Abstract
The clinical course of five patients with partial dearterialization of their hepatic allografts is described. One patient died and three others suffered serious morbidity as a direct or indirect result of this complication. Partial dearterialization of the liver allograft is a serious and potentially life-threatening complication for which preservation of the complete hepatic arterial supply is important, even if this requires reconstruction of the aberrant vessels.
Keywords: Liver transplantation, partial dearterialization, Arterial supply, in liver transplantation
The arterial blood supply of the liver is extremely variable, and the interruption of the hepatic artery is a feared complication in hepatobiliary or pancreatic surgical procedures, as well as in liver transplantation [2, 7, 10, 11]. Although the clinical consequences of complete dearterialization of an allograft in orthotopic liver transplantation (OLTx) are well documented [10], little is known about the behavior of a partially dearterialized liver graft. We studied the clinical course of five patients who were known to have had this complication in order to clarify its significance.
Patients and results
Case 1
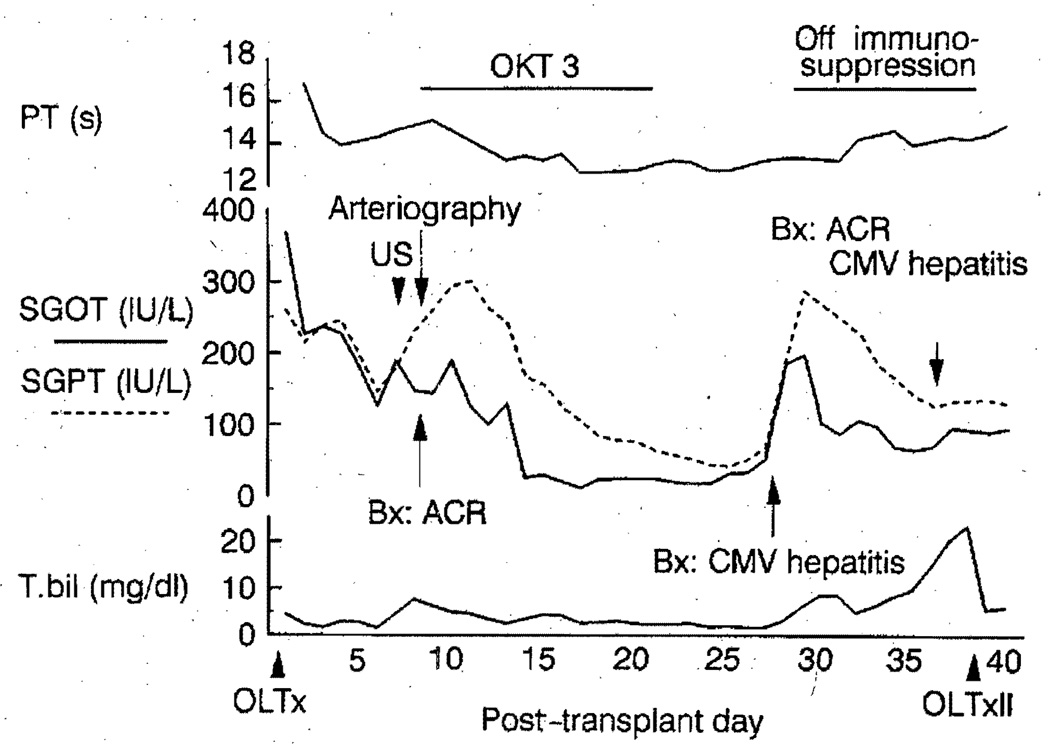
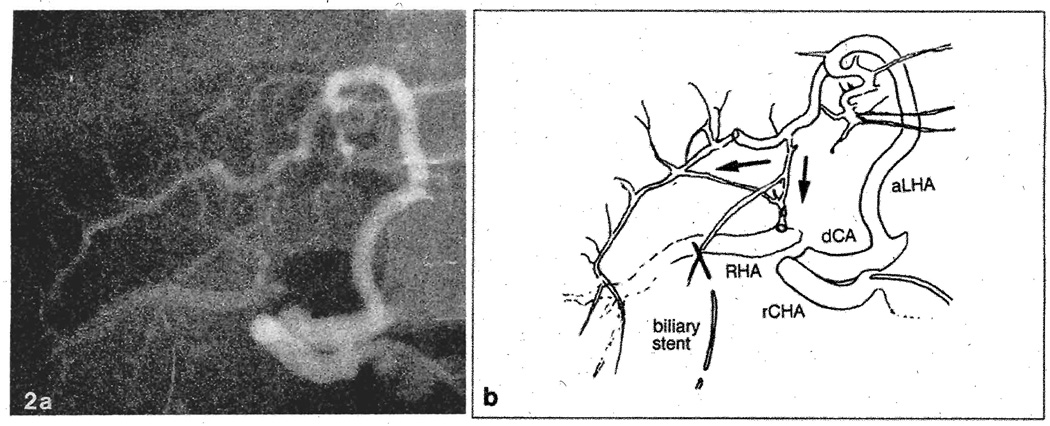
A 20-year-old male underwent OLTx for sclerosing cholangitis. The graft had an aberrant left hepatic artery (HA) originating from the left gastric artery (LGA) and a proper HA that supplied the right hepatic lobe. Figure 1 demonstrates the postoperative course. On day 7 post-transplantation, he underwent Doppler ultrasonography of the graft, which failed to demonstrate arterial flow. Emergency hepatic arteriography revealed thrombosis of the proper HA, which was reconstituted through intrahepatic collaterals from the aberrant left HA (Fig. 2). A needle liver biopsy at that time revealed acute cellular rejection, for which OKT3 was administered for 2 weeks. The patient developed cytomegalovirus (CMV) hepatitis and subsequently required retransplantation. The failed allograft revealed no evidence of hepatic abscesses or lobar ischemia. The patient was discharged 3 months after the initial transplant and is doing well.
