Abstract
Despite the recognition that humoral rejection is an important cause of allograft injury, the mechanism of antibody-mediated injury to allograft parenchyma is not well understood. We used a well-characterized murine hepatocellular allograft model to determine the mechanism of antibody-mediated destruction of transplanted liver parenchymal cells. In this model allogeneic hepatocytes are transplanted into CD8-deficient hosts in order to focus on CD4-dependent, alloantibody-mediated rejection. Host serum alloantibody levels correlated with in vivo allospecific cytotoxic activity in CD8 KO hepatocyte rejector mice. Host macrophage depletion, but not CD4+ T cell, NK cell, neutrophil, or complement depletion, inhibited in vivo allocytotoxicity. Recipient macrophage deficiency delayed CD4-dependent hepatocyte rejection and inhibited in vivo allocytotoxicity without influencing alloantibody production. Furthermore, hepatocyte coincubation with alloantibody and macrophages resulted in antibody-dependent hepatocellular cytotoxicity in vitro. These studies are consistent with a paradigm of acute humoral rejection in which CD4+ T cell-dependent alloantibody production results in the targeting of transplanted allogeneic parenchymal cells for macrophage-mediated cytotoxic immune damage. Consequently, strategies to eliminate recipient macrophages during CD4-dependent rejection pathway may prolong allograft survival.
Keywords: macrophages, alloantibody, hepatocytes, antibody-mediated cellular cytotoxicity, transplantation
Introduction
Acute antibody-mediated allograft rejection is an important cause of solid organ graft dysfunction and failure. Complement split product deposition at the graft site has become an important component of the pathologic diagnostic criteria for antibody-mediated graft damage (1). C4d deposition correlates with the occurrence of renal (2–5), lung (6, 7), liver (8–10), and cardiac (11–13) allograft loss, suggesting complement is a key mediator of graft tissue destruction. The concurrent presence of innate and adaptive immune cellular infiltrates at the graft site reported in many studies in which complement deposition is observed and humoral immunity is operative (14–20), as well as known antibody-dependent cellular cytotoxicity (ADCC) mechanisms (21) bring into question the role of graft infiltrating cells in alloantibody-mediated damage to the graft parenchyma.
We have previously reported that liver parenchymal cell (hepatocellular) allografts initiate a robust humoral immune response (especially when CD8+ T cell-mediated immunity is perturbed) which is sufficient to mediate hepatocyte rejection in a dose-dependent fashion. Acute rejection in CD8-deficient hosts is CD4+ T cell-dependent, B cell-dependent, and allospecific (22). The histology associated with alloantibody-mediated hepatocellular allograft rejection is remarkable for absence of injury to the native liver tissue. Hepatocellular allografts are distinguished from solid organ allografts by the absence of donor endothelium intervening between the vasculature and graft parenchymal cells. Consequently, the target of humoral alloimmunity after hepatocyte transplant is not donor endothelium, suggesting alloantibody is able to target donor liver parenchymal cells specifically and clear them efficiently. A possible mechanism for this selective targeting and destruction of donor parenchymal cells include complement activation and resultant membrane attack complex mediated lysis of the donor cells. Another mechanism, ADCC, involves other immune cell mediators, such as NK cells, macrophages, and neutrophils. These mechanisms are relevant to clinical cell (islet (23, 24), bone marrow (25)) and solid organ allografts in which alloantibodies are a known barrier to early and long term graft survival (14–20).
The current studies were performed to determine the in vivo mechanism of alloantibody-mediated damage of transplanted liver parenchymal cells. We found that host macrophages, and not complement, CD4+ T cells, NK cells, or neutrophils, were critical for in vivo allospecific cytotoxic effector function generated during CD4-dependent antibody-mediated hepatocyte allograft rejection (CD8 KO recipients). The role of host macrophages as cellular effectors of antibody-mediated graft rejection was supported using three experimental approaches including the CD8-depleted macrophage “deficient” op/op host, macrophage depletion of a CD8 KO host, and an in vitro cytotoxicity assay in which hepatocellular cytotoxicity was determined in the presence of alloantibody, macrophages, or both alloantibody and macrophages. Therapies designed to limit or block interactions between alloantibody and host macrophages could prevent graft injury by humoral mechanisms which can occur despite effective control of T cell-mediated rejection responses.
Materials and Methods
Experimental animals
FVB/N (H-2q, Taconic), CD8 KO (H-2b, C57BL/6Cd8atm1Mak, Jackson Laboratory), and op/op osteopetrosis (B6C3Fe a/a-Csf1op/Csf1op, H-2b, a gift from Dr. Clay Marsh, The Ohio State University) mouse strains were used in this study. Transgenic FVB/N mice expressing human alpha-1 antitrypsin (hA1AT-FVB/N, H-2q) were the source of “donor” hepatocytes. This strain was created, bred, and maintained at the Biotechnology Center and Transgenic Animal Facility (The Ohio State University) (26, 27). Mice which were 6–9 weeks of age were used in experiments. All experiments were performed in compliance with the guidelines of the Institutional Laboratory Animal Care and Use Committee of The Ohio State University (Protocol 2007A0071).
Hepatocyte isolation and purification
Hepatocyte isolation and purification was performed as described previously (26, 27). Briefly, the liver was perfused with 0.09% EGTA-containing calcium-free salt solution followed by a 0.05% collagenase solution (type IV, Sigma, Saint Louis, Missouri) in 1% albumin. Liver tissue was minced, filtered, and washed in RPMI-1640 with 10% FBS. Hepatocytes were purified on a 50% Percoll gradient (Pharmacia Biotech, Uppsala, Sweden). Hepatocyte viability and purity were both consistently >99%.
Hepatocyte transplantation and monitoring of hepatocyte graft function
Donor hepatocytes were retrieved from transgenic mice expressing hA1AT under control of the liver-specific hA1AT promoter and transplanted into recipients by intrasplenic injection with circulation of donor hepatocytes to the host liver, as previously described (27). Graft function was determined by detection of the secreted transgenic reporter product, hA1AT, in serial recipient serum samples. Graft survival was reflected by stable and persistent serum hA1AT levels, whereas graft rejection was reflected by rapidly decreasing serum hA1AT (usually over 5–7 days) to undetectable levels (less than 0.5 μg/mL) (26, 27).
In vivo host treatment
Recipients were depleted of circulating CD4+ T cells, CD8+ T cells, NK cells (NK1.1+) or neutrophils (Ly6G+) cells using monoclonal antibodies (mAbs). Anti-CD4 (GK1.5), anti-CD8 (53.6.72), and anti-Ly6G (RB6-8C5) were obtained from Bioexpress Cell Culture Services (West Lebanon, New Hampshire). Anti-NK1.1 (PK136) mAb was purified from ascites produced in pristane primed mice. Hepatocyte recipients (as well as nontransplanted control mice) were depleted of CD4+ T cells, NK cells or neutrophils by intraperitoneal (i.p.) treatment with 250 μg of anti-CD4, 300 μg of anti-NK1.1 or 100 μg of anti-Ly6G mAbs respectively 48 hours prior to the in vivo cytotoxicity assay. Depletion was confirmed through flow cytometric analysis of recipient splenocytes. MCSF−/− (op/op) and wild type littermate recipient mice were depleted of CD8+ T cells using anti-CD8 mAb (300 μg, i.p.) on days −4, −2, 7, and 14 relative to hepatocyte transplant. Depletion was confirmed through flow cytometric analysis of peripheral blood lymphocytes (PBLs).
Recipient macrophages were depleted through intraperitoneal injection of liposome-encapsulated clodronate. To determine the contribution of host macrophages to in vivo cytotoxic effector function, hepatocyte recipients were depleted of host macrophages (0.2 mL liposome clodronate, i.p.) 48 hours prior to the in vivo cytotoxicity assay. To determine the role of host macrophages in the effector phase of hepatocyte rejection, CD8 KO hepatocyte recipients were depleted of host macrophages (0.2 mL liposome clodronate, i.p.) on days 5, 9, 13, 17, 21 post transplant while monitoring graft survival. Liposome clodronate and control liposomes containing only PBS were prepared as previously described (28). Clodronate was a kind gift of Roche Diagnostics GmbH, Mannheim, Germany. Depletion of macrophages was confirmed through flow cytometric analysis of F4/80+ (CI:A3-1, Caltag Laboratories, Burlingame, California) cells in recipient splenocytes.
Host complement was depleted through intraperitoneal treatment of 25 μg of cobra venom factor (Naja melanoleuca Venom Supplies, Tanunda, South Australia). Host depletion of complement was confirmed through reduction in hemolysis of antibody sensitized sheep erythrocytes in Gelatin Veronal buffer according to manufacturer’s instructions (Sigma).
