During the last decade, newly acquired diabetes mellitus has been reported to follow kidney transplantation in 10% to 46% of the patients.1–5 Although prednisone was thought to be a major factor in azathioprine regimens long before the advent of cyclosporine (CyA),1,4,6 the diabetogenic influence of CyA has received much recent attention.2,3,5,7 Diabetogenic effects also have been ascribed to FK 506, both in animals8,9 and in humans.10–13 In this study, the changes in glucose metabolism were recorded in 72 liver transplant recipients who were changed from CyA to FK 506 therapy because of either uncontrolled rejection or toxicity of the conventional immunosuppressive regimens, or for both reasons.
MATERIALS AND METHODS
The original case list included all of the first 81 liver recipients changed from CyA to FK 506. Nine of these were excluded from the analysis herein reported because of previous total pancreatectomy (1), a prolonged need for total insulin-containing parenteral infusions (1), and multisystem failure with early death (7). Of the 72 patients who were included, 63 were adults: 39 were men and 24 women. The mean age of the adults were 44.5 ± 13.7 SD (range, 18 to 66). The nine children were 7.81 ± 6.23 SD years old (range, 0.8 to 17.5).
The patients were changed from CyA to FK 506 between March 1, 1989 and November 15, 1989: follow-ups continued until January 15, 1990. If the interval between liver transplantation and the institution of FK 506 therapy was less than 4 weeks, the patients were classified as having “early rescue” (Table 1). These recipients tended to be very ill because of severe acute and/or beginning chronic liver rejection, which had not responded to additional steroids and OKT3 therapy. The “late rescue” group faced retransplantation because of chronic rejection or because the patients had serious CyA or steroid toxicity (Table 1).
Table 1.
Indications and Outcome of Rescue Therapy in 72 Liver Allograft Recipients
| Early Rescue |
Late Rescue |
Total |
||||
|---|---|---|---|---|---|---|
| Indications | No. of Patients |
No. of Success |
No. of Patients |
No. of Success |
No. of Patients |
No. of Success |
| Chronic rejection | 2 | 1 | 31 | 22 | 33 | 23 |
| Acute rejection | 10 | 10 | 14 | 11 | 24 | 21 |
| Acute and chronic rejection | 0 | 0 | 2 | 2 | 2 | 2 |
| Chronic rejection and other | 0 | 0 | 3 | 2 | 3 | 2 |
| Acute rejection and other | 1 | 1 | 2 | 0 | 3 | 1 |
| CyA and/or steroid toxicity | — | — | 6 | 5 | 6 | 5 |
| Primary nonfunction | 1 | 0 | — | — | 1 | 0 |
| Total | 14 | 12 (85.7%) | 58 | 42 (72.4%) | 72 | 54(74.4%) |
The records of all patients were reviewed for past or present evidence of insulin-dependent diabetes mellitus (IDDM) or non–insulin-dependent diabetes mellitus (NIDDM). In recipients who did not require insulin therapy, NIDDM was diagnosed if three or more fasting blood sugars were found above 140 mg/dL. The nonparametric sign test was used to evaluate the meaning of changes in the diabetic classification of the patients before and after treatment with FK 506, using a significance level of P < .05 (SPSS, Statistical Software, Chicago, Ill).
In addition, for 10 weeks before and 17 weeks after the drug switch, correlations were made between the level of blood glucose, serum total bilirubin, alanine aminotransferase (ALT), serum creatinine, dosages of prednisone, CyA, and FK 506. Whole blood CyA levels were followed with TDx (Abbot Laboratories, Chicago) radioimmunoassay.14 FK 506 plasma levels were determined with the enzyme immunoassay technique of Tamura et al.15 but these were obtained only sporadically during this early phase of our rescue trials.
RESULTS
Outcome of Rescue Therapy
Rejection was present in 65 of the 72 (90%) patients (Table 1). In seven patients (9.7%) CyA and/or steroid toxicity was the reason for the switch. The original diagnosis of acute rejection in one patient was later revised to primary nonfunction. The 74.4% success rate of the rescue therapy for the entire group in the present study (Table 1) was 5% higher than previously reported for the same group of patients because the nine exclusions from the present study were included in the study of Fung et al.13 Seven of these nine were recorded as failed rescues.
