Abstract
Between January 1, 1989, and December 31, 1994, we have treated 122 primary heart recipients with FK 506 (group I) and 121 with cyclosporine (group II). Fifty patients in the cyclosporine (CyA) group received no lympholytic induction (CyA alone) and 71 others received lympholytic induction with either rabbit antithymocyte globulin or OKT3 (CyA + LI). The mean follow-up was longer in the FK 506 group than in the CyA groups (3.2 ± 1.3 vs 2.3 ± 1.8 years; p < 0.01). Patient survival did not differ on the basis of the type of immunosuppression used. At 3 months after transplantation, the freedom from rejection in the FK 506 group was higher than that of the CyA-alone group (47% vs 22%, p < 0.01) but similar to that of the CyA + LI group (47% vs 53%). The linearized rejection rate (episodes/100 patient-days) of the FK 506 group (0.09 episodes) was lower (p < 0.05) than that of the CyA-alone group (0.26) and the CyA + LI group (0.13). The requirement for pulsed steroids to treat rejection was less in common in the FK 506 group than in either CyA group. Eighteen patients in the CyA group had refractory rejections; all resolved with FK 506 rescue. Two patients in the FK 506 group had refractory rejection that resolved with total lymphoid irradiation (n = 1) and methotrexate therapy (n = 1). Patients receiving FK 506 had a lower risk of hypertension and required a lower dose of steroids. Although the mean serum creatinine concentration at 1 year was higher in the FK 506 group, this difference disappeared after 2 years. No patients required discontinuation of FK 506 because of its side effects. Our intermediate-term results indicate that FK 506 compares favorably with CyA as a primary immunosuppressant in heart transplantation.
Tacrolimus (FK 506), a macrolide lactone derived from the fungus Streptomyces tsurubaensis, was recently approved by the Food and Drug Administration for use as an immunosuppressive agent in liver transplantation. In vitro FK 506 has been found to be 10 to 100 times more potent than cyclosporine (CyA) in its immunosuppressive properties. 1, 2 We first introduced FK 506 into clinical heart transplantation in October 1989 and have since treated 122 primary heart recipients with this drug. This report summarizes our 5-year experience with the use of FK 506 as a primary immunosuppressant in heart transplantation. We will compare the clinical outcome of heart recipients treated with FK 506 with that of a concurrent cohort treated with a CyA-based regimen.
Patients and methods
Patient population
The patient population in this study consisted of patients who had an initial heart transplantation and survived for more than 7 days after transplantation. Patients who had second transplants or multiple organ transplants or who died within 7 days after transplantation because of primary graft failure were excluded from the analysis.
Between January 1, 1989, and December 31, 1994, 243 heart transplant recipients at our center met these criteria. A total of 122 patients received an FK 506–based immunosuppression protocol, and 121 were treated with a CyA-based regimen. From January to September, 1989 all patients received CyA-based immunosuppression. When FK 506 was available for clinical trial in heart transplantation at our center in October 1989, all heart transplant recipients were considered as potential candidates for the FK 506 protocol. CyA was used only when informed consent could not be obtained or when there were restrictions from third-party payers on the use of experimental drugs.
The use of FK 506 was approved by the Institutional Review Board at the University of Pittsburgh, and informed consent was obtained from every patient.
The demographic data for these patients are summarized in Table I. The FK 506 group had a lower mean age than the CyA group (34.2 ± 22.3 vs 47.8 ± 14.5 years; p < 0.05). The mean duration of follow-up was longer in the FK 506 group (3.2 ± 1.3 vs 2.3 ± 1.8 years; p < 0.01). No significant difference was observed in the mean ischemic time between the CyA (206.9 ± 63.2 minutes) and the FK 506 groups (215.1 ± 63.2 minutes).
Table I.
Demographics of cardiac transplant recipients receiving FK 506 and CyA
| Immunosuppression | ||
|---|---|---|
| FK 506 | CyA | |
| No. of patients | 122 | 121 |
| Age (yr) | ||
| Mean ± SD | 34.2 ± 22.3 | 47.8 ± 14.5* |
| Range | 0–65.2 | 1.8–66.3 |
| <18 yr | 42 | 10† |
| >18 yr | 80 | 111 |
| Gender | ||
| Male | 97 | 104 |
| Female | 25 | 17 |
| Underlying disease | ||
| Ischemic | 37 | 53 |
| Idiopathic | 31 | 49 |
| Congenital | 27 | 3 |
| Other | 27 | 16 |
| Ischemic time (min) | ||
| Mean ± SD | 215.1 ± 63.0 | 206.9 ± 63.2 |
| Duration of follow-up (yr) | ||
| Mean ± SD | 3.2 ± 1.3 | 2.8 ± 1.9† |
SD, Standard deviation.
p < 0.05.
p < 0.01.