In vivo cytotoxicity assay
An in vivo cytotoxicity assay, initially designed to detect cytolytic T cell function in vivo through clearance of CFSE stained allogeneic and syngeneic target cells, has been previously described (29). Syngeneic target splenocytes from C57BL/6 mice were stained with 0.2μM Carboxyfluorescein Diacetate Succinimidyl Ester (CFSElow; Molecular Probes, Eugene, OR). Allogeneic target splenocytes from FVB/N mice were stained with 2.0μM CFSE (CFSEhigh). Equal numbers of CFSE-labeled syngeneic and allogeneic target splenocytes (20×106 each, mixed 1:1) were injected into the tail veins of allograft recipient and control untransplanted mice. Eighteen hours after CFSE-labeled target cell injection, splenocytes from hepatocyte recipients were retrieved and analyzed by flow cytometry, gating on CFSE-positive splenocytes. Percent allospecific cytotoxicity was calculated using the following formula where #CFSEhigh represents the number of allogeneic target cells and #CFSElow represents the number of syngeneic target cells recovered from either untransplanted or experimental mice:
Donor-reactive antibody
Host serum was assayed for the presence of donor-specific IgG alloantibodies by incubating serum (diluted 1:10) obtained 14 days post transplant with FVB/N target splenocytes followed by incubation with FITC-conjugated goat F(ab′)2 anti-mouse IgG Fc (Organon Teknika Corp, West Chester, PA) and analysis by FACS. Alloantibody level is represented as the percentage of target cells labeled by secondary fluorescent antibody as described previously (22).
Immunohistochemistry
Tissue was snap frozen in liquid nitrogen, fixed in cold acetone, quenched in hydrogen peroxide solution to block endogenous peroxidase activity, and endogenous biotin was blocked using a biotin blocking system (DakoCytomation, Carpinteria CA). For detection of donor hepatocytes engrafted within recipient liver parenchyma, the polyclonal rabbit anti-human alpha-1 antitrypsin antibody was used (DakoCytomation, Carpinteria, CA). Biotinylated goat anti-rabbit IgG (H+L; Vector Laboratories BA-5000) secondary antibody (1:200) was used prior to the Vectastain Elite ABC Kit (Vector Laboratories PK-7100) for detection. Liver macrophages (F4/80+ cells) were detected with rat anti-mouse F4/80 antibody (1:25, Serotec Ltd, Kindlington, Oxford, UK) and goat anti-rat IgG, horseradish peroxidase (mouse adsorbed; 1:25, Serotec Ltd). Both anti-hA1AT or anti-F4/80 antibodies were visualized with liquid DAB+ substrate (DakoCytomation). Slides were counterstained with Richard-Allen Hematoxylin, and the histology was assessed by blinded analysis.
In vitro cytotoxicity assay
Purified mouse FVB/N allogeneic hepatocytes (H-2q) were incubated for 30 minutes (37°C) with serum from naïve or rejecter CD8 KO mice (H-2b; 14 days after hepatocyte transplantation). Aliquots of hepatocytes were then added to 12-well plates with 10% FBS medium. Macrophages were harvested from C57BL/6 mice (H-2b) as previously described (30). Briefly, 1 mL of 4% Brewer thioglycollate medium was injected into the peritoneal cavity of wild type mice. Five days later, the peritoneal cavity was washed with 10 mL of PBS. Subsequently, the peritoneal exudates cells were plated for 1 hour and the adherent macrophages were collected (85 – 95% purity by CD68 flow cytometry, data not shown). Macrophages were then added to the wells at various E:T ratios (10:1 or 40:1). Following the addition of macrophages, hepatocytes were cultured for an additional 8 hours at 37°C. Supernatants were then collected and analyzed for alanine transaminase (ALT) release (The Ohio State University, Department of Clinical Laboratories).
Statistical analysis
Graft survival between experimental groups was compared using Kaplan Meier survival curves and log-rank statistics (SPSS, Chicago, IL). Other statistical calculations were performed using Student’s t test to analyze differences between experimental groups. P<0.05 was considered significant.
Results
Recipient serum alloantibody levels correlate with the magnitude of in vivo cytotoxic effector function in CD8 KO hepatocyte rejector mice
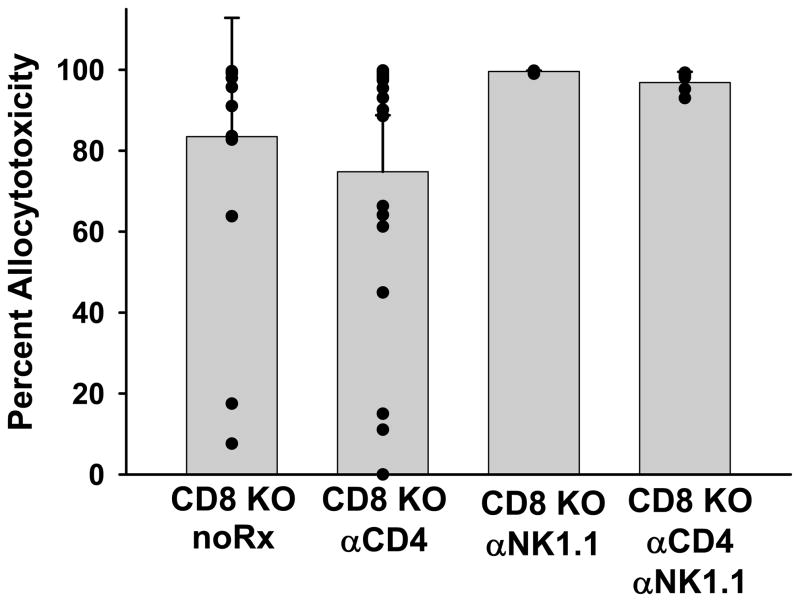
We have previously reported that in the absence of host CD8+ T cells (CD8 KO, CD8+ T cell depleted C57BL/6, and SCID hosts reconstituted with CD8-depleted splenocytes) rejection of hepatocellular allografts is CD4+ T cell-dependent and mediated by alloantibody (22, 26). These studies prompted further analysis of the mechanism of antibody-mediated allogeneic parenchymal cell damage. Untreated CD8 KO (H-2b) recipients were transplanted with FVB/N (H-2q) hepatocellular allografts and monitored for graft rejection which occurred, as in prior studies, with median survival time (MST) of 14 days (26). Following rejection, the hosts were tested for in vivo cytotoxic effector function using an in vivo cytotoxicity assay by the adoptive transfer of syngeneic and allogeneic target splenocytes. This assay was originally designed to detect CD8+ T cell or NK cell-mediated cytotoxic activity in vivo (31, 32). Despite the absence of the CD8+ cytotoxic T cell subset, CD8 KO hepatocellular allograft rejector mice develop potent cytotoxic effector activity (n=16; Figure 1). All CD8 KO hepatocyte rejector mice generated detectable levels of alloantibody in recipient serum. In order to determine if this cytotoxic activity was mediated by immune cells capable of cytotoxic effector function, such as CD4+ T cells or NK cells, CD8 KO hepatocyte hosts that had rejected hepatocyte allografts were depleted of CD4+ T cells (GK1.5) and/or NK cells (PK136) 48 hours prior to the in vivo cytotoxicity assay. The high magnitude cytotoxic activity generated in CD8 KO transplant rejectors was not significantly decreased in the absence of host CD4+ T cells (n=19), NK cells (n=4), or both CD4+ T cells and NK cells (n=5), in comparison to nondepleted rejectors (p=ns; Figure 1). We noted a small decrease in cytotoxicity with CD4-depletion, although the effect was not statistically significant. Nevertheless, in subsequent cytotoxicity experiments all hosts were depleted of CD4+ T cells to remove any potential cytotoxic contributions from CD4+ T cells and to clarify interpretation of data.
Figure 1. CD8 KO rejector mice have high magnitude in vivo cytotoxic effector function which is not mediated by CD4+ T or NK cellular effectors.
FVB/N (H-2q) hepatocytes were transplanted into untreated CD8 KO (H-2b) mice. After graft rejection occurred (MST = 14 days), CD8 KO hosts were left untreated (no Rx), or were depleted of CD4+ T cells (CD8 KO αCD4), NK cells (CD8 KO αNK1.1), or both CD4+ T cells and NK cells (CD8 KO αCD4 αNK1.1) 48 hours prior to the in vivo cytotoxicity assay. The bars represent average cytotoxicity ± standard deviation for each group. Individual cytotoxicity values are represented by the circles. Allocytotoxicity in all groups was statistically similar (p=ns).