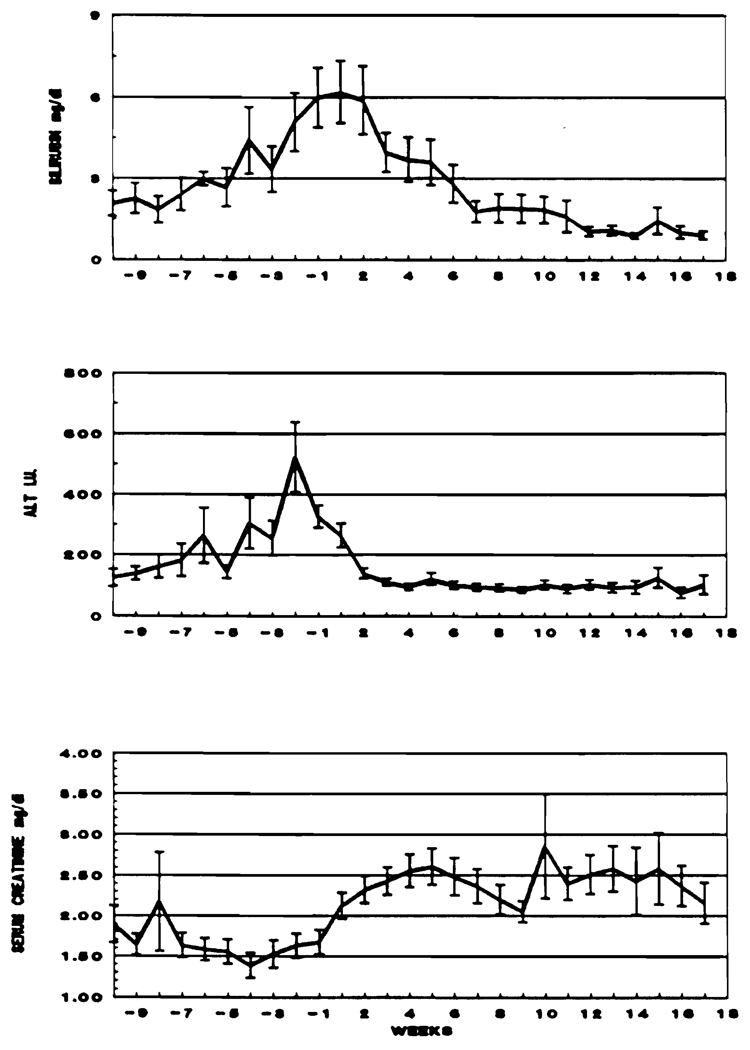
During the 10 weeks preceding the drug switch, serum bilirubin and ALT slowly rose from week −10 to the time of the FK 506 switch. This reflected the deterioration that provided the reason for changing therapy. After the switch, these values declined to or toward normal (Fig 1).
Fig 1.
Liver and kidney function during the 10 weeks preceding and the 17 weeks following rescue therapy in 72 liver allograft recipients.
The mean serum creatinine had begun a progressive rise before the FK 506 switch. This continued to increase for 10 weeks after the drug change, before a tendency to decline was noticed. This reversible deterioration of renal function preceding and following the FK 506 switch was similar to that described earlier.13,16
Incidence of Diabetes Before and After Drug Switch
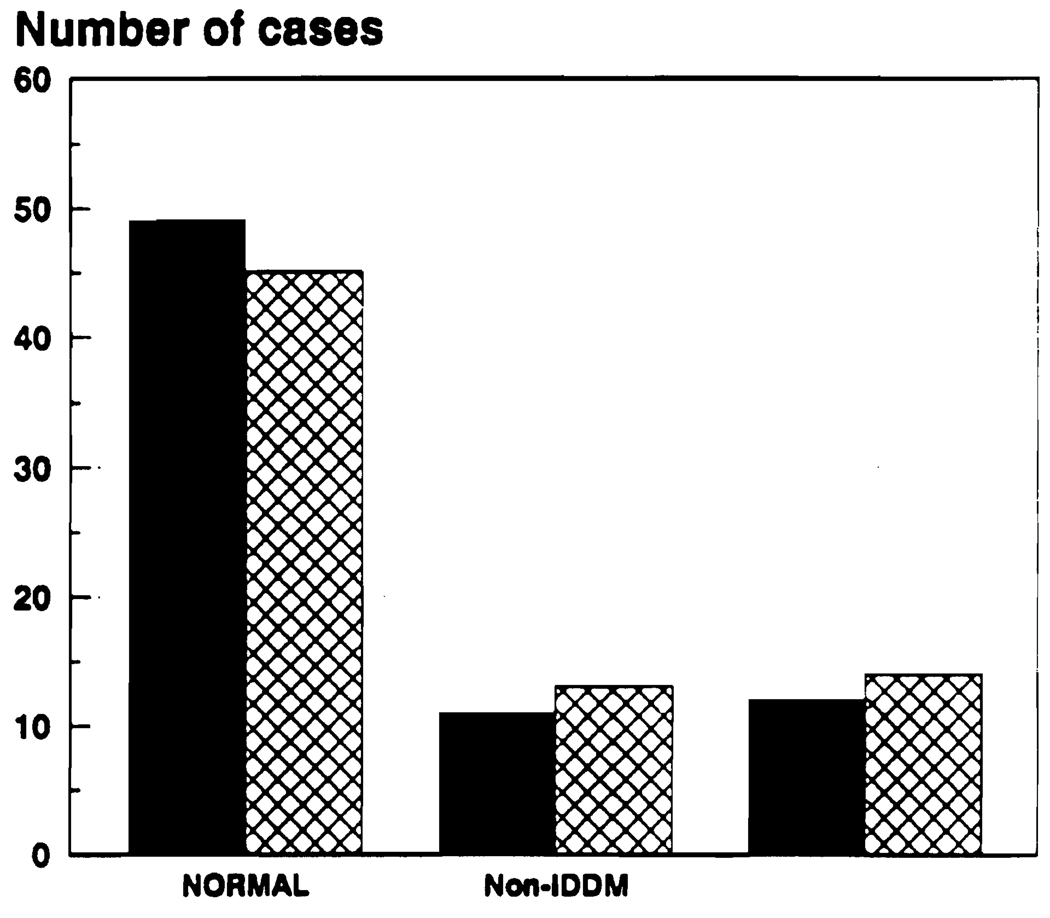
Overall, there was a small but not statistically significant (sign test P > .12) increase from 32% to 37.5% of impaired glucose metabolism (Fig 2). Before the drug change, 49 patients were classified as normal, 11 had NIDDM, and 12 had IDDM. Afterwards 45 were classified as normal, 13 had NIDDM, and 14 had IDDM.