Immunosuppression protocols
In the FK 506 (Prograf ®, Fujisawa USA, Deerfield, Ill.), group (n = 122), patients were given methylprednisolone in a dose of 15 mg/kg during the operation, 5 mg/kg per day in three divided doses on postoperative day 1, and 0.3 mg/kg per day as a single dose thereafter. Methylprednisolone was converted to prednisone when the patient was able to tolerate a diet, and weaning from steroids was begun 2 months after transplantation. In the early phase of this study, FK 506 was administered 6 to 12 hours after transplantation, at a dose of 0.15 mg/kg per day in two divided doses, each over 4 hours, for a duration of 24 to 72 hours. Because renal dysfunction was prevalent with this regimen, we have modified this protocol. 3 Since August 1990, FK 506 has been given intravenously at a dose of 0.05 mg/kg per day as a continuous infusion 6 to 12 hours after transplantation. As soon as the patient’s gastrointestinal function returned, oral FK 506 was commenced at a dosage of 0.2 to 0.3 mg/kg per day in two divided doses. The plasma level (12-hour trough = 0.5 to 2.0 ng/ml) has been replaced by a whole blood FK 506 level,4 which was maintained at 5 to 30 ng/ml. In the first 2 months after transplantation, the FK 506 level was kept in the range of 15 to 30 ng/ml. This level was gradually decreased to 5 to 15 ng/ml according to the pattern of rejection and the status of the renal function. Azathioprine (2 mg/kg per day) was added if the serum creatinine level was higher than 2.0 mg/dl (to allow a reduction in the FK 506 dosage) or if there was persistent rejection. When a combination of FK 506, steroids, and azathioprine was required, endomyocardial biopsies were performed monthly, and patients have been aggressively weaned from steroids (by 5 mg per month) to avoid major infections.
The immunosuppression protocol for CyA plus lympholytic induction (CyA + LI; n = 71) with either OKT3 (Orthoclone, Ortho Pharmaceutical Corp., Raritan, N.J.) (n = 11) or rabbit antithymocyte globulin (ATG; n = 60) has been described in details elsewhere.5 ATG was prepared locally by Dr. Charles P. Bieber according to the method of Davis, Cooperband, and Mannick.6 In this protocol, steroids were administered as previously described for the FK 506 group. Azathioprine (4 mg/kg) was administered intraoperatively and continued postoperatively at a dosage of 2 mg/kg per day so long as the white blood cell count was greater than 3500 cells/mm3. Cyclosporine was started within 24 hours after the operation, and the target trough whole blood level was maintained at 800 to 1200 ng/ml (TDx method, Sandoz Pharmaceutical Corp., East Hannover, N.J.). OKT3 was administered intravenously at 5 mg/day for 14 days beginning on the second or third postoperative day, and ATG was given intramuscularly at 1.5 mg/kg per day during the first 5 days after the transplantation.
In the group receiving CyA without lympholytic induction (CyA alone) (n = 50), a modified triple drug regimen, as previously described by Bolman and associates,7 was used. In brief, a preoperative loading dose of CyA (6 to 10 mg/kg) was administered orally 2 hours before the operation. On postoperative day 1, oral CyA was commenced twice a day at appropriate dosages (2 to 6 mg/kg per day) to maintain a trough whole blood (TDx method, Sandoz) level of 800 to 1200 ng/ml. Methylprednisolone at a dose of 15 mg/kg was given during the operation, followed by 3 mg/kg per day in four divided doses on postoperative day 1, and reduced to 0.4 mg/kg per day by postoperative day 6. Methylprednisone was converted to prednisone on the return of the gastrointestinal function. By 1 month after transplantation, the dose of prednisone was reduced to 0.3 mg/kg, and steroids were weaned further after 6 months. Azathioprine was administered as in the CyA + LI group.
Monitoring for rejection
Surveillance for rejection involves weekly endomyocardial biopsies during the first month, monthly for the next 3 months, and every 3 months for the remaining first year. Thereafter, biopsies are performed semiannually. In addition, endomyocardial biopsies are performed whenever clinically indicated. In infants and small children, endomyocardial biopsies are performed less frequently because of the inherent technical difficulty. Biopsy specimens are graded according to the criteria of the International Society for Heart and Lung Transplantation.8
Treatment of rejection
Acute rejection (grade 3A or higher) is treated with boluses of methylprednisolone (1 gm/day for adults and 10 to 20 mg/kg per day for pediatric patients, for 3 days). Grade 1B to 2 rejections are treated by augmenting the baseline doses of the primary immunosuppressant (FK 506 or CyA) or steroids. OKT3 or ATG is reserved for steroid-resistant rejections.
Monitoring for infection
Pretransplantation titers for herpesvirus, hepatitis A, B, and C viruses, and Toxoplasma gondii were obtained on every patient. After transplantation, all patients were followed up by an infectious disease specialist. Infections were diagnosed according to previously established criteria.9 Only major infections, as previously defined,5 that necessitated hospitalization for intravenous drug therapy were included for analysis.
Statistical analysis
Actuarial survival, freedom from acute rejection, and freedom from allograft coronary arteriopathy were computed by means of life table analysis. These analyses were based on both the intention-to-treat method (data were analyzed according to the initial treatment assignment) and the censoring crossover method (crossovers were censored when treatment changed).10, 11 Survival curves were compared by means of the log-rank (Mantel-Cox) test. Differences in group means were compared by the t test, and analysis of variance for repeated measures with adjustment was made for comparisonwise error.12 Differences in proportions were compared by the χ2 test. A p value less than 0.05 was considered statistically significant. For the calculation of incidence rates (episodes per 100 patient-days) of rejection, infection, requirement for steroid bolus, and lympholytic treatments of patients in the CyA group who were converted to FK 506, the rates were calculated from the time of the transplantation to the time of conversion to FK 506. Incidence rates were compared with the use of the two-sample test for incidence-density measure.13 A software package (CSS Statistica, Release 4.5, Statsoft, Tulsa, Okla.) was used for statistical analyses.