Fig. 1.
Clinical course of case 1 after orthotopic liver transplantation. Studies performed for a rise in serum liver chemistries revealed thrombosis of the right hepatic artery and acute cellular rejection. Discontinuation of immunosuppression for cytomegalovirus hepatitis resulted in recurrent and irreversible acute cellular rejection, which necessitated retransplantation, PT, Prothrombin time; T. bil, total serum bilirubin, oLTx, orthotopic liver transplantation; US, ultrasonography; Bx, percutaneous liver biopsy; ACR, acute cellular rejection; CMV, cytomegalovirus; OLTxII, second orthotopic liver transplantation
Fig. 2.
a Selective hepatic arteriogram of case 1 on post-transplant day 7. b Schematic view of the arteriogram. The right hepatic artery is thrombosed at its origin from the proper hepatic artery. The arterial flow to the right hepatic lobe is reconstituted through intrahepatic collaterals from the aberrant left hepatic artery originating from the left gastric artery. rCHA, Recipient common hepatic artery; dCA, donor celiac axis; RHA, right hepatic artery; aLHA, aberrant left hepatic artery
Case 2
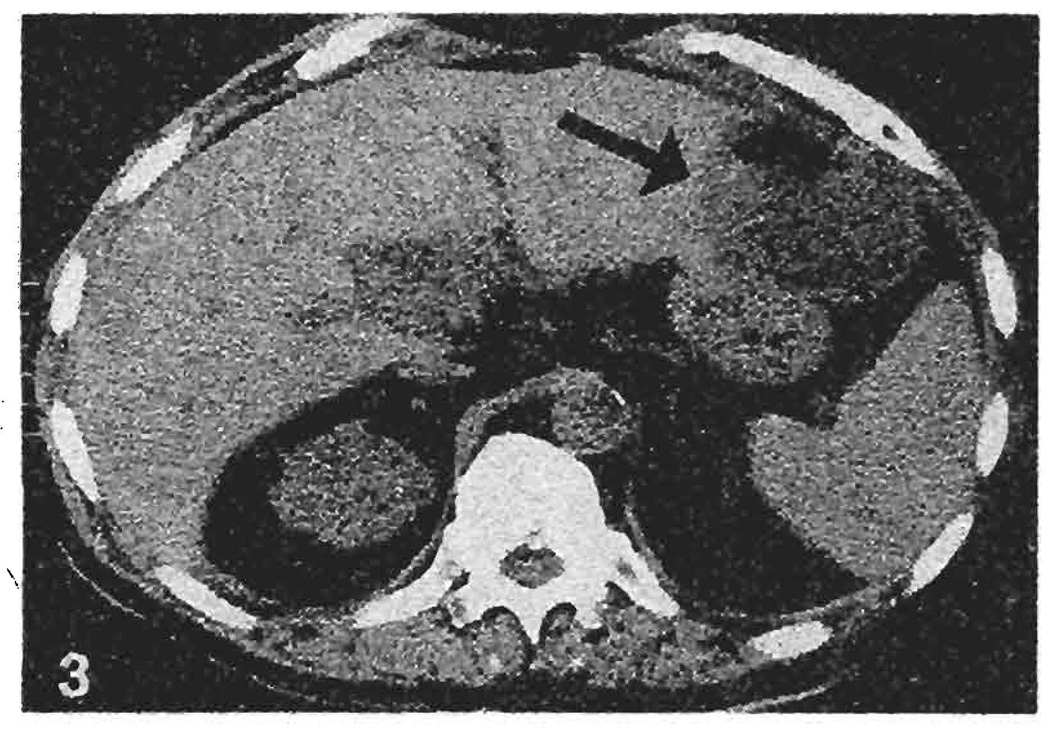
A 62-year-old male with postnecrotic cirrhosis underwent OLTx. The aberrant left HA of the allograft originating from the LGA was erroneously injured during organ procurement and was ligated. His postoperative course was complicated by systemic bacterial and CMV infection. At 6 months post-transplantation, he developed a fever. Computed tomography revealed a 6.0 × 6.0 cm collection with an air-fluid level in the left lateral segment of the hepatic graft (Fig. 3), which was drained. The aspirate grew enterococcus and diphtheloids, for which appropriate antibiotics were given. The main HA was patent on Doppler ultrasonographs, and a percutaneous transhepatic cholangiogram was normal. The drainage catheter was removed 47 days later, when the cavity collapsed completely. He remains alive and well 13 months later.