Fig 2.
Diabetic status before and after treatment with FK 506, ■, before FK 506;  , after FK 506.
, after FK 506.
Correlations in the Nondiabetic Subgroup
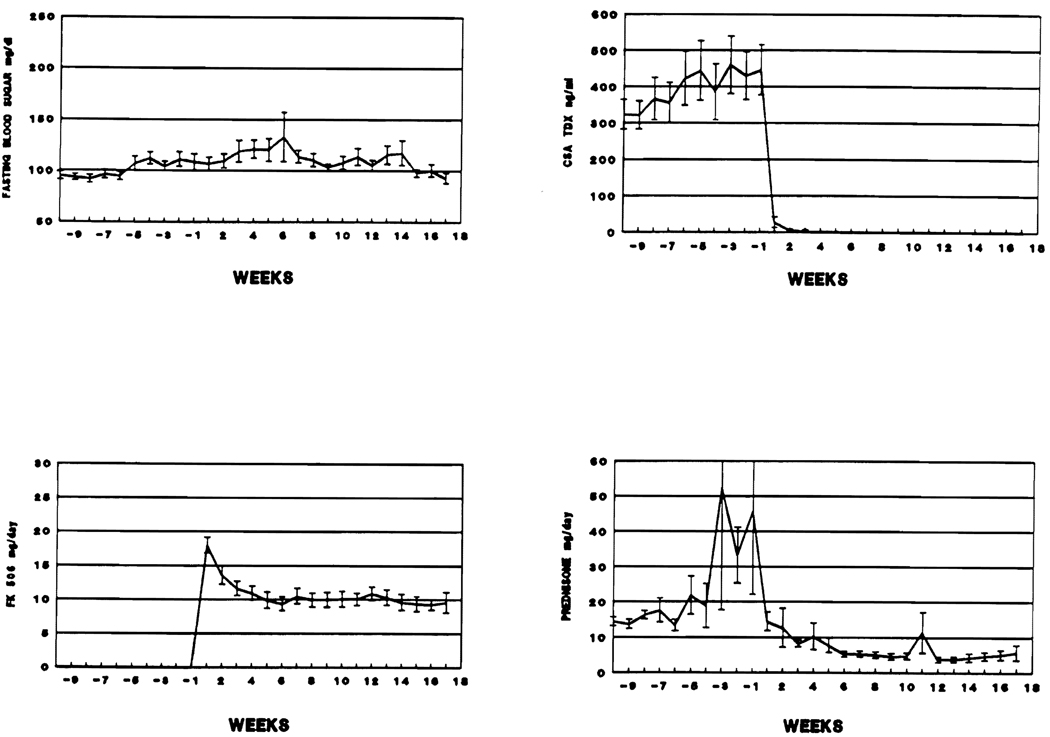
In the 49 patients who were classified as normal before the switch to FK 506, the average fasting blood sugar slowly increased from 95 mg/dL ± 3.86 SE on week −10 to 111 mg/dL ± 7.32 SE on week − 2, and then to 131 ± 24.25 SE on week +6. After this, there was a steady decline to 93 mg/dL ± 4.96 SE by week + 17. During the pre-switch interval there were also major increases in the average prednisone doses and less dramatic rises in CyA blood levels. This pattern reflected attempts to control rejection as the day of the drug switch approached. After patients were switched to FK 506, prednisone treatment was decreased and after week +6 the average daily dose was 5.37 mg ± 2.11 SE (Fig 3). Since the majority of patients require less FK 506 once rejection is controlled, the FK 506 doses were also progressively reduced.