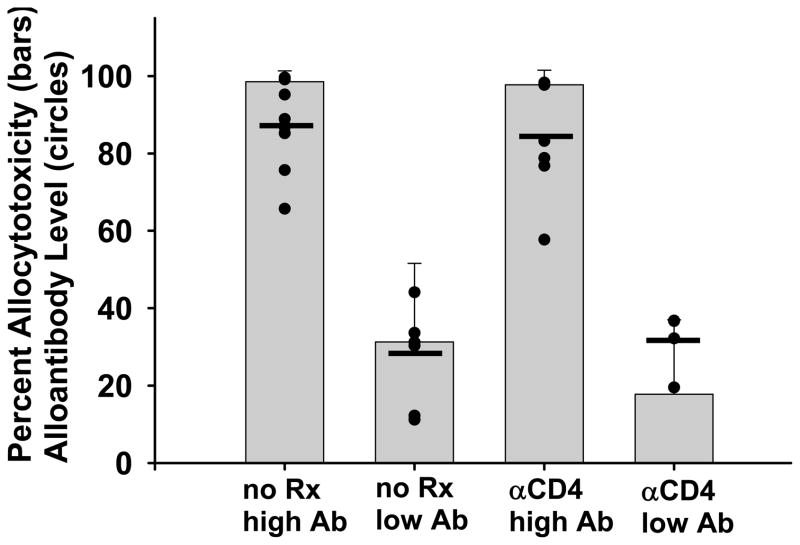
In previous studies we reported that CD4-dependent rejection in CD8 KO recipients is accompanied by a variable level of alloantibody production (22). In the current studies we also detected a variable level of alloantibody in CD8 KO hepatocyte rejectors (Figure 1). In order to determine the correlation between recipient serum alloantibody and the magnitude of in vivo allocytotoxicity, we measured the serum alloantibody levels (day 14 post transplant) and in vivo cytotoxic activity in CD8 KO hepatocyte rejectors (1 week post-rejection). A group of these CD8 KO rejector recipients were depleted of CD4+ T cells 48 hours prior to the cytotoxicity assay. Figure 2 shows that CD8 KO hepatocyte rejectors (with or without CD4+ T cell depletion) could be separated into groups with “high” (87±12%) or “low” (28±11%) serum alloantibody levels (p<0.0001). Alloantibody levels were consistent between days 14 and 28 within individual mice (not shown). Recipients with “high” serum alloantibody levels had significantly higher magnitude of cytotoxicity (98±3% for both untreated and CD4-depleted groups) than recipients with “low” serum alloantibody levels (26±20% allocytotoxicity for both untreated and CD4-depleted groups) as shown in Figure 2 (p<0.0001). Thus in vivo allospecific cytotoxic effector function in CD8 KO hepatocyte rejectors is not mediated by CD4+ T cells or NK cells but strongly correlates with recipient alloantibody level, supporting a causal relationship between antibody and in vivo cytotoxic effector function.
Figure 2. Recipient serum alloantibody levels correlate with the magnitude of in vivo allospecific cytotoxic effector function in CD8 KO hepatocyte rejectors.
FVB/N (H-2q) hepatocytes were transplanted into untreated (no Rx) CD8 KO (H-2b) mice. After graft rejection occurred (MST = 14 days), CD8 KO hosts were assessed for both alloantibody and in vivo cytotoxic effector function post rejection. Hepatocyte rejector mice were divided into groups with “high” (>60%, n=10) or “low” (<60%, n=6) serum alloantibody levels. Some hepatocyte rejectors with “high” (n=8) or “low” (n=4) alloantibody levels were also depleted of CD4+ T cells (αCD4) prior to the in vivo cytotoxicity assay to eliminate the potential contribution of CD4+ T cells to allocytotoxicity. Serum alloantibody levels in “high” compared to “low” alloantibody groups were statistically different (p<0.0001), and are represented by the circles with the black lines indicating the averages. The average cytotoxicity ± standard deviation for hepatocyte rejector mice with “high” or “low” serum alloantibody levels (with or without recipient CD4+ T cell depletion) is represented by the bars. Hosts with high levels of alloantibody demonstrated significantly higher levels of cytotoxicity than hosts with low antibody levels (p<0.0001 for undepleted as well as CD4-depleted groups).
In vivo cytotoxic effector function in CD8 KO rejector mice does not require complement
Activation of the classical complement cascade through cross-linking antibodies on the surface of a target cell can result in lysis of the target cell through formation of the membrane attack complex. This potential mechanism in addition to evidence that complement is involved in antibody-mediated rejection in other experimental models (17, 33–39) prompted our investigation of whether complement is critical to the in vivo cytotoxic effector mechanism observed in CD8 KO hepatocyte rejector mice. Early and/or late phases of the classical complement cascade could contribute to target cell destruction through opsonization or direct lysis of allogeneic target cells, respectively, therefore cobra venom factor was chosen as a reagent to physiologically exhaust functional complement in recipient hosts [reviewed in (40)]. Hepatocellular allografts were transplanted into CD8 KO hosts without immunosuppression. Alloantibody was assayed in CD8 KO rejector mice and only recipients with “high” serum alloantibody levels (>40% binding of target splenocytes) previously shown to correlate with maximal in vivo cytotoxic effector function (Figure 2) were utilized for subsequent experimentation. Following graft rejection, hosts were depleted of CD4+ T cells to focus on alloantibody-mediated immunity and treated with cobra venom factor (CVF) 24–36 hours prior to the cytotoxicity assay. Complement depleted hosts (n=6) did not show any loss of in vivo allocytotoxicity in comparison to hosts not treated with CVF (n=10; p=ns; Figure 3). The two host groups had similar high levels of alloantibody (p=ns). Therefore, complement activity is not required to effect the observed high magnitude in vivo cytotoxic effector function which is generated in CD8 KO rejector mice.
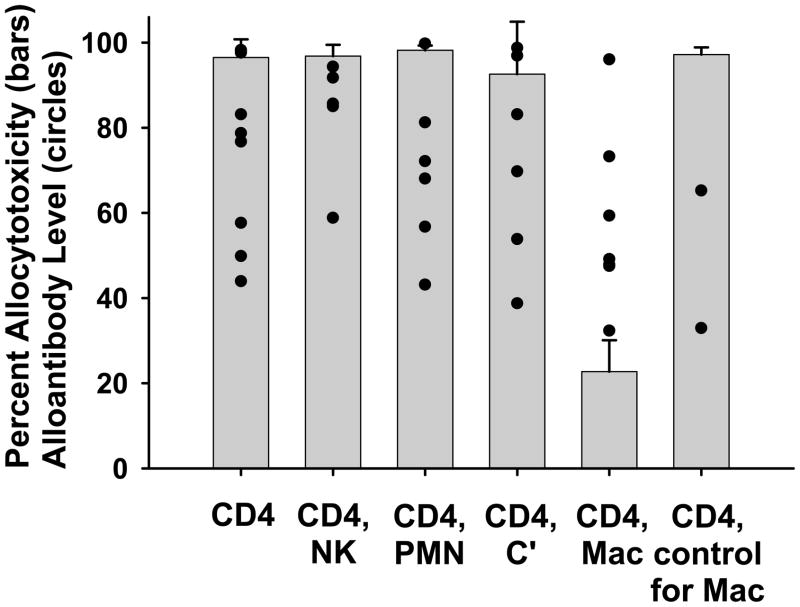
Figure 3. In vivo allospecific cytotoxic effector function generated in CD8 KO rejector mice is mediated by macrophages.
FVB/N (H-2q) hepatocytes were transplanted into untreated CD8 KO (H-2b) recipients. Following graft rejection, hosts were depleted of CD4+ T cells alone (n=10) or CD4+ T cells in addition to NK cells (NK, n=5), neutrophils (PMN, n=6), macrophages (Mac, n=7), or complement (C′, n=6). In vivo cytotoxicity was assessed within 48 hours of host treatment. The average ± standard deviation of allocytotoxicity for each treatment group is shown by the bars. Serum alloantibody level for recipients within the treatment groups is represented by the circles. Alloantibody levels were similar between treatment groups (p=ns). Depletion of host macrophages significantly reduces in vivo allocytotoxicity in comparison to all other groups (p<0.00001). Treatment of a separate cohort with PBS liposomes as a vehicle control for liposome clodronate did not interfere with cytotoxic activity (n=2).