Fig. 3.
Computed tomogram of case 2 at 6 months following orthotopic liver transplantation. An intrahepatic fluid collection with an air-fluid level measuring 6.0 × 6.0 cm is visible in the left lateral segment of the transplant liver (arrow). Aspiration of the collection yielded 120 cc of pus, which grew enterococcus and diphtheloids
Case 3
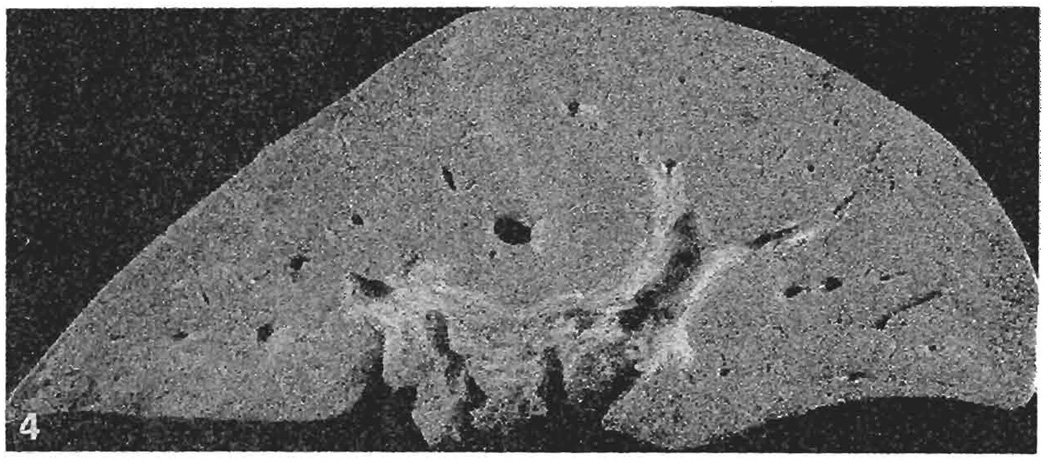
A 31-year-old male underwent uneventful OLTx for postnecrotic cirrhosis due to hepatitis B. The allograft had a small aberrant left HA originating from the LGA and a proper HA from the common HA, both of which exhibited good pulsation following arterial revascularization. The liver allograft exhibited primary nonfunction. OKT3 was started on postoperative day 1. Retransplantation was performed on day 4. The aberrant left HA was thrombosed and a clear demarcation line was demonstrated between the two hepatic lobes (Fig. 4). Histology of the failed allograft exhibited hepatocellular atrophy in the left lobe and diffuse extensive periportal necrosis. The postoperative course was complicated by a bile leak as well as by mediastinitis and empyema thorax, due to esophageal perforation from the preoperative sclerotherapy. The patient died 45 days later.
Fig. 4.
Coronal section of the failed allograft in case 3. A distinct demarcation line is visible between the two hepatic lobes
Case 4
A 43-year-old male with Laennec’s cirrhosis underwent OLTx using a graft with a transected, small, aberrant left HA originating from the LGA. The aberrant artery was ligated. His postoperative course was essentially unremarkable except for an episode of liver dysfunction, for which OKT3 was given for a clinical impression of acute cellular rejection starting on day 7 post-transplantation. The patient was discharged 23 days post-transplantation and remains a febrile and well 11 months later.