Fig 3.
Immunosuppression and average fasting blood sugar, before and after the drug switch, in 49 nondiabetic patients.
Correlations in the NIDDM Subgroup
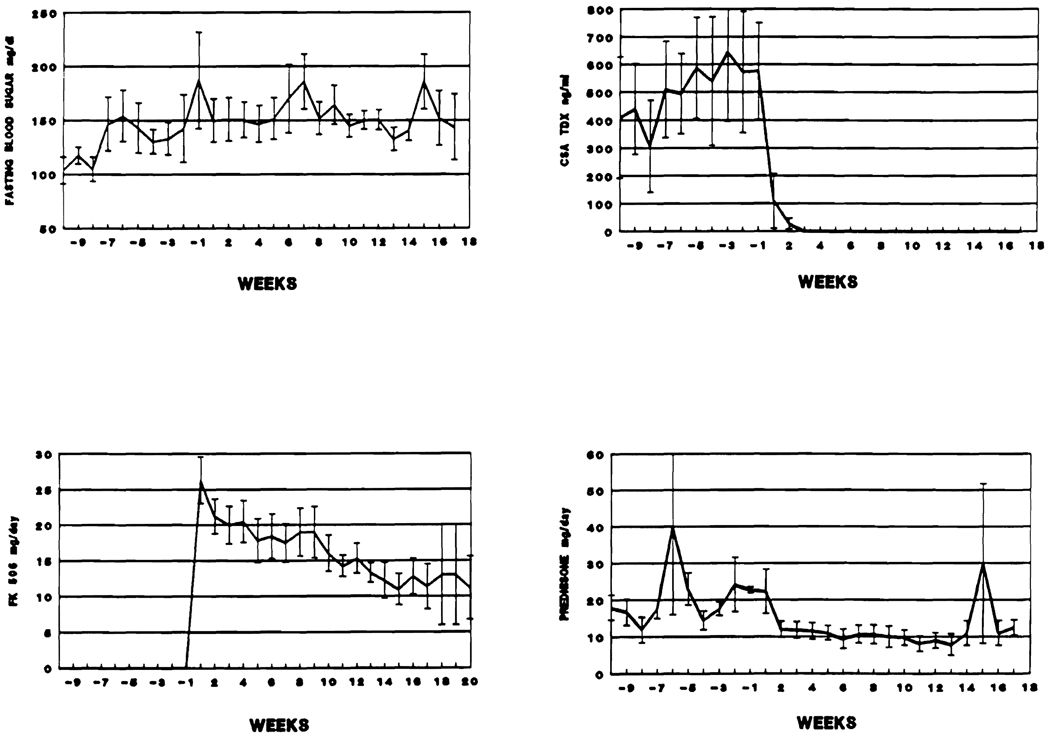
With increased conventional immunosuppression, the average fasting blood sugars in these 11 patients rose from 103.6 mg/dL ± 12.24 SE to 187 ± 44.81 SE during the 10 weeks preceding FK 506 therapy. These remained high thereafter (Fig 4). Insulin was given to six of these patients for several days before and/or after the drug change, and in one the need for insulin eventually became permanent.
Fig 4.
Immunosuppression and average fasting blood sugar, before and after the drug switch, in the 11 NIDDM diabetics.