Host macrophages (but not NK cells or neutrophils) mediate in vivo allospecific cytotoxic effector function in CD8 KO rejector mice
Innate immune cells can contribute to antibody-bound target cell destruction by binding alloantibody through their Fc receptors and triggering cytotoxicity [reviewed in (41)]. To determine if ADCC by innate immune cells is involved in the allocytotoxicity observed in CD8 KO rejector mice, CD8 KO recipients of FVB/N allogeneic hepatocytes were monitored for graft rejection and alloantibody production. Following rejection, the hosts were depleted of CD4+ T cells (GK1.5) and neutrophils (RB6-8C5), NK cells (PK136), or macrophages (liposome enclosed clodronate), 48 hours prior to the in vivo cytotoxicity assay. As shown in Figure 3, in vivo allocytotoxicity was not influenced by the depletion of CD4+ T cells alone (n=10) or CD4+ T cells in conjunction with neutrophils (n=6) or NK cells (n=5). In contrast, in the absence of macrophages (n=7), all hosts showed significantly decreased allocytotoxicity (p<0.0001), despite similar levels of alloantibody (p=ns between groups). PBS filled liposomes were used as a control for the liposome treatment, and did not deplete host macrophages or reduce the cytotoxic activity of CD8 KO rejector mice (p=ns, PBS liposome treated hosts compared to untreated hosts). Collectively, these data support the conclusion that humoral immune damage of transplanted liver parenchymal cells in CD8 KO hepatocyte rejector mice occurs by an ADCC mechanism mediated by recipient macrophages. We therefore hypothesized that CD8-deficient recipients with genetically impaired macrophage populations or which were macrophage depleted would exhibit prolonged hepatocellular allograft survival.
Host macrophages are required to effect rapid CD4-dependent, alloantibody-mediated hepatocyte rejection
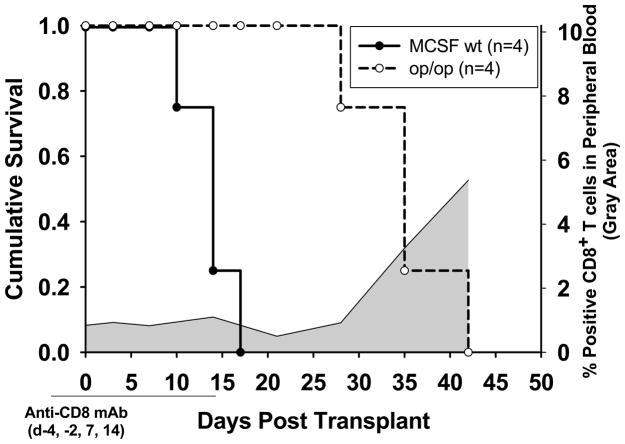
To determine if macrophages are necessary for CD4-dependent, alloantibody-mediated allogeneic hepatocyte rejection, FVB/N hepatocytes (H-2q) were transplanted into op/op recipient mice (H-2b), which are macrophage “deficient” due to the genetic absence of a macrophage growth and differentiation factor (macrophage colony stimulating factor, MCSF). This genetic defect is known to result in a general reduction of resident macrophages, including in the spleen and liver (42). These recipients were transiently depleted of CD8+ T cells to focus on the CD4-dependent, antibody-mediated rejection pathway. MCSF+/+ littermates depleted of CD8+ T cells were used as controls, and graft survival was compared between the groups (Figure 4). The CD8-depleted MCSF+/+ hosts rejected hepatocyte allografts with a median survival time of 14 days (n=4), which is equivalent to the rejection kinetics in untreated CD8 KO hosts (shown in Figure 5 as ‘no Treatment’; p=ns). However, transiently CD8-depleted macrophage “deficient” op/op (MCSF−/−) hosts demonstrated delayed rejection of hepatocellular allografts with MST of 35 days (n=4; p=0.007). Rejection at this time point coincided with reconstitution of CD8+ T cells in the periphery (Figure 4). The op/op hosts generated similar levels of alloantibody as the MCSF+/+ control recipients (60±12% in op/op vs. 65±36% in MCSF+/+; p=ns), suggesting that host macrophage “deficiency” did not affect priming of alloantibody production after hepatocyte transplant. Rather, host macrophages appear important to the efferent phase of CD4-dependent, antibody-mediated hepatocyte rejection.
Figure 4. Hepatocyte allograft rejection in CD8-depleted recipients is significantly delayed in macrophage-deficient hosts.
FVB/N (H-2q) hepatocytes were transplanted into CD8+ T cell depleted (day −4, day −2, day 7, day 14) op/op M-CSF deficient hosts, (which have generally reduced numbers of host macrophages including in the liver and spleen), or into CD8+ T cell depleted M-CSF wild type (wt) littermate controls (both H-2b). CD8-depleted M-CSF wt controls (n=4) rejected hepatocyte allografts with median survival time of 14 days, similar to CD8 KO hosts (p=ns). CD8-depleted, macrophage-deficient op/op hosts (n=4) rejected hepatocyte allografts with MST of 35 days (p=0.007). Flow cytometric analysis of peripheral blood lymphocytes was performed to monitor circulating CD8+ T cells in recipient mice. CD8+ T cell reconstitution in transiently CD8-depleted recipient mice is shown by the gray shaded area.
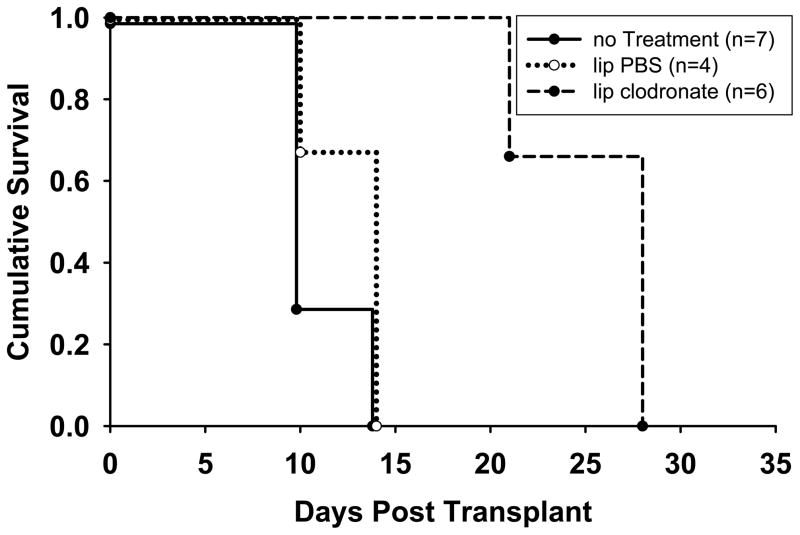
Figure 5. Depletion of host macrophages during the efferent immune phase significantly delays rejection in CD8 KO recipients.
FVB/N (H-2q) hepatocytes were transplanted into CD8 KO (H-2b) recipients. Recipients were depleted of macrophages (liposome clodronate) or treated with control liposome enclosed PBS on days 5, 9, 13, 17, 21 post hepatocyte transplant. A third group of CD8 KO hosts remained untreated. Hepatocyte allografts were rejected with MST of 10 days in untreated hosts and 14 days in control PBS liposome treated hosts (p=ns). Macrophage depletion during the effector phase of CD4-dependent, alloantibody-mediated graft rejection significantly prolonged hepatocyte allograft survival (MST = 25 days, p< .001 relative to untreated hosts, p=0.003 relative to control liposome PBS treated hosts).
An additional experimental approach to focus on the role of macrophages during the effector arm of CD4-dependent antibody-mediated hepatocyte rejection was utilized. CD8 KO recipients were transplanted with allogeneic FVB/N hepatocytes and rejection permitted to ensue for 5 days post transplant. Then host macrophages were depleted from untreated CD8 KO recipients during the efferent immune phase with clodronate liposomes, a macrophage depletion treatment technology developed by Van Rooijen and Sanders (28) which is reported to be effective for approximately 5–6 days per treatment. The effect of host macrophage depletion in CD8 KO recipients by treatment with clodronate liposomes was compared to CD8 KO recipients treated with control PBS liposomes. Serial host macrophage depletion beginning day 5 post transplant did not affect the level of alloantibody production compared to control liposome treated hosts or untreated hosts (alloantibody level: 68±24% in liposome clodronate treated hosts, n=6; 62±39% in PBS liposome treated hosts, n=4; 61±26% in untreated CD8 KO hosts, n=7). Hepatocyte allograft rejection was similar in untreated hosts (MST = 10 days; n=7) and control PBS liposome treated hosts (MST = 14 days; n=4; p=ns). In contrast, in hosts depleted of macrophages, hepatocellular allograft survival was prolonged with MST of 25 days (n=6; p<0.001 compared to untreated hosts, p=0.003 compared to liposome clodronate treated hosts; Figure 5).