Results
Patient survivals
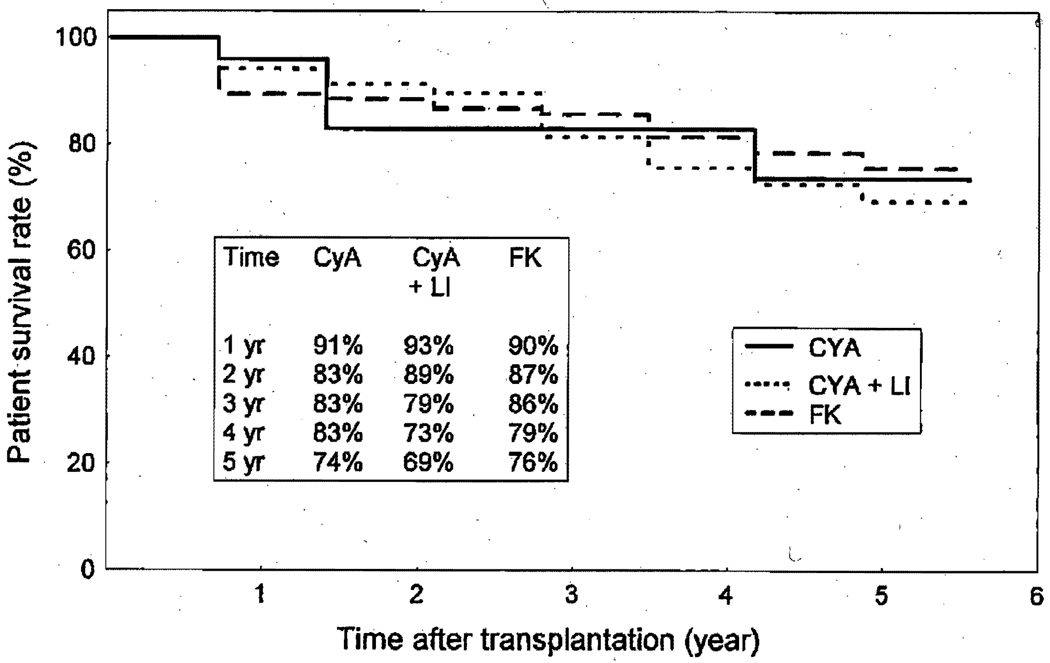
According to the intention-to-treat analysis, the 1- and 5-year actuarial patient survivals did not differ significantly on the basis of the type of immunosuppressive protocols (CyA alone, 91% and 74%; CyA + LI, 93% and 69%; FK 506,90% and 76%) (Fig. 1). Because the intention-to-treat analyses have been criticized for not evaluating the true effect of the treatment in studies in which there are significant crossovers (15% of patients receiving CyA initially were converted to FK 506 [see below]), we have analyzed the survival data using both the intention-to-treat and the censoring crossover methods.10, 11 In the former method, data are analyzed according to the initial treatment assignment; in the latter, data are censored when there is a change in treatment (crossover). There was no difference in patient survivals according to the types of immunosuppressive protocols when either method was used.
Fig. 1.
Actuarial survival of cardiac transplant recipients according to different immunosuppression protocols.
Causes of death
Twenty-four patients in the FK 506 and 23 in the CyA groups (CyA alone and CyA + LI) died during this study (p = not significant; Table II). The most common causes of death for both groups were rejection and infection. Three patients died of disseminated posttransplant lymphoproliferative disease, one in the FK 506 group and two in the CyA group. Four patients in the CyA group died of other malignancies including lung tumor (n = 1), testicular tumor (n = 1), osteosarcoma (n = 1), and recurrent cardiac rhabdomyosarcoma (n = 1).
Table II.
Causes of death during FK 506 and CyA immunosuppression
| Immunosuppression | ||
|---|---|---|
| FK 506 | CyA | |
| No. of patients | 122 | 121 |
| No. of deaths | 24 (20%) | 23 (19%)* |
| Causes of death | ||
| Acute rejection | 2 (2%) | 4 (3%) |
| Allograft CAD | 3 (2%) | 5 (4%) |
| Infection | 6 (5%) | 3 (2%) |
| Malignancy | ||
| PTLD | 1 (0.8%) | 2 (2%) |
| Others | 0 (0%) | 4 (3%) |
| Miscellaneous | 12 (10%) | 7 (6%) |
CAD, Coronary artery disease; PTLD, posttransplant lymphoproliferative disease.
p = Not Significant.