Correlations in the IDDM Subgroup
The same pattern of increasing mean blood glucose levels was seen in association with increasing conventional immunosuppression leading to FK 506. Multiple adjustments of daily insulin made further correlations impossible.
DISCUSSION
It is well known that whole organ recipients are susceptible to new onset diabetes in the postoperative period. When azathioprine and prednisone were used together in kidney recipients, the incidence of diabetes seemed related to the quantities of steroids that were required for rejection control.1,4,6 This same association was noted using CyA,2,3 but with growing realization that CyA is itself intrinsically diabetogenic.2,7,17–20 CyA inhibits the release of insulin,19 may cause permanent structural and functional damage to animals,21 and also has been reported to increase peripheral insulin resistance.22
There have been no previous studies of the incidence and features of diabetes mellitus after liver transplantation. Of the 72 liver recipients under CyA-steroid therapy herein reported, 23 (32%) already had diabetes when they entered the study and 12 (17%) were being given insulin daily. This rate is comparable with that after kidney transplantation.1–5 In treating these patients, we attempted to define the effect, if any, a change from CyA to FK 506 would have on this preexisting complication, and to see if there would be new examples of diabetes.
The analysis was complicated by the increased immunosuppression (especially with prednisone) that was given prior to the switch in an effort to treat hepatic graft dysfunction. Presumably, this change contributed to the increases in blood sugar and rises in serum creatinine that were noted before and after the institution of FK 506. Reversal of these undesirable changes required weeks or months after the switch. However, the basic diabetic profile changed only minimally. Most of the patients who did not have diabetes remained that way and patients who already had diabetes remained so. One patient who was insulin free before the switch required this treatment permanently afterwards. In the meanwhile, the central objectives of salvaging the liver grafts or ameliorating drug toxicity were met in three fourths of the patients.
The mechanism of either CyA or FK 506 toxicity is not known, but a plausible hypothesis can be advanced from recent advances in enzyme chemistry. Cyclophilin, the ubiquitously distributed binding site of CyA is rich in peptidyl propyl isomerase (PPIase).23,24 an enzyme that facilitates protein folding and the catalysis of oligopeptide bonds.25 The cytosolic binding site of FK 506 also contains PPIase.26,27 Because both CyA and FK 506 cause strikingly similar immunologic as well as metabolic alterations of multiple organ systems, it has been speculated that these chemically unrelated drugs act through PPIase inhibition.28,29 If this were true, FK 506 also should be diabetogenic, and we have reported clinical evidence to support this.10–13 Studies of cultured rodent and human islets have been confirmatory.30,31 Although the experimental studies have demonstrated a better therapeutic to toxic ratio for FK 506, the differences between the drugs were not fundamental.
At a practical level, it is clear that double diabetogenic jeopardy is the price for using either CyA or FK 506 in combination with prednisone. The balance should be favorably altered if the use of steroids can be minimized as usually is possible with FK 506.11–13 In the study herein reported, the diabetes problem was peripheral to the main purpose of salvaging rejecting liver grafts or ameliorating toxicity from immunosuppression regimens.
Further basic and clinical investigations of the diabetogenic effects of FK 506 will have implications beyond the field of transplantation. The potency and safety of this drug are great enough to envision its use as the sole therapy for a variety of T-cell mediated autoimmune disorders including insulin-dependent diabetes. BB rat diabetes, which is analogous to type I human diabetes, can be prevented completely with nontoxic doses of FK 50632 the same way as previously demonstrated with CyA.33 What remains to be determined fully is the clinical liability of such therapy. An encouraging note is that the previously unattainable objective of human pancreatic islet cell transplantation has been achieved with FK 506 in several patients of whom one is insulin free more than 6 months later.34