Case 5
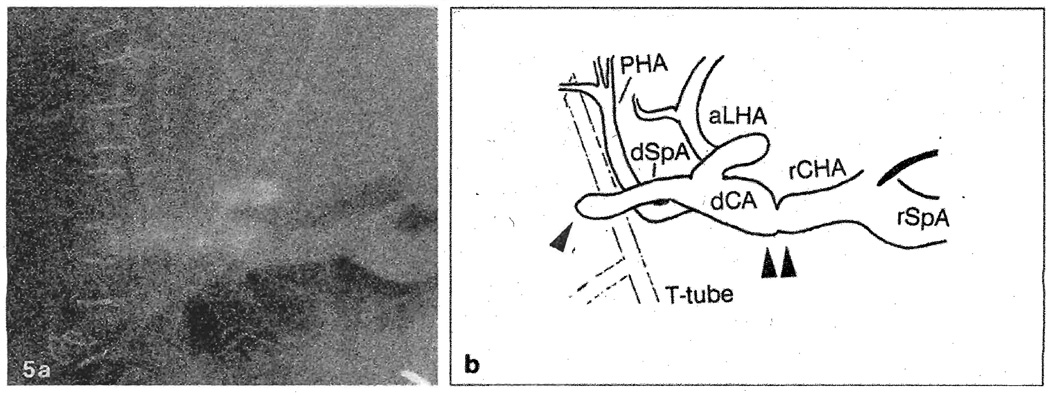
A 29-year-old male underwent OLTx for postnecrotic cirrhosis due to non-A, non-B hepatitis. The allograft had a triple arterial supply with an aberrant left HA originating from the LGA, an aberrant right HA from the superior mesenteric artery, and a middle artery from the normal origin. The aberrant right HA was anastomosed end-to-end to the donor splenic artery [6]. After revascularization, pulsation of each branch was thought to be adequate. On post-transplant day 3, emergency arteriography was performed for absent arterial Doppler signals. There was thrombosis of the aberrant right HA distal to its anastomosis to the donor splenic artery, and the main HA anastomosis was strictured (Fig. 5). No arterial perfusion of the posterior segment was identified. The patient underwent retransplantation on post-transplant day 9 due to sudden graft failure, when all branches of the HA were thrombosed. The failed graft exhibited severe and diffuse centrilobular hemorrhagic necrosis. The patient was discharged on post-transplant day 52 in good condition and remains well.
Fig. 5.
a Selective hepatic arteriogram of case 5 on post-transplant day 3. b Schematic view of the arteriogram; The aberrant right hepatic artery is thrombosed at its anastomosis site with the donor splenic artery (single arrow). There is also a stricture at the main anastomosis site (double arrow). rSpA, Recipient splenic artery; rCHA, recipient common hepatic artery; dCA, donor celiac axis; aLHA, aberrant left hepatic artery, dSpA, donor splenic artery; PHA, proper hepatic artery
All five patients received liver allografts of the same blood type and were given immunosuppression with cyclosporin and corticosteroids. Four of the five patients received OKT3 treatment for rejection, clinically suspected in two and biopsy-proven in the other two. Three of the four developed systemic CMV infection after the treatment. In the two patients who had injury to the aberrant left HA originating from the LGA, backbleeding through the cut stump was thought to be satisfactory. No bile leak was noted from the partially dearterialized hepatic grafts.
Discussion
Clinical features of complete hepatic artery thrombosis (HAT) in native and transplant livers are quite different. In native livers, intact ligamentous attachments allow rapid development of various arterial collaterals [1, 4]. On the other hand, the hepatic allograft is connected to the recipient with vascular and biliary anastomoses, and HAT after OLTx results in rapid formation of a graft gangrene or delayed bile leak [10].
Partial dearterialization of the hepatic allograft was recognized in some of the first clinical trials of OLTx, children being particularly susceptible to this complication [8,9]. The syndrome of regional hepatic gangrene resulted often, with retention of good hepatic function in the face of the septic complication that caused death several months later.
In the group of five patients described herein, two exhibited what could be considered as specific complications of partial dearterialization of the hepatic allograft. In patient 2, intrahepatic abscess developed in the corresponding part of the hepatic graft. Patient 5 initially developed partial HAT, which was followed by thrombosis of the other HA branches, and the liver failed. In contrast, patients 1 and 4 apparently had adequate arterial collaterals to prevent ischemic hepatic necrosis or abscess formation. In patient 3, the partial dearterialization may or may not have contributed to primary graft nonfunction and the need for retransplantation.