Acute rejection
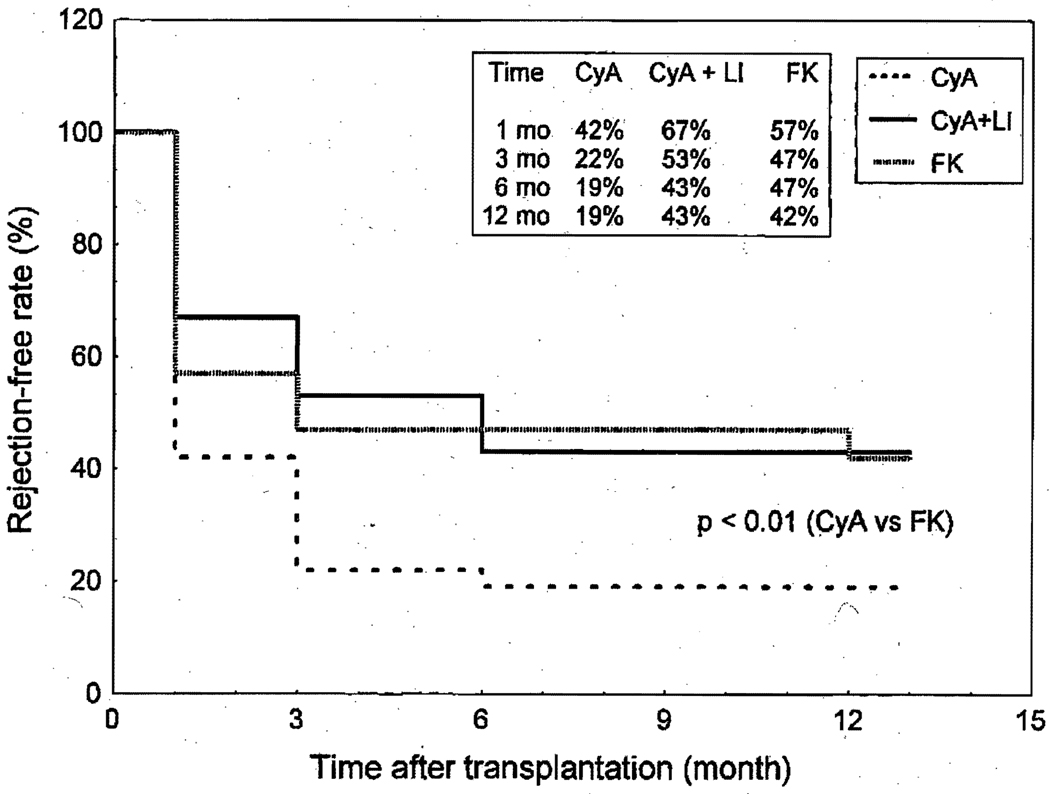
The actuarial freedom from rejection at 3 months for the CyA-alone group was 22%, significantly lower (p < 0.01) than that of the CyA + LI (53%) and FK 506 groups (47%) (Fig. 2). The incidence rate of rejection (episodes per 100 patient-days) in the FK 506 group was significantly lower (p < 0.05) than that of the CyA-alone or CyA + LI group (Table III). Steroid boluses used to treat rejection were lower in the FK 506 group than in the CyA-alone (p < 0.01) and the CyA + LI (p < 0.05) groups. There were fewer rejection episodes that necessitated lympholytic treatment in the FK 506 group than in the CyA-alone group (p < 0.01).
Fig. 2.
Freedom from acute rejection (grade 3A or higher) according to different immunosuppression protocols. The actuarial freedom from rejection in patients who received triple-drug therapy without lympholytic induction (CyA alone) was lower (p < 0.01) than that of the FK 506 group. No difference in the freedom from rejection between the FK 506–treated patients and the CyA-treated patients who received lympholytic induction.
Table III.
Rejection, and requirement for steroid boluses and lympholytic treatments under different protocols
| Immunosuppression | |||
|---|---|---|---|
| FK 506 | CyA | CyA+LI | |
| Rejection-free rate at 30 days | 47% | 22%† | 53% |
| Episodes of rejection (No.)* | 0.09 | 0.26† | 0.13‡ |
| Steroid bolus (No.)* | 0.09 | 0.20‡ | 0.11‡ |
| Lympholytic treatment (No.)* | 0.009 | 0.06† | 0.02 |
Number per 100 patient-days.
p < 0.01 compared with FK 506 group.
p < 0.05 compared with FK 506 group.
Intractable rejection
Thirteen patients in the CyA-alone group and five in the CyA + LI group (four with ATG and one with OKT3) had refractory rejection that was resistant to conventional therapy with at least one course of pulsed steroids and one course of lympholytic treatment. All 18 of these patients were successfully treated by conversion to FK 506. Only two pediatric patients in the FK 506 group had refractory rejection. One required total lymphoid irradiation and the other was treated successfully with methotrexate.