Acknowledgments
Supported by research grants from the Veterans Administration and project grant no. DK 29961 from the National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.David DS, Cheigh JS, Braun DW, et al. JAMA. 1980;243:532. [PubMed] [Google Scholar]
- 2.Yoshimura N, Nakai I, Ohmori Y, et al. Am J Kidney Dis. 1988;12:11. doi: 10.1016/s0272-6386(88)80065-9. [DOI] [PubMed] [Google Scholar]
- 3.Boudreaux JP, McHugh L, Canafax DM, et al. Transplantation. 1987;44:376. doi: 10.1097/00007890-198709000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Gunnarsson R, Lundgren G, Magnusson G, et al. Scand J Urol Nephrol. 1980;54:135. [PubMed] [Google Scholar]
- 5.Roth D, Milgrom M, Esquenzai V, et al. Transplantation. 1989;47:278. [PubMed] [Google Scholar]
- 6.Starzl TE. Experience in Renal Transplantation. Philadelphia: WB Saunders; 1964. pp. 117–161.pp. 221 [Google Scholar]
- 7.Ost L, Tyden T, Fehrman I. Transplantation. 1988;370:46. doi: 10.1097/00007890-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Nalesnik MA, Todo S, Murase N, et al. Transplant Proc. 1987;19:79. [PMC free article] [PubMed] [Google Scholar]
- 9.Collier DSJ, Calne R, Thiru S, et al. Transplant Proc. 1987;19:3975. [PubMed] [Google Scholar]
- 10.Mieles L, Todo S, Fung JJ, et al. Transplant Proc. 1990;22:41. [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE, Fung J, Jordan M, et al. JAMA. 1990;264:63. [PMC free article] [PubMed] [Google Scholar]
- 12.Todo S, Fung JJ, Starzl TE, et al. Ann Surg. 1990;212:245. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung JJ, Todo S, Tzakis A, et al. Transplantation. (in press) [Google Scholar]
- 14.Schroeder TJ, Brunson ME, Pesce AJ, et al. Transplantation. 1989;47:262. doi: 10.1097/00007890-198902000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Tamura K, Kobayashi M, Hasimoto K, et al. Transplant Proc. 1987;19 suppl 6:23. [PubMed] [Google Scholar]
- 16.McCauley J, Fung JJ, Jain A, et al. Transplant Proc. 1990;22:17. [PMC free article] [PubMed] [Google Scholar]
- 17.Engfeldt P, Tyden G, Gunnarsson R, et al. Transplant Proc. 1986;18:65. [Google Scholar]
- 18.Yagisawa T, Takahashi K, Teraoka S, et al. Transplant Proc. 1986;18:1548. [Google Scholar]
- 19.Alejandro R, Feldman EC, Bloom AD, et al. Diabetes. 1989;38:698. doi: 10.2337/diab.38.6.698. [DOI] [PubMed] [Google Scholar]
- 20.Stegall MD, Chabot J, Weber C, et al. Transplantation. 1989;48:944. [PubMed] [Google Scholar]
- 21.Holmechan U, Schmidt WE, Siegel FG, et al. Diabetologia. 1984;27:416. doi: 10.1007/BF00304861. [DOI] [PubMed] [Google Scholar]
- 22.Yale JB, Chamelian M, Courchosno S, et al. Transplant Proc. 1988;20:985. [PubMed] [Google Scholar]
- 23.Takahashi N, Hayano T, Suzuki M. Nature. 1989;337:473. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- 24.Fischer G, Wittmann-Liebold B, Lang K, et al. Nature. 1989;337:476. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- 25.Fischer G, Bang H. Biochim Biophys Acta. 1984;828:39. doi: 10.1016/0167-4838(85)90006-8. [DOI] [PubMed] [Google Scholar]
- 26.Siekierka JJ, Hung SHY, Poe M, et al. Nature. 1989;341:755. doi: 10.1038/341755a0. [DOI] [PubMed] [Google Scholar]
- 27.Harding MW, Galat A, Uehling DE, et al. Nature. 1989;341:758. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- 28.Starzl TE, Fung JJ, Todo S. JAMA. 1990;263:2686. [PMC free article] [PubMed] [Google Scholar]
- 29.Starzl TE, Porter KA, Mazzaferro V, et al. Transplantation. doi: 10.1097/00007890-199101000-00010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tze WJ, Tai J, Cheung S. Transplantation. 1989;49:1172. doi: 10.1097/00007890-199006000-00030. [DOI] [PubMed] [Google Scholar]
- 31.Carroll PB, Boschero AC, Li MY, et al. Transplantation. (in press) [Google Scholar]
- 32.Murase N, Lieberman I, Nalesnik M, et al. Lancet. 1990;336:373. doi: 10.1016/0140-6736(90)91913-u. [DOI] [PubMed] [Google Scholar]
- 33.Stiller CR, Laupacis A, Keowyn PA, et al. Metabolism. 1983;32 suppl 1:69. doi: 10.1016/s0026-0495(83)80014-6. [DOI] [PubMed] [Google Scholar]
- 34.Tzakis AG, Ricordi C, Alejandro R, et al. Lancet. 1990;336:402. doi: 10.1016/0140-6736(90)91946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]