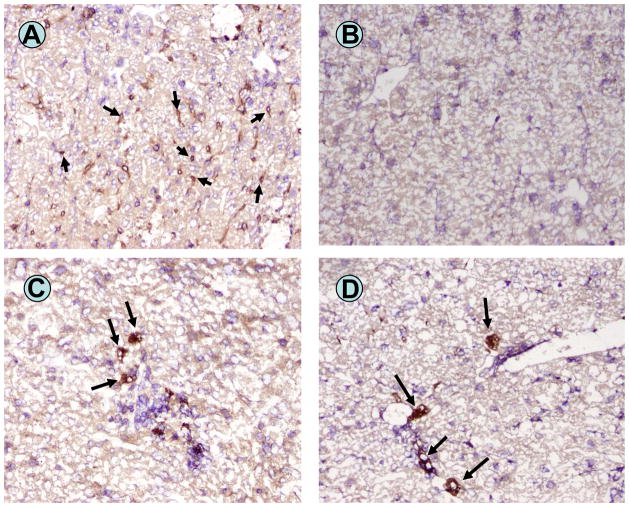
Immunohistochemistry of recipient livers (day 8–9 post transplant) confirmed the depletion of host macrophages (F4/80+ cells) in hepatocyte recipients treated with liposome clodronate (Figure 6A and 6B). Donor hepatocytes, detected by staining for the transgenic reporter product hA1AT, were present in the recipient liver in both macrophage-replete and macrophage-depleted hosts (Figure 6C and 6D). No evidence for C4d staining of recipient liver tissue was noted (not shown). Cumulative histologic assessment of successive sections of the host livers on day 8–9 post transplant showed, even at this early time point following macrophage depletion, more abundant donor hepatocytes in the macrophage-depleted hosts in comparison to the undepleted hosts, concordant with the extended graft survival in macrophage-depleted hosts. In macrophage-replete (untreated) mouse liver sections 44±13.7 donor hepatocytes were detected (20× high power field) while significantly more donor (hA1AT-positive) hepatocytes were found in liver sections from macrophage-depleted mice (91±13.2 hepatocytes; p=0.0032).
Figure 6. Liver immunohistochemical staining in liposome clodronate treated CD8 KO recipients.
Immunohistochemical staining for F4/80+ (macrophage marker) cells (arrows) in the liver of an untreated CD8 KO recipient nine days post FVB/N hepatocyte transplantation (A). Staining for F4/80+ cells in the liver of liposome clodronate treated hosts eight days following hepatocyte transplantation shows the complete absence of liver macrophages (B). The presence of donor hepatocytes engrafted within the recipient liver parenchyma (arrows) are detectable in untreated CD8 KO recipients (C), as well as in liposome clodronate treated (macrophage depleted) hosts (D) on days 8–9 post transplant. Serial section analysis revealed more clusters of donor hepatocytes present in the livers of macrophage-depleted recipients in comparison to untreated recipients.
Alloantibody and macrophages together effect in vitro hepatocellular cytotoxicity
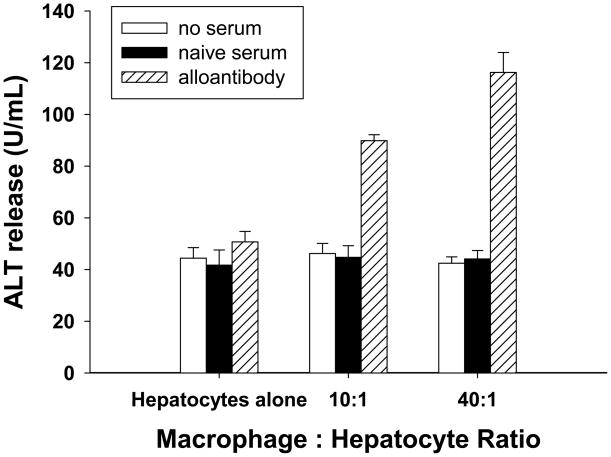
To further explore the role of macrophages in humoral immune damage of allogeneic hepatocytes, hepatocytotoxicity was evaluated using an in vitro cellular cytotoxicity assay. Alloantibody in serum from CD8 KO (H-2b) mice was collected 14 days after allogeneic (FVB/N, H-2q) hepatocyte transplantation. Hepatocytes (FVB/N) were cultured with alloantibody, serum from untransplanted (H-2b) mice, or left untreated (37°C, 30 minutes). Following incubation with antibody, hepatocytes were plated into culture dishes and cultured alone or co-cultured with macrophages for 8 hours. Macrophages (H-2b) were added to the appropriate wells with hepatocytes at specific effector to target ratios (E:T = 10:1 or 40:1). ALT release in the culture supernatant was measured as a reflection of hepatocellular injury. As a positive control, hepatocytes were mechanically disrupted in de-ionized H2O which resulted in a “maximum” ALT release of 202.0±9.5 U/mL (data not shown). Hepatocytes that were cultured alone and hepatocytes cultured with serum containing alloantibody, showed similar baseline ALT release (50.7±4.1 U/mL vs. 44.4±4.1 U/mL (Figure 7). Untreated hepatocytes that were co-cultured with macrophages (E:T = 10:1 and 40:1) also showed similar baseline ALT release (41.7±5.9 U/mL, 44.7±4.5 U/mL, respectively) when compared to the untreated control. However, hepatocytes that were treated with serum containing alloantibody and co-cultured with macrophages (E:T = 10:1 and 40:1) exhibited a significant increase in ALT release (89.8±2.3 U/mL, 116.2±7.7 U/mL, respectively; p<0.0001 for both E:T ratios) compared to untreated hepatocytes or hepatocytes cultured with alloantibody or macrophages alone.
Figure 7. Alloantibody and macrophages effect in vitro hepatocellular cytotoxicity.
The role of macrophages in effecting antibody-dependent immune injury to hepatocytes was examined using an in vitro hepatocytotoxicity assay based on the release of intracellular ALT into the culture supernatant. Purified FVB/N (H-2q) mouse hepatocytes which were untreated or incubated with serum from untransplanted (naïve) mice (H-2b) or serum containing alloantibody (from CD8 KO, H-2b, hepatocyte rejectors), were subsequently co-cultured with macrophages (H-2b) at various effector to target ratios (E:T = hepatocytes alone, 10:1, or 40:1). Hepatocytotoxicity was measured by supernatant quantification of ALT release from injured hepatocytes. Hepatocytes that were treated with alloantibody and co-cultured with macrophages (E:T = 10:1 and 40:1) exhibited a significant increase in ALT release (p<0.0001 for both E:T ratios; “*” indicates significance) compared to hepatocytes untreated or treated with serum from untransplanted (naïve) mice as well as hepatocytes incubated with alloantibody or macrophages alone. Error bars denote the standard deviations of triplicate wells. The data in this figure is representative of three experiments.
Discussion
Both clinical and experimental studies highlight the barrier that acute antibody-mediated allograft rejection (AAMR) poses to successful allograft survival [reviewed in (1, 25, 43–46)]. Acute antibody-mediated rejection occurs despite the use of maintenance immunosuppression to prevent rejection and is associated with worse graft outcome than T cell-mediated rejection (47). The detection of C4d complement split product in graft tissue is an integral component of the recently established standards used to diagnose AAMR in kidney and cardiac allograft recipients (48). Complement deposition is usually detected perivascularly, supporting the inference that antibody and complement primarily target graft endothelial cells with subsequent ischemic graft damage [reviewed in (1, 21, 44, 48)]. The current studies which focus on humoral immune damage of transplanted allogeneic liver parenchymal cells add an additional dimension to existing studies which focus on donor endothelial cell damage.
Our studies employed a well-characterized hepatocellular allograft model that is known to initiate brisk alloantibody production and CD4-dependent rejection in the absence of CD8+ T cells (26). Alloantibody is sufficient to mediate acute rejection of hepatocellular allografts engrafted in the liver in the absence of host T or B cells and without nonspecific tissue or vascular injury to the native liver (22). In the current studies, CD4-dependent (CD8-independent) rejection of allogeneic liver parenchymal cells in CD8 KO recipients was accompanied by alloantibody production and the development of high magnitude in vivo cytotoxic effector activity which is not mediated by CD4+ T cells or NK cells. In vivo cytotoxic effector activity is highly correlated with graft rejection in this transplant model since rejection is always accompanied by the detection of in vivo allocytotoxicity and in vivo allocytotoxicity is always absent with graft acceptance (induced by effective immunotherapy) (29). Since in vivo allocytotoxicity in CD8 KO rejector mice is not mediated by CD4+ T cells, it is likely that the allospecificity of the observed cytotoxic effector function is attributable to serum alloantibody. The observation that recipient serum alloantibody levels correlated with the magnitude of in vivo allospecific cytotoxic activity is consistent with this conclusion. Furthermore, we have previously reported that alloantibodies in rejector mice bind to allogeneic but not third party or syngeneic hepatocytes by flow cytometric analysis (22). Additionally the observed in vivo cytotoxicity is allospecific since differential target cell clearance is only observed when allogeneic and syngeneic targets are injected into recipient mice. Rejector mice do not differentially clear third party targets when co-injected with syngeneic targets (32).