Allograft coronary arteriopathy
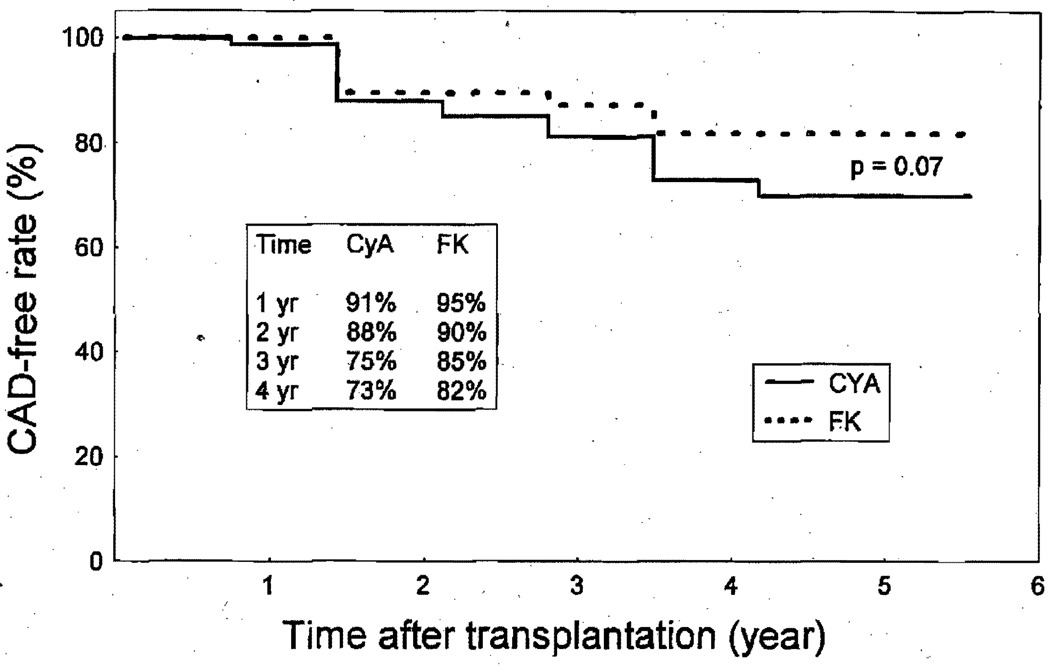
Allograft coronary arteriopathy after transplantation was defined as any luminal irregularity and any coronary stenosis seen on the coronary angiogram or any diffuse coronary artery disease at autopsy. In 183 patients whose allograft coronary arteries could be evaluated, the actuarial freedom from allograft coronary arteriopathy at 4 years for the FK 506 (103 patients) and CyA (80 patients) groups was 82% and 73%, respectively (p = not significant) (Fig. 3).
Fig. 3.
Actuarial freedom from allograft coronary artery disease (CAD) in patients who received FK 506 versus those who received CyA-based regimen (with and without lympholytic induction). No difference was observed between the two groups (p = 0.07).
Requirement for steroids and azathioprine
At most recent follow-up examination or at the time of death, 40 of 83 adults receiving FK 506 (48%) were free of steroids as compared with 16 of 95 adults receiving CyA (17%) (p < 0.01). Among those who were still receiving steroids, the average daily dose of prednisone was 5.8 ± 2.6 mg in the FK 506 group and 8.0 ± 4.5 mg in the CyA group (p < 0.01). The most significant impact of FK 506 has been in the pediatric patients; 76% of the 42 pediatric recipients treated with FK 506 were free of steroids. Of the 10 pediatric patients (age = 1.8 to 17 years) who were initially given CyA, eight required conversion to FK 506 because they could not be weaned from steroids; all of these were eventually free of steroids.
Azathioprine was added to the steroid/FK 506 regimen when serum creatinine concentration was higher than 2 mg/dl or when there were at least two consecutive episodes of acute rejection that necessitated treatments. At latest follow-up, 43% of patients receiving FK 506 were also receiving azathioprine.
Infection
A total of 70 episodes (0.05 episodes per 100 patient-days) of major infections occurred in the FK 506 group as compared with 59 episodes (0.06 episodes per 100 patient-days) in the CyA groups (p = not significant). The prevalence of bacterial, viral, and fungal infection was not significantly different between the FK 506 and CyA groups.
Nephrotoxicity
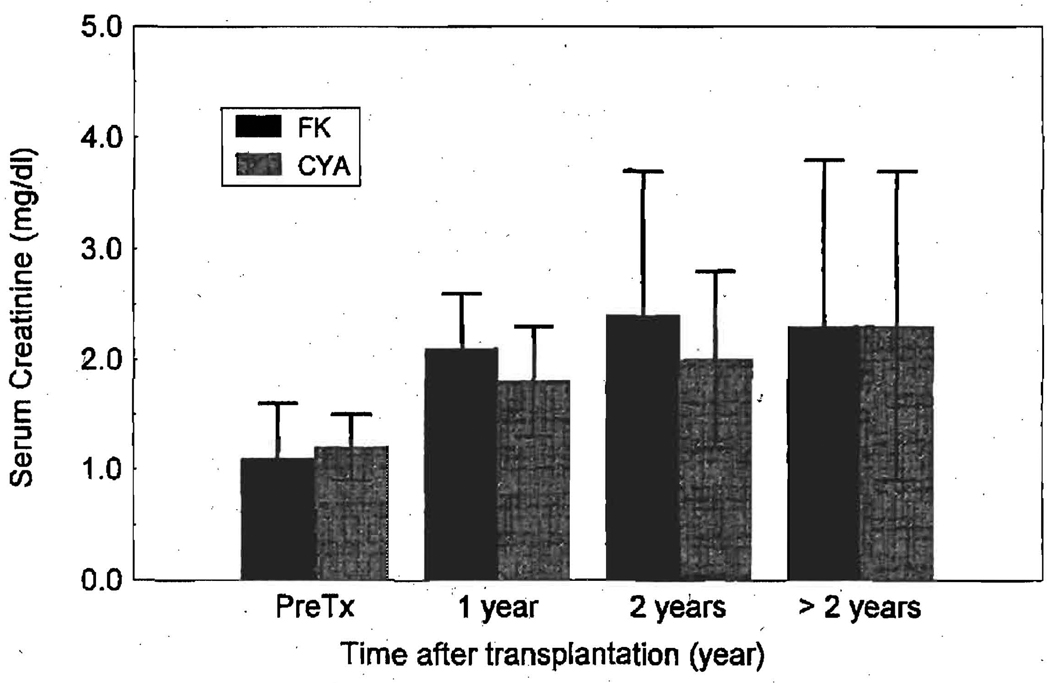
The effect of FK 506 on renal function was most pronounced during the first year after transplantation in both adult and pediatric patients, inasmuch as the mean serum creatinine concentration was much higher (p < 0.01) after the first year (adult = 2.1 ± 0.5 mg/dl; pediatric = 0.9 ± 0.5 mg/dl) when compared with the pretransplantation values (adolt = 1.1 ± 0.5 mg/dl; pediatric = 0.6 ± 0.3 mg/dl). The rise in serum creatinine concentration leveled off after the first postoperative year. In the adult patients (age > 18 years) mean serum creatinine levels at 1 and 2 years after transplantation were higher in the FK 506 group than in the CyA groups (Fig. 4). However; this difference did not reach statistical significance (by the analysis of variance for repeated measures).
