher-Sonnenberg E, Kane D, Theill L E, Birchmeier C. Development (Cambridge, UK) 1997;124:3575–3586. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- 12.Wolpowitz D, Mason T B, Dietrich P, Mendelsohn M, Talmage D A, Role L W. Neuron. 2000;25:79–91. doi: 10.1016/s0896-6273(00)80873-9. [DOI] [PubMed] [Google Scholar]
- 13.Golding N L, Jung H Y, Mickus T, Spruston N. J Neurosci. 1999;19:8789–8798. doi: 10.1523/JNEUROSCI.19-20-08789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao L R, Halvorsrud R, Borg-Graham L, Storm J F. J Physiol (London) 1999;521:135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dryer S E, Dourado M M, Wisgirda M E. J Physiol (London) 1991;443:601–627. doi: 10.1113/jphysiol.1991.sp018854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dourado M M, Brumwell C, Wisgirda M E, Jacob M H, Dryer S E. J Neurosci. 1994;14:3156–3165. doi: 10.1523/JNEUROSCI.14-05-03156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dryer S E. J Neurobiol. 1998;37:23–36. doi: 10.1002/(sici)1097-4695(199810)37:1<23::aid-neu3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 18.Dourado M M, Dryer S E. J Physiol (London) 1992;449:411–428. doi: 10.1113/jphysiol.1992.sp019093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramony P, Dryer S E. Dev Brain Res. 1996;91:149–152. doi: 10.1016/0165-3806(95)00174-3. [DOI] [PubMed] [Google Scholar]
- 20.Corfas G, Rosen K M, Aratake H, Krauss R, Fischbach G D. Neuron. 1995;14:103–115. doi: 10.1016/0896-6273(95)90244-9. [DOI] [PubMed] [Google Scholar]
- 21.Subramony P, Dryer S E. Proc Natl Acad Sci USA. 1997;94:5934–5938. doi: 10.1073/pnas.94.11.5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berghard A, Buck L B. J Neurosci. 1996;16:909–918. doi: 10.1523/JNEUROSCI.16-03-00909.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramony P, Raucher S, Dryer L, Dryer S E. Neuron. 1996;17:115–124. doi: 10.1016/s0896-6273(00)80285-8. [DOI] [PubMed] [Google Scholar]
- 24.Cameron J S, Lhuillier L, Subramony P, Dryer S E. Neuron. 1998;21:1045–1053. doi: 10.1016/s0896-6273(00)80622-4. [DOI] [PubMed] [Google Scholar]
- 25.Cameron J S, Dryer L, Dryer S E. Development (Cambridge, UK) 1999;126:4157–4164. doi: 10.1242/dev.126.18.4157. [DOI] [PubMed] [Google Scholar]
- 26.Lhuillier L, Dryer S E. J Neurosci. 2000;20:5616–5622. doi: 10.1523/JNEUROSCI.20-15-05616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn B E, Fitzharris T P, Barnett B D. Anat Rec. 1981;199:33–43. doi: 10.1002/ar.1091990105. [DOI] [PubMed] [Google Scholar]
- 28.Finn T P, Kim S, Nishi R. J Neurobiol. 1998;34:283–293. [PubMed] [Google Scholar]
- 29.Hamburger V, Hamilton H L. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 30.Chiappinelli V A, Fairman K, Giacobini E. Dev Neurosci. 1978;1:191–202. doi: 10.1159/000112565. [DOI] [PubMed] [Google Scholar]
- 31.Brauer P R, Yee J A. Dev Biol. 1993;155:281–285. doi: 10.1006/dbio.1993.1026. [DOI] [PubMed] [Google Scholar]
- 32.Loeb J A, Fischbach G D. J Cell Biol. 1995;130:127–135. doi: 10.1083/jcb.130.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cameron, J. S. & Dryer, S. E. (2000) J. Neurophys., in press.