Different mechanisms of antibody-mediated graft endothelial cell injury have been described. These include both complement-dependent (MAC lysis, recruitment of innate inflammatory cells, and opsonization by complement proteins with subsequent phagocytosis), and complement-independent cytotoxic mechanisms (ADCC) (21). Our studies did not support a complement-dependent cytotoxic mechanism of allogeneic parenchymal cell damage since complement depleted hosts retained high cytotoxic effector function. Rather, our studies are consistent with a paradigm of acute humoral rejection by an ADCC mechanism in which CD4+ T cell-dependent alloantibody production results in targeting of transplanted allogeneic parenchymal cells for macrophage-mediated cytotoxic immune damage. This paradigm is supported by the observation that only depletion of host macrophages (and not NK cells or neutrophils) in CD8 KO rejector mice markedly reduces in vivo cytotoxic effector function. The remaining low level in vivo allocytotoxicity could be attributed to incomplete macrophage depletion or to the activity of CD4−CD8− TCR+ killer cells and/or eosinophils in CD8 KO mice. Furthermore, genetic “deficiency” of recipient macrophages in op/op hosts, or transient depletion of host macrophages with liposome clodronate following the onset of CD4-dependent allograft rejection in CD8 KO recipients resulted in significant prolongation of allograft survival in comparison to macrophage replete controls. Prolongation of graft survival in macrophage “deficient” recipients could not be attributed to differences in serum alloantibody since alloantibody levels were equivalent in both macrophage “deficient” and macrophage replete hosts. The data also demonstrates that host macrophages were critical during the effector phase of rejection in CD8 KO hosts since transient macrophage depletion after the onset of rejection effectively delayed rejection. Furthermore, macrophages are sufficient to effect alloantibody-dependent hepatocyte cytotoxicity in vitro. Resident liver Kupffer cells are presumably a primary macrophage population involved in ADCC-associated humoral rejection in this model since hepatocytes are transplanted to the liver. Human Kupffer cells have been shown to express FcγRI in areas of liver inflammation, as well as FcγRII and FcγRIII, all of which participate in ADCC (49, 50). The donor-directed antibody developing in response to hepatocellular allografts is polyclonal, developing high titers of IgG1, IgG2a, IgG2b, and IgG3 isotypes (Horne PH, unpublished observation); consequently, all Fc receptor types may be relevant. However, if further experimentation revealed that one receptor was used predominantly, therapy targeting the individual receptor could be developed to avoid systemic macrophage depletion.
The role of ADCC in allograft rejection in humans has been proposed, but limited clinical data is available to support this as an effector mechanism during AAMR [reviewed in (21, 44)]. Many clinical and experimental studies of AAMR after cardiac (14, 15, 17), renal (18, 51), and liver (8, 52) transplantation have been notable for increased macrophage infiltration in concert with C4d staining in the graft tissue. However in these studies the role of macrophages as effectors of acute humoral rejection was not examined. To our knowledge the current report is the first to demonstrate a role of macrophages as effectors of CD4/alloantibody-mediated in vivo cytotoxicity and acute rejection of allogeneic parenchymal cells. Results of the current studies have direct implications for humoral immune damage of clinical cellular allografts, such as hepatocyte, bone marrow, or islet allografts; alloantibody in sensitized bone marrow or islet allograft human recipients is a known barrier for successful cellular engraftment (23, 25). These results also suggest that the macrophage infiltration noted in solid organ allografts during antibody-mediated rejection may be active participants in the humoral rejection process.
Although the current studies did not show a role for complement for in vivo cytotoxic effector function or evidence of C3d staining during hepatocyte allograft rejection, allograft rejection is a complex process requiring recruitment of inflammatory cells to the graft site, recognition of target cells by host immune cells, and local activation of effector mechanisms. Complement can contribute to macrophage-dependent infiltration of the graft tissue, as has been reported in clinical [reviewed in (11, 43, 53)] and experimental studies (34, 54). Experimental studies have also demonstrated a role for complement in the pathogenicity of alloantibody for acute rejection of cardiac allografts (55). Another study demonstrated that recipient macrophages provide an important source of terminal complement protein C6, which is necessary for MAC formation and humoral rejection of cardiac allografts (56). Thus, complement may be important for recruitment of macrophages, alloantibody pathogenicity and/or have a role as a macrophage effector product. Perhaps in solid organ allografts, which possess donor-type vasculature, complement activation on donor endothelial cells with membrane attack complex formation results in damage to the graft blood vessels with subsequent inflammatory cell and alloantibody influx into the parenchyma. Once the vascular bed is compromised, macrophages can more readily access the parenchyma, and together with native tissue macrophages exert complement-independent humoral immune injury (ADCC) to graft parenchymal cells. In allograft tissues such as the liver, which possesses fenestrated endothelial barriers, damage to the graft endothelium may not be necessary to allow antibody access to graft parenchyma. In this situation, immune damage to engrafted allogeneic parenchymal cells could occur in the absence of endothelial damage and complement activity.
In conclusion, acute humoral rejection of organ allografts is marked in severe cases by vasculitis, thrombosis, and hemorrhage. In the absence of these advanced hallmark histologic characteristics, the presence of C4d in concert with relevant clinical indicators is diagnostic for antibody-mediated rejection. The current studies support a novel paradigm of humoral immune injury to allogeneic parenchymal cells, which occurs through a macrophage-mediated ADCC mechanism. Depletion or impairment of recipient macrophages significantly reduced in vivo allocytotoxicity and enhanced hepatocellular allograft survival in two models, suggesting a promising therapeutic target for CD4-dependent, antibody-mediated rejection of graft parenchymal cells. Targeting macrophage-mediated mechanisms of graft parenchymal destruction could afford time to attempt more permanent therapies to inhibit alloantibody production.
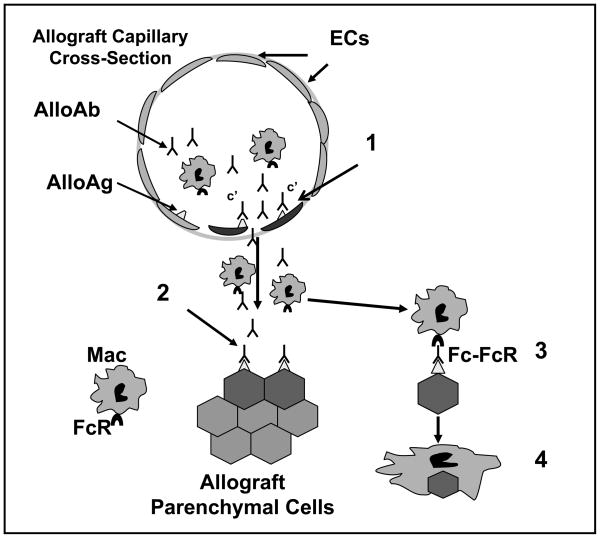
Figure 8. Proposed paradigm for antibody-mediated rejection of parenchymal cells in solid organ allografts.
Circulating alloantibody (AlloAb) binds alloantigen (AlloAg), presumably but not limited to donor MHC molecules, on donor vascular endothelial cells (ECs). Activation of the complement cascade results in injury to capillary endothelial cells with subsequent efflux of alloantibody and recruited inflammatory cells (1). Within the surrounding graft parenchymal tissue, alloantibody binding to MHC alloantigen (and/or non MHC antigens) on parenchymal cells allows for specific humoral targeting of donor cells (2). Fc receptor (FcR) interaction of recruited and resident macrophages (Mac) with antibody bound allogeneic parenchymal cells (3) initiates macrophage-mediated antibody-dependent cellular cytotoxicity and destruction of graft parenchyma (4).
Acknowledgments
The authors would like to thank Dr. Gregg Hadley (OSU Comprehensive Transplant Center) for critical review of the manuscript and helpful comments and Lori Fiessinger for assistance with manuscript preparation.
Grant Support: This work was supported in part by grants from the American Society of Transplantation Basic Science Physician Scientist Award, Roche Organ Transplantation Research Foundation, the American Society of Transplant Surgeons, and NIH DK072262.