Fig. 4.
Serum creatinine levels at various time points after transplantation in adult cardiac recipients receiving FK 506 and CyA. One year after transplantation, the serum creatinine levels were significantly higher than the pretransplant (PreTx) values for both groups (p < 0.01). There were no statistically significant difference in the serum creatinine concentrations between FK 506 and CyA groups.
Renal failure necessitating dialysis or kidney transplantation developed in five patients in the FK 506 group and in seven in the CyA groups 2 to 5 years after transplantation. In the CyA groups, three patients required dialysis while being treated with CyA alone and four required dialysis after being converted to FK 506. Of these four patients switched to FK 506, two had infections (one mucormycosis and one tuberculosis) that necessitated antibiotics with nephrotoxicity.
Other side effects
Hypertension
The prevalence of new-onset hypertension in adults in the FK 506 and CyA groups was 47% (39/83) and 84% (80/95), respectively (p < 0.01). In the pediatric group, 10% of the patients treated with FK 506 had hypertension.
Hyperkalemia
Twenty-five adults and one pediatric patient (21%) who were receiving FK 506 had persistent hyperkalemia (K+ >5.0 mEq/L) that necessitated treatment. This was easily controlled with a low dose of ftudrocortisone (0.2 mg/day). No patient died of hyperkalemia in this trial.
Diabetes mellitus
The prevalence of new-onset insulin-dependent diabetes mellitus was the same in adults in both the FK 506 (26%) and CyA (22%) groups. New-onset insulin-dependent diabetes mellitus developed in one of 50 pediatric patients receiving FK 506 (both as a primary agent and after conversion from CyA).
Posttransplant lymphoproliferative disease and other malignant diseases
Posttransplant lymphoproliferative disease developed in tow pediatric patients in the FK 506 group at 4 and 5 months after transplantation. In one patient the disease resolved with reduction in immunosuppression; the other patient died of disseminated posttransplantation lymphoproliferative disease. Another pediatlic patient, who initially received CyA, died of disseminated posttransplantation lymphoproliferative disease after conversion to FK 506. Of the adult patients, one in the CyA group died of brain lymphoma; four in the CyA group died of other malignancies, including osteosarcoma (n = 1), recunent cardiac rhabdomyosarcoma (n = 1), lung carcinoma (n = 1), and testicular embryonal cell carcinoma (n = 1).
Other side effects of FK 506, which have been reported elsewhere16 and included extremity paresthesis, akinetic mutism, myalgia, and tremor, were infrequent and transient. Notably absent in the FK 506 group were gingival hyperplasia, hirsutism, and coarsening of facial features. No patients in the FK 506 group had severe and persistent adverse events that required discontinuation of this drug.
Discussion
Although chemically unrelated, FK 506 and CyA both inhibit the immune response via their ability to prevent the transcription of lymphokine genes after the activation of T-cell receptors.14 Both act early in the cell cycle and thus are very effective as immunosuppressants. Since its discovery by Ochia and associates15 in 1987, FK 506 has been extensively studied. In vitro, FK 506 suppressed the proliferative response in mixed lymphocyte cultures by inhibiting interleukin-2 synthesis after alloactivation.1, 2, 16 In vivo, FK 506 effectively prevented and reversed rejection to various allografts in different animal models.16–18 FK 506 was initially introduced into clinical use in 1989 by Starzl and colleagues19 for the treatment of liver, kidney, and pancreas recipients. After the initial success of FK 506 as a primary and rescue agent in clinical organ transplantation,20–22 randomized multicenter trials both in the United States and in Europe with liver transplant recipients have indicated that FK 506 is effective and superior to CyA as an immunosuppressive agent.23–25 FK 506 was finally approved by the Food and Drug Administration for use in clinical liver transplantation in 1994. We first introduced FK 506 into clinical heart transplantation in October 1989. Our initial experience with this drug as a primary and rescue agent indicated that FK 506 was an effective immunosuppressant and was well tolerated.3 The intermediate-term data reported herein confirm our initial findings. Although the survival is the same in both groups, an FK 506–based regimen results in a lower rate of acute and refractory rejection than does a CyA regimen. The effect of FK 506 was most pronounced when it was compared with a CyA-based regimen that included no lympholytic induction. In addition, FK 506 was associated with a lower risk for hypertension and fewer requirement for steroids.