Michels [7] reported in 1960 the incidence of hepatic arterial anomalies in autopsies of 200 cadavers. The incidence of aberrant right and left HA was 17% and 25%, respectively. Of these, half of the aberrant left and 24 of 34 aberrant right HA were the only arteries to the corresponding areas of the liver. In such cases, the interruption of the aberrant HA to a hepatic allograft would result in ischemic necrosis.
In 1983, Mays and Mays. [6] used arteriography to demonstrate the presence of intrahepatic collaterals in 20 patients who had selective ligation of the right, left, or common HA, contrary to anatomical investigations of cadaver livers in which all arteries have been reported as end-arteries [3, 6, 7]. Mays and Mays speculate that these contradicting findings in livers during life and after death might be explained by neurohumoral factors that would not be operative postmortem. However, their observation could merely be the demonstration of accessory, rather than replacing, aberrant HA [7]. In fact, Lurie [5], in 1987, described the capricious behavior of the liver with partial dearterialization of the aberrant left HA after gastric surgery and ascribed it to variations in the size and number of intrahepatic arterial anastomoses.
In a hepatic allograft, it is usually impossible to differentiate if the aberrant HA is replacing or accessory. The aberrant HA should, therefore, be preserved during procurement of the liver [11]. If it is injured or interrupted, an attempt should be made to reconstruct the artery.
Complete HAT can simulate acute cellular rejection (ACR) early after OLTx [10]. Partial dearterialization of the hepatic allograft could also mimic ACR and lead to an ill-advised increase in immunosuppression and consequent opportunistic infection, as occurred in most of our patients.
ACR itself has been known to be a contributing factor to the development of complete HAT [10]. Although merely speculative, ACR in case 1 could have contributed to the development of partial HAT.
Acknowledgements
This study was supported by research grant from the Veterans .Administration and by project grant no. AM 29961 from the National Institute of Health, Bethesda, Maryland.
References
- 1.Bengmark S, Rosengren K. Angiographic study of the collateral circulation to the liver after ligation of the hepatic artery in man. Am J Surg. 1970;119:620–624. doi: 10.1016/0002-9610(70)90228-x. [DOI] [PubMed] [Google Scholar]
- 2.Brittain RS, Marchioro TL, Hermann G, Waddell WR, Starzl TE. Accidental hepatic artery ligation in humans. Am J Surg. 1964;107:822–832. doi: 10.1016/0002-9610(64)90169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glauser F. Studies on intrahepatic arterial circulation. Surgery. 1953;33:333–341. [PubMed] [Google Scholar]
- 4.Koehler RE, Korobkin M, Lewis F. Arteriographic demonstration of collateral arterial supply to the liver after hepatic artery ligation. Radiology. 1975;117:49–54. doi: 10.1148/117.1.49. [DOI] [PubMed] [Google Scholar]
- 5.Lurie AS. The significance of the variant left accessory hepatic artery in surgery for proximal gastric cancer. Arch Surg. 1987;122:725–728. doi: 10.1001/archsurg.1987.01400180107021. [DOI] [PubMed] [Google Scholar]
- 6.Mays ET, II, Mays ET. Are hepatic arteries end-arteries? J Anat. 1983;137:637–644. [PMC free article] [PubMed] [Google Scholar]
- 7.Michels NA. Newer anatomy of liver-variant blood supply and collateral circulation. JAMA. 1960;172:125–132. doi: 10.1016/0002-9610(66)90201-7. [DOI] [PubMed] [Google Scholar]
- 8.Starzl TE, Putnam CW. Experience in hepatic transplantation. Philadelphia: Saunders; 1969. pp. 3pp. 130–133.pp. 174pp. 323–327. [Google Scholar]
- 9.Starzl TE, Groth CG, Brettschneider L, Penn I, Fulginiti VA, Moon JB, Blanchard H, Martin AJ, Jr, Porter KA. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392–415. doi: 10.1097/00000658-196809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzakis AG, Gordon RD, Shaw BW, Iwatsuki S, Starzl TE. Clinical presentation of hepatic artery thrombosis after liver transplantation in the cyclosporin era. Transplantation. 1985;40:667–671. doi: 10.1097/00007890-198512000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanaga K, Tzakis AG, Starzl TE. Personal experience with the procurement of 132 hepatic allografts. Transplant Int. 1989;2:137–142. doi: 10.1007/bf02414600. [DOI] [PMC free article] [PubMed] [Google Scholar]