Footnotes
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology(The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyrightto this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI(online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
References
- 1.Terasaki PI, Cai J. Humoral theory of transplantation: further evidence. Curr Opin Immunol. 2005;17:541–545. doi: 10.1016/j.coi.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Koo DD, I, Roberts S, Quiroga I, Procter J, Barnardo MC, Sutton M, Cerundolo L, Davies DR, Friend PJ, Morris PJ, Fuggle SV. C4d deposition in early renal allograft protocol biopsies. Transplantation. 2004;78:398–403. doi: 10.1097/01.tp.0000128328.68106.54. [DOI] [PubMed] [Google Scholar]
- 3.Poduval RD, Kadambi PV, Josephson MA, Cohn RA, Harland RC, Javaid B, Huo D, Manaligod JR, Thistlethwaite JR, Meehan SM. Implications of immunohistochemical detection of C4d along peritubular capillaries in late acute renal allograft rejection. Transplantation. 2005;79:228–235. doi: 10.1097/01.tp.0000148987.13199.10. [DOI] [PubMed] [Google Scholar]
- 4.Bohmig GA, Exner M, Habicht A, Schillinger M, Lang U, Kletzmayr J, Saemann MD, Horl WH, Watschinger B, Regele H. Capillary C4d deposition in kidney allografts: a specific marker of alloantibody-dependent graft injury. J Am Soc Nephrol. 2002;13:1091–1099. doi: 10.1681/ASN.V1341091. [DOI] [PubMed] [Google Scholar]
- 5.Vargha R, Mueller T, Arbeiter K, Regele H, Exner M, Csaicsich D, Aufricht C. C4d in pediatric renal allograft biopsies: a marker for negative outcome in steroid-resistant rejection. Pediatr Transplant. 2006;10:449–453. doi: 10.1111/j.1399-3046.2006.00492.x. [DOI] [PubMed] [Google Scholar]
- 6.Girnita AL, Duquesnoy R, Yousem SA, Iacono AT, Corcoran TE, Buzoianu M, Johnson B, Spichty KJ, Dauber JH, Burckart G, Griffith BP, McCurry KR, Zeevi A. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant. 2005;5:131–138. doi: 10.1111/j.1600-6143.2004.00650.x. [DOI] [PubMed] [Google Scholar]
- 7.Magro CM, Abbas AE, Seilstad K, Pope-Harman AL, Nadasdy T, Ross P., Jr C3d and the septal microvasculature as a predictor of chronic lung allograft dysfunction. Hum Immunol. 2006;67:274–283. doi: 10.1016/j.humimm.2005.11.001. Epub 2006 Mar 2031. [DOI] [PubMed] [Google Scholar]
- 8.Sawada T, Shimizu A, Kubota K, Fuchinoue S, Teraoka S. Lobular damage caused by cellular and humoral immunity in liver allograft rejection. Clin Transplant. 2005;19:110–114. doi: 10.1111/j.1399-0012.2004.00310.x. [DOI] [PubMed] [Google Scholar]
- 9.Schmeding M, Dankof A, Krenn V, Krukemeyer MG, Koch M, Spinelli A, Langrehr JM, Neumann UP, Neuhaus P. C4d in acute rejection after liver transplantation--a valuable tool in differential diagnosis to hepatitis C recurrence. Am J Transplant. 2006;6:523–530. doi: 10.1111/j.1600-6143.2005.01180.x. [DOI] [PubMed] [Google Scholar]
- 10.Lorho R, Turlin B, Aqodad N, Triki N, de Lajarte-Thirouard AS, Camus C, Lakehal M, Compagnon P, Dupont-Bierre E, Meunier B, Boudjema K, Messner M. C4d: a marker for hepatic transplant rejection. Transplant Proc. 2006;38:2333–2334. doi: 10.1016/j.transproceed.2006.06.120. [DOI] [PubMed] [Google Scholar]
- 11.Abrams J, Amir O, Etheridge WB, Frazier OH. Histologic findings proving the existence of humoral rejection in a cardiac allograft. Cardiovasc Pathol. 2007;16:38–42. doi: 10.1016/j.carpath.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Vasilescu ER, Ho EK, de la Torre L, Itescu S, Marboe C, Cortesini R, Suciu-Foca N, Mancini D. Anti-HLA antibodies in heart transplantation. Transpl Immunol. 2004;12:177–183. doi: 10.1016/j.trim.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Smith RN, Brousaides N, Grazette L, Saidman S, Semigran M, Disalvo T, Madsen J, Dec GW, Perez-Atayde AR, Collins AB. C4d deposition in cardiac allografts correlates with alloantibody. J Heart Lung Transplant. 2005;24:1202–1210. doi: 10.1016/j.healun.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Ratliff NB, McMahon JT. Activation of intravascular macrophages within myocardial small vessels is a feature of acute vascular rejection in human heart transplants. J Heart Lung Transplant. 1995;14:338–345. [PubMed] [Google Scholar]
- 15.Lones MA, Czer LS, Trento A, Harasty D, Miller JM, Fishbein MC. Clinical-pathologic features of humoral rejection in cardiac allografts: a study in 81 consecutive patients. J Heart Lung Transplant. 1995;14:151–162. [PubMed] [Google Scholar]
- 16.Michaels PJ, Espejo ML, Kobashigawa J, Alejos JC, Burch C, Takemoto S, Reed EF, Fishbein MC. Humoral rejection in cardiac transplantation: risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. J Heart Lung Transplant. 2003;22:58–69. doi: 10.1016/s1053-2498(02)00472-2. [DOI] [PubMed] [Google Scholar]
- 17.Qian Z, Lee CY, Murata K, Liu J, Fox-Talbot K, Wasowska BA, Baldwin WM., 3rd Antibody and complement-mediated injury in transplants following sensitization by allogeneic blood transfusion. Transplantation. 2006;82:857–864. doi: 10.1097/01.tp.0000232335.06792.35. [DOI] [PubMed] [Google Scholar]
- 18.Magil AB, Tinckam K. Monocytes and peritubular capillary C4d deposition in acute renal allograft rejection. Kidney Int. 2003;63:1888–1893. doi: 10.1046/j.1523-1755.2003.00921.x. [DOI] [PubMed] [Google Scholar]
- 19.Mauiyyedi S, Crespo M, Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Tolkoff-Rubin NE, Williams WW, Delmonico FL, Cosimi AB, Colvin RB. Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol. 2002;13:779–787. doi: 10.1681/ASN.V133779. [DOI] [PubMed] [Google Scholar]
- 20.Trpkov K, Campbell P, Pazderka F, Cockfield S, Solez K, Halloran PF. Pathologic features of acute renal allograft rejection associated with donor-specific antibody, analysis using the Banff grading schema. Transplantation. 1996;61:1586–1592. doi: 10.1097/00007890-199606150-00007. [DOI] [PubMed] [Google Scholar]
- 21.Cai J, Terasaki PI. Humoral theory of transplantation: mechanism, prevention, and treatment. Hum Immunol. 2005;66:334–342. doi: 10.1016/j.humimm.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Horne PH, Lunsford KE, Eiring AM, Wang Y, Gao D, Bumgardner GL. CD4+ T-cell-dependent immune damage of liver parenchymal cells is mediated by alloantibody. Transplantation. 2005;80:514–521. doi: 10.1097/01.tp.0000168342.57948.68. [DOI] [PubMed] [Google Scholar]
- 23.Mohanakumar T, Narayanan K, Desai N, Ramachandran S, Shenoy S, Jendrisak M, Susskind BM, Olack B, Benshoff N, Phelan DL, Brennan DC, Fernandez LA, Odorico JS, Polonsky KS. A significant role for histocompatibility in human islet transplantation. Transplantation. 2006;82:180–187. doi: 10.1097/01.tp.0000226161.82581.b2. [DOI] [PubMed] [Google Scholar]
- 24.Rickels MR, Kamoun M, Kearns J, Markmann JF, Naji A. Evidence for allograft rejection in an islet transplant recipient and effect on beta-cell secretory capacity. J Clin Endocrinol Metab. 2007;92:2410–2414. doi: 10.1210/jc.2007-0172. Epub 2007 May 2418. [DOI] [PubMed] [Google Scholar]
- 25.Xu H, Chilton PM, Tanner MK, Huang Y, Schanie CL, Dy-Liacco M, Yan J, Ildstad ST. Humoral immunity is the dominant barrier for allogeneic bone marrow engraftment in sensitized recipients. Blood. 2006;108:3611–3619. doi: 10.1182/blood-2006-04-017467. Epub 2006 Aug 3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bumgardner GL, Gao D, Li J, Baskin J, Heininger M, Orosz CG. Rejection responses to allogeneic hepatocytes by reconstituted SCID mice, CD4 KO, and CD8 KO mice. Transplantation. 2000;70:1771–1780. doi: 10.1097/00007890-200012270-00017. [DOI] [PubMed] [Google Scholar]
- 27.Bumgardner GL, Heininger M, Li J, Xia D, Parker-Thornburg J, Ferguson RM, Orosz CG. A functional model of hepatocyte transplantation for in vivo immunologic studies. Transplantation. 1998;65:53–61. doi: 10.1097/00007890-199801150-00011. [DOI] [PubMed] [Google Scholar]