The fact that 15% of the patients in the CyA groups were successfully converted to FK 506 for refractory rejection reflects, in part, the more potent immunosuppressive properties of FK 506 and, in part, our philosophy to minimize the use of multiple courses of high-dose steroids and lympholytic therapy. In our earlier experience with FK 506 as a “rescue” drug for refractory rejection, we observed a very high incidence of posttransplantation lymphoproliferative disease, especially in patients who had received multiple courses of steroids and lympholytic agents before being converted to FK 506.26 Excessive use of lympholytic treatments is a primary risk factor in the development of posttransplantation lymphoproliferative disease.27 We therefore strongly believe that early conversion to FK 506 in patients with refractory rejection who are being treated with CyA should reduce this risk.
Renal toxicity is a major side effect of both FK 506 and CyA. Studies in liver recipients have shown that nephrotoxicity was comparable between CyA and FK 506 and that the mean serum creatinine levels at various time points after transplantation were similar in the two groups.23, 25 In the current study, 1 year after transplantation, patients in the FK 506 group had slightly higher mean serum creatinine levels than those in the CyA group (p = not significant). However, this difference disappeared after 2 years. The higher serum creatinine level at 1 year after transplantation in the FK 506 group probably reflects our learning curve in the use of this drug. With experience, we believe that this difference will disappear. Hyperkalemia is a known side effect of FK 506 and has been reported to be independent of renal function.28, 29 This side effect was, however, easily treated with a small dose of fludrocortisone. Possible mechanisms responsible for the hyperkalemia include the inability of the distal renal tubules to respond to aldosterone (type IV renal tubular acidosis) and a decrease in the level of plasma renin caused by a depressed cellular activity of the juxtaglomerular system.30, 31
The risks of infection and posttransplantation lymphoproliferative diseases were similar with both FK 506 and CyA. Other advantages of FK 506 over CyA include a lower risk of the development of new-onset hypertension, the lack of gingival hyperplasia, and the lack of coarsening of facial features.
Although the current study is limited by a lack of randomization and involves a different organ, it yields results similar to those of the randomized studies with liver and lung transplantation. In the European multicenter trial, which consisted of 545 primary liver recipients, the rate of patient and graft survival was similar between FK 506 and, CyA groups; however, patients receiving FK 506 had significantly fewer episodes of acute, refractory acute, and chronic rejection.25 The multicenter trial in the United States consisting of 529 primary liver recipients reported similar results.23 The 1-year actuarial survivals were similar between FK 506 and CyA arms, and patients receiving FK 506 had fewer episodes of acute, steroid-resistant, and refractory rejection. Our own randomized trial of FK 506 in lung transplantation,32 which involved 74 lung recipients (38 received FK 506 and 36 CyA), reported the same findings. The 1-year survival was similar between the two groups; however, patients in the FK 506 arm had significantly fewer episodes of acute and refractory rejection.
In summary, FK 506 has proved to be an effective immunosuppressive agent in clinical cardiac transplantation. The intermediate-term results indicate that the patient survival is similar with FK 506 and CyA immunosuppression. However, patients receiving FK 506 have fewer episodes of acute and refractory rejection, require less treatment for rejection, and need lower doses of maintenance steroids. Major side effects of FK 506 and CyA are similar. FK 506, therefore, is a useful drug in clinical heart transplantation.
Supplementary Material
Acknowledgments
We thank Howard R. Doyle, MD, and Alfred Cecchetti, MSc, for their advice on statistical methods.
Footnotes
Read at the Seventy-fifth Annual Meeting of The American Association for Thoracic Surgery, Boston, Mass., April 23–26, 1995.
REFERENCES
- 1.Kino T, Hataraka H, Miyata S, et al. FK506, a novel immunosuppression isolated from a streptomyces: immuosuppressive effect of FK506 in vitro. J Antibiotics. 1987;40:1256–1260. doi: 10.7164/antibiotics.40.1256. [DOI] [PubMed] [Google Scholar]
- 2.Zeevi A, Duquesnoy R, Eiras G, et al. Immunosuppressive effect of FK 506 on in vitro lymphocyte alloactivation: synergism with cyclosporine A. Transplant Proc. 1987;19:40–44. [PMC free article] [PubMed] [Google Scholar]
- 3.Armitage JM, Kormos RL, Fung J, Starzl TE. The clinical trial of FK506 as primary and rescue immunosuppression in adult cardiac transplantation. Transplant Proc. 1992;23:3054–3057. [PMC free article] [PubMed] [Google Scholar]
- 4.Greiner FC, Luczkiw J, Bergmann M, et al. A whole blood FK506 assay for the IMx analyzer. Transplant Proc. 1991;23:2748–2749. [PubMed] [Google Scholar]
- 5.Kormos RL, Armitage JM, Dummer JS, et al. Optimal perioperative immunosuppression in cardiac transplantation using rabbit antithymocyte globulin. Transplantation. 1990;49:306–311. doi: 10.1097/00007890-199002000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Davis RC, Cooperband SR, Mannick JA. Preparation and in vitro assay of effective and ineffective antithymocyte sera. Surgery. 1969;66:58–64. [PubMed] [Google Scholar]
- 7.Bolman RM, Elick B, Olivari MT, Ring WS, Arentzen CE. Improved immunosuppression for cardiac transplantation. J Heart Transplant. 1985;4:315–318. [PubMed] [Google Scholar]
- 8.Billingham ME, Cary NRB, Hammond ME, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. J Heart Lung Transplant. 1990;9:587–593. [PubMed] [Google Scholar]
- 9.Kusne S, Dummer JS, Singh N, et al. Infection after liver transplantation: an analysis of 101 consecutive cases. Medicine. 1988;67:132. doi: 10.1097/00005792-198803000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Detre K, Peduzzi P. The problem of attributing deaths of nonadherers: the VA coronary bypass experience. Controlled Clin Trials. 1982;3:355–364. doi: 10.1016/0197-2456(82)90025-3. [DOI] [PubMed] [Google Scholar]
- 11.Peduzzi P, Detre K, Wittes J, Holford T. Intent-to-treat analysis and the problem of crossovers. J THORAC CARDIOVASC SURG. 1991;101:481–487. [PubMed] [Google Scholar]
- 12.Wiener BJ. Statistical principles in experimental design. New York: McGraw-Hill; 1971. pp. 196–201. [Google Scholar]
- 13.Rosner B. Fundamentals of biostatics. 3rd ed. Belmont, California: Duxbury Press; 1990. pp. 336–372. [Google Scholar]
- 14.Tocci MS, Matkovich DA, Collier KA, et al. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J Immunol. 1989;143:718–726. [PubMed] [Google Scholar]
- 15.Ochiai T, Nakajima K, Nagata M, et al. Effect of a new immunosuppressive agent, FK506, on heterotopic cardiae allotransplantation in the rat. Transplant Proc. 1987;19:1284–1286. [PubMed] [Google Scholar]
- 16.Murase N, Kim DG, Todo S, et al. Suppression'of allograft rejection with FK506. I. Prolonged cardiac and liver survival in rats following short course therapy. Transplantation. 1990;50:1986–1989. doi: 10.1097/00007890-199008000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murase N, Kim DG, Todo S, Cramer DV, Fung J, Statzl TE. FK506 suppression of heart and liver allograft rejection. II. The induction of graft acceptance in rats. Transplantation. 1990;50:739–744. doi: 10.1097/00007890-199011000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Todo S, Ueda Y, Demetris AJ, et al. Immunosuppression of canine, monkey, and baboon allografts by FK506 with special reference to synergism with other drugs and tolerance induction. Surgery. 1988;104:239–240. [PMC free article] [PubMed] [Google Scholar]
- 19.Starzl TE, Todo S, Fung JJ, et al. FK506 for liver, kidney, and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todo S, Fung JJ, Tzakis A, et al. Early trials with FK506 as primary treatment in liver transplantation. Transplant Proc. 1990;22:13–16. [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan ML, Shapiro R, Vivas CA, et al. FK506 “rescue” for resistant rejection of renal allografts under primary cyclosporine immunosuppression. Transplantation. 1994;57:860–865. doi: 10.1097/00007890-199403270-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armitage JM, Kormos RL, Morita S, et al. Clinical trial of FK506 immunosuppression in adult cardiac transplantation. Ann Thorae Surg. 1992;54:205–211. doi: 10.1016/0003-4975(92)91371-f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The U.S. Multicenter FK506 Liver Study Group. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med. 1994;331:1110–1115. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 24.The US Multicenter FK506 Study Group. Prognostic factors for successful conversion from cyclosporine to FK506-based immunosuppressive therapy for refractory rejection after liver transplantation. Transplant Proc. 1993;23:2987–2990. [PubMed] [Google Scholar]
- 25.European FK506 Multicentre Liver Study Group. Randomized trial comparing tacrolimus (FK506) and cyclosporine in prevention of liver allograft rejection. Lancet. 1994;344:423–424. [PubMed] [Google Scholar]
- 26.Pham SM, Armitage JM, Kormos RL, et al. Rescue therapy with FK-506 in cardiac transplantation. Circulation. 1993;88 Suppl:l94. [Google Scholar]
- 27.Swinnen LJ, Costanzo-Nordin M, Fisher SG, et al. Increased incidence of lymphoproliferative disorder after immunosuppression with the monoclonal antibody OKT3 in cardiac transplant recipients. N Engl J Med. 1990;323:1723–1728. doi: 10.1056/NEJM199012203232502. [DOI] [PubMed] [Google Scholar]
- 28.Jain AB, Fung JJ, Todo S, et al. One thousand consecutive prima orthotopic liver transplants under FK 506: survival and adverse events. Transplant Proc. 1995;27:1099–1104. [PMC free article] [PubMed] [Google Scholar]
- 29.US Multicenter Liver Study Group. Comparing nephrotoxicity of FK506 and cyclosporine regimens after liver transplantation: preliminary results from US Multicenter Trial. Transplant Proc. 1995;27:1114–1116. [PubMed] [Google Scholar]
- 30.Berg KJ, Forre O, Bjerkhoel F, et al. Side effects of cyclosporine A treatment in patients with rheumatoid arthritis. Kidney Int. 1986;29:1180–1187. doi: 10.1038/ki.1986.125. [DOI] [PubMed] [Google Scholar]
- 31.Bantle JP, Nath KA, Sutherland DE, Najarian JS, Ferris TF. Effects of cyclosporine on the renin-angiotensin-aldosterone system and potassium excretion in renal transplant recipients. Arch Intern Med. 1985;145:505–508. [PubMed] [Google Scholar]
- 32.Griffith BP, Bando K, Hardesty RL, et al. A prospective randomized trial of FK506 versus cyclosporine after human pulmonary transplantation. Transplantation. 1994;57:848–851. doi: 10.1097/00007890-199403270-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.