- 28.Van Rooijen N, Sanders A. Liposome-mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 29.Lunsford KE, Koester MA, Eiring AM, Gao D, Horne PH, Bumgardner GL. Targeting LFA-1 and CD154 suppresses the in vivo activation and development of cytolytic (CD4-independent) CD8+ T cells. J Immunol. 2005;175:7855–7866. doi: 10.4049/jimmunol.175.12.7855. [DOI] [PubMed] [Google Scholar]
- 30.Luo Y, Yamada H, Evanoff DP, Chen X. Role of Th1-stimulating cytokines in bacillus Calmette-Guerin (BCG)-induced macrophage cytotoxicity against mouse bladder cancer MBT-2 cells. Clin Exp Immunol. 2006;146:181–188. doi: 10.1111/j.1365-2249.2006.03191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kean LS, Hamby K, Koehn B, Lee E, Coley S, Stempora L, Adams AB, Heiss E, Pearson TC, Larsen CP. NK cells mediate costimulation blockade-resistant rejection of allogeneic stem cells during nonmyeloablative transplantation. Am J Transplant. 2006;6:292–304. doi: 10.1111/j.1600-6143.2005.01172.x. [DOI] [PubMed] [Google Scholar]
- 32.Horne PH, Koester MA, Jayashankar K, Lunsford KE, Dziema HL, Bumgardner GL. Disparate primary and secondary allospecific CD8+ T cell cytolytic effector function in the presence or absence of host CD4+ T cells. J Immunol. 2007;179:80–88. doi: 10.4049/jimmunol.179.1.80. [DOI] [PubMed] [Google Scholar]
- 33.Amano H, Bickerstaff A, Orosz CG, Novick AC, Toma H, Fairchild RL. Absence of recipient CCR5 promotes early and increased allospecific antibody responses to cardiac allografts. J Immunol. 2005;174:6499–6508. doi: 10.4049/jimmunol.174.10.6499. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Jiang J, Liu W, Kubelik D, Chen G, Gies D, Garcia B, Zhong R, Rother RP. Prevention of acute vascular rejection by a functionally blocking anti-C5 monoclonal antibody combined with cyclosporine. Transplantation. 2005;79:1121–1127. doi: 10.1097/01.tp.0000161218.58276.9a. [DOI] [PubMed] [Google Scholar]
- 35.Li R, Chen G, Guo H, Wang DW, Xie L, Wang SS, Wang WY, Xiong YL, Chen S. Prolonged cardiac allograft survival in presensitized rats after a high activity Yunnan-cobra venom factor therapy. Transplant Proc. 2006;38:3263–3265. doi: 10.1016/j.transproceed.2006.10.125. [DOI] [PubMed] [Google Scholar]
- 36.Minami K, Murata K, Lee CY, Fox-Talbot K, Wasowska BA, Pescovitz MD, Baldwin WM., 3rd C4d deposition and clearance in cardiac transplants correlates with alloantibody levels and rejection in rats. Am J Transplant. 2006;6:923–932. doi: 10.1111/j.1600-6143.2006.01281.x. [DOI] [PubMed] [Google Scholar]
- 37.Qian Z, Jakobs FM, Pfaff-Amesse T, Sanfilippo F, Baldwin WM., 3rd Complement contributes to the rejection of complete and class I major histocompatibility complex--incompatible cardiac allografts. J Heart Lung Transplant. 1998;17:470–478. [PubMed] [Google Scholar]
- 38.Nakashima S, Qian Z, Rahimi S, Wasowska BA, Baldwin WM., 3rd Membrane attack complex contributes to destruction of vascular integrity in acute lung allograft rejection. J Immunol. 2002;169:4620–4627. doi: 10.4049/jimmunol.169.8.4620. [DOI] [PubMed] [Google Scholar]
- 39.Lin T, Zhou W, Farrar CA, Hargreaves RE, Sheerin NS, Sacks SH. Deficiency of C4 from donor or recipient mouse fails to prevent renal allograft rejection. Am J Pathol. 2006;168:1241–1248. doi: 10.2353/ajpath.2006.050360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan BP, Harris CL. Complement therapeutics; history and current progress. Mol Immunol. 2003;40:159–170. doi: 10.1016/s0161-5890(03)00111-1. [DOI] [PubMed] [Google Scholar]
- 41.Siberil S, Dutertre CA, Fridman WH, Teillaud JL. FcgammaR: The key to optimize therapeutic antibodies? Crit Rev Oncol Hematol. 2007;62:26–33. doi: 10.1016/j.critrevonc.2006.12.003. Epub 2007 Jan 2019. [DOI] [PubMed] [Google Scholar]
- 42.Naito M, Hayashi S, Yoshida H, Nishikawa S, Shultz LD, Takahashi K. Abnormal differentiation of tissue macrophage populations in ‘osteopetrosis’ (op) mice defective in the production of macrophage colony-stimulating factor. Am J Pathol. 1991;139:657–667. [PMC free article] [PubMed] [Google Scholar]
- 43.Moll S, Pascual M. Humoral rejection of organ allografts. Am J Transplant. 2005;5:2611–2618. doi: 10.1111/j.1600-6143.2005.01086.x. [DOI] [PubMed] [Google Scholar]
- 44.Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5:807–817. doi: 10.1038/nri1702. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu A, Colvin RB. Pathological features of antibody-mediated rejection. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:199–214. doi: 10.2174/1568006054064744. [DOI] [PubMed] [Google Scholar]
- 46.Vongwiwatana A, Tasanarong A, Hidalgo LG, Halloran PF. The role of B cells and alloantibody in the host response to human organ allografts. Immunol Rev. 2003;196:197–218. doi: 10.1046/j.1600-065x.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 47.Lorenz M, Regele H, Schillinger M, Exner M, Rasoul-Rockenschaub S, Wahrmann M, Kletzmayr J, Silberhumer G, Horl WH, Bohmig GA. Risk factors for capillary C4d deposition in kidney allografts: evaluation of a large study cohort. Transplantation. 2004;78:447–452. doi: 10.1097/01.tp.0000128344.94808.03. [DOI] [PubMed] [Google Scholar]
- 48.Takemoto SK, Zeevi A, Feng S, Colvin RB, Jordan S, Kobashigawa J, Kupiec-Weglinski J, Matas A, Montgomery RA, Nickerson P, Platt JL, Rabb H, Thistlethwaite R, Tyan D, Delmonico FL. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004;4:1033–1041. doi: 10.1111/j.1600-6143.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 49.Wu JZ, Ogle CK, Ogle JD, Alexander JW. A comparison of hepatic, splenic, peritoneal and alveolar macrophages with respect to PGE2, TXB2, production and ADCC function. Prostaglandins Leukot Essent Fatty Acids. 1993;48:149–153. doi: 10.1016/0952-3278(93)90103-4. [DOI] [PubMed] [Google Scholar]
- 50.Tomita M, Yamamoto K, Kobashi H, Ohmoto M, Tsuji T. Immunohistochemical phenotyping of liver macrophages in normal and diseased human liver. Hepatology. 1994;20:317–325. [PubMed] [Google Scholar]
- 51.Tinckam KJ, Djurdjev O, Magil AB. Glomerular monocytes predict worse outcomes after acute renal allograft rejection independent of C4d status. Kidney Int. 2005;68:1866–1874. doi: 10.1111/j.1523-1755.2005.00606.x. [DOI] [PubMed] [Google Scholar]
- 52.Dankof A, Schmeding M, Morawietz L, Gunther R, Krukemeyer MG, Rudolph B, Koch M, Krenn V, Neumann U. Portal capillary C4d deposits and increased infiltration by macrophages indicate humorally mediated mechanisms in acute cellular liver allograft rejection. Virchows Arch. 2005;447:87–93. doi: 10.1007/s00428-005-1245-z. Epub 2005 Jun 2010. [DOI] [PubMed] [Google Scholar]
- 53.Fahim T, Bohmig GA, Exner M, Huttary N, Kerschner H, Kandutsch S, Kerjaschki D, Brambock A, Nagy-Bojarszky K, Regele H. The cellular lesion of humoral rejection: predominant recruitment of monocytes to peritubular and glomerular capillaries. Am J Transplant. 2007;7:385–393. doi: 10.1111/j.1600-6143.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- 54.Rahimi S, Qian Z, Layton J, Fox-Talbot K, Baldwin WM, 3rd, Wasowska BA. Non-complement- and complement-activating antibodies synergize to cause rejection of cardiac allografts. Am J Transplant. 2004;4:326–334. doi: 10.1111/j.1600-6143.2004.00334.x. [DOI] [PubMed] [Google Scholar]
- 55.Wasowska BA, Qian Z, Cangello DL, Behrens E, Tran KV, Layton J, Sanfilippo F, Baldwin WM. Passive transfer of alloantibodies restores acute cardiac rejection in IgKO mice. Transplantation. 2001;71:727–736. doi: 10.1097/00007890-200103270-00007. [DOI] [PubMed] [Google Scholar]
- 56.Qian Z, Wasowska BA, Behrens E, Cangello DL, Brody JR, Kadkol SS, Horwitz L, Liu J, Lowenstein C, Hess AD, Sanfilippo F, Baldwin WM., 3rd C6 produced by macrophages contributes to cardiac allograft rejection. Am J Pathol. 1999;155:1293–1302. doi: 10.1016/S0002-9440(10)